Temperature Field-Assisted Ultraviolet Nanosecond Pulse Laser Processing of Polyethylene Terephthalate (PET) Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laser Processing System
2.3. Experimental Design
2.4. Characterizations
3. Results and Discussion
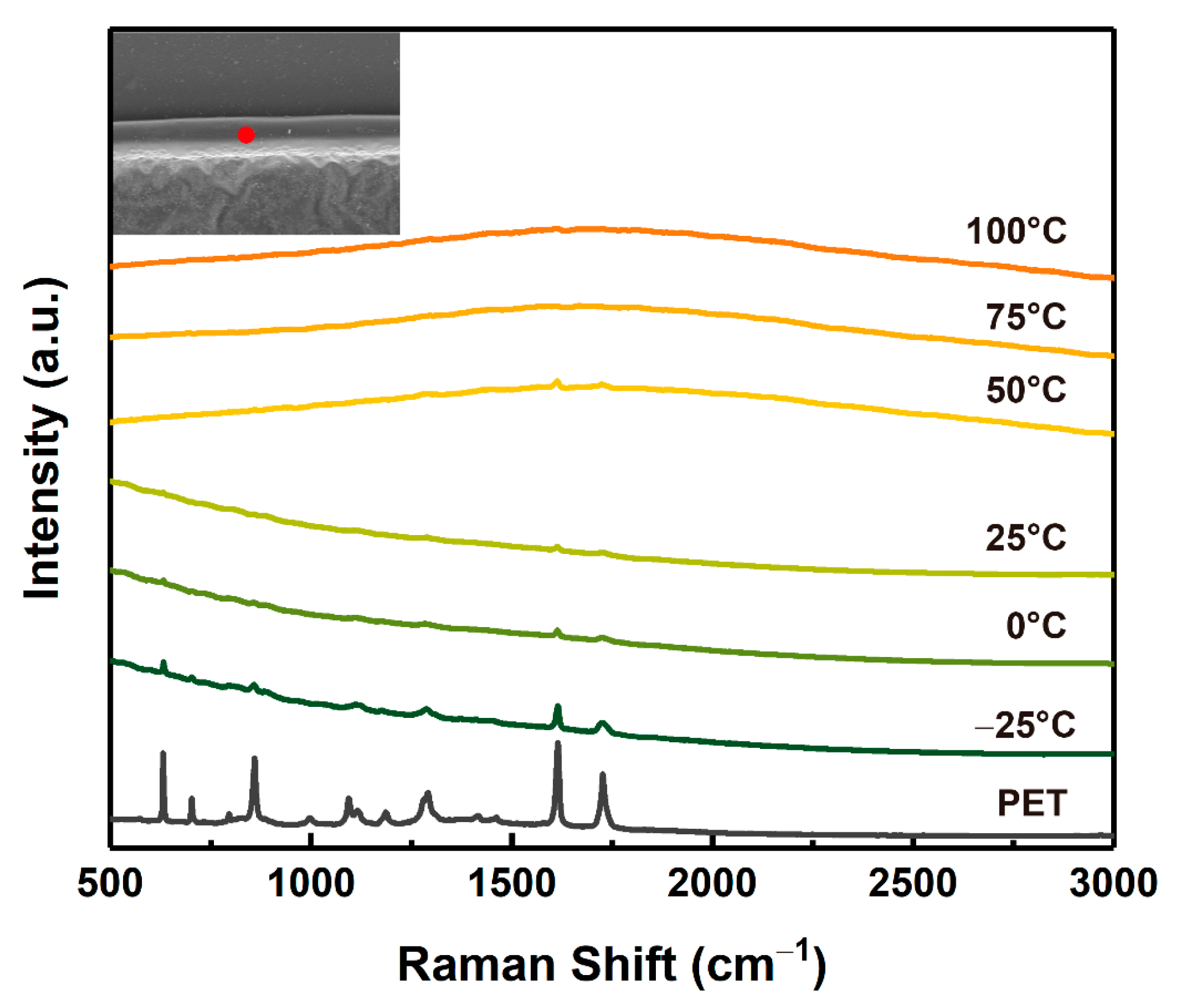
3.1. Laser Ablation Mechanism of PET Film
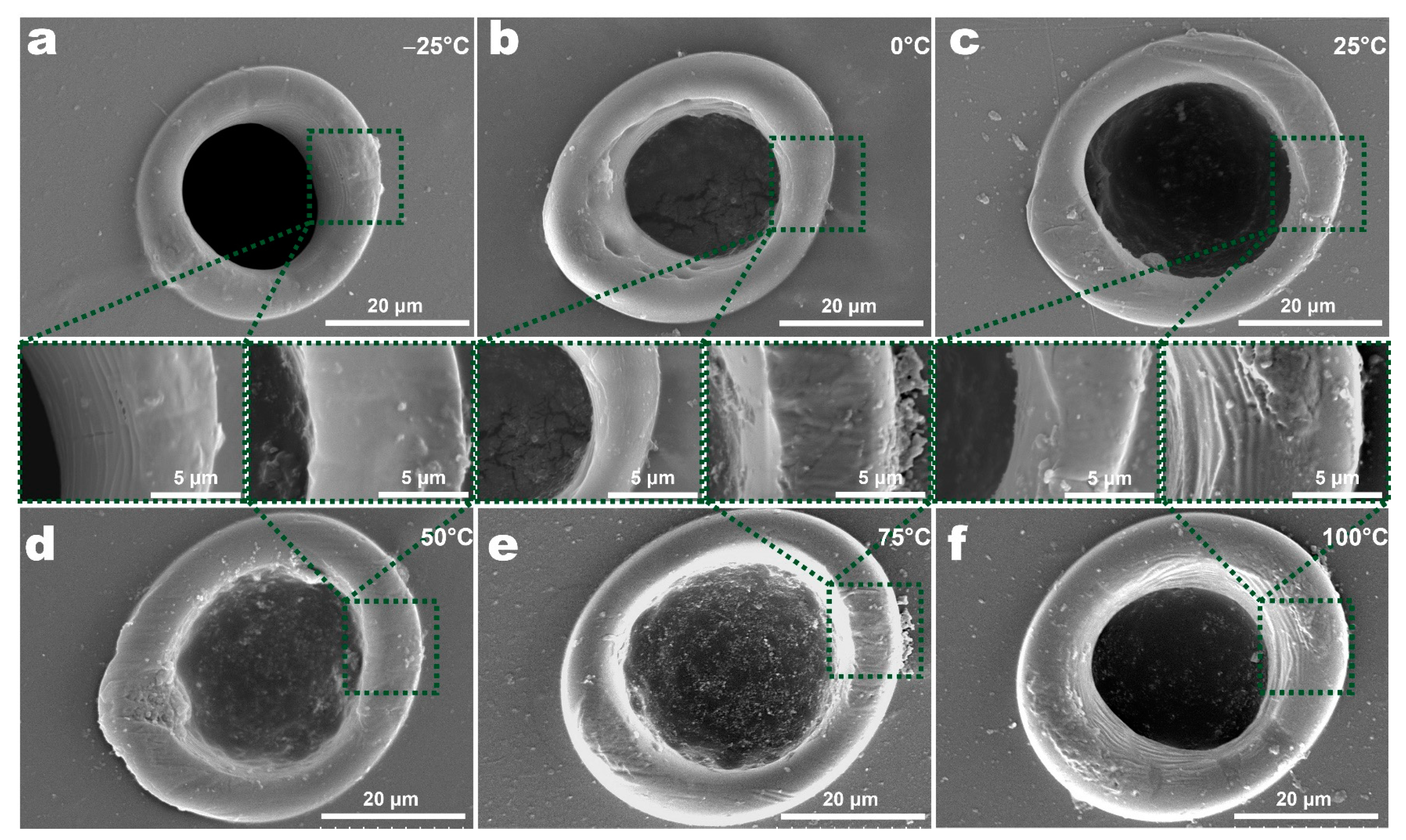
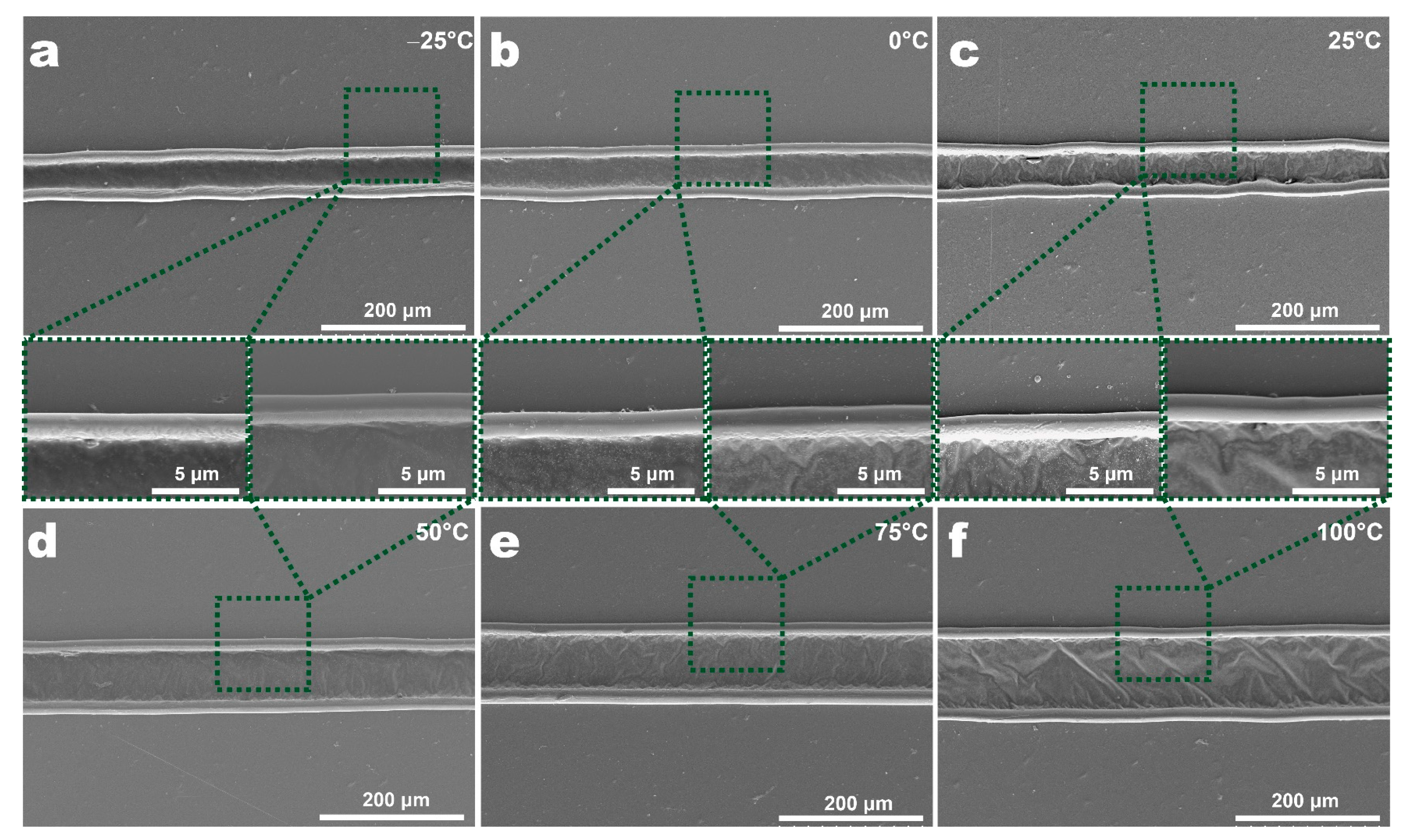
3.2. The Morphology of Features at Different Temperatures
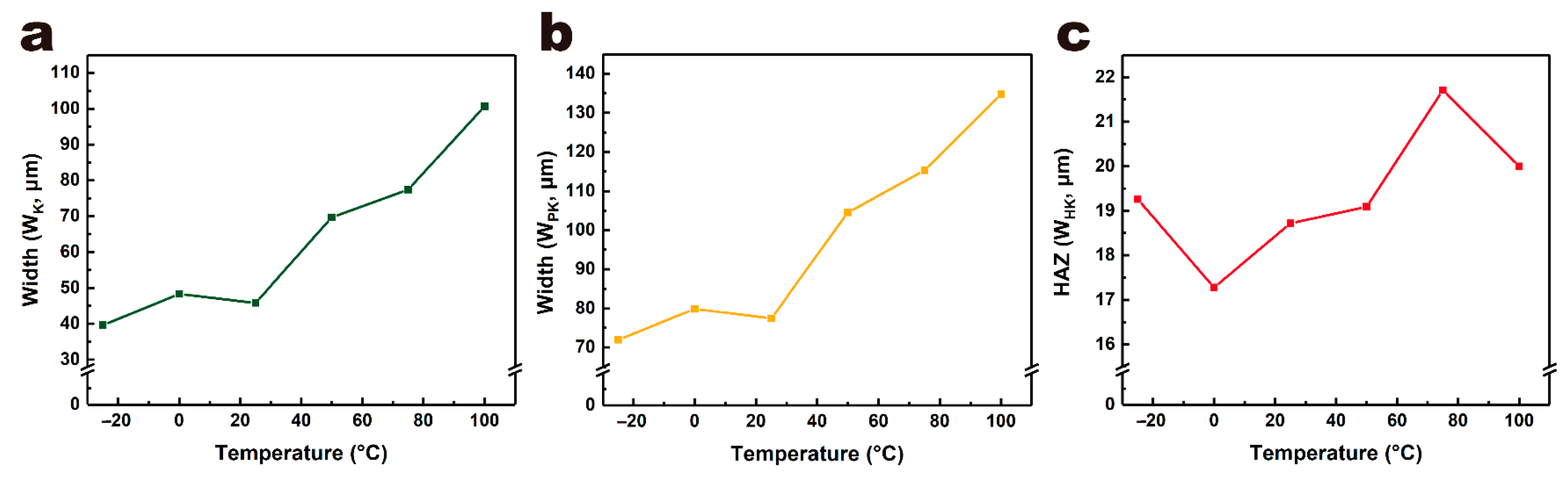
3.3. The Effects of Temperature on Laser Processing of PET Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zang, Y.; Shen, H.; Huang, D.; Di, C.A.; Zhu, D. A dual-organic-transistor-based tactile-perception system with signal-processing functionality. Adv. Mater. 2017, 29, 1606088. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, Y.; Lai, W.Y.; Huang, W. Stretchable thin-film electrodes for flexible electronics with high deformability and stretchability. Adv. Mater. 2015, 27, 3349–3376. [Google Scholar] [CrossRef]
- Kim, M.P.; Ahn, C.W.; Lee, Y.; Kim, K.; Park, J.; Ko, H. Interfacial polarization-induced high-k polymer dielectric film for high-performance triboelectric devices. Nano Energy 2021, 82, 105697. [Google Scholar] [CrossRef]
