An Automatic Platform Based on Nanostructured Microfluidic Chip for Isolating and Identification of Circulating Tumor Cells
Abstract
1. Introduction
2. Methods
2.1. Cell Line Preparation
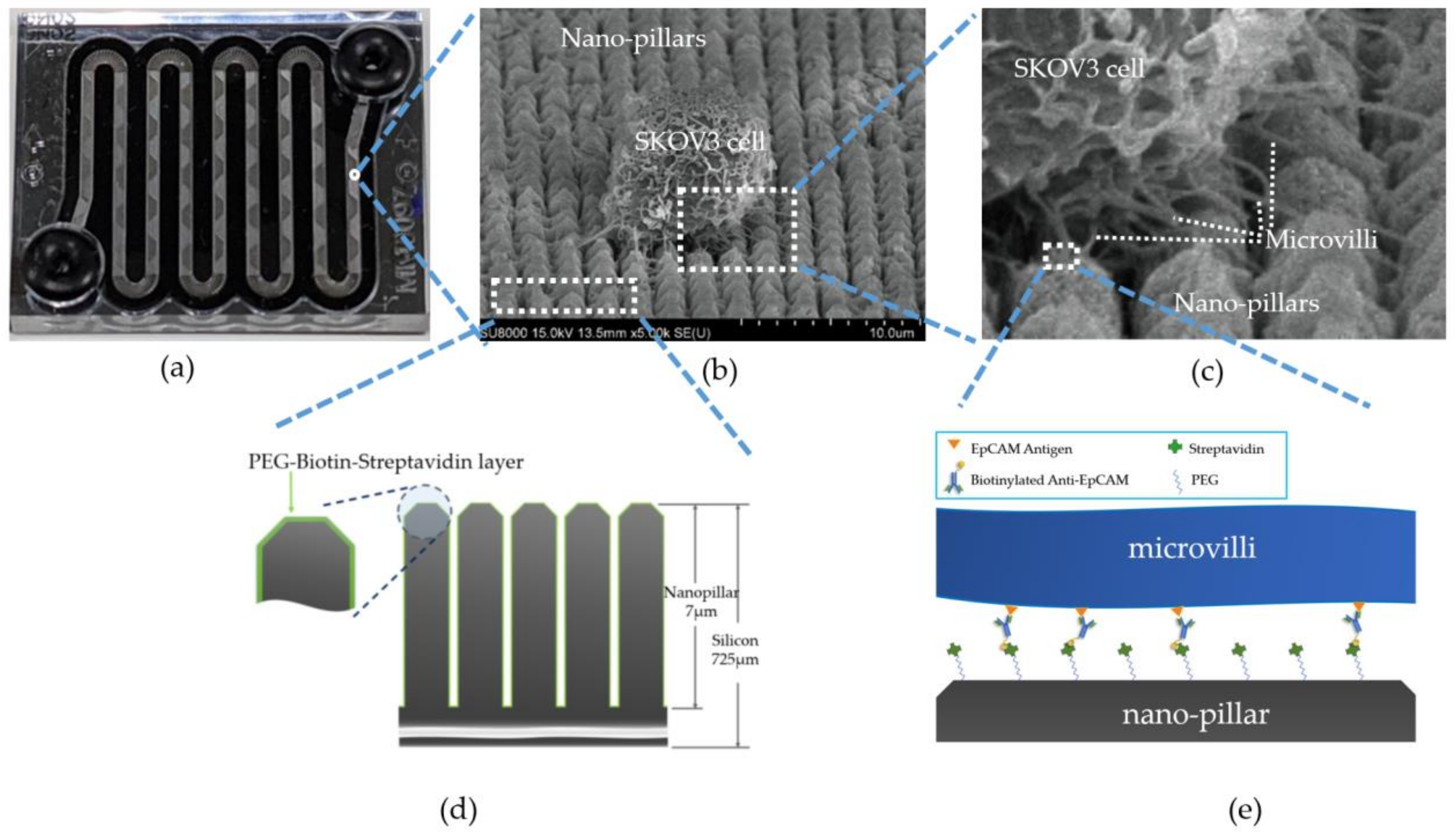
2.2. Microfluid Chip
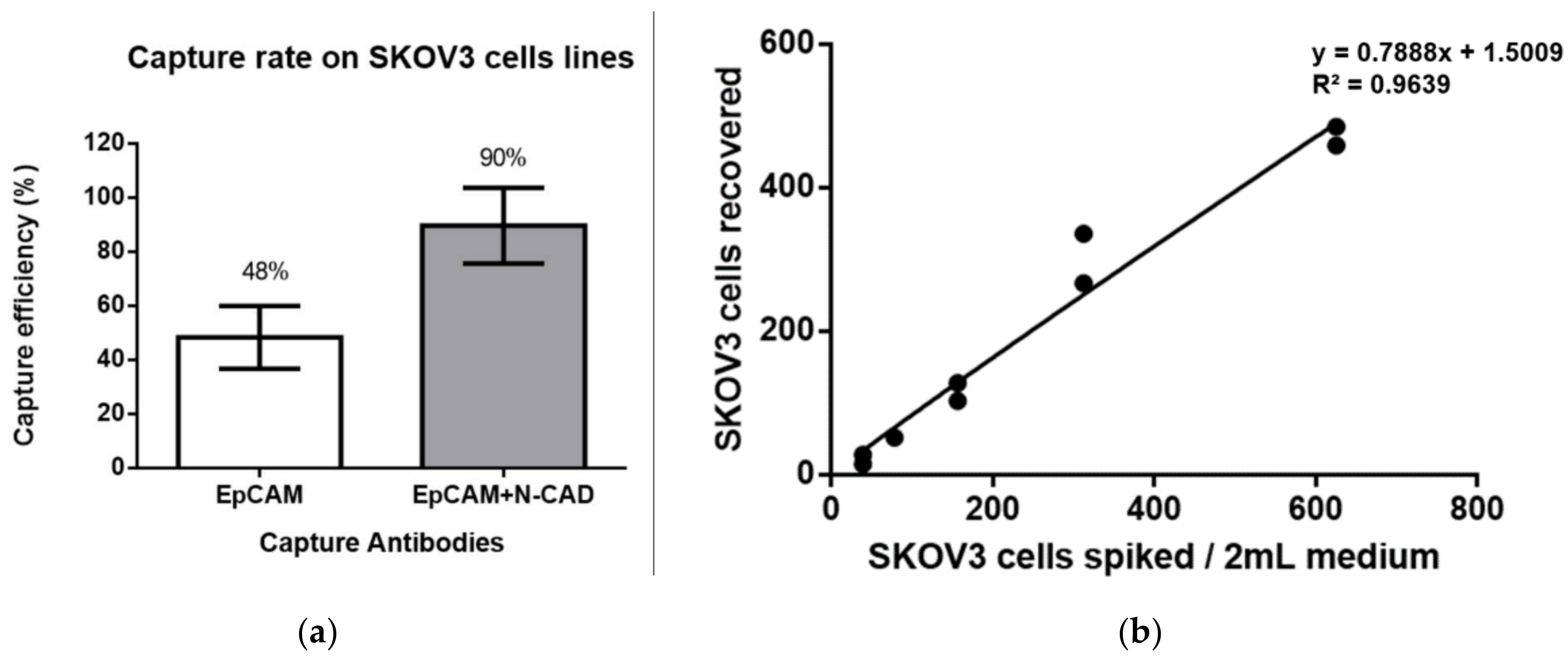
2.3. Cell Spiking Test
2.4. Linearity between the Numbers of Captured Cells and Spiked Cells
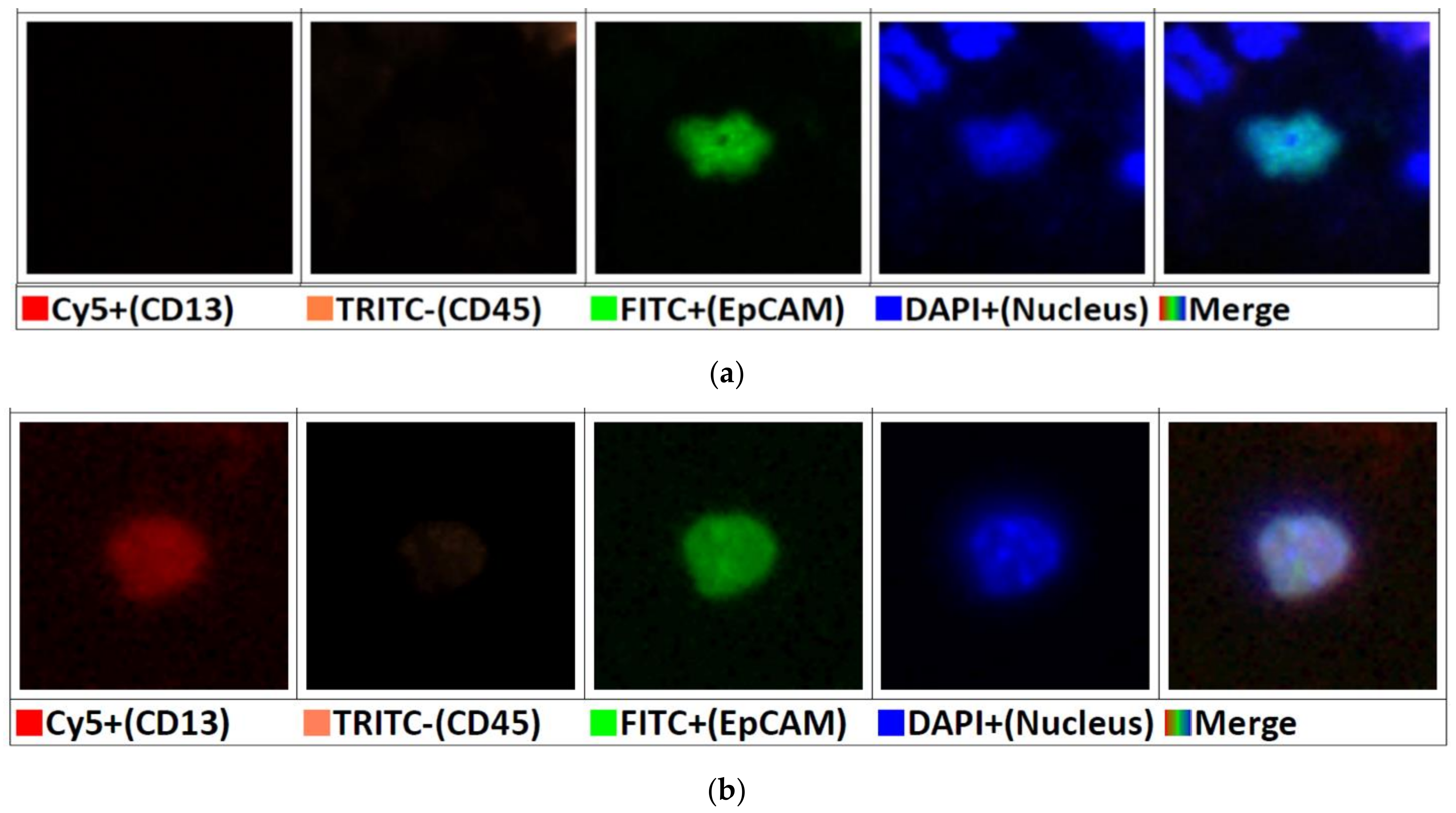
2.5. Feasibility Study
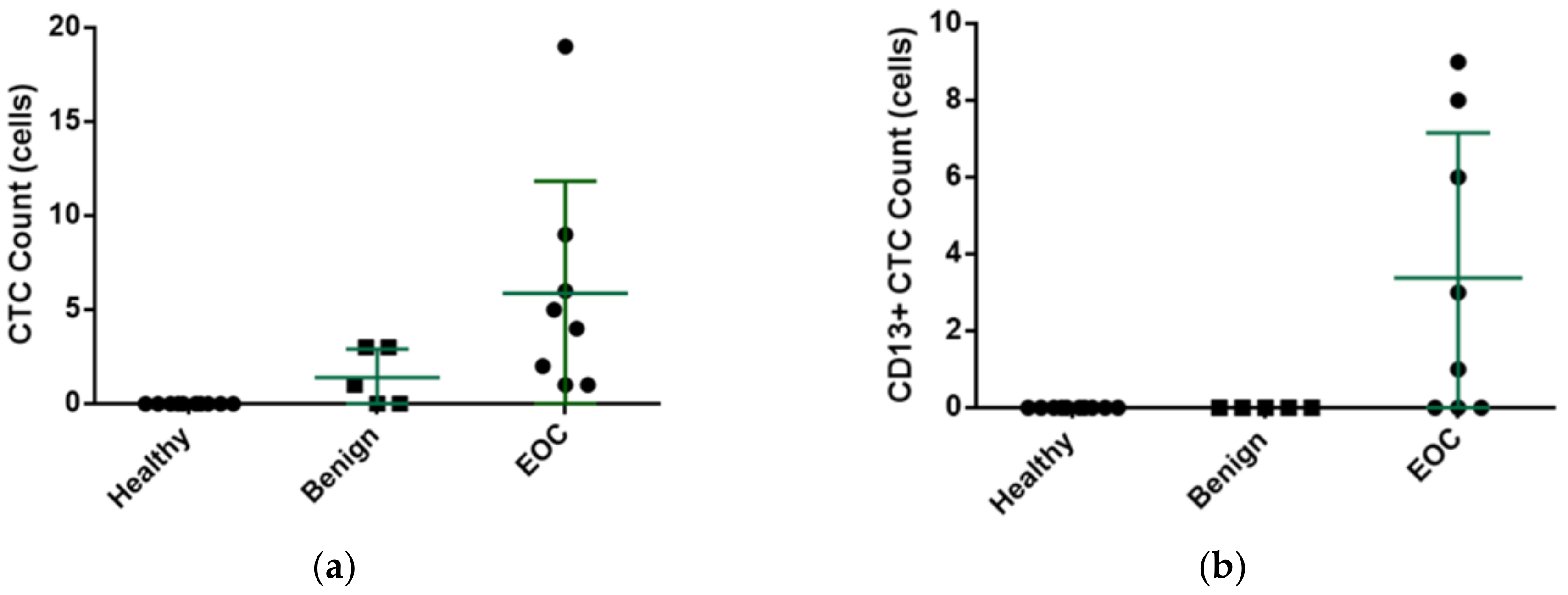
3. Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quandt, D.; Zucht, H.D.; Amann, A.; Wulf-Goldenberg, A.; Borrebaeck, C.; Cannarile, M.; Lambrechts, D.; Oberacher, H.; Garrett, J.; Nayak, T.; et al. Implementing liquid biopsies into clinical decision making for cancer immunotherapy. Oncotarget 2017, 8, 48507–48520. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Lorente, D.; Omlin, A.; Olmos, D.; De Bono, J.S. Circulating Tumor Cells: A Multifunctional Biomarker. Clin. Cancer Res. 2014, 20, 2553–2568. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.; Yu, M. Circulating tumor cells as “liquid biopsies” to understand cancer metastasis. Transl. Res. 2018, 201, 128–135. [Google Scholar] [CrossRef]
- Zieglschmid, V.; Hollmann, C.; Böcher, O. Detection of Disseminated Tumor Cells in Peripheral Blood. Crit. Rev. Clin. Lab. Sci. 2005, 42, 155–196. [Google Scholar] [CrossRef]
- Dan, Z.; Daxiang, C.; Zou, D.; Cui, D. Advances in isolation and detection of circulating tumor cells based on microfluidics. Cancer Biol. Med. 2018, 15, 335–353. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Jackson, J.M.; Witek, M.A.; Kamande, J.W.; Soper, S.A. Materials and microfluidics: Enabling the efficient isolation and analysis of circulating tumour cells. Chem. Soc. Rev. 2017, 46, 4245–4280. [Google Scholar] [CrossRef]
