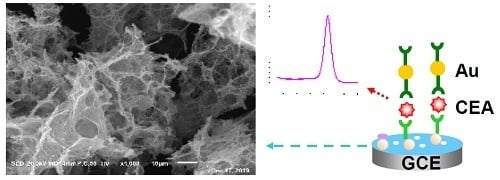
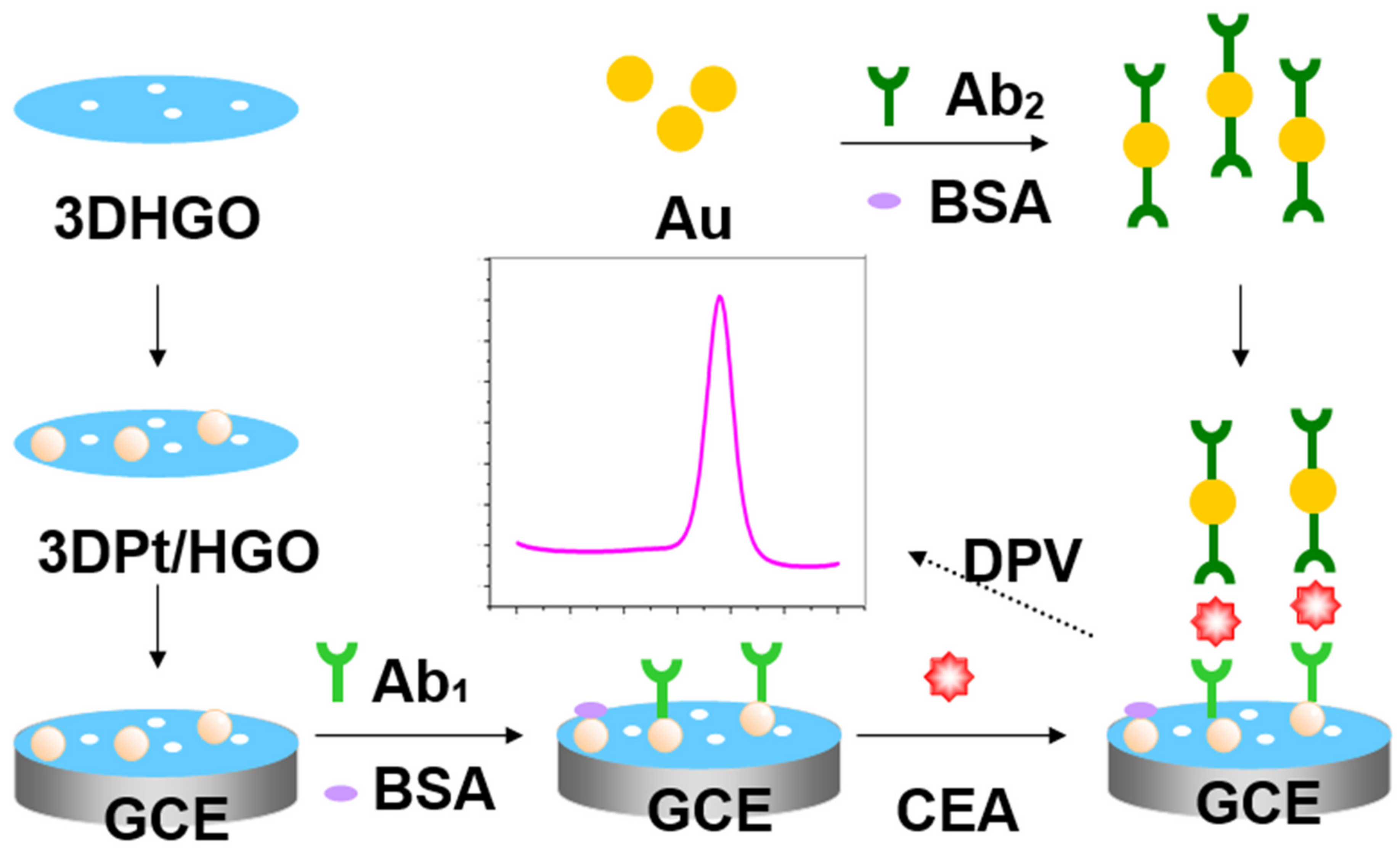
An Electrochemical Immunosensor for Sensitive Detection of the Tumor Marker Carcinoembryonic Antigen (CEA) Based on Three-Dimensional Porous Nanoplatinum/Graphene
Abstract
1. Introduction
2. Experimental Sections
2.1. Instruments and Reagents
2.2. Preparation of 3D Porous Graphene Oxide (3DHGO)
2.3. Preparation of 3DPt/HGO Composites
2.4. Preparation of Ab2-HRP/Au Bioconjugate
2.5. Fabrication of the Electrochemical Immunosensor
2.6. Electrochemical Detection
3. Results and Discussion
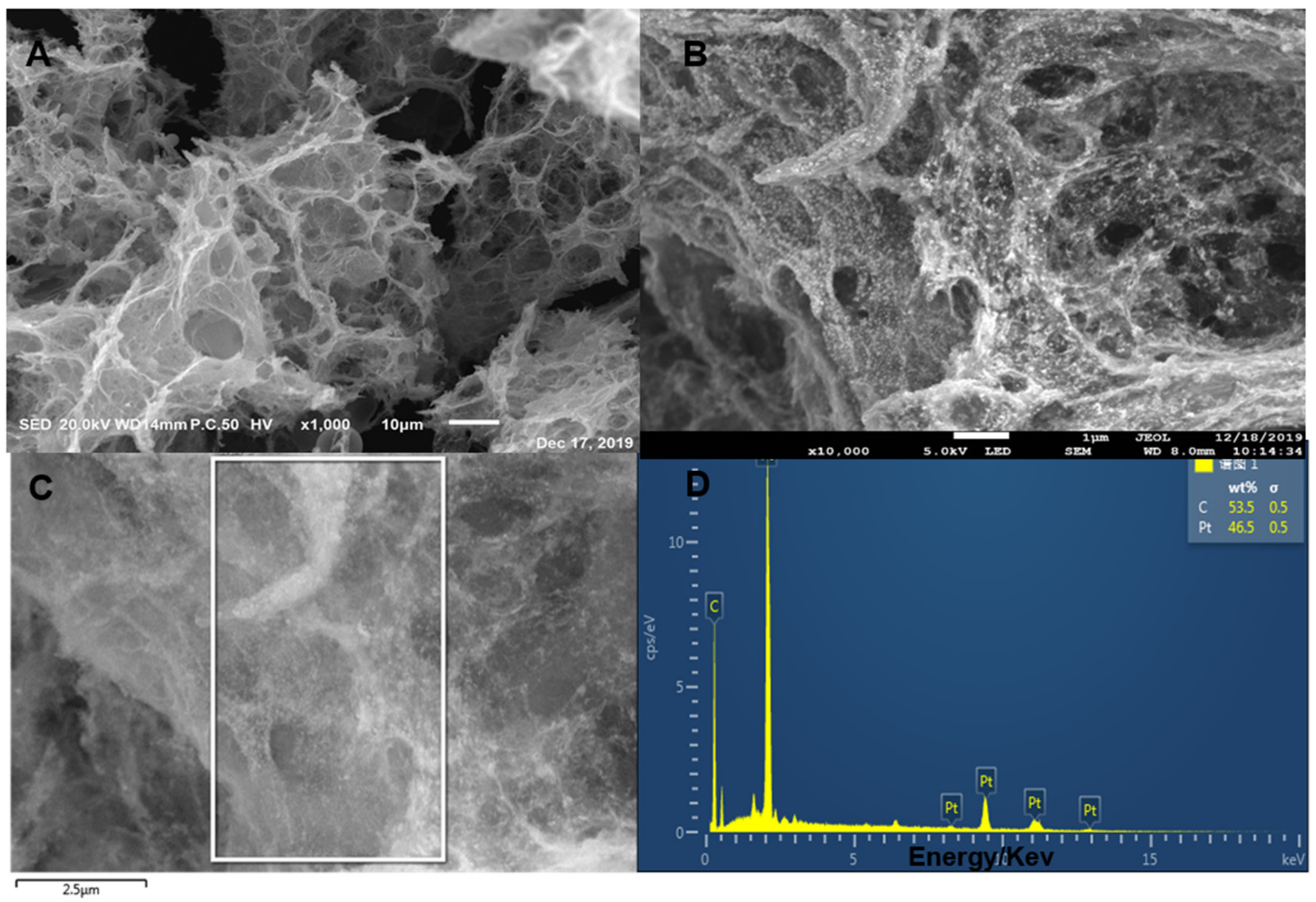
3.1. Characterization of Materials
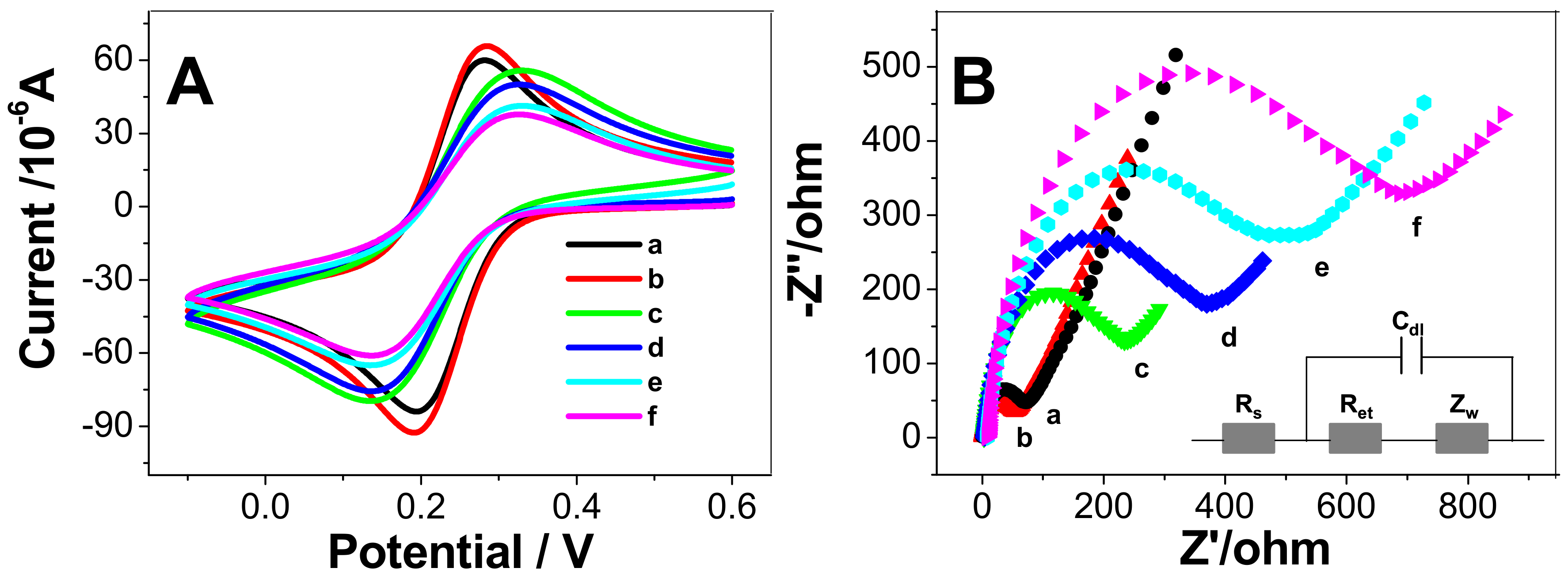
3.2. Electrochemical Behavior of Modified Electrodes
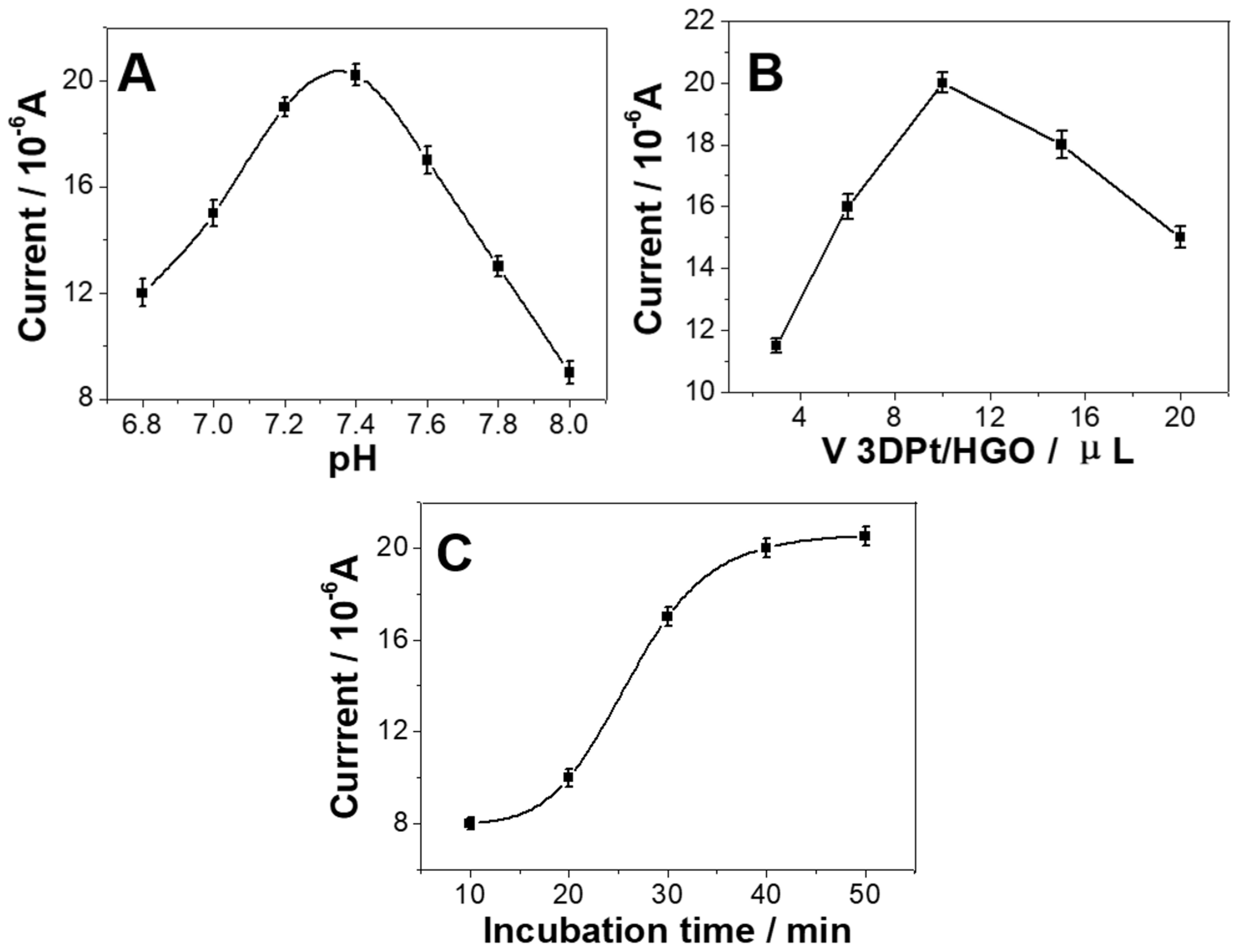
3.3. Optimization of Experimental Conditions
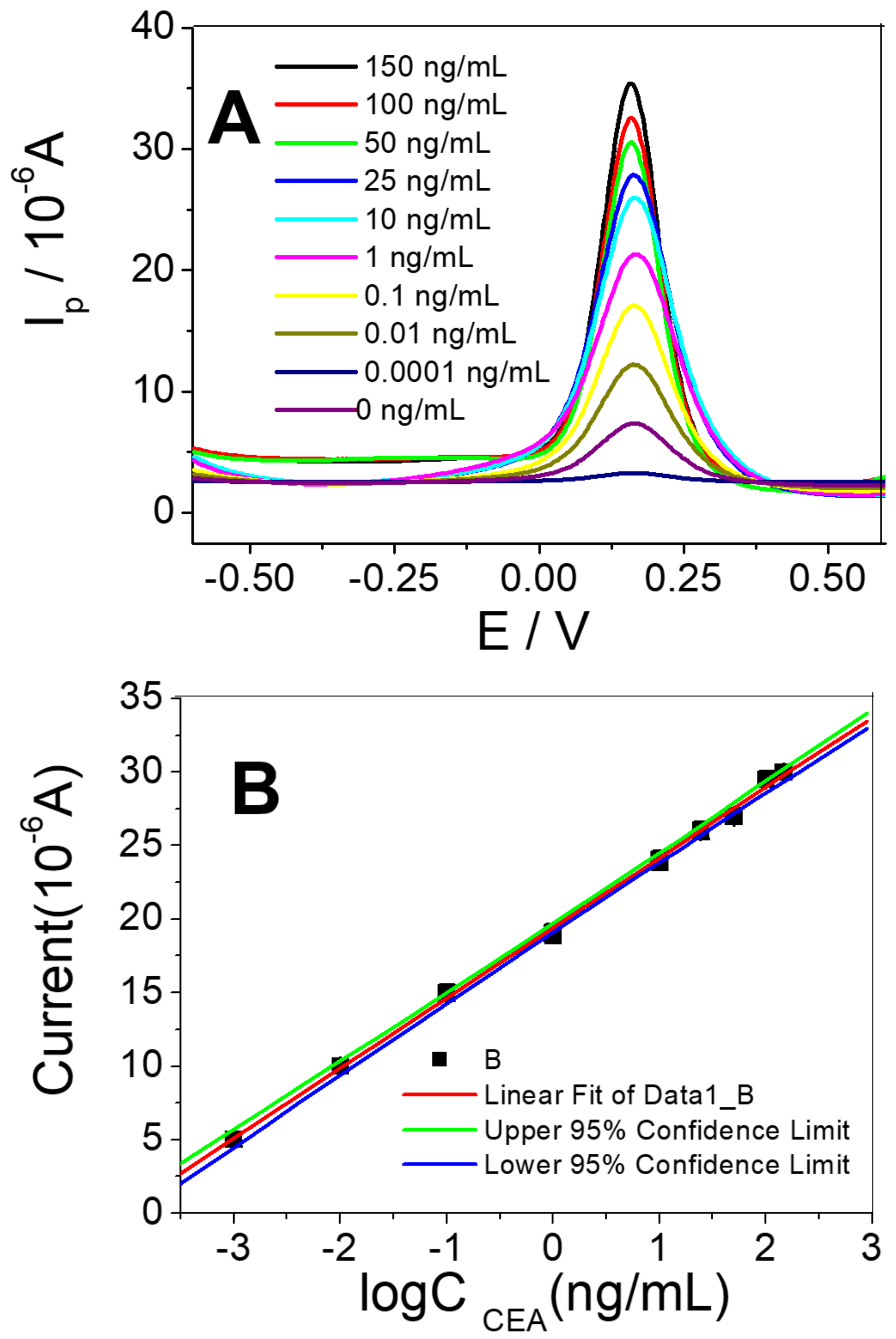
3.4. Sensor Response Characteristics
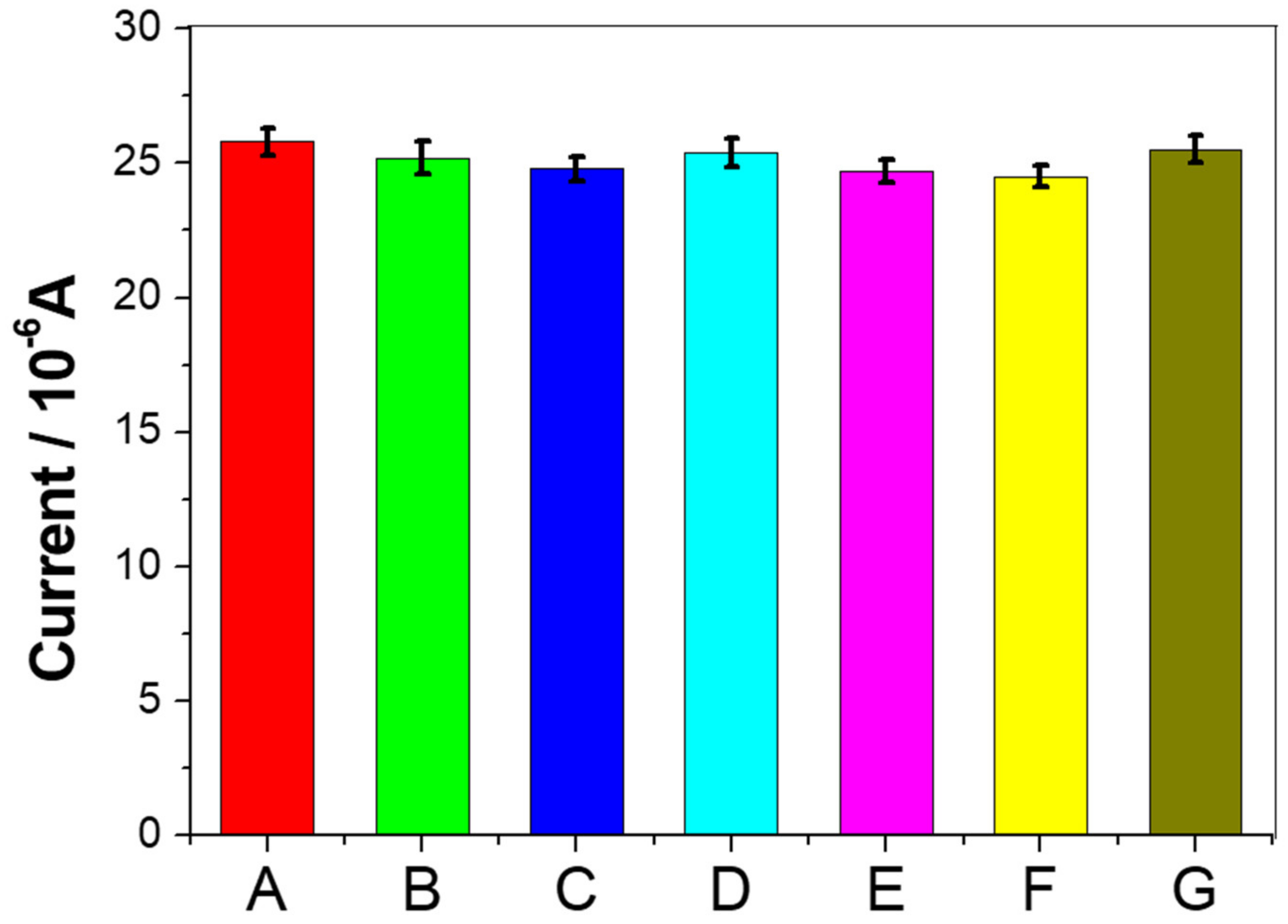
3.5. Selectivity, Reproducibility, and Stability of Immunosensors
3.6. Real Sample Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Turkyilmaz, A.; Eroglu, A.; Aydin, Y.; Karaoglanoglu, N. The relationship of serum CEA and CA 19-9 levels to liver metastasis and pancreatic invasion in esophageal cancer. Turk. J. Med. Sci. 2009, 39, 895–899. [Google Scholar]
- Van Manen, L.; Groen, J.V.; Putter, H.; Vahrmeijer, A.L.; Swijnenburg, R.-J.; Bonsing, B.A.; Mieog, J.S.D. Elevated CEA and CA19-9 serum levels independently predict advanced pancreatic cancer at diagnosis. Biomarkers 2020, 25, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Amri, R.; Berger, D.L. Elevation of Pretreatment Carcinoembryonic Antigen Level as a Prognostic Factor for Colon Cancer Incorporating a C Stage in the AJCC TNM Classification. JAMA Surg. 2015, 150, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnology 2020, 18, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Maccio, G.; Goussot, V.; Berriolo-Riedinger, A.; Riedinger, J.