- Singha, S.S.; Khareb, A.; Joshi, S.N. Fabrication of microchannel on polycarbonate below the laser ablation threshold by repeated scan via the second harmonic of Q-switched Nd:YAG laser. J. Mater. Process. Technol. 2020, 55, 359–372. [Google Scholar] [CrossRef]
- Dahotre, N.B.; Harimkar, S. Laser Fabrication and Machining of Material; Springer: Boston, MA, USA, 2001. [Google Scholar] [CrossRef]
- Chryssolouris, G. Laser Machining—Theory and Practice, Mechanical Engineering Series; Springer: New York, NY, USA, 1991. [Google Scholar] [CrossRef]
- Kamlage, G.; Bauer, T.; Ostendorf, A.; Chichkov, B.N. Deep drilling of metals by femtosecond laser pulses. Appl. Phys. A. 2003, 77, 307–310. [Google Scholar] [CrossRef]
- Jia, W.; Yu, J.; Chai, L.; Wang, C.Y. Micromachining soda-lime glass by femtosecond laser pulses. Front. Phys. 2015, 10, 1–4. [Google Scholar] [CrossRef]
- Frank, P.; Shaw-Stewart, J.; Lippert, T.; Boneberg, J.; Leiderer, P. Laser-induced ablation dynamics and flight of thin polymer films. Appl. Phys. A 2011, 104, 579–582. [Google Scholar] [CrossRef][Green Version]
- Jiang, H.; Ma, C.; Li, M.; Cao, Z. Femtosecond laser drilling of cylindrical holes for carbon fiber-reinforced polymer (CFRP) composites. Molecules 2021, 26, 2953. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, S.A.J.; Chua, K.L.; Black, I. Experimental development of a machining database for the CO2 laser cutting of ceramic tile. J. Laser Appl. 1997, 9, 233–241. [Google Scholar] [CrossRef]
