A Magnetorheological Fluids-Based Robot-Assisted Catheter/Guidewire Surgery System for Endovascular Catheterization
Abstract
1. Introduction
1.1. Related Work
1.2. Our Contribution
2. System Description
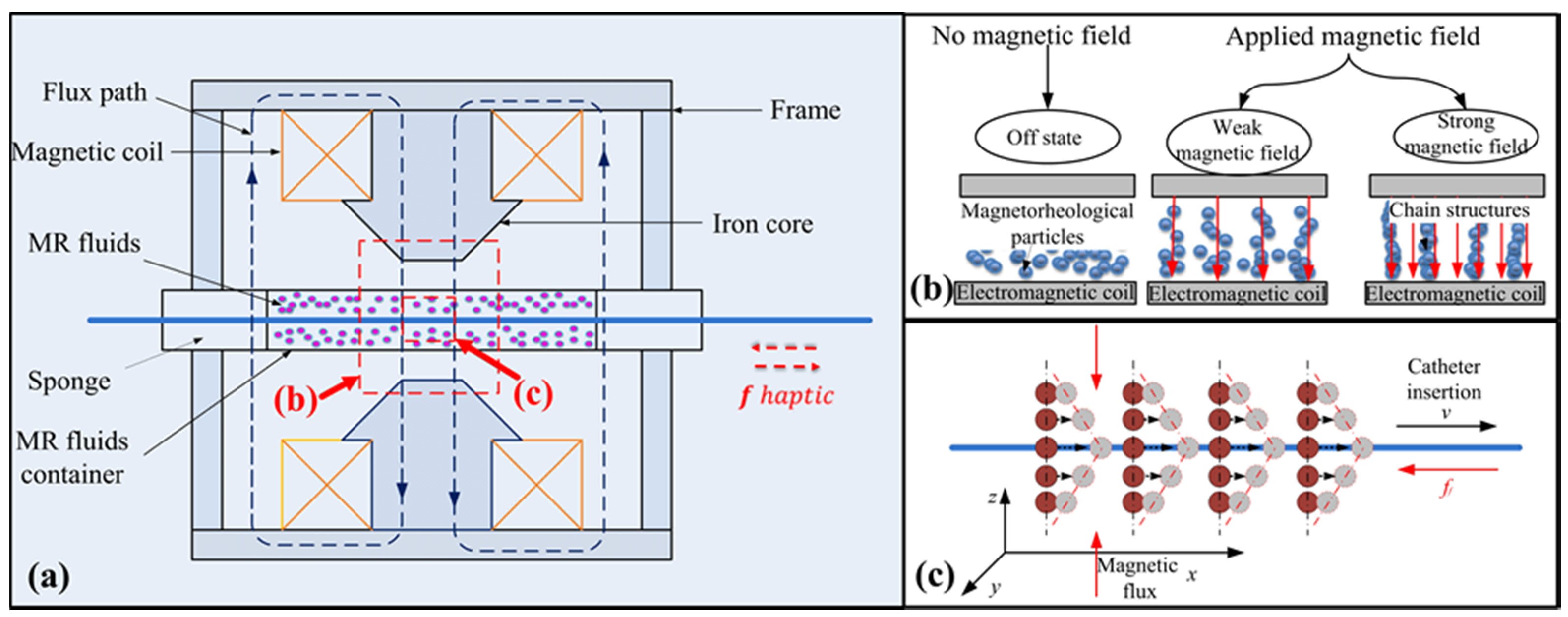
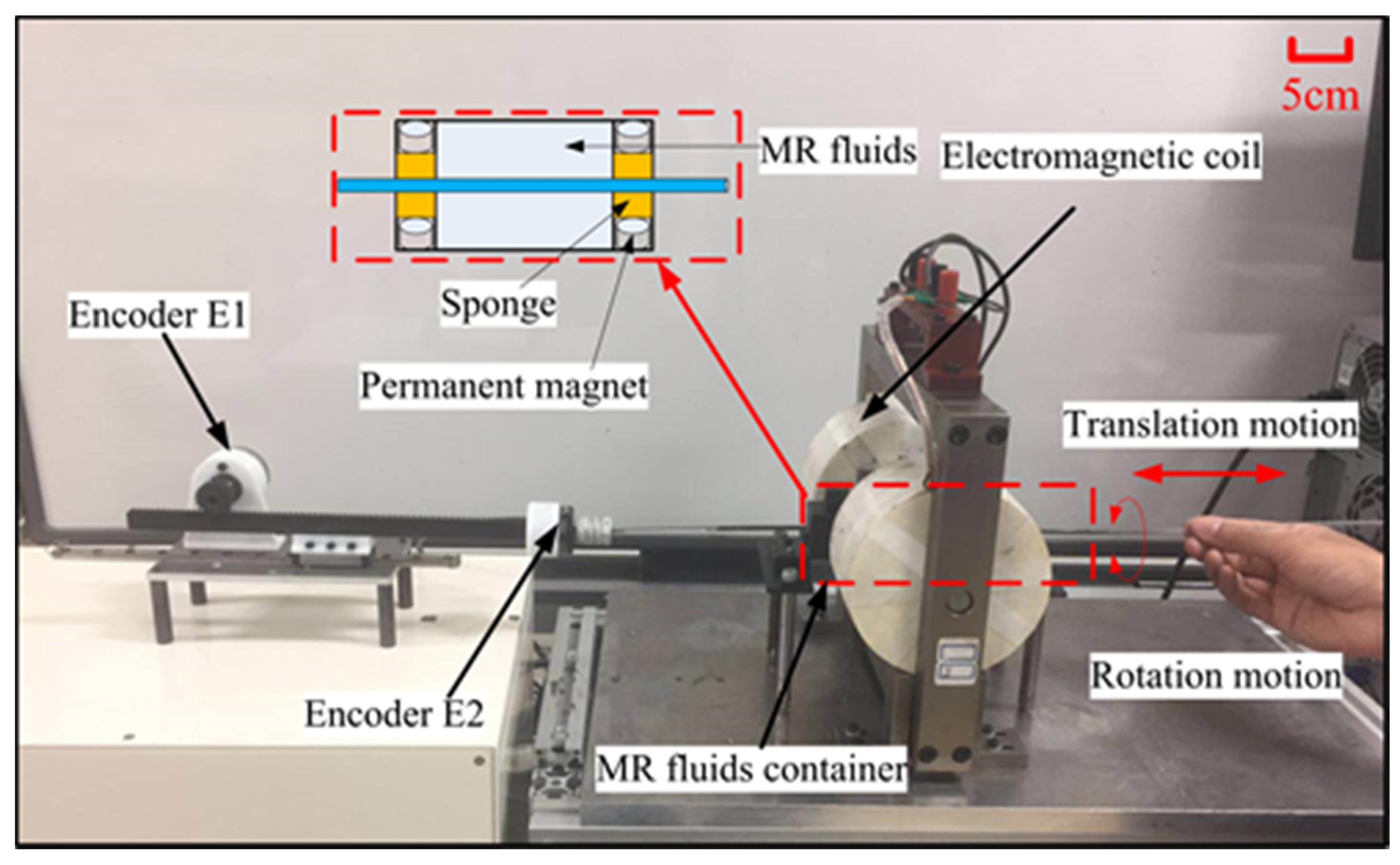
2.1. Design of the Master Haptic Interface
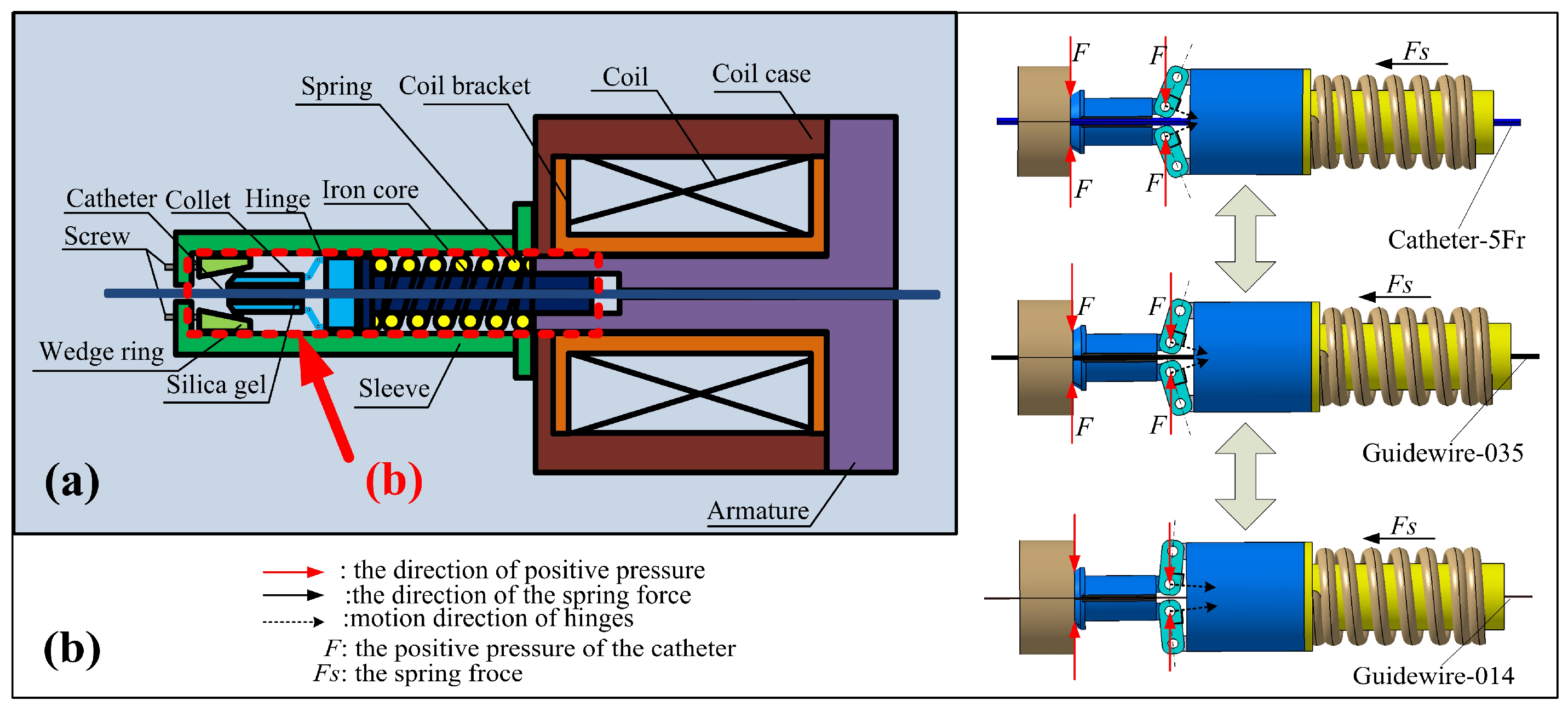
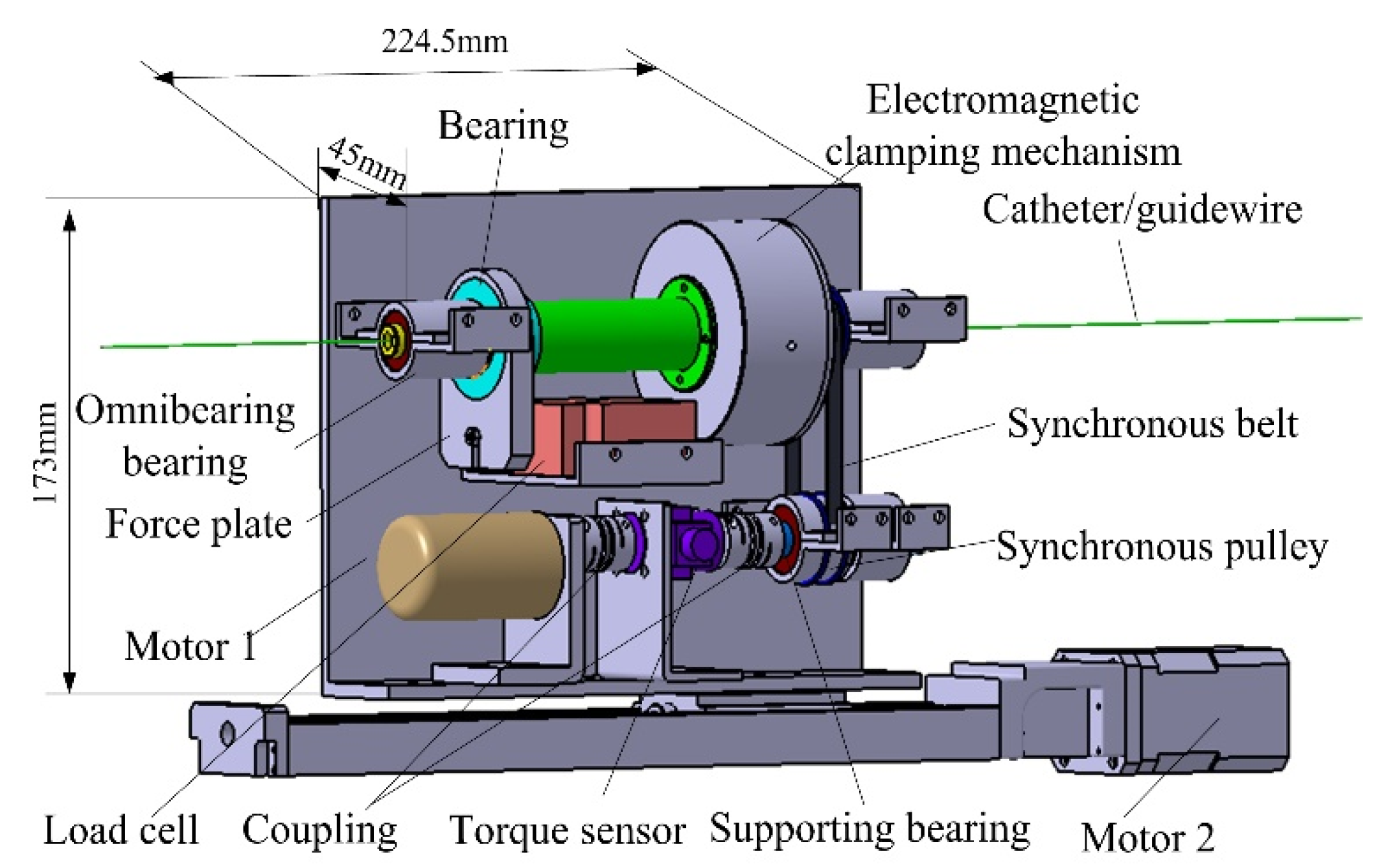
2.2. Design of the Slave Manipulator
3. Safety Mechanism
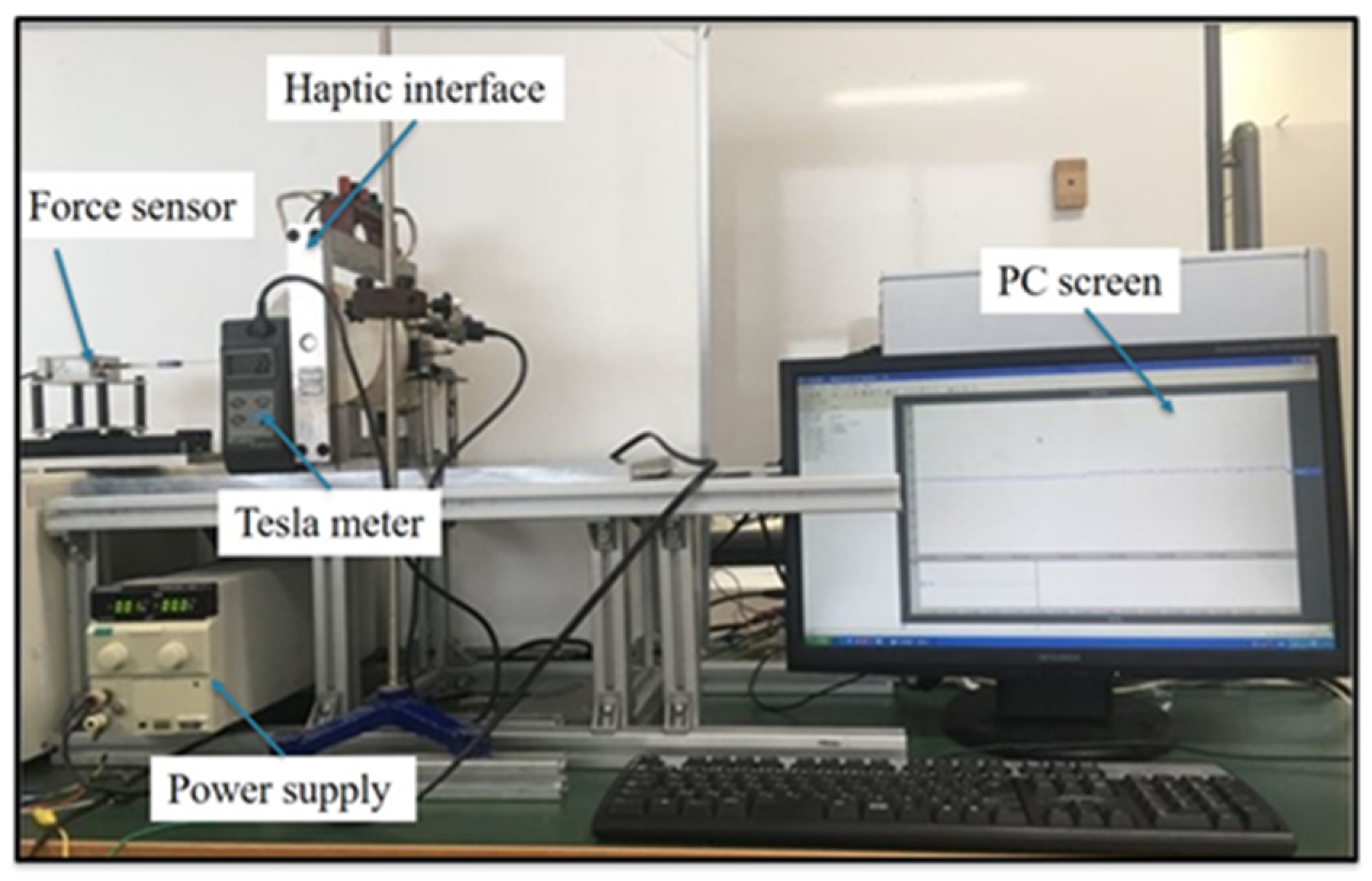
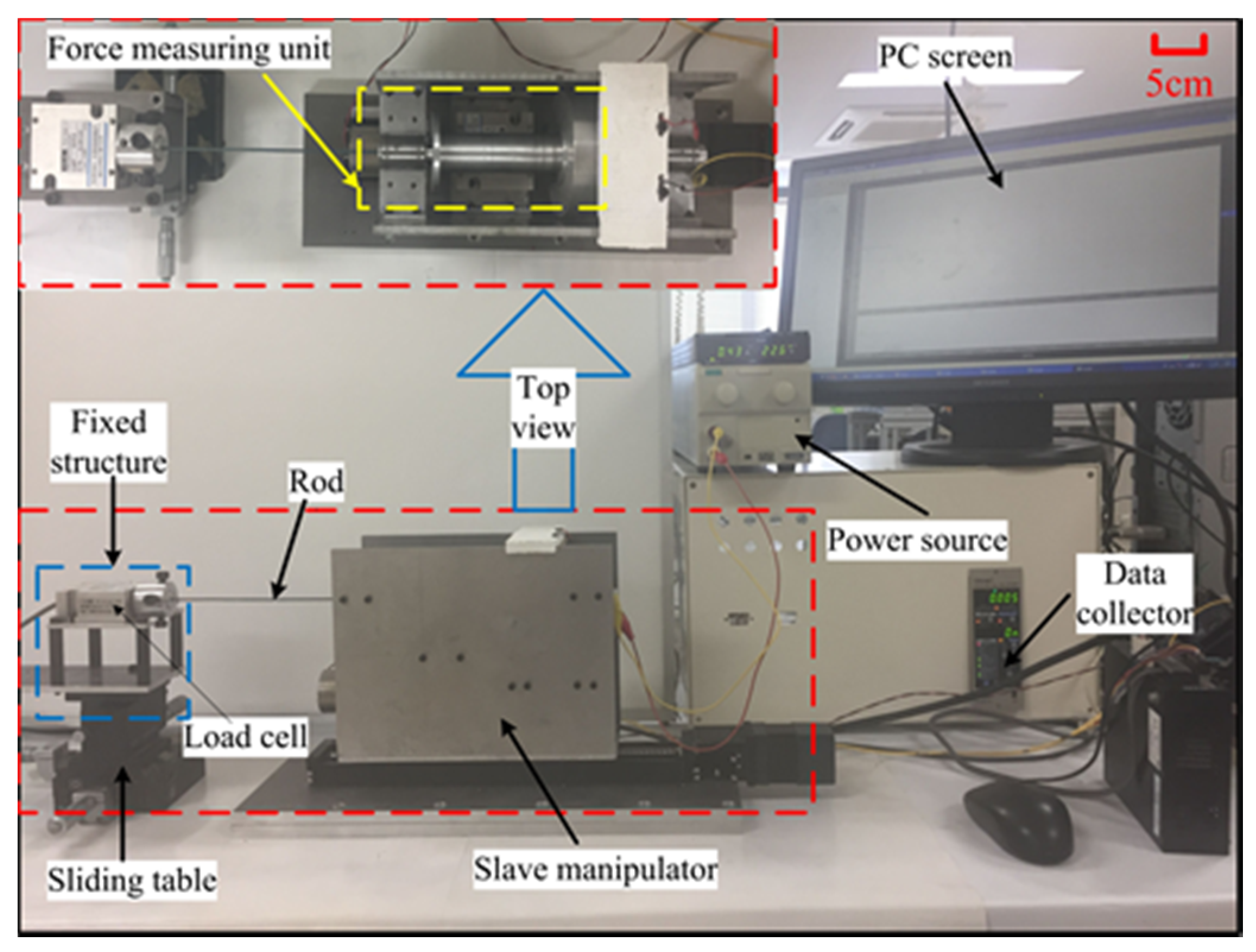
4. Experimental Evaluation
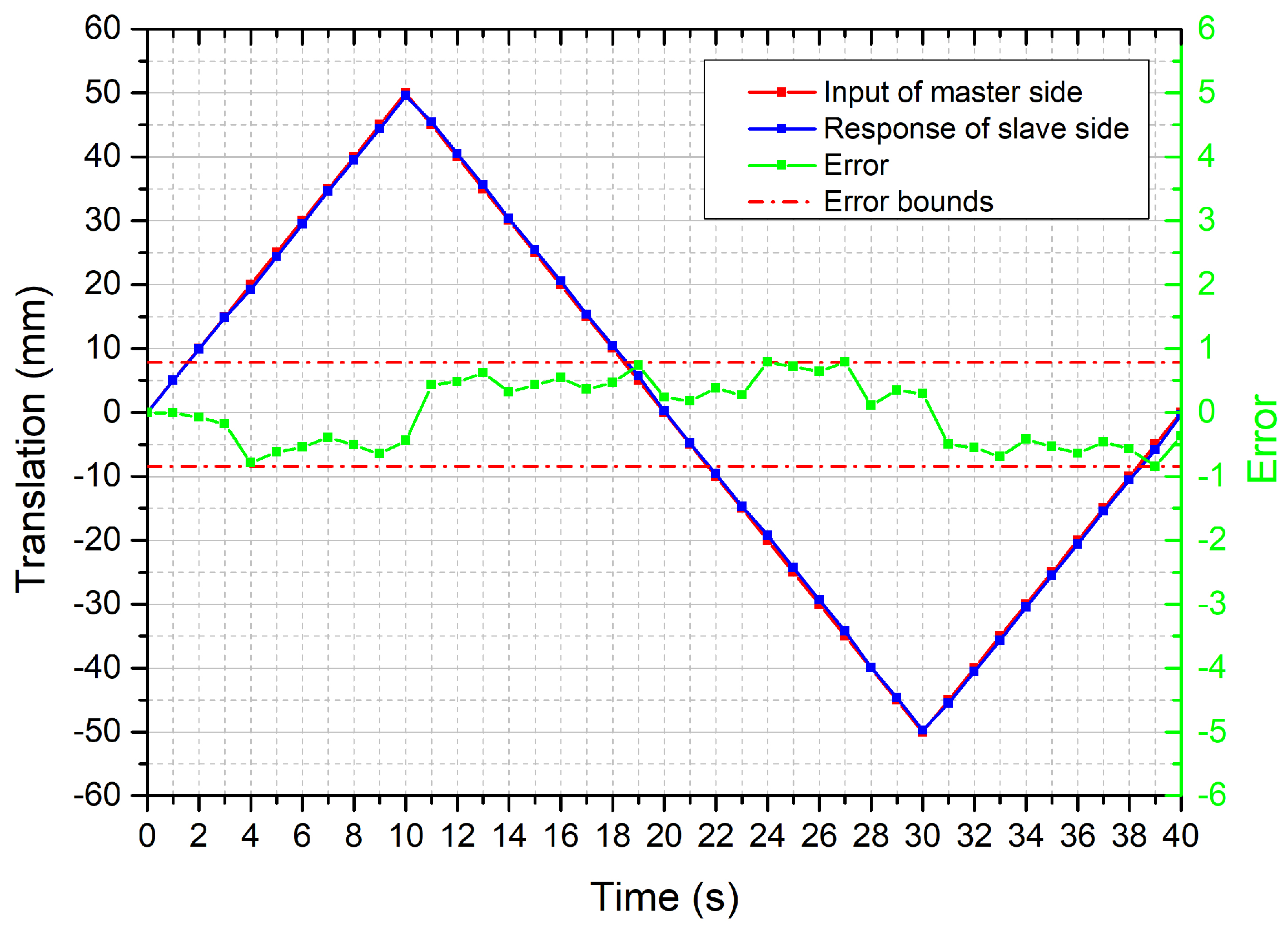
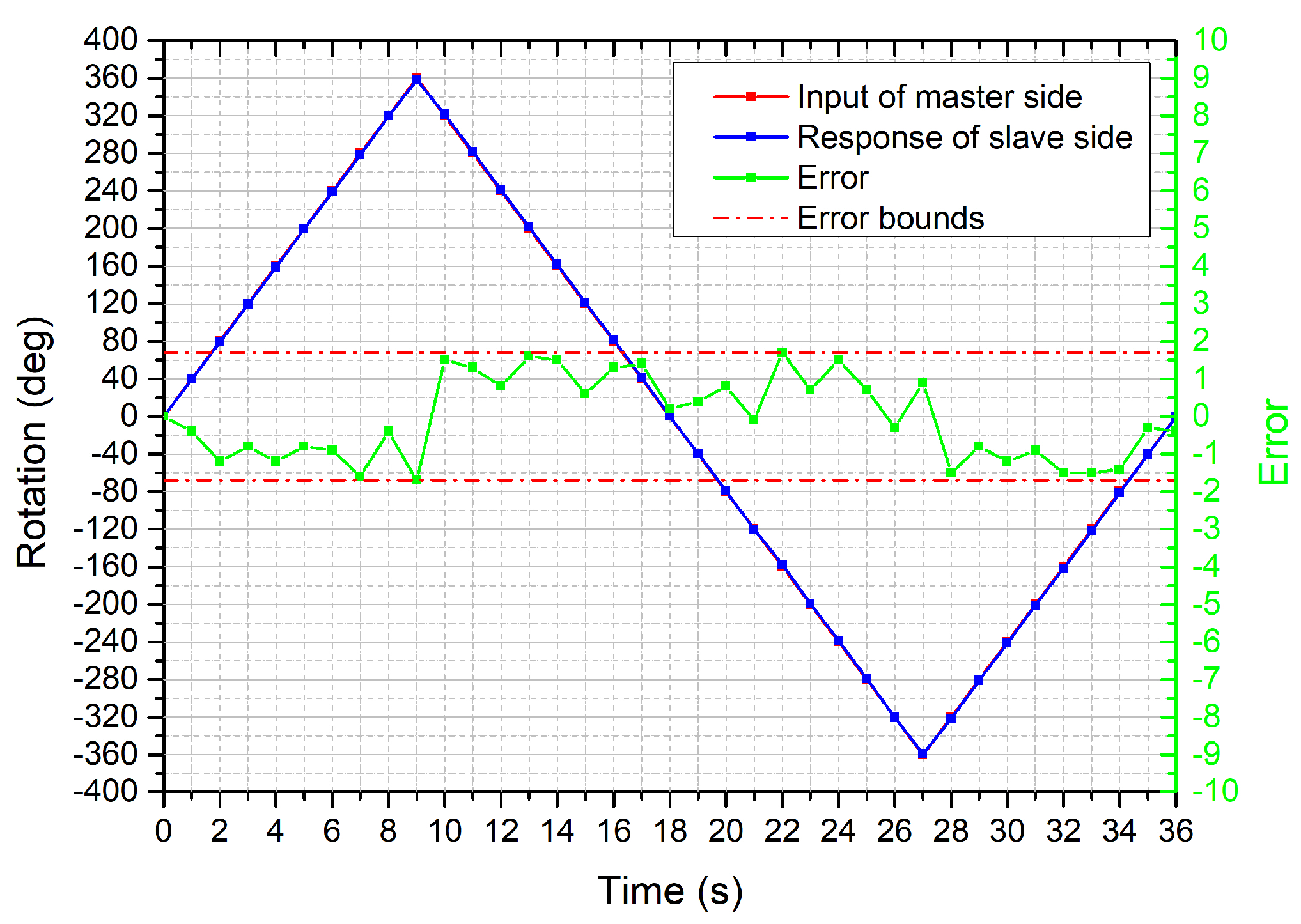
4.1. Performance Evaluation of the Bilateral Operation
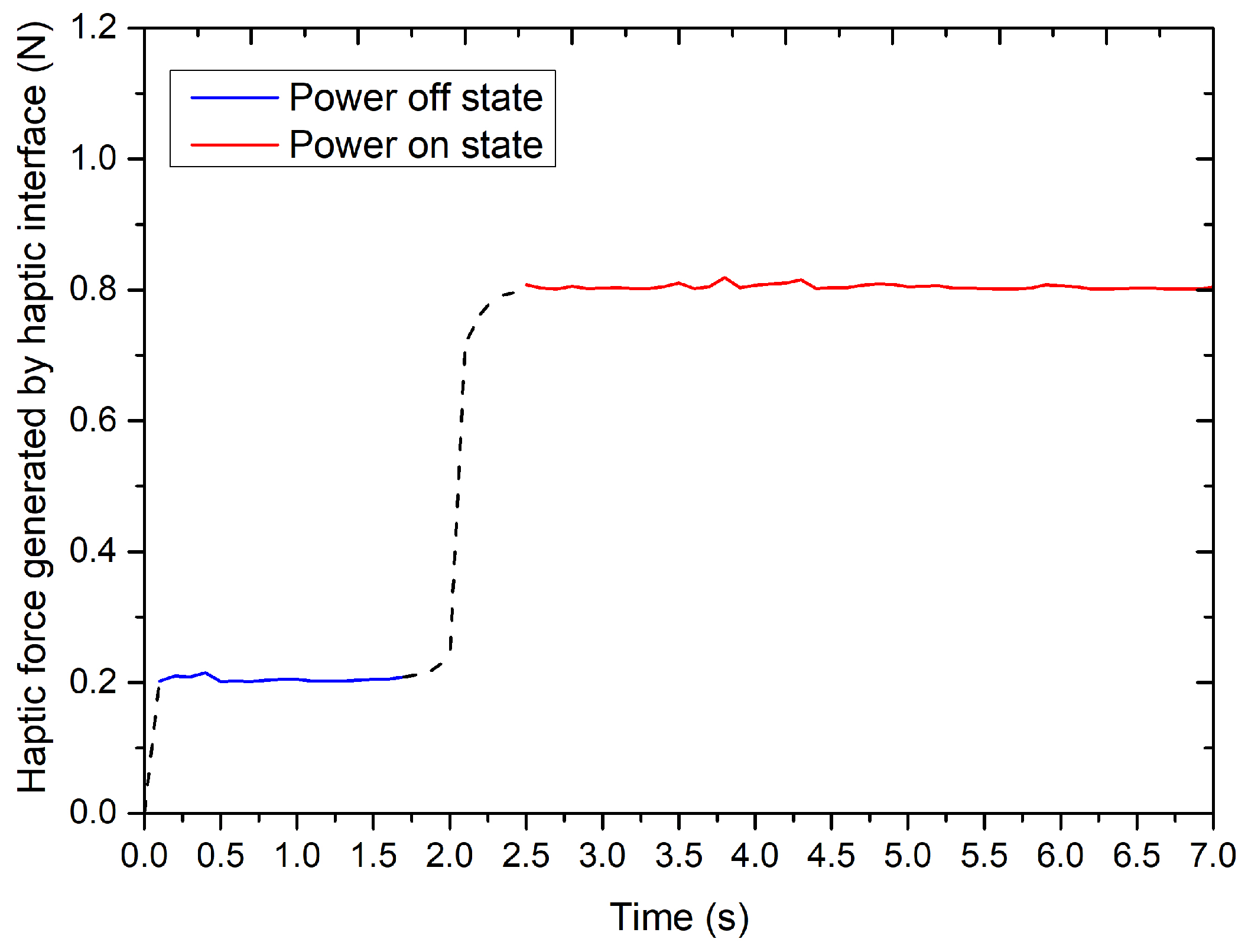
4.2. Performance Evaluation of the Force-Measuring Unit
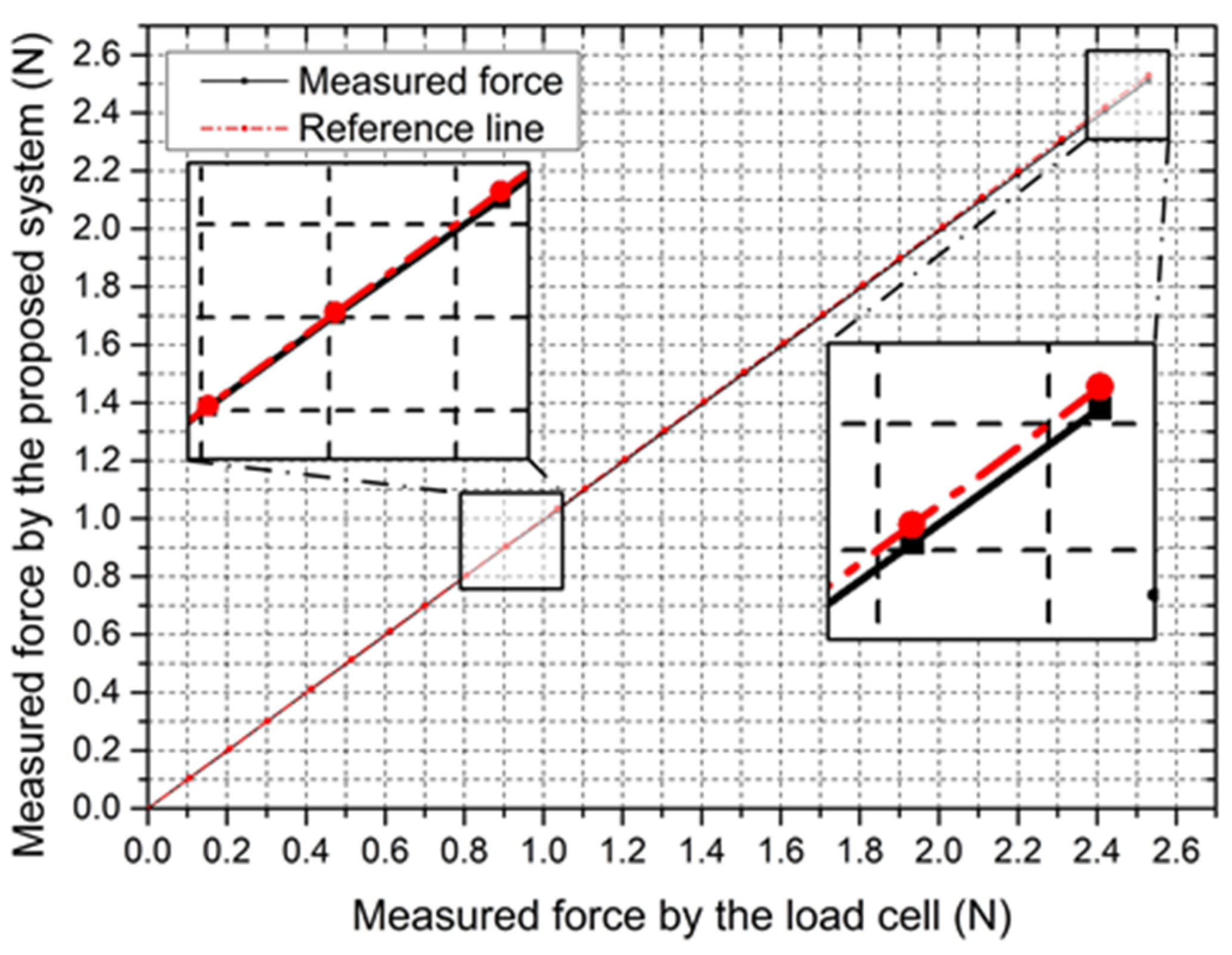
4.2.1. Static Characteristic for the Force-Measuring Unit
4.2.2. Dynamic Characteristic for the Force-Measuring Unit
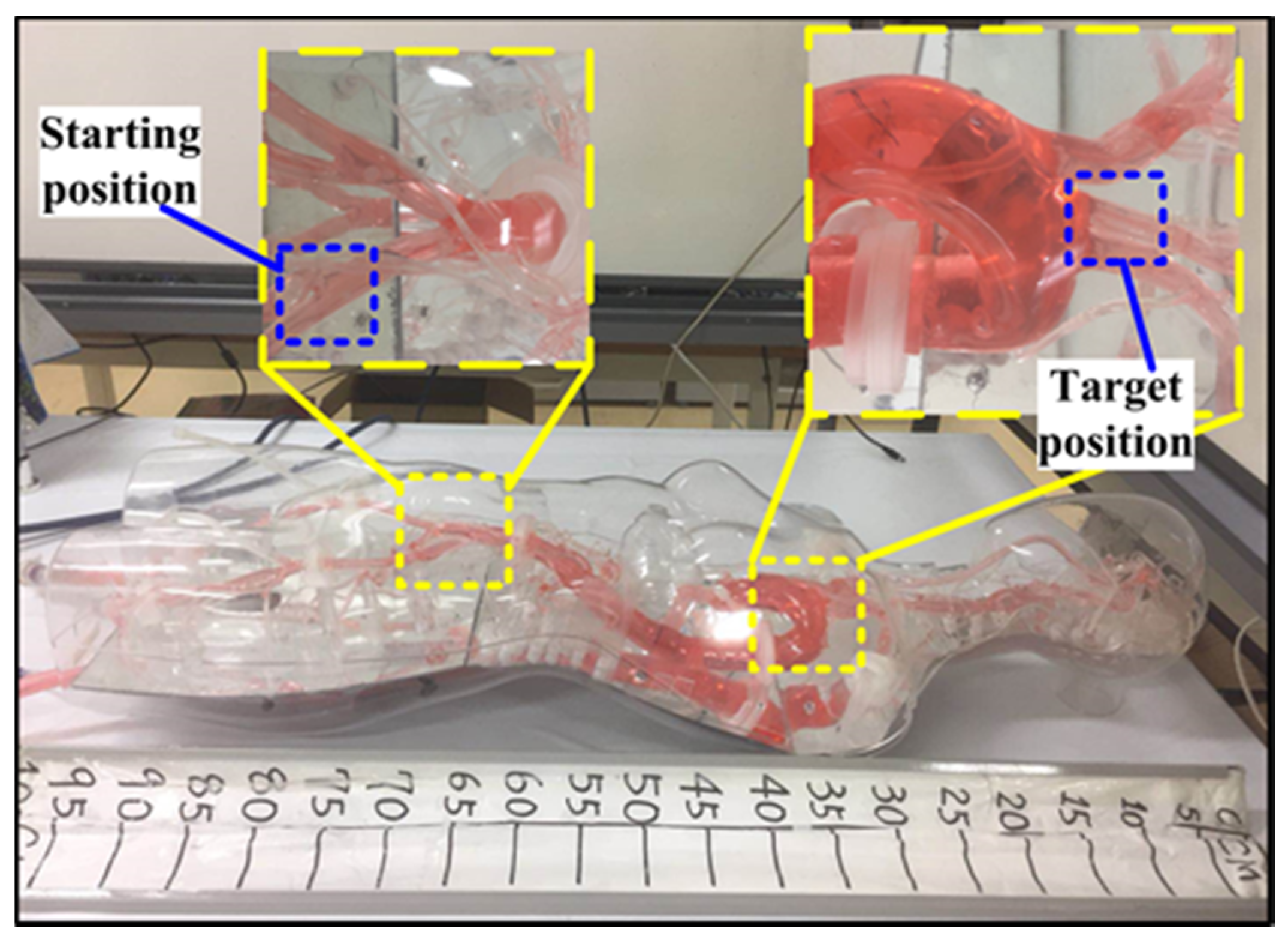
5. In Vitro Evaluation
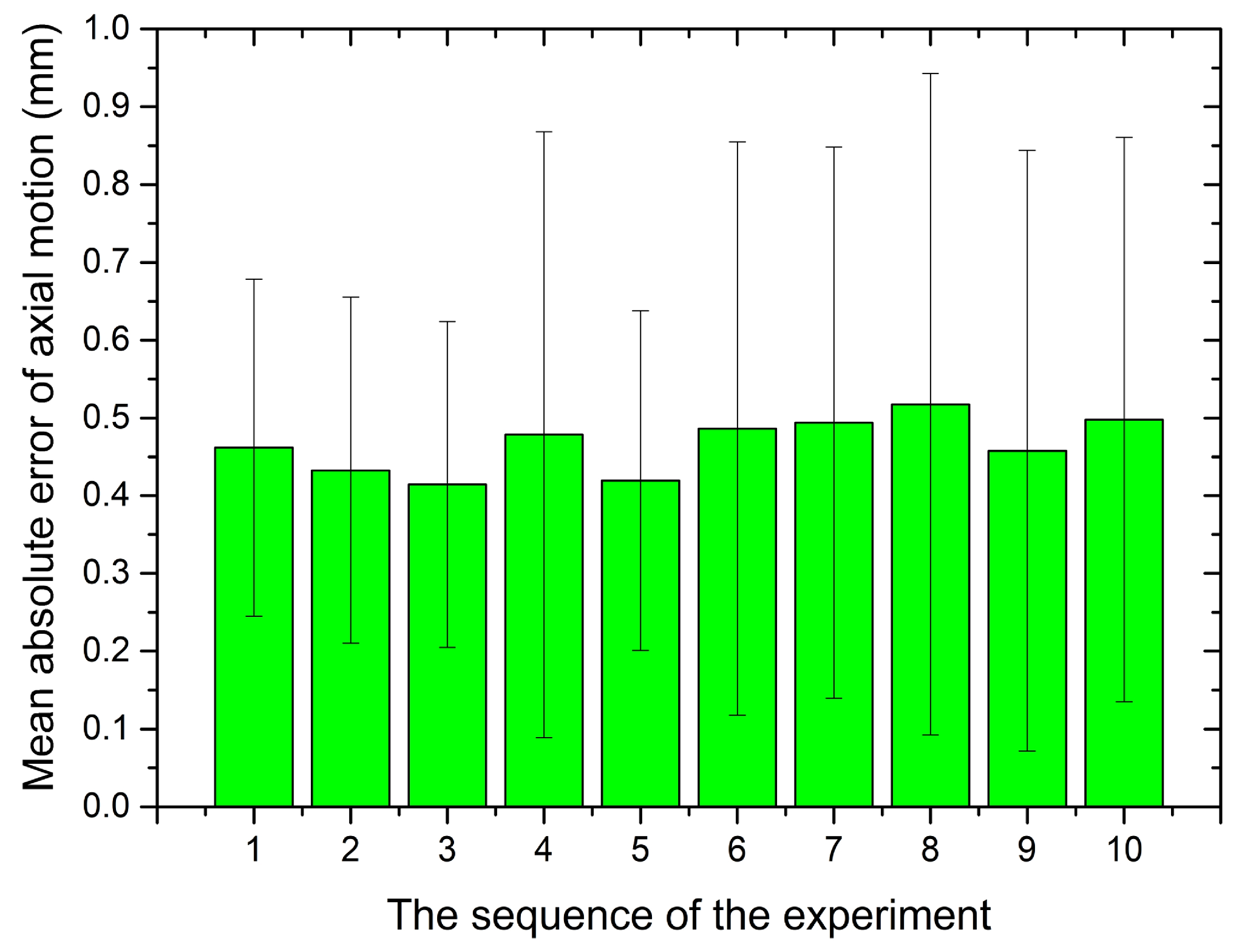
5.1. Performance Evaluation
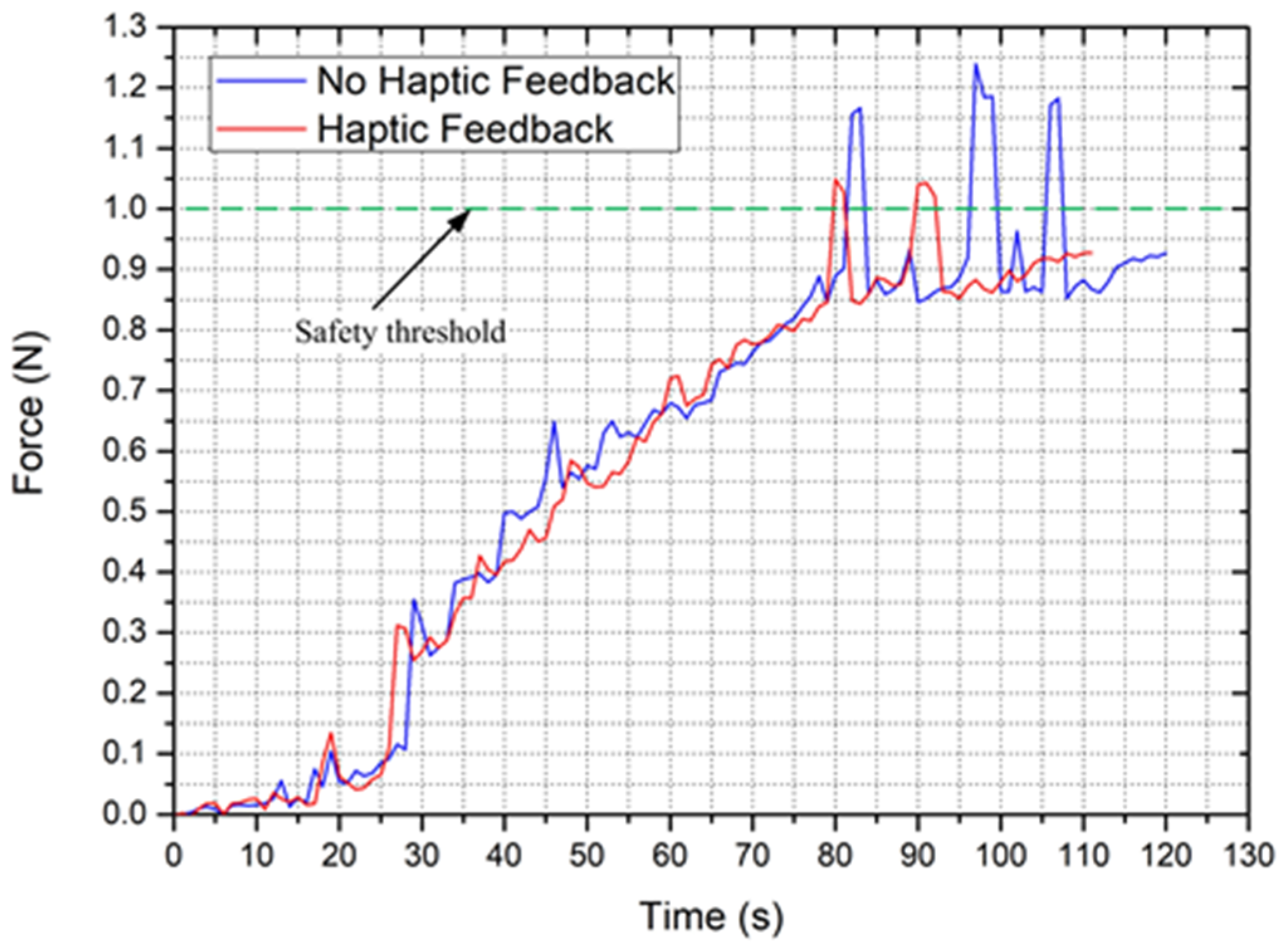
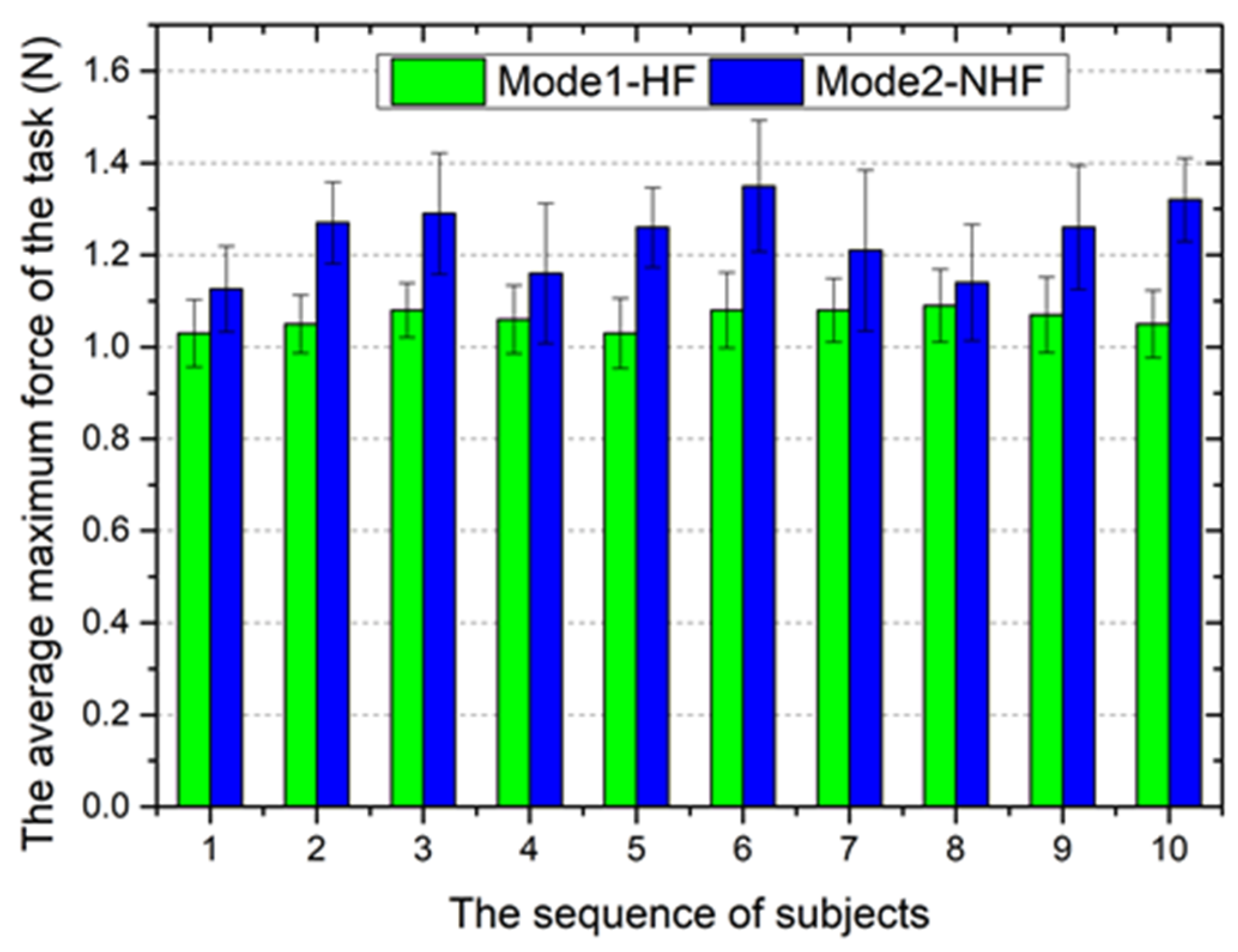
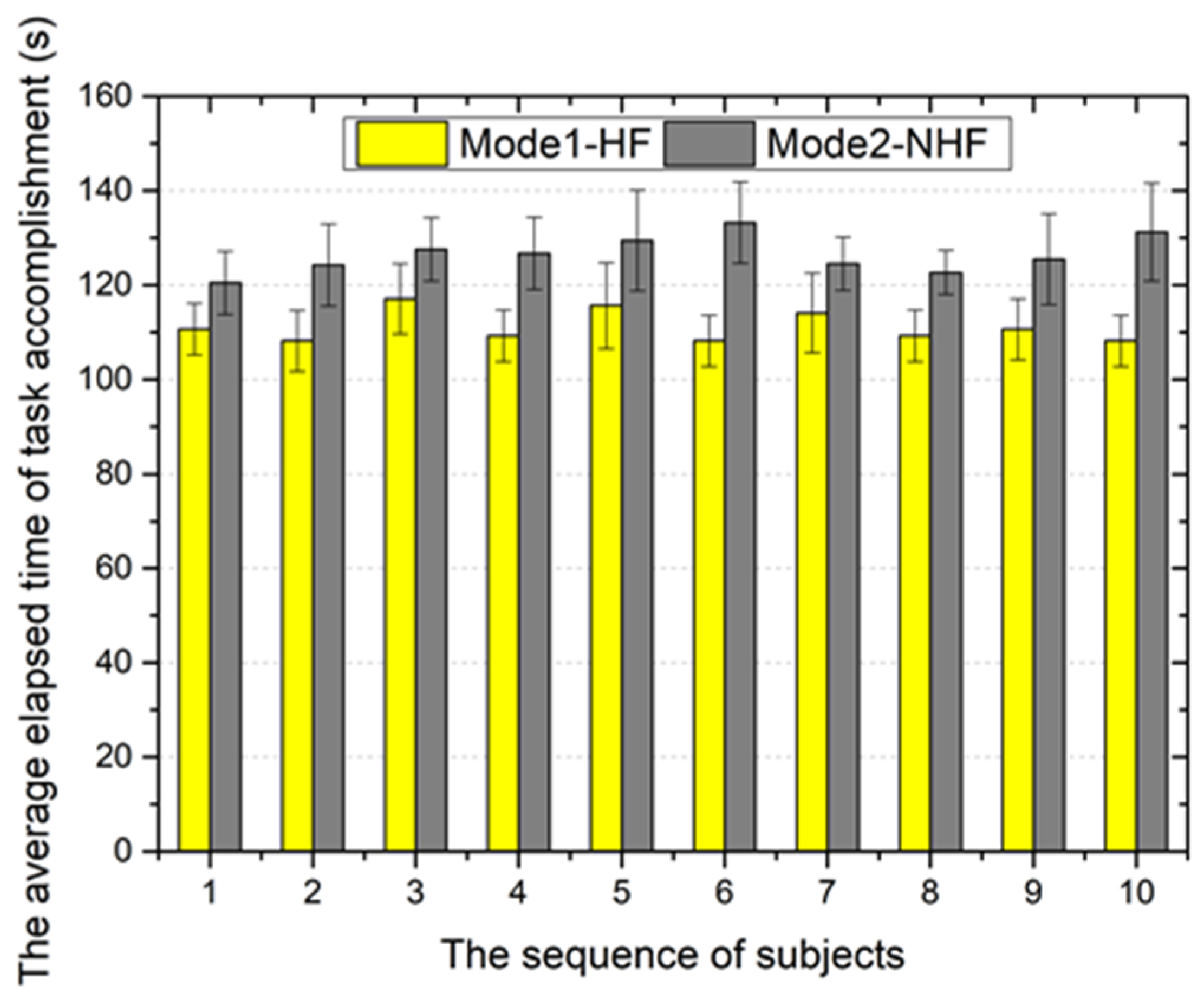