-M. Clinical value of CEA for detection of distant metastases in newly diagnosed breast cancer: Comparison with CA 15-3. Annales De Biologie Clinique 2017, 75, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, L.; Shen, L.; Shen, X.; Ju, S.; Cong, H. Diagnostic Value of Serum Concentration and Integrity of Circulating Cell-Free DNA in Breast Cancer: A Comparative Study With CEA and CA 15-3. Lab. Med. 2018, 49, 323–328. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, H.; Han, Z.; Gao, N.; Xue, J.; Wang, Y. Clinical significance of joint detection of serum CEA, SCCA, and bFGF in the diagnosis of lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9506–9511. [Google Scholar]
- Wu, L.-X.; Li, X.-F.; Chen, H.-F.; Zhu, Y.-C.; Wang, W.-X.; Xu, C.-W.; Xie, D.-F.; Wan, Y.; Du, K.-Q. Combined detection of CEA and CA125 for the diagnosis for lung cancer: A meta-analysis. Cell. Mol. Biol. 2018, 64, 67–70. [Google Scholar] [CrossRef]
- Lee, E.-C.; Yang, J.-Y.; Lee, K.-G.; Oh, S.-Y.; Suh, Y.-S.; Kong, S.-H.; Yang, H.-K.; Lee, H.-J. The value of postoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the early detection of gastric cancer recurrence after curative resection. J. Gastric Cancer 2014, 14, 221–228. [Google Scholar] [CrossRef][Green Version]
- Gong, X.; Zhang, H. Diagnostic and prognostic values of anti-helicobacter pylori antibody combined with serum CA724, CA19-9, and CEA for young patients with early gastric cancer. J. Clin. Lab. Anal. 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Tao, Z.; Du, J.; Cheng, Y.; Li, Q. Electrochemical Immune Analysis System for Gastric Cancer Biomarker Carcinoembryonic Antigen (CEA) Detection. Int. J. Electrochem. Sci. 2018, 13, 1413–1422. [Google Scholar] [CrossRef]