- Wu, C.; Li, M.; Huang, Y.; Rong, Y. Cutting of polyethylene terephthalate (PET) film by 355 nm nanosecond laser. Opt. Laser Technol. 2021, 133, 106565. [Google Scholar] [CrossRef]
- Ravi-Kumar, S.; Lies, B.; Lyu, H.; Qin, H. Laser ablation of polymers: A review. Procedia Manuf. 2019, 34, 316–327. [Google Scholar] [CrossRef]
- Rong, Y.; Huang, Y.; Lin, C.; Liu, Y.; Shi, S.; Zhang, G.; Wu, C. Stretchability improvement of flexible electronics by laser micro-drilling array holes in PDMS film. Opt. Lasers Eng. 2020, 134, 106307. [Google Scholar] [CrossRef]
- Beinhorn, F.; Ihlemann, J.; Luther, K.; Troe, J. Micro-lens arrays generated by UV laser irradiation of doped PMMA. Appl. Phys. A 1999, 68, 709–713. [Google Scholar] [CrossRef]
- Yong, J.; Fang, Y.; Chen, F.; Huo, J.; Yang, Q.; Bian, H.; Du, G.; Hou, X. Femtosecond laser ablated durable superhydrophobic PTFE films with micro-through-holes for oil/water separation: Separating oil from water and corrosive solutions. Appl. Surf. Sci. 2016, 389, 1148–1155. [Google Scholar] [CrossRef]
- Caiazzo, F.; Curcio, F.; Daurelio, G.; Minutolo, F.M.C. Laser cutting of different polymeric plastics (PE, PP and PC) by a CO2 laser beam. J. Mater. Process. Technol. 2005, 159, 279–285. [Google Scholar] [CrossRef]