5.2. Validation Trials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lloyd-Jones, D.; Adams, R.; Brown, T.; Carnethon, M.; Dai, S.; De Simone, G.; Go, A. Executive summary: Heartdisease and stroke statistics-2010 update: A report from the American Heart Association. Circulation 2010, 121, 948–954. [Google Scholar] [PubMed]
- Khoshnam, M.; Patel, R.V. A Pseudo-Rigid-Body 3R Model for a Steerable Ablation Catheter. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 4427–4432. [Google Scholar]
- Cha, H.; Yi, B.; Won, J. An assembly-type master-slave catheter and guidewire driving system for vascular intervention. Part H J. Eng. Med. 2017, 231, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Greenberg, R.; Mastracci, T.; Eagleton, M.; Thornsberry, B. Radiation exposure to operating room personnel and patients during endovascular procedures. J. Vasc. Surg. 2013, 58, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jin, X.; Guo, S. Study of the Operational Safety of a Vascular Interventional Surgical Robotic System. Micromachines 2018, 9, 119. [Google Scholar] [CrossRef]
- Willems, S.; Steven, D.; Servatius, H.; Hoffmann, B.A.; Drewitzet, I. Persistence of Pulmonary Vein Isolation After Robotic Remote-Navigated Ablation for Atrial Fibrillation and its Relation to Clinical Outcome. J. Cardiovasc. Electr. 2010, 21, 1079–1084. [Google Scholar] [CrossRef]
