A Sensitivity-Enhanced Electrolyte-Gated Graphene Field-Effect Transistor Biosensor by Acoustic Tweezers
Abstract
:1. Introduction
2. Methods
2.1. Mechanism Analysis
2.2. Measurement Setup
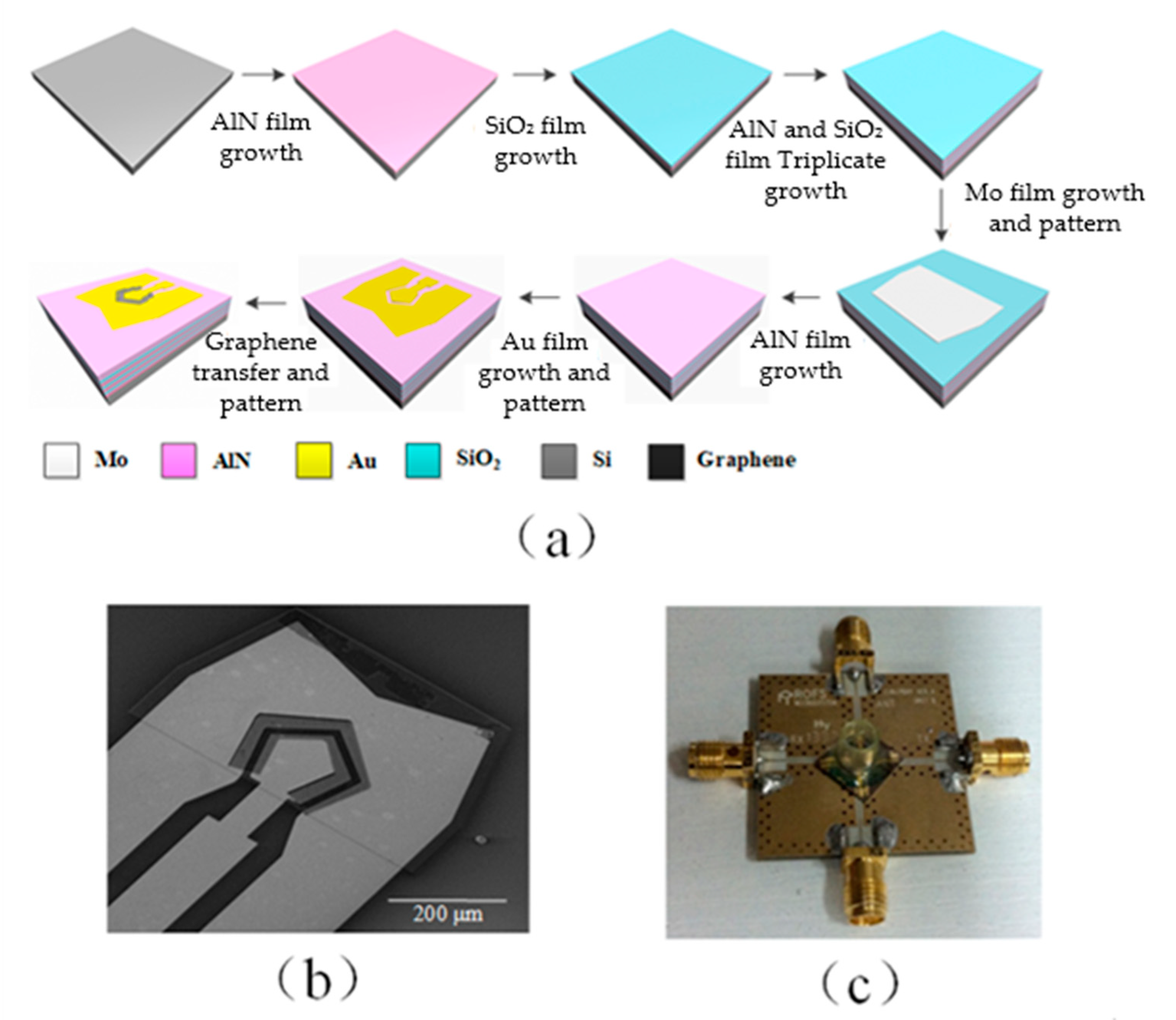
2.3. Fabrication of Device
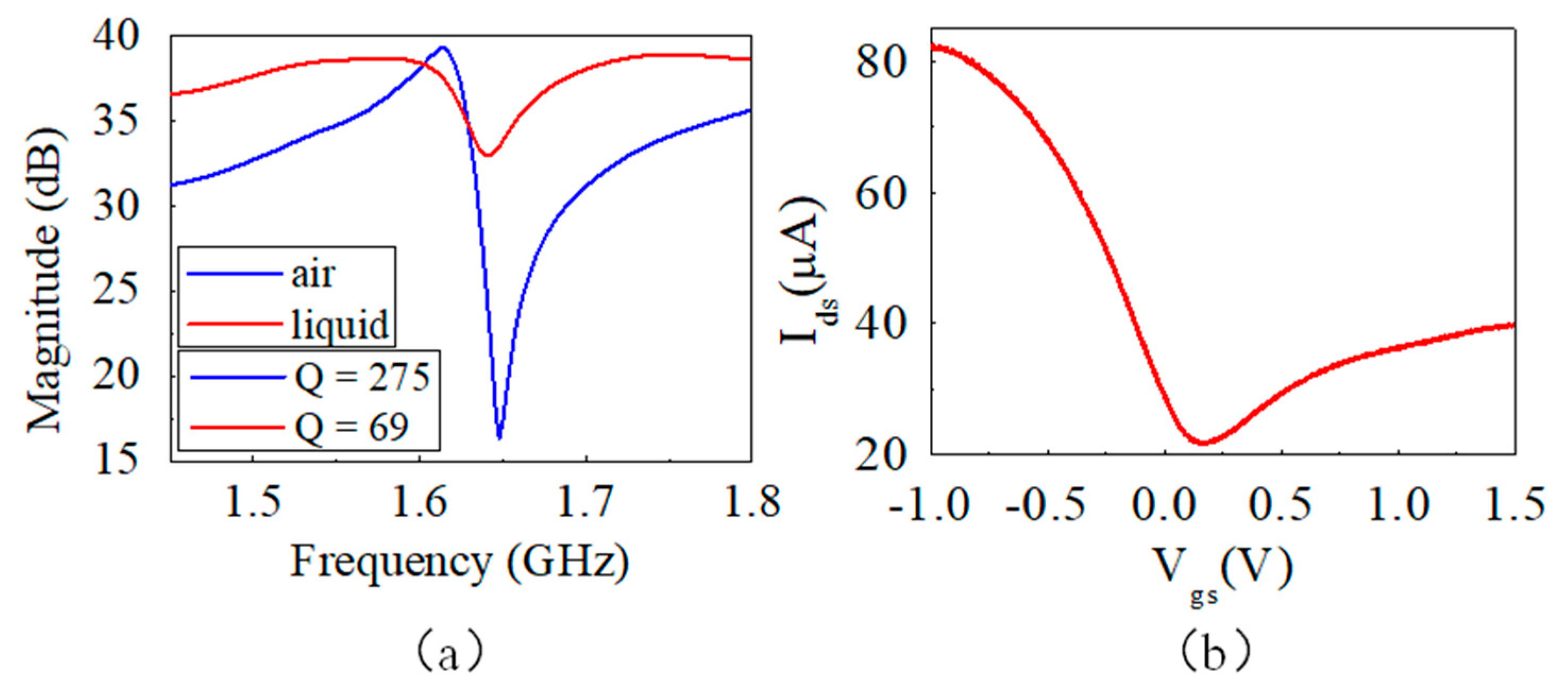
2.4. Characterization of Device
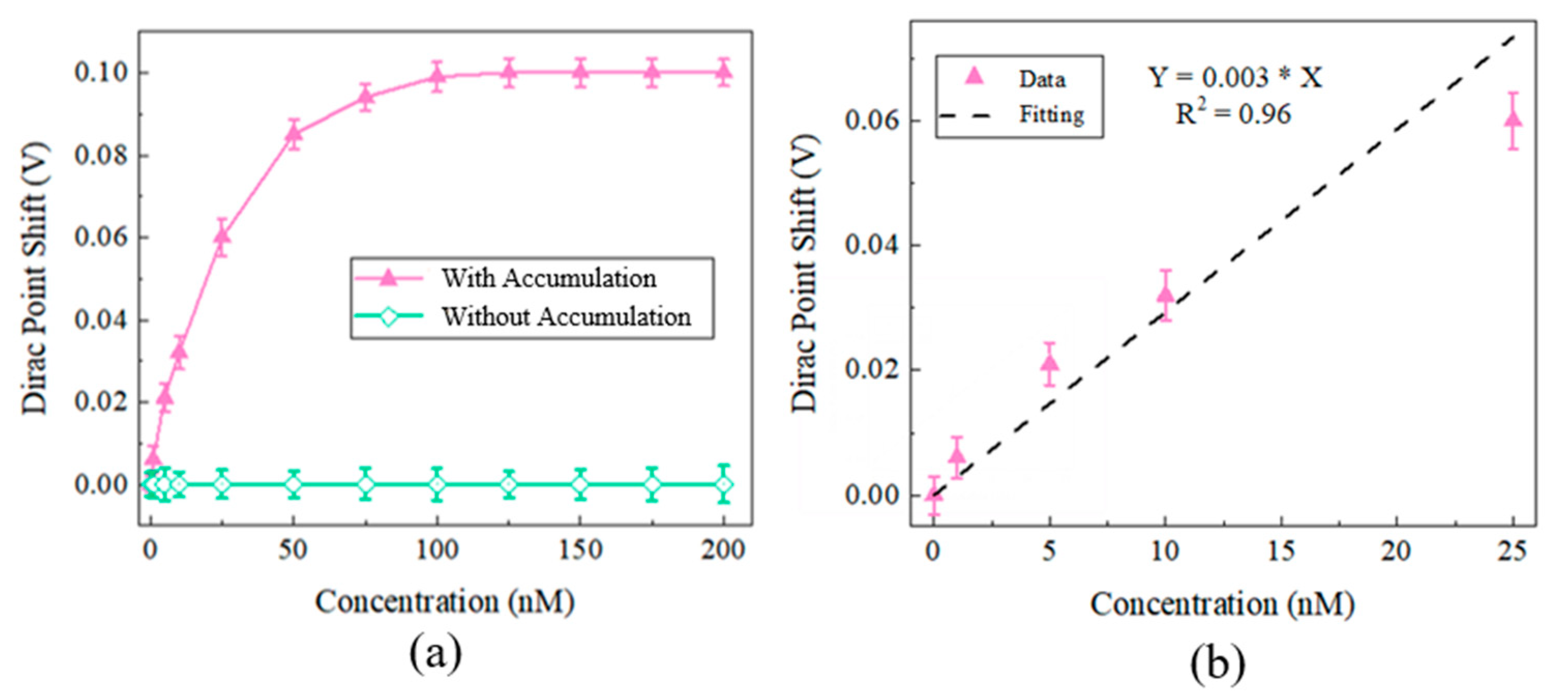
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahshid, S.S.; Camire, S.; Ricci, F.; Vallee-Belisle, A. A Highly Selective Electrochemical DNA-Based Sensor That Employs Steric Hindrance Effects to Detect Proteins Directly in Whole Blood. J. Am. Chem. Soc. 2015, 137, 15596–15599. [Google Scholar] [CrossRef]
- Kosaka, P.; Pini, V.; Ruz, J.J.; da Silva, R.; Ujue, M.; Ramos, D.; Calleja, M.; Tamayo, J. Detection of cancer biomarkers in serum using a hybrid mechanical and optoplasmonic nanosensor. Nat. Nanotechnol. 2014, 9, 1047–1053. [Google Scholar] [CrossRef]
- Meister, S.; Plouffe, D.M.; Kuhen, K.L.; Bonamy, G.M.C.; Wu, T.; Barnes, S.W.; Bopp, S.E.; Borboa, R.; Bright, A.T.; Che, J.W.; et al. Imaging of Plasmodium Liver Stages to Drive Next-Generation Antimalarial Drug Discovery. Science 2011, 334, 1372–1377. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.P.; Pan, S.T.; Zhang, H.X.; Tang, Z.F.; Liang, J.; Wang, Y.Y.; Zhang, M.L.; Hu, X.D.; Pang, W.; Duan, X.X. A Universal Biomolecular Concentrator To Enhance Biomolecular Surface Binding Based on Acoustic NEMS Resonator. ACS Cent. Sci. 2018, 4, 899–908. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Yamashiro, Y.; Matsumoto, K. Electrolyte-Gated Graphene Field-Effect Transistors for Detecting pH Protein Adsorption. Nano Lett. 2009, 9, 3318–3322. [Google Scholar] [CrossRef]