- Schmidt, H.; Ihlemann, J.; Wolff-Rottke, B.; Luther, K.; Troe, J. Ultraviolet laser ablation of polymers: Spot size, pulse duration, and plume attenuation effects explained. J. Appl. Phys. 1998, 83, 5458–5468. [Google Scholar] [CrossRef]
- Stankova, N.E.; Atanasov, P.A.; Nedyalkov, N.N.; Stoyanchov, T.R.; Kolev, K.N.; Valova, E.I.; Georgieva, J.S.; Armyanov, S.A.; Amoruso, S.; Wang, X.; et al. fs- and ns-laser processing of polydimethylsiloxane (PDMS) elastomer: Comparative study. Appl. Surf. Sci. 2015, 336, 321–328. [Google Scholar] [CrossRef]
- Lipperta, T.; Wei, J.; Wokaun, A.; Hoogen, N.; Nuyken, O. Polymers designed for laser microstructuring. Appl. Surf. Sci. 2000, 168, 270–272. [Google Scholar] [CrossRef]
- Ahmed, N.; Darwish, S.; Alahmari, A.M. Laser ablation and laser-hybrid ablation processes: A review. Mater. Manuf. Process. 2016, 31, 1121–1142. [Google Scholar] [CrossRef]
- Lippert, T.; Yabe, A.; Wokaun, A. Laser ablation of doped polymer systems. Adv. Mater. 1997, 9, 105–119. [Google Scholar] [CrossRef]
- Shen, H.; Liu, J.; Chen, Y.; Zhang, J.; Zhang, Z.; Guan, N.; Zhang, F.; Huang, L.; Zhao, D.; Jin, Z.; et al. Investigation on time stability of laser-textured patterned surfaces under different temperatures. Surf. Coat. Technol. 2020, 400, 126225. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, R.; Jiao, H.; Bao, J.; Liu, Q.; Long, Y. Study on the mechanism of ultrasonic-assisted water confined laser micromachining of silicon. Opt. Lasers Eng. 2020, 132, 106118. [Google Scholar] [CrossRef]