- Ma, G.-C.; Lin, W.-H.; Huang, C.-E.; Chang, T.-Y.; Liu, J.-Y.; Yang, Y.-J.; Lee, M.-H.; Wu, W.-J.; Chang, Y.-S.; Chen, M. A Sili-con-based Coral-like Nanostructured Microfluidics to Isolate Rare Cells in Human Circulation: Validation by SK-BR-3 Cancer Cell Line and Its Utility in Circulating Fetal Nucleated Red Blood Cells. Micromachines 2019, 10, 132. [Google Scholar] [CrossRef]
- Lakshmipriya, T.; Gopinath, S.C.; Tang, T.H. Biotin-Streptavidin Competition Mediates Sensitive Detection of Biomole-cules in Enzyme Linked Immunosorbent Assay. PLoS ONE 2016, 11, e0151153. [Google Scholar] [CrossRef] [PubMed]
- Dundas, C.M.; Demonte, D.; Park, S. Streptavidin-biotin technology: Improvements and innovations in chemical and bio-logical applications. Appl. Microbiol. Biotechnol. 2013, 97, 9343–9353. [Google Scholar] [CrossRef] [PubMed]
- Stayton, P.S.; Freitag, S.; Klumb, L.A.; Chilkoti, A.; Chu, V.; Penzotti, J.E.; To, R.; Hyre, D.; Le Trong, I.; Lybrand, T.P.; et al. Streptavidin–biotin binding energetics. Biomol. Eng. 1999, 16, 39–44. [Google Scholar] [CrossRef]
- Haraguchi, N.; Ishii, H.; Mimori, K.; Tanaka, F.; Ohkuma, M.; Kim, H.M.; Akita, H.; Takiuchi, D.; Hatano, H.; Nagano, H.; et al. CD13 is a therapeutic target in human liver cancer stem cells. J. Clin. Investig. 2010, 120, 3326–3339. [Google Scholar] [CrossRef]
- Colvin, H.; Mori, M. Getting to the heart of the matter in cancer: Novel approaches to targeting cancer stem cells. Proc. Jpn. Acad. Ser. B 2017, 93, 146–154. [Google Scholar] [CrossRef]