- Scarano, S.; Mascini, M.; Turner, A.P.F.; Minunni, M. Surface plasmon resonance imaging for affinity-based biosensors. Biosens. Bioelectron. 2010, 25, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Mannelli, I.; Minunni, M.; Tombelli, S.; Mascini, M. Quartz crystal microbalance (QCM) affinity biosensor for genetically modified organisms (GMOs) detection. Biosens. Bioelectron. 2003, 18, 129–140. [Google Scholar] [CrossRef]
- Guo, X.F.; Kulkarni, A.; Doepke, A.; Halsall, H.B.; Iyer, S.; Heineman, W.R. Carbohydrate-Based Label-Free Detection of Escherichia coli ORN 178 Using Electrochemical Impedance Spectroscopy. Anal. Chem. 2012, 84, 241–246. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Han, M.Y.; Ozyilmaz, B.; Zhang, Y.B.; Kim, P. Energy band-gap engineering of graphene nanoribbons. Phys. Rev. Lett. 2007, 98, 206805. [Google Scholar] [CrossRef] [Green Version]
- Meric, I.; Han, M.Y.; Young, A.F.; Ozyilmaz, B.; Kim, P.; Shepard, K.L. Current saturation in zero-bandgap, topgated graphene field-effect transistors. Nat. Nanotechnol. 2008, 3, 654–659. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of Graphene's Properties by Reversible Hydrogenation: Evidence for Graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef] [Green Version]