- Hlivák, P.; Mlčochová, H.; Peichl, P.; Čihák, R.; Wichterle, D.; Kautzner, J. Robotic Navigation in Catheter Ablation for Paroxysmal Atrial Fibrillation: Midterm Efficacy and Predictors of Postablation Arrhythmia Recurrences. J. Cardiovasc. Electr. 2011, 22, 534–540. [Google Scholar] [CrossRef]
- Granada, J.; Delgado, J.; Uribe, M.P.; Fernandez, A.; Blanco, G.; Leon, M.B.; Weisz, G. First-Human Evaluation of a Novel Robotic-Assisted Coronary Angioplasty System. JACC Cardiovasc. Interv. 2011, 4, 460–465. [Google Scholar] [CrossRef]
- Swaminathan, R.; Rao, S. Robotic-assisted transradial diagnostic coronary angiography. Catheter. Cardio. Interv. 2018, 92, 54–57. [Google Scholar] [CrossRef]
- Khan, E.M.; Frumkin, W.; Ng, G.A.; Neelagaru, S.; Abi-Samra, F.M.; Lee, J.; Giudici, M.; Gohn, D.; Winkle, R.A.; Sussman, J.; et al. First experience with a novel robotic remote catheter system: AmigoTM mapping trial. J. Interv. Card. Electr. 2013, 37, 124–129. [Google Scholar] [CrossRef]
- Tavallaei, M.; Thakur, Y.; Haider, S.; Drangova, M. A Magnetic-Resonance-Imaging-Compatible Remoto Catheter Navigation System. IEEE Trans. Bio-Med. Eng. 2013, 60, 899–905. [Google Scholar] [CrossRef][Green Version]
- Tavallaei, M.; Gelman, D.; Lavdas, M.; Skanes, A.; Jones, D.; Bax, J.; Drangova, M. Design, development and evaluation of a compact telerobotic catheter navigation system. Int. J. Med. Robot. Comp. 2015, 12, 442–452. [Google Scholar] [CrossRef]