- Chen, W.-C.; Hsu, H.-P.; Li, C.-Y.; Yang, Y.-J.; Hung, Y.-H.; Cho, C.-Y.; Wang, C.-Y.; Weng, T.-Y.; Lai, M.-D. Cancer stem cell marker CD90 inhibits ovarian cancer formation via β3 integrin. Int. J. Oncol. 2016, 49, 1881–1889. [Google Scholar] [CrossRef]
- Gonzalez, V.D.; Samusik, N.; Chen, T.J.; Savig, E.S.; Aghaeepour, N.; Quigley, D.A.; Huang, Y.-W.; Giangarrà, V.; Borowsky, A.D.; Hubbard, N.E.; et al. Commonly Occurring Cell Subsets in High-Grade Serous Ovarian Tumors Identified by Single-Cell Mass Cytometry. Cell Rep. 2018, 22, 1875–1888. [Google Scholar] [CrossRef]
- Schmelzer, E.; Reid, L.M. EpCAM expression in normal, non-pathological tissues. Front. Biosci. 2008, 13, 3096–3100. [Google Scholar] [CrossRef]
- Went, P.T.; Lugli, A.; Meier, S.; Bundi, M.; Mirlacher, M.; Sauter, G.; Dirnhofer, S. Frequent EpCam protein expression in human carcinomas. Hum. Pathol. 2004, 35, 122–128. [Google Scholar] [CrossRef]
- Osta, W.A.; Chen, Y.; Mikhitarian, K.; Mitas, M.; Salem, M.; Hannun, Y.A.; Cole, D.J.; Gillanders, W.E. EpCAM is overex-pressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004, 64, 5818–5824. [Google Scholar] [CrossRef]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef]
- Jie, X.-X.; Zhang, X.-Y.; Xu, C.-J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget 2017, 8, 81558. [Google Scholar] [CrossRef]
- Mohtar, M.A.; Syafruddin, S.E.; Nasir, S.N.; Low, T.Y. Revisiting the Roles of Pro-Metastatic EpCAM in Cancer. Biomolecules 2020, 10, 255. [Google Scholar] [CrossRef]