- Bityurin, N.; Luk’yanchuk, B.S.; Hong, M.H.; Chong, T.C. Models for laser ablation of polymers. Chem. Rev. 2003, 103, 519–552. [Google Scholar] [CrossRef]
- Kim, W.G.; Kim, D.W.; Tcho, I.W.; Kim, J.K.; Kim, M.S.; Choi, Y.K. Triboelectric nanogenerator: Structure, mechanism, and applications. ACS Nano 2021, 15, 258–287. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriege, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Shin, B.; Oh, J.Y. Photothermal and photochemical investigation on laser ablation of the polyimide by 355nm UV laser processing. J. Korean Soc. Precis. Eng. 2007, 24, 147–152. [Google Scholar]
- Parvinzadeh, M.; Moradian, S.; Rashidi, A.; Yazdanshenas, M.E. Surface characterization of polyethylene terephthalate/silica nanocomposites. Appl. Surf. Sci. 2010, 256, 2792–2802. [Google Scholar] [CrossRef]
- Mohan, J. Organic Spectroscopy: Principle & Application; Mehra: New Delhi, India, 2000. [Google Scholar]
- Bistričić, L.; Borjanović, V.; Leskovac, M.; Mikac, L.; McGuire, G.E.; Shenderova, O.; Nunn, N. Raman spectra, thermal and mechanical properties of poly(ethylene terephthalate) carbon-based nanocomposite films. J. Polym. Res. 2015, 22, 39. [Google Scholar] [CrossRef]