- Khoo, S.K.; Mackay, E.V. Carcinoembryonic antigen by radioimmunoassay in the detection of recurrence during long-term followup of female genital cancer. Cancer 1974, 34, 542–548. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, S.; Huang, Y.; Qin, G.; Ye, F. Highly sensitive immunoassay of carcinoembryonic antigen by capillary electrophoresis with gold nanoparticles amplified chemiluminescence detection. J. Chromatogr. A 2013, 1282, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.-Q.; Du, Y.; Liu, Y.-W.; Wang, Y.-Z.; He, Y.-Q.; Yang, C.-X.; Wang, W.-J.; Gao, F. Clinical and experimental studies regarding the expression and diagnostic value of carcinoembryonic antigen-related cell adhesion molecule 1 in non-small-cell lung cancer. BMC Cancer 2013, 13, 359–368. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, C.L.; Yang, Y.X.; Cao, K.H.; Qiao, X.W.; Hong, C.L. Ag-Co3O4 @ Nr GO Material Synthesized by One-pot Hydrothermal Method for Carcinoembryonic Antigen (CEA) Detection of Enzyme-mimetic Electrochemical Immunosensor. Electroanalysis 2020, 32, 1329–1336. [Google Scholar] [CrossRef]
- Shi, W.T.; Ma, Z.F. A novel label-free amperometric immunosensor for carcinoembryonic antigen based on redox membrane. Biosens. Bioelectron. 2011, 26, 3068–3071. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, M.; Cao, K.; Wu, M.; Zhao, C.; Li, H.; Hong, C. An electrochemical immunosensor for CEA detection based on Au-Ag/rGO@PDA nanocomposites as integrated double signal amplification strategy. Microchem. J. 2019, 151, 104223–104230. [Google Scholar] [CrossRef]