- Gorges, T.M.; Tinhofer, I.; Drosch, M.; Röse, L.; Zollner, T.M.; Krahn, T.; von Ahsen, O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Po, J.W.; Roohullah, A.; Lynch, D.; DeFazio, A.; Harrison, M.; Harnett, P.R.; Kennedy, C.; de Souza, P.; Becker, T.M. Im-proved ovarian cancer EMT-CTC isolation by immunomagnetic targeting of epithelial EpCAM and mesenchymal N-cadherin. J. Circ. Biomark. 2018, 7, 1849454418782617. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Lee, S.; Chang, H.J.; Lee, E.S.; Cho, Y. Multifunctional magnetic nanowires: A novel breakthrough for ultrasensi-tive detection and isolation of rare cancer cells from non-metastatic early breast cancer patients using small volumes of blood. Biomaterials 2016, 106, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Avelar, C.; Soto-García, B.; Aráiz-Hernández, D.; León, J.F.Y.-D.; Esparza, M.; Chacón, F.; Delgado-Balderas, J.R.; Alvarez, M.M.; Santiago, G.T.-D.; Gómez-Guerra, L.S.; et al. High-Throughput Automated Microscopy of Circulating Tumor Cells. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Kindelberger, D.; Doyle, C.; Lowe, A.; Barry, W.T.; Matulonis, U.A. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol. Oncol. 2013, 131, 352–356. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, M.; Choi, J.Y.; Bu, J.; Kang, Y.-T.; Kwon, B.S.; Lee, B.; Kim, K.; No, J.H.; Kim, Y.-B.; et al. Circulating tumor cells in the differential diagnosis of adnexal masses. Oncotarget 2017, 8, 77195–77206. [Google Scholar] [CrossRef]
- Obermayr, E.; Bednarz-Knoll, N.; Orsetti, B.; Weier, H.-U.; Lambrechts, S.; Castillo-Tong, D.C.; Reinthaller, A.; Braicu, E.I.; Mahner, S.; Sehouli, J.; et al. Circulating tumor cells: Potential markers of minimal residual disease in ovarian cancer? A study of the OVCAD consortium. Oncotarget 2017, 8, 106415–106428. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Z.; Xu, S.; Li, X.; Yang, X.; Jin, P.; Liu, Y.; Zhou, X.; Zhang, T.; Gong, C.; et al. Heterotypic CAF-tumor sphe-roids promote early peritoneal metastasis of ovarian cancer. J. Exp. Med. 2019, 216, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Van Berckelaer, C.; Brouwers, A.; Peeters, D.; Tjalma, W.; Trinh, X.; van Dam, P. Current and future role of circulating tumor cells in patients with epithelial ovarian cancer. Eur. J. Surg. Oncol. 2016, 42, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Yu, X.; Li, S.; Lei, Z.; Li, C.; Zhang, Q.; Han, Q.; Li, Y.; Zhang, K.; et al. Analysis of Circulating Tumor Cells in Ovarian Cancer and Their Clinical Value as a Biomarker. Cell. Physiol. Biochem. 2018, 48, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, E.J.; Cho, Y.; Kim, S.; Chung, H.H.; Park, N.H.; Song, Y.-S. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol. Oncol. 2017, 145, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Zhang, Q.; Zheng, C.; Dai, W.; Zhang, B.; Ionescu-Zanetti, C.; Lin, Z.; Zhang, L. Detection of circulating tumour cells in patients with epithelial ovarian cancer by a microfluidic system. Int. J. Clin. Exp. Pathol. 2017, 10, 9599–9606. [Google Scholar]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Lim, S.; Becker, T.; Chua, W.; Caixeiro, N.; Ng, W.; Kienzle, N.; Tognela, A.; Lumba, S.; Rasko, J.; De Souza, P.; et al. Circulating tumour cells and circulating free nucleic acid as prognostic and predictive biomarkers in colorectal cancer. Cancer Lett. 2014, 346, 24–33. [Google Scholar] [CrossRef]