- Sattmann, R.; Mönch, I.; Krause, H.; Noll, R.; Couris, S.; Hatziapostolou, A.; Mavromanolakis, A.; Fotakis, C.; Larrauri, E.; Miguel, R. Laser-Induced Breakdown Spectroscopy for Polymer Identification. Appl. Spectrosc. 1998, 52, 456–461. [Google Scholar] [CrossRef]
- Mansour, N.; Ghaleh, K.J. Ablation of polyethylene terephthalate at 266 nm. Appl. Phys. A 2002, 74, 63–67. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamamoto, M. Laser ablation of poly(ethylene terephthalate). J. Appl. Polym. Sci. 1997, 64, 1203–1209. [Google Scholar] [CrossRef]
- Klose, S.; Arenholz, E.; Heitz, J.; Bäuerle, D. Laser-induced dendritic structures on PET (polyethylene- terephthalate): The importance of redeposited ablation products. Appl. Phys. A 1999, 69, 487–490. [Google Scholar] [CrossRef]
- Wu, C.; Xu, J.; Zhang, T.; Xin, G.; Li, M.; Rong, Y.; Zhang, G.; Huang, Y. Precision cutting of PDMS film with UV-nanosecond laser based on heat generation-diffusion regulation. Opt. Laser Technol. 2022, 145, 107462. [Google Scholar] [CrossRef]
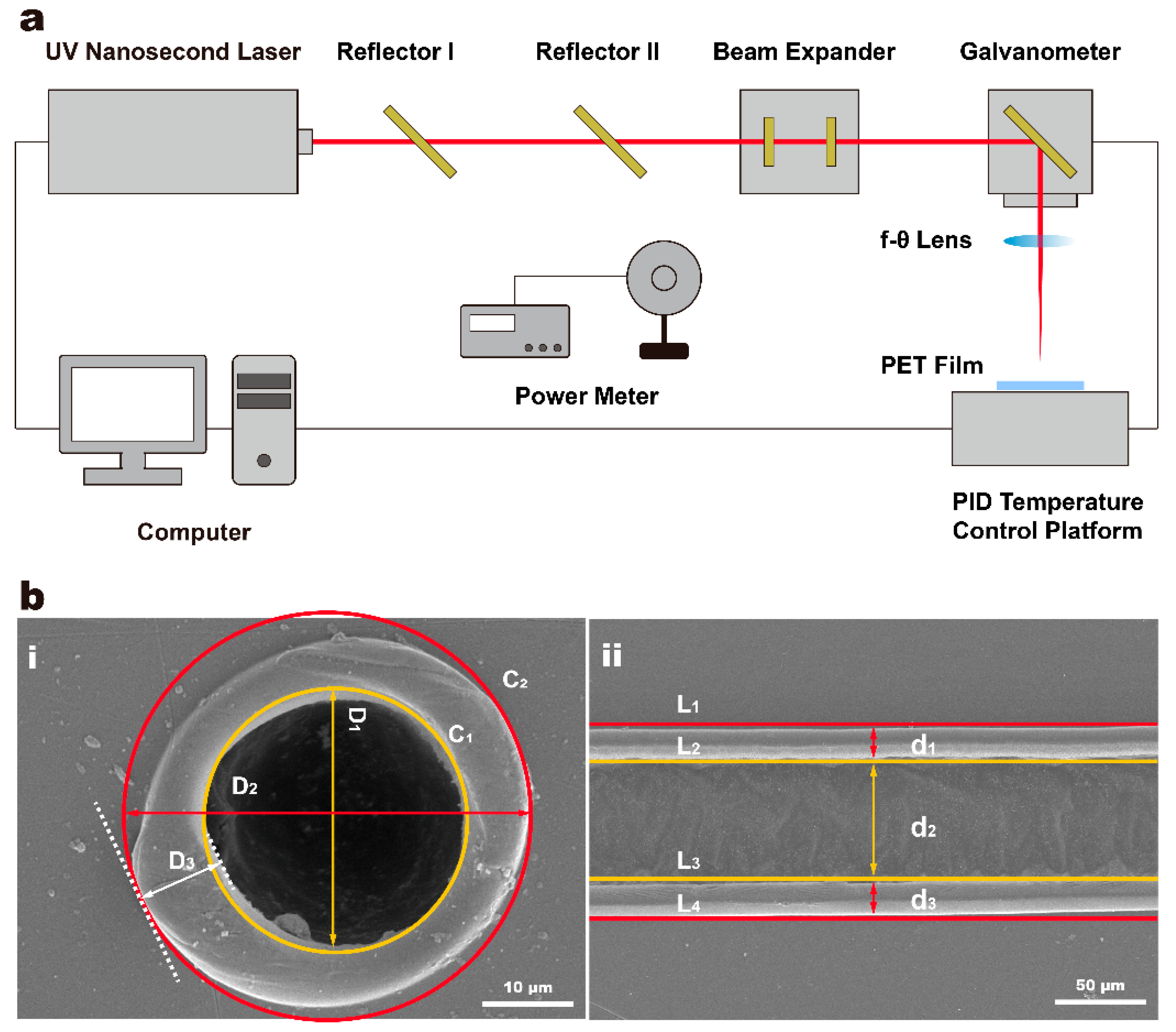
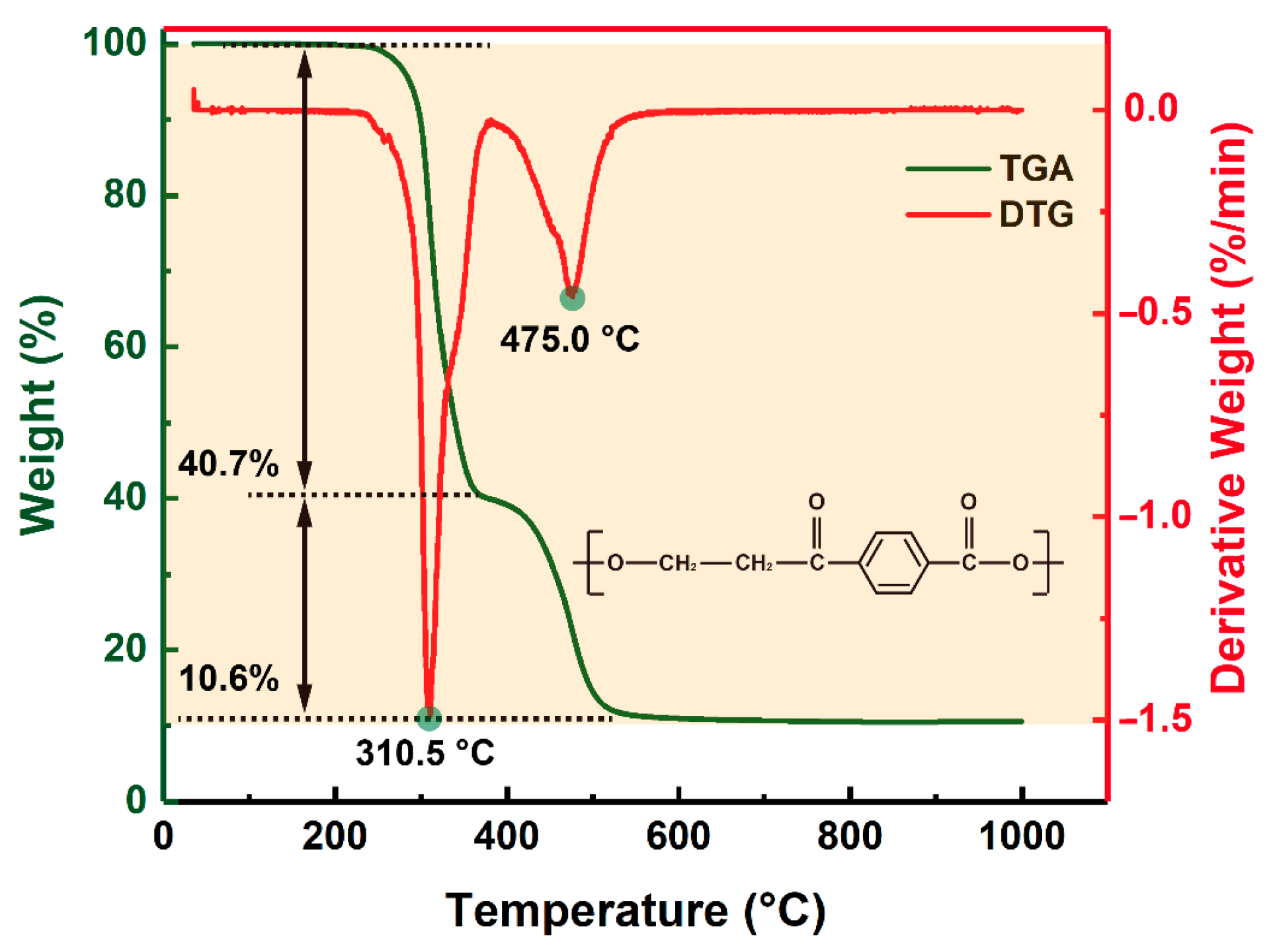
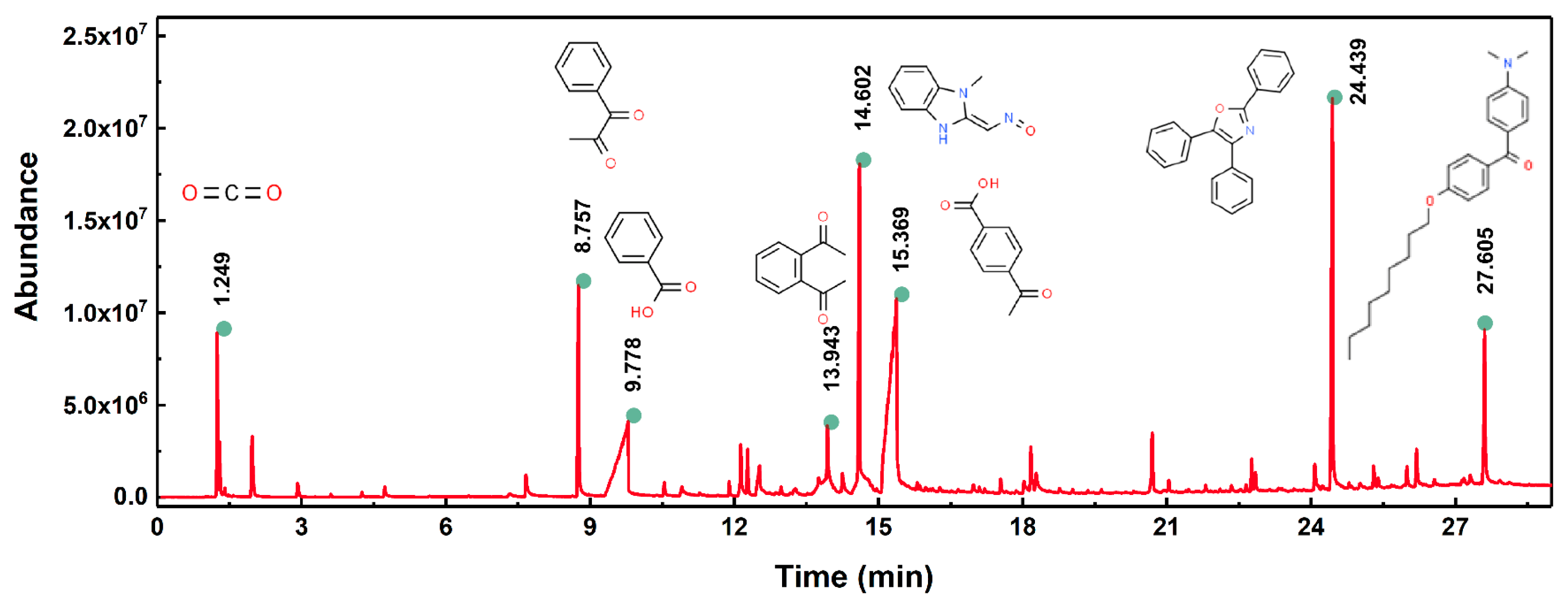

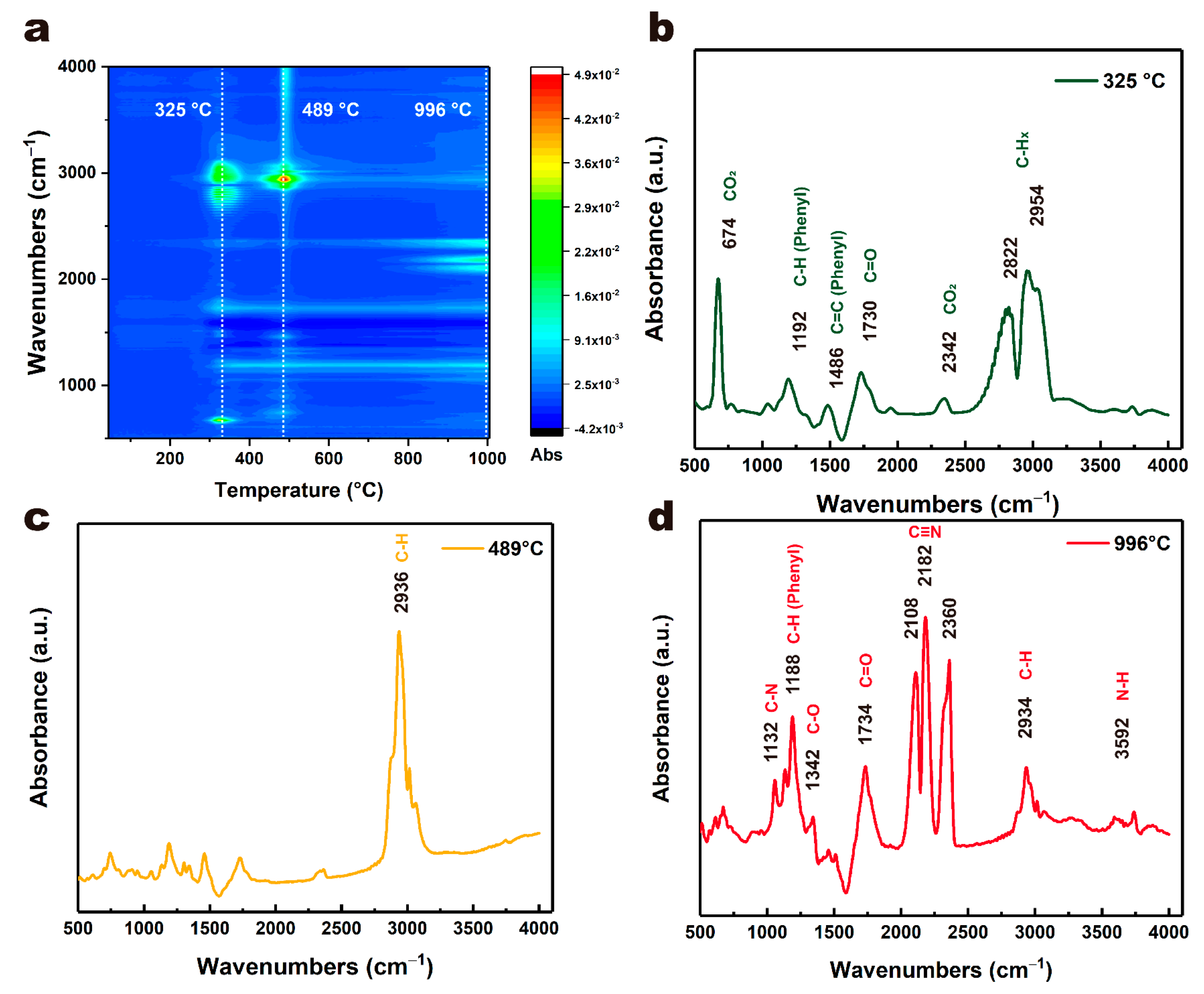



| Parameters | Values |
|---|---|
| Wavelength | 355 nm |
| Pulse width | 16 ± 2 ns@50 kHz |