- Tian, J.N.; Zhou, L.J.; Zhao, Y.C.; Wang, Y.; Peng, Y.; Zhao, S.L. Multiplexed detection of tumor markers with multicolor quantum dots based on fluorescence polarization immunoassay. Talanta 2012, 92, 72–77. [Google Scholar] [CrossRef]
- Zheng, X.; Mo, G.; He, Y.; Qin, D.; Jiang, X.; Mo, W.; Deng, B. An electrochemiluminescence immunosensor based on ZnSe@ZnS QDs composite for CEA detection in human serum. J. Electroanal. Chem. 2019, 844, 132–141. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Gao, Z.; Zhang, C.; Zhang, S.; Dong, Y. Enhanced peroxidase-like properties of Au@Pt DNs/NG/Cu2+ and application of sandwich-type electrochemical immunosensor for highly sensitive detection of CEA. Biosens. Bioelectron. 2018, 112, 1–7. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Jia, Y.; Li, Y.; Wang, P.; Liu, Q.; Xu, Z.; Li, X.; Dong, Y. Sandwich-type electrochemical immunosensor for sensitive detection of CEA based on the enhanced effects of Ag NPs@CS spaced Hemin/rGO. Biosens. Bioelectron. 2019, 126, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Liu, L.; Li, Y.; Wei, Q.; Cao, W. Ultrasensitive sandwich-type electrochemical immunosensor based on trimetallic nanocomposite signal amplification strategy for the ultrasensitive detection of CEA. Sci. Rep. 2016, 6, 30849–30856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wu, D.; Ma, H.; Zhang, Y.; Fan, D.; Pang, X.; Du, B.; Wei, Q. Label-free electrochemical immunosensor based on flower-like Ag/MoS2/rGO nanocomposites for ultrasensitive detection of carcinoembryonic antigen. Sens. Actuators B-Chem. 2018, 255, 125–132. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Ling, C.; Chen, Z.; Deng, Y.; He, N. Electrochemical sensor for Cd2+ and Pb2+ detection based on nano-porous pseudo carbon paste electrode. Chin. Chem. Lett. 2019, 30, 2211–2215. [Google Scholar] [CrossRef]