- Nagashio, K.; Nishimura, T.; Kita, K.; Toriumi, A. Mobility Variations in Mono- and Multi-Layer Graphene Films. Appl. Phys. Express 2009, 2, 025003. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.C.; Shi, Y.M.; Huang, W.; Chen, P.; Li, L.J. Electrical Detection of DNA Hybridization with Single-Base Specificity Using Transistors Based on CVD-Grown Graphene Sheets. Adv. Mater. 2010, 22, 1649–1653. [Google Scholar] [CrossRef]
- Kakatkar, A.; Abhilash, T.S.; De Alba, R.; Parpia, J.M.; Craighead, H.G. Detection of DNA and poly-l-lysine using CVD graphene-channel FET biosensors. Nanotechnology 2015, 26, 125502. [Google Scholar] [CrossRef] [Green Version]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef]
- Isaacs, J.S.; Xu, W.P.; Neckers, L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 2003, 3, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Shangary, S.; Wang, S.M. Small-Molecule Inhibitors of the MDM2-p53 Protein-Protein Interaction to Reactivate p53 Function: A Novel Approach for Cancer Therapy. Annu. Rev. Pharmacol. 2009, 49, 223–241. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Junkin, M.; Kim, D.H.; Kwon, S.; Shin, Y.S.; Wong, P.K.; Gale, B.K. Applications, techniques, and microfluidic interfacing for nanoscale biosensing. Microfluid. Nanofluid. 2009, 7, 149–167. [Google Scholar] [CrossRef]
- Kim, D.R.; Zheng, X.L. Numerical Characterization and Optimization of the Microfluidics for Nanowire Biosensors. Nano Lett. 2008, 8, 3233–3237. [Google Scholar] [CrossRef]
- Liao, K.T.; Chou, C.F. Nanoscale Molecular Traps and Dams for Ultrafast Protein Enrichment in High-Conductivity Buffers. J. Am. Chem. Soc. 2012, 134, 8742–8745. [Google Scholar] [CrossRef]