| Beam diameter | >11.8 μm |
| Focal length | 167 mm |
| Experiment | No. | Temperature (°C) | Parameters |
|---|---|---|---|
| Single-Point Laser Processing | 1 | −25 | Laser Power = 0.9 W Repetition Frequency = 200 kHZ Duration Time = 0.05s |
| 2 | 0 | ||
| 3 | 25 | ||
| 4 | 50 | ||
| 5 | 75 | ||
| 6 | 100 | ||
| Line Laser Processing | 1 | −25 | Laser Power = 0.9 W Repetition Frequency = 200 kHz Scanning Speed = 50 mm/s Scanning Times = 30 |
| 2 | 0 | ||
| 3 | 25 | ||
| 4 | 50 | ||
| 5 | 75 | ||
| 6 | 100 |
| Peak | RT (min) | Area (%) | ID | CAS# | 2D | 3D |
|---|---|---|---|---|---|---|
| 1# | 1.249 | 3.90 | Carbon dioxide | 000124-38-9 | 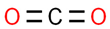 |  |
| 2# | 8.757 | 4.65 | 1-Phenyl-1,2-propanedione | 000579-07-7 | 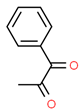 |  |
| 3# | 9.778 | 12.56 | Benzoic acid | 000065-85-0 | 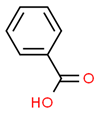 |  |
| 4# | 13.943 | 3.90 | o-Diacetylbenzene | 000704-00-7 | 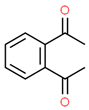 |  |
| 5# | 14.602 | 11.56 | Benzimidazole-2-carboxaldehyde, 1-methyl-, oxime | 003013-07-8 |  | 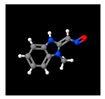 |
| 6# | 15.369 | 26.17 | 4-Acetylbenzoic acid | 000586-89-0 |  | 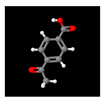 |
| 7# | 24.439 | 11.67 | 2,4,5-triphenyl-1,3-oxazole | 000573-34-2 | 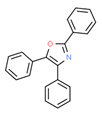 | 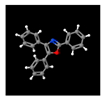 |