- Karim, M.R.; Hatakeyama, K.; Matsui, T.; Takehira, H.; Taniguchi, T.; Koinuma, M.; Matsumoto, Y.; Akutagawa, T.; Nakamura, T.; Noro, S.-I.; et al. Graphene Oxide Nanosheet with High Proton Conductivity. J. Am. Chem. Soc. 2013, 135, 8097–8100. [Google Scholar] [CrossRef]
- Fugallo, G.; Cepellotti, A.; Paulatto, L.; Lazzeri, M.; Marzari, N.; Mauri, F. Thermal Conductivity of Graphene and Graphite: Collective Excitations and Mean Free Paths. Nano Lett. 2014, 14, 6109–6114. [Google Scholar] [CrossRef]
- Jing, A.; Liang, G.; Shi, H.; Yuan, Y.; Zhan, Q.; Feng, W. Three-Dimensional Holey-Graphene Architectures for Highly Sensitive Enzymatic Electrochemical Determination of Hydrogen Peroxide. J. Nanosci. Nanotechnol. 2019, 19, 7404–7409. [Google Scholar] [CrossRef]
- Sheng, Y.; Miao, H.; Jing, J.; Yao, W.; Zhu, Y. Perylene diimide anchored graphene 3D structure via pi-pi interaction for enhanced photoelectrochemical degradation performances. Appl. Catal. B-Environ. 2020, 272, 118897–118902. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.Y.; Zhao, Z.; Lin, Z.; Lee, C.; Xu, X.; Wang, C.; Huang, Y.; Shakir, M.I.; Duan, X. Solution Processable Holey Graphene Oxide and Its Derived Macrostructures for High-Performance Supercapacitors. Nano Lett. 2015, 15, 4605. [Google Scholar] [CrossRef]
- Jing, A.; Liang, G.; Yuan, Y.; Feng, W. Three-Dimensional Au/Holey-Graphene as Efficient Electrochemical Interface for Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. Micromachines 2019, 10, 84. [Google Scholar] [CrossRef]
- Uzundurukan, A.; Devrim, Y. Carbon nanotube-graphene hybrid supported platinum as an effective catalyst for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2019, 44, 26773–26782. [Google Scholar] [CrossRef]