- Eijkel, J.C.T.; van den Berg, A. NANOFLUIDICS Tiny electrostatic traps. Nature 2010, 467, 666–667. [Google Scholar] [CrossRef]
- Lien, K.Y.; Lee, W.C.; Lei, H.Y.; Lee, G.B. Integrated reverse transcription polymerase chain reaction systems for virus detection. Biosens. Bioelectron. 2007, 22, 1739–1748. [Google Scholar] [CrossRef]
- Lee, S.; Hohng, S. An Optical Trap Combined with Three-Color FRET. J. Am. Chem. Soc. 2013, 135, 18260–18263. [Google Scholar] [CrossRef]
- Grier, D.G. A revolution in optical manipulation. Nature 2003, 424, 810–816. [Google Scholar] [CrossRef]
- Wang, K.; Schonbrun, E.; Steinvurzel, P.; Crozier, K.B. Trapping and rotating nanoparticles using a plasmonic nano-tweezer with an integrated heat sink. Nat. Commun. 2011, 2, 469. [Google Scholar] [CrossRef] [Green Version]
- Nyborg, W.L. Acoustic Streaming Due to Attenuated Plane Waves. J. Acoust. Soc. Am. 1953, 25, 68–75. [Google Scholar] [CrossRef]
- Mitome, H. The mechanism of generation of acoustic streaming. Electron. Comm. Jpn. 1998, 81, 1–8. [Google Scholar] [CrossRef]
- Pan, S.T.; Zhang, H.X.; Liu, W.P.; Wang, Y.Y.; Pang, W.; Duan, X.X. Biofouling Removal and Protein Detection Using a Hypersonic Resonator. ACS Sens. 2017, 2, 1175–1183. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Feng, Z.H.; Zhang, H.X.; Zhang, R.; Yu, Y.Y.; Tao, J.; Zhao, H.Y.; Guo, W.L.; Pang, W.; et al. Chemiresistive and Gravimetric Dual-Mode Gas Sensor toward Target Recognition and Differentiation. ACS Appl. Mater. Interfaces 2016, 8, 21742–21749. [Google Scholar] [CrossRef]