- Macdonald, S.; Lee, R.; Williams, R.; Stansby, G. Towards safer carotid artery stenting: A scoring system for anatomic suitability. Stroke 2009, 40, 1698–1703. [Google Scholar] [CrossRef]
- Payne, C.J.; Rafii-Tari, H.; Yang, G. A Force Feedback System for Endovascular Catheterisation. In Proceedings of the 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems, Vilamoura-Algarve, Portugal, 7–12 October 2012; pp. 1298–1304. [Google Scholar]
- Fu, Y.; Gao, A.; Liu, H.; Guo, S. The master-slave catheterization system for positioning the steerable catheter. Int. J. Mechatron. Autom. 2011, 1, 143–152. [Google Scholar] [CrossRef]
- Guo, J.; Guo, S.; Xiao, N.; Ma, X.; Yoshida, S.; Tamiya, T.; Kawanishi, M. A novel robotic catheter system with force and visual feedback for vascular interventional surgery. Int. J. Mechatron. Autom. 2012, 2, 15–24. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Jiang, Y. A cooperation of catheters and guidewires-based novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 20. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Shen, R.; Cui, J.; Jiang, Y.; Liu, X.; Liu, K. Operation evaluation in-human of a novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 34. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, S.; Yu, H.; Song, Y. Performance evaluation of a strain-gauge force sensor for a haptic robot-assisted catheter operating system. Microsyst. Technol. 