- Wang, X.; Shang, L.; Zhang, W.; Jia, L.P.; Ma, R.N.; Jia, W.L.; Wang, H.S. An ultrasensitive luminol cathodic electrochemiluminescence probe with highly porous Pt on ionic liquid functionalized graphene film as platform for carcinoembryonic antigen sensing. Biosens. Bioelectron. 2019, 141, 111436–111443. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Liu, Q.; Liu, Y.; Cui, J.J.; Liu, H.; Wang, P.; Li, Y.Y.; Chen, L.; Zhao, Z.D.; Dong, Y.H. A novel label-free electrochemical immunosensor based on functionalized nitrogen-doped graphene quantum dots for carcinoembryonic antigen detection. Biosens. Bioelectron. 2017, 90, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Feng, F.; Liu, Z.M.; Ma, Z.F. Porous platinum nanoparticles and PdPt nanocages for use in an ultrasensitive immunoelectrode for the simultaneous determination of the tumor markers CEA and AFP. Microchim. Acta 2015, 182, 1143–1151. [Google Scholar] [CrossRef]
- Jia, X.L.; Chen, X.; Han, J.M.; Ma, J.; Ma, Z.F. Triple signal amplification using gold nanoparticles, bienzyme and platinum nanoparticles functionalized graphene as enhancers for simultaneous multiple electrochemical immunoassay. Biosens. Bioelectron. 2014, 53, 65–70. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, A.-J.; Yuan, P.-X.; Luo, X.; Xue, Y.; Feng, J.-J. Three dimensional sea-urchin-like PdAuCu nanocrystals/ferrocene-grafted-polylysine as an efficient probe to amplify the electrochemical signals for ultrasensitive immunoassay of carcinoembryonic antigen. Biosens. Bioelectron. 2019, 132, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, G. Preparation of Highly Conductive Graphene Hydrogels for Fabricating Supercapacitors with High Rate Capability. J. Phys. Chem. C 2011, 115, 17206–17212. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Pan, X.; Zhang, N.; Xu, Y.