- Chung, C.-J.; Chen, Y.-C.; Cheng, C.-C.; Kao, K.-S. Fabrication and frequency response of solidly mounted resonators with 1/4λ mode configuration. Thin Solid Films 2008, 516, 5277–5281. [Google Scholar] [CrossRef]
- Ibsen, S.D.; Wright, J.; Lewis, J.M.; Kim, S.; Ko, S.Y.; Ong, J.; Manouchehri, S.; Vyas, A.; Akers, J.; Chen, C.C.; et al. Rapid Isolation and Detection of Exosomes and Associated Biomarkers from Plasma. ACS Nano 2017, 11, 6641–6651. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.J.; Fine, D.; Schmulen, J.; Hu, Y.; Godin, B.; Zhang, J.X.; Liu, X. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip 2013, 13, 2879–2882. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, W.; Zhang, H.; Zhang, D.; Guo, X. A Sensitivity-Enhanced Electrolyte-Gated Graphene Field-Effect Transistor Biosensor by Acoustic Tweezers. Micromachines 2021, 12, 1238. https://doi.org/10.3390/mi12101238
Chen Y, Liu W, Zhang H, Zhang D, Guo X. A Sensitivity-Enhanced Electrolyte-Gated Graphene Field-Effect Transistor Biosensor by Acoustic Tweezers. Micromachines. 2021; 12(10):1238. https://doi.org/10.3390/mi12101238
Chicago/Turabian StyleChen, Yan, Wenpeng Liu, Hao Zhang, Daihua Zhang, and Xiaoliang Guo. 2021. "A Sensitivity-Enhanced Electrolyte-Gated Graphene Field-Effect Transistor Biosensor by Acoustic Tweezers" Micromachines 12, no. 10: 1238. https://doi.org/10.3390/mi12101238
APA StyleChen, Y., Liu, W., Zhang, H., Zhang, D., & Guo, X. (2021). A Sensitivity-Enhanced Electrolyte-Gated Graphene Field-Effect Transistor Biosensor by Acoustic Tweezers. Micromachines, 12(10), 1238. https://doi.org/10.3390/mi12101238