- Goldkorn, A.; Ely, B.; Quinn, D.I.; Tangen, C.M.; Fink, L.M.; Xu, T.; Twardowski, P.; Van Veldhuizen, P.J.; Agarwal, N.; Carducci, M.A.; et al. Circulating Tumor Cell Counts Are Prognostic of Overall Survival in SWOG S0421: A Phase III Trial of Docetaxel With or Without Atrasentan for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2014, 32, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Behbakht, K.; Sill, M.W.; Darcy, K.M.; Rubin, S.C.; Mannel, R.S.; Waggoner, S.; Schilder, R.J.; Cai, K.Q.; Godwin, A.K.; Alpaugh, R.K. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor bi-omarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: A Gynecologic Oncology Group study. Gynecol. Oncol. 2011, 123, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Poveda, A.; Kaye, S.B.; McCormack, R.; Wang, S.; Parekh, T.; Ricci, D.; Lebedinsky, C.A.; Tercero, J.C.; Zintl, P.; Monk, B.J. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol. Oncol. 2011, 122, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.; Cabezas-Sainz, P.; Alonso-Alconada, L.; Ferreirós, A.; Mondelo-Macía, P.; Lago-Lestón, R.M.; Abalo, A.; Díaz, E.; Palacios-Zambrano, S.; Rojo-Sebastian, A.; et al. Circulating Tumor Cells Characterization Revealed TIMP1 as a Potential Therapeutic Target in Ovarian Cancer. Cells. 2020, 14, 1218. [Google Scholar] [CrossRef] [PubMed]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tu-mor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalig-nant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Banys-Paluchowski, M.; Fehm, T.; Neubauer, H.; Paluchowski, P.; Krawczyk, N.; Meier-Stiegen, F.; Wallach, C.; Kaczerowsky, A.; Gebauer, G. Clinical relevance of circulating tumor cells in ovarian, fallopian tube and peritoneal cancer. Arch. Gynecol. Obstet. 2020, 301, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Massard, C.; Borget, I.; Farace, F.; Aspeslagh, S.; Le Deley, M.