-J. A facile one-step way to anchor noble metal (Au, Ag, Pd) nanoparticles on a reduced graphene oxide mat with catalytic activity for selective reduction of nitroaromatic compounds. Crystengcomm 2013, 15, 6819–6828. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-N.; Zou, J.; Teng, J.; Liu, Q.; Yuan, M.-M.; Jiao, F.-P.; Jiang, X.-Y.; Yu, J.-G. A novel electrochemical sensor based on self-assembled platinum nanochains—Multi-walled carbon nanotubes-graphene nanoparticles composite for simultaneous determination of dopamine and ascorbic acid. Ecotoxicol. Environ. Saf. 2019, 172, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Cao, J.; Li, L.; Rana, R.K.; Zhu, J.-J. Carboxymethyl chitosan-functionalized graphene for label-free electrochemical cytosensing. Carbon 2013, 51, 124–133. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Pei, L.; Yue, S.; Ma, L.; Zhou, L.; Huang, Z.; He, Y.; Gao, J. Silver nanoparticles modified two-dimensional transition metal carbides as nanocarriers to fabricate acetycholinesterase-based electrochemical biosensor. Chem. Eng. J. 2018, 339, 547–556. [Google Scholar] [CrossRef]
- Cui, Z.; Wu, D.; Zhang, Y.; Ma, H.; Li, H.; Du, B.; Wei, Q.; Ju, H. Ultrasensitive electrochemical immunosensors for multiplexed determination using mesoporous platinum nanoparticles as nonenzymatic labels. Anal. Chim. Acta 2014, 807, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Akbari Nakhjavani, S.; Afsharan, H.; Khalilzadeh, B.; Ghahremani, M.H.; Carrara, S.; Omidi, Y. Gold and silver bio/nano-hybrids-based electrochemical immunosensor for ultrasensitive detection of carcinoembryonic antigen. Biosens. Bioelectron. 2019, 141, 111439. [Google Scholar] [CrossRef] [PubMed]
| Electrode | Detection Range | Detection Limit | Method | Ref. |
|---|---|---|---|---|
| Pt/ionic liquid/graphene | 0.001 fg/mL−1 ng/mL | 0.0003 fg/mL | ECL | [32] |