2017, 23, 5041–5050. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, S.; Xiao, N.; Wang, Y.; Li, Y.; Jiang, Y. Operating force information on-line acquisition of a novel slave manipulator for vascular interventional surgery. Biomed. Microdevices 2018, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Guo, S.; Hirata, H.; Ishihara, H. Design and experimental evaluation of teleoperated haptic robot-assisted catheter operating system. J. Intell. Mater. Syst. Struct. 2016, 27, 3–16. [Google Scholar] [CrossRef]
- Yin, X.; Guo, S.; Xiao, N.; Tamiya, T. Safety Operation Consciousness Realization of a MR Fluids-based Novel Haptic Interface for Teleoperated Catheter Minimally Invasive Neuro Surgery. IEEE/ASME Trans. Mech. 2016, 21, 1043–1054. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, S.; Li, Y.; Tamiya, T.; Song, Y. Design and evaluation of safety operation VR training system for robotic catheter surgery. Med. Biol. Eng. Comput. 2018, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, S.; Yu, H.; Song, Y.; Tamiya, T.; Hirata, H.; Ishihara, H. Design and performance evaluation of collision protection-based safety operation for a haptic robot-assisted catheter operating system. Biomed. Microdevices 2018, 20, 22. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, S.; Yu, H.; Song, Y. Design and Principle Analysis for Electromagnetic Brake Clamping Mechanism of a Novel Slave Manipulator. In Proceedings of the 2016 IEEE International Conference on Mechatronics and Automation, Harbin, China, 7–10 August 2016; pp. 490–495. [Google Scholar]
- Ahmadkhanlou, F.; Washington, G.N.; Bechtel, S.E. Modeling and control of single and two degree of freedom magnetorheological fluid-based haptic systems for telerobotic surgery. J. Intell. Mater. Syst. Struct. 2009, 20, 1171–1186. [Google Scholar] [CrossRef]
- Tsujita, T.; Sase, K.; Konno, A.; Nakayama, M.; Chen, X.; Abe, K.; Uchiyama, M. Design and evaluation of an encountered-type haptic interface using MR fluid for surgical simulators. Adv. Robot. 2013, 27, 525–540. [Google Scholar] [CrossRef]
- Guo, J.; Guo, S.; Yu, Y. Design and characteristics evaluation of a novel teleoperated robotic catheterization system with force feedback for vascular interventional surgery. Biomed. Microdevices 2016, 18, 76. [Google Scholar] [CrossRef]
- Park, B.J.; Fang, F.F.; Choi, H.J. Magnetorheology: Materials and application. Soft Matter 2010, 6, 5246–5253. [Google Scholar] [CrossRef]
- Song, Y.; Guo, S.; Yin, X.; Zhang, L.; Hirata, H.; Ishihara, H.; Tamiya, T. Performance evaluation of a robot-assisted catheter operating system with haptic feedback. Biomed. Microdevices 2018, 20, 50. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, S.; Yu, H.; Gu, S.; Song, Y.; Yu, M. Electromagnetic Braking-Based Collision Protection of a Novel Catheter Manipulator. In Proceedings of the 2017 IEEE International Conference on Mechatronics and Automation, Takamatsu, Japan, 6–9 August 2017; pp. 1726–1731. [Google Scholar]
- Jones, L.A.; Tan, H.Z. Application of psychophysical techniques to haptic research. IEEE Trans. Haptics 2013, 6, 268–284. [Google Scholar] [CrossRef]
- Niemeyer, G.; Preusche, C.G. Hirzinger, Telerobotics. In Spring Handbook of Robotics; Springer: New York, NY, USA, 2008; pp. 741–757. [Google Scholar]
- Gwilliam, J.C.; Mahvash, M.; Vagvolgyi, B.; Vacharat, A.; Yuh, D.D.; Okamura, A.M. Effects of Haptic and Graphical Force Feedback on Teleoperated Palpation. In Proceedings of the 2009 IEEE International Conference on Robotics and Automation, Kobe, Japan, 12–17 May 2009; pp. 677–682. [Google Scholar]
- Lu, W.; Xu, W.; Pan, F.; Liu, D.; Tian, Z.; Zeng, Y. Clinical application of a vascular interventional robot in cerebral angiography. Int. J. Med. Robot. Comp. 2016, 12, 132–136. [Google Scholar] [CrossRef]
- Okumura, Y.; Johnson, S.B.; Bunch, T.J.; Henz, B.D.; O’brien, C.J.; Packer, D.L. A Systematical Analysis of In Vivo Contact Forces on Virtual Catheter Tip/Tissue Surface Contact during Cardiac Mapping and Intervention. J. Cardiovasc. Electr. 2008, 19, 632–640. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Shi, L. Compensatory force measurement and multimodal force feedback for remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 74. [Google Scholar] [CrossRef] [PubMed]
- Che Zakaria, N.A.; Komeda, T.; Low, C.Y.; Mahadhir, K. Development of Foolproof Catheter Guide System Based on Mechatronic Design. Prod. Eng. 2013, 7, 81–90. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Gu, S.; Guo, S.; Tamiya, T. A Magnetorheological Fluids-Based Robot-Assisted Catheter/Guidewire Surgery System for Endovascular Catheterization. Micromachines 2021, 12, 640. https://doi.org/10.3390/mi12060640
Zhang L, Gu S, Guo S, Tamiya T. A Magnetorheological Fluids-Based Robot-Assisted Catheter/Guidewire Surgery System for Endovascular Catheterization. Micromachines. 2021; 12(6):640. https://doi.org/10.3390/mi12060640
Chicago/Turabian StyleZhang, Linshuai, Shuoxin Gu, Shuxiang Guo, and Takashi Tamiya. 2021. "A Magnetorheological Fluids-Based Robot-Assisted Catheter/Guidewire Surgery System for Endovascular Catheterization" Micromachines 12, no. 6: 640. https://doi.org/10.3390/mi12060640
APA StyleZhang, L., Gu, S., Guo, S., & Tamiya, T. (2021). A Magnetorheological Fluids-Based Robot-Assisted Catheter/Guidewire Surgery System for Endovascular Catheterization. Micromachines, 12(6), 640. https://doi.org/10.3390/mi12060640