-C.; Le Tourneau, C.; Bidard, F.-C.; Pierga, J.-Y.; Dieras, V.; Hofman, P.; et al. RECIST response and variation of circulating tumour cells in phase 1 trials: A prospective multicentric study. Eur. J. Cancer 2017, 83, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-X.; Neoh, K.H.; Chang, X.-H.; Sun, Y.; Cheng, H.-Y.; Ye, X.; Ma, R.-Q.; Han, R.P.; Cui, H. Diagnostic value of HE4+ circulating tumor cells in patients with suspicious ovarian cancer. Oncotarget 2018, 9, 7522–7533. [Google Scholar] [CrossRef]
- Surowiak, P.; Drąg, M.; Materna, V.; Suchocki, S.; Grzywa, R.; Spaczyński, M.; Dietel, M.; Oleksyszyn, J.; Zabel, M.; Lage, H. Expression of aminopeptidase N/CD13 in human ovarian cancers. Int. J. Gynecol. Cancer 2006, 16, 1783–1788. [Google Scholar] [CrossRef]
- Wickström, M.; Larsson, R.; Nygren, P.; Gullbo, J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef]
- Van Hensbergen, Y.; Broxterman, H.J.; Rana, S.; Van Diest, P.J.; Duyndam, M.C.A.; Hoekman, K.; Pinedo, H.M.; Boven, E. Reduced Growth, Increased Vascular Area, and Reduced Response to Cisplatin in CD13-Overexpressing Human Ovarian Cancer Xenografts. Clin. Cancer Res. 2004, 10, 1180–1191. [Google Scholar] [CrossRef][Green Version]


| Case Number | Age | Diagnosis | Staging | Number of CTCs | Number of CD13+ CTCs |
|---|---|---|---|---|---|
| Benign | |||||
| 1 | 45 | Endometrioma | - | 3 | 0 |
| 2 | 48 | Endometrioma | - | 1 | 0 |
| 3 | 42 | Mature teratoma | - | 0 | 0 |
| 4 | 32 | Endometrioma | - | 3 | 0 |
| 5 | 34 | Endometrioma | - | 0 | 0 |
| Malignant | |||||
| 1 | 56 | High grade serous cystadenocarcinoma | I | 6 | 6 |
| 2 | 56 | High grade serous cystadenocarcinoma | I | 1 | 1 |