| Nitrogen-doped graphene/PtPd | 5 fg/mL–50 ng/mL | 2 fg/mL | A.C. impedance | [33] |
| NiAuPt nanoparticles on graphene | 0.001–100 ng/mL | 0.27 pg/mL | DPV | [22] |
| Platinum nanoparticles /PdPt nanocages | 0.05–200 ng/mL | 1.4 pg/mL | A.C. impedance | [34] |
| Graphene/platinum nanoparticles | 0.01–100 ng/mL | 1.64 pg/mL | DPV | [35] |
| 3DPt/HGO | 0.001–150 ng/mL | 0.0006 ng/mL | DPV | this work |
| Samples | Added (ng/mL) | Found (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0.10 | 0.107 | 107.00 | 2.86 |
| 2 | 1.00 | 0.942 | 94.20 | 2.33 |
| 3 | 10.00 | 10.480 | 104.80 | 1.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, A.; Xu, Q.; Feng, W.; Liang, G. An Electrochemical Immunosensor for Sensitive Detection of the Tumor Marker Carcinoembryonic Antigen (CEA) Based on Three-Dimensional Porous Nanoplatinum/Graphene. Micromachines 2020, 11, 660. https://doi.org/10.3390/mi11070660
Jing A, Xu Q, Feng W, Liang G. An Electrochemical Immunosensor for Sensitive Detection of the Tumor Marker Carcinoembryonic Antigen (CEA) Based on Three-Dimensional Porous Nanoplatinum/Graphene. Micromachines. 2020; 11(7):660. https://doi.org/10.3390/mi11070660
Chicago/Turabian StyleJing, Aihua, Qiong Xu, Wenpo Feng, and Gaofeng Liang. 2020. "An Electrochemical Immunosensor for Sensitive Detection of the Tumor Marker Carcinoembryonic Antigen (CEA) Based on Three-Dimensional Porous Nanoplatinum/Graphene" Micromachines 11, no. 7: 660. https://doi.org/10.3390/mi11070660
APA StyleJing, A., Xu, Q., Feng, W., & Liang, G. (2020). An Electrochemical Immunosensor for Sensitive Detection of the Tumor Marker Carcinoembryonic Antigen (CEA) Based on Three-Dimensional Porous Nanoplatinum/Graphene. Micromachines, 11(7), 660. https://doi.org/10.3390/mi11070660