| 8# | 27.605 | 4.28 | (4-Dimethylamino-phenyl)-(4-nonyloxy-phenyl)-methanone | 300382-52-9 | 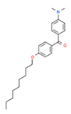 | 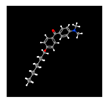 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Rong, Y.; Liu, W.; Zhang, T.; Xin, G.; Huang, Y.; Wu, C. Temperature Field-Assisted Ultraviolet Nanosecond Pulse Laser Processing of Polyethylene Terephthalate (PET) Film. Micromachines 2021, 12, 1356. https://doi.org/10.3390/mi12111356
Xu J, Rong Y, Liu W, Zhang T, Xin G, Huang Y, Wu C. Temperature Field-Assisted Ultraviolet Nanosecond Pulse Laser Processing of Polyethylene Terephthalate (PET) Film. Micromachines. 2021; 12(11):1356. https://doi.org/10.3390/mi12111356
Chicago/Turabian StyleXu, Jun, Youmin Rong, Weinan Liu, Tian Zhang, Guoqiang Xin, Yu Huang, and Congyi Wu. 2021. "Temperature Field-Assisted Ultraviolet Nanosecond Pulse Laser Processing of Polyethylene Terephthalate (PET) Film" Micromachines 12, no. 11: 1356. https://doi.org/10.3390/mi12111356
APA StyleXu, J., Rong, Y., Liu, W., Zhang, T., Xin, G., Huang, Y., & Wu, C. (2021). Temperature Field-Assisted Ultraviolet Nanosecond Pulse Laser Processing of Polyethylene Terephthalate (PET) Film. Micromachines, 12(11), 1356. https://doi.org/10.3390/mi12111356