| 3 | 21 | Endometrioid carcinoma | I | 5 | 0 |
| 4 | 61 | Endometrioid carcinoma | I | 19 | 8 |
| 5 | 67 | High grade serous cystadenocarcinoma | II | 2 | 0 |
| 6 | 60 | Clear cell carcinoma | III | 1 | 0 |
| 7 | 48 | High grade serous cystadenocarcinoma | III | 4 | 3 |
| 8 | 58 | Endometrioid carcinoma | III | 9 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jou, H.-J.; Chou, L.-Y.; Chang, W.-C.; Ho, H.-C.; Zhang, W.-T.; Ling, P.-Y.; Tsai, K.-H.; Chen, S.-H.; Chen, T.-H.; Lo, P.-H.; et al. An Automatic Platform Based on Nanostructured Microfluidic Chip for Isolating and Identification of Circulating Tumor Cells. Micromachines 2021, 12, 473. https://doi.org/10.3390/mi12050473
Jou H-J, Chou L-Y, Chang W-C, Ho H-C, Zhang W-T, Ling P-Y, Tsai K-H, Chen S-H, Chen T-H, Lo P-H, et al. An Automatic Platform Based on Nanostructured Microfluidic Chip for Isolating and Identification of Circulating Tumor Cells. Micromachines. 2021; 12(5):473. https://doi.org/10.3390/mi12050473
Chicago/Turabian StyleJou, Hei-Jen, Li-Yun Chou, Wen-Chun Chang, Hsin-Cheng Ho, Wan-Ting Zhang, Pei-Ying Ling, Ko-Hsin Tsai, Szu-Hua Chen, Tze-Ho Chen, Pei-Hsuan Lo, and et al. 2021. "An Automatic Platform Based on Nanostructured Microfluidic Chip for Isolating and Identification of Circulating Tumor Cells" Micromachines 12, no. 5: 473. https://doi.org/10.3390/mi12050473
APA StyleJou, H.-J., Chou, L.-Y., Chang, W.-C., Ho, H.-C., Zhang, W.-T., Ling, P.-Y., Tsai, K.-H., Chen, S.-H., Chen, T.-H., Lo, P.-H., Chen, M., & Hsu, H.-T. (2021). An Automatic Platform Based on Nanostructured Microfluidic Chip for Isolating and Identification of Circulating Tumor Cells. Micromachines, 12(5), 473. https://doi.org/10.3390/mi12050473








