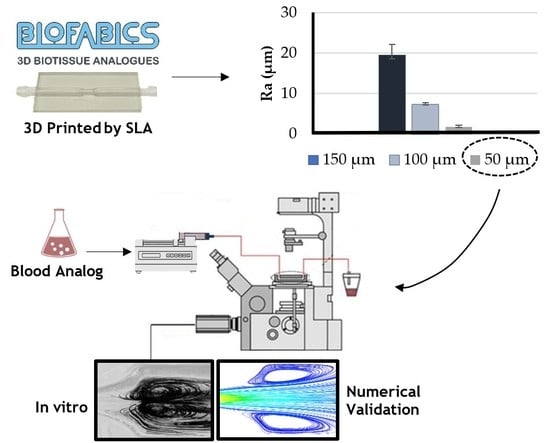
3D Printed Biomodels for Flow Visualization in Stenotic Vessels: An Experimental and Numerical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Coronary Artery 3D Biomodels Design and Fabrication
2.2. Blood Analogue Fluid and Flow Rate
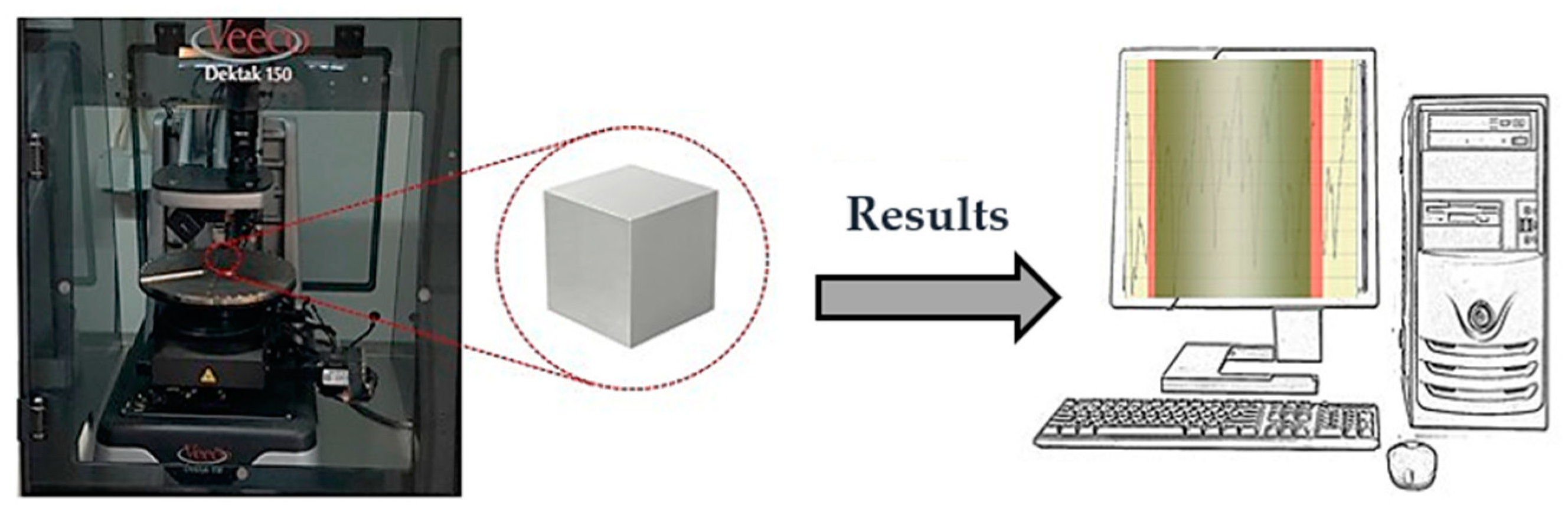
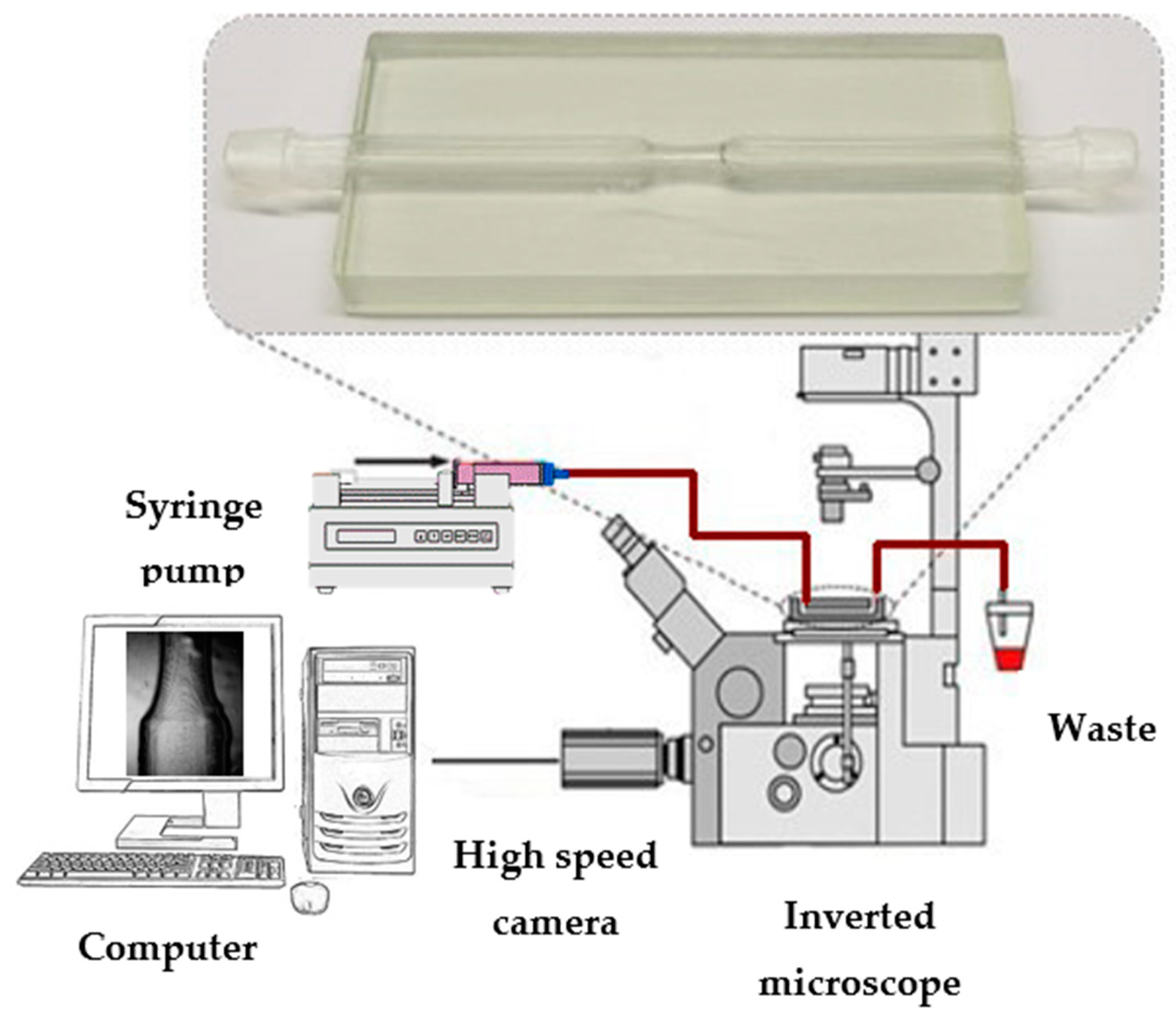
2.3. Experimental Setup for Roughness Measurements and Flow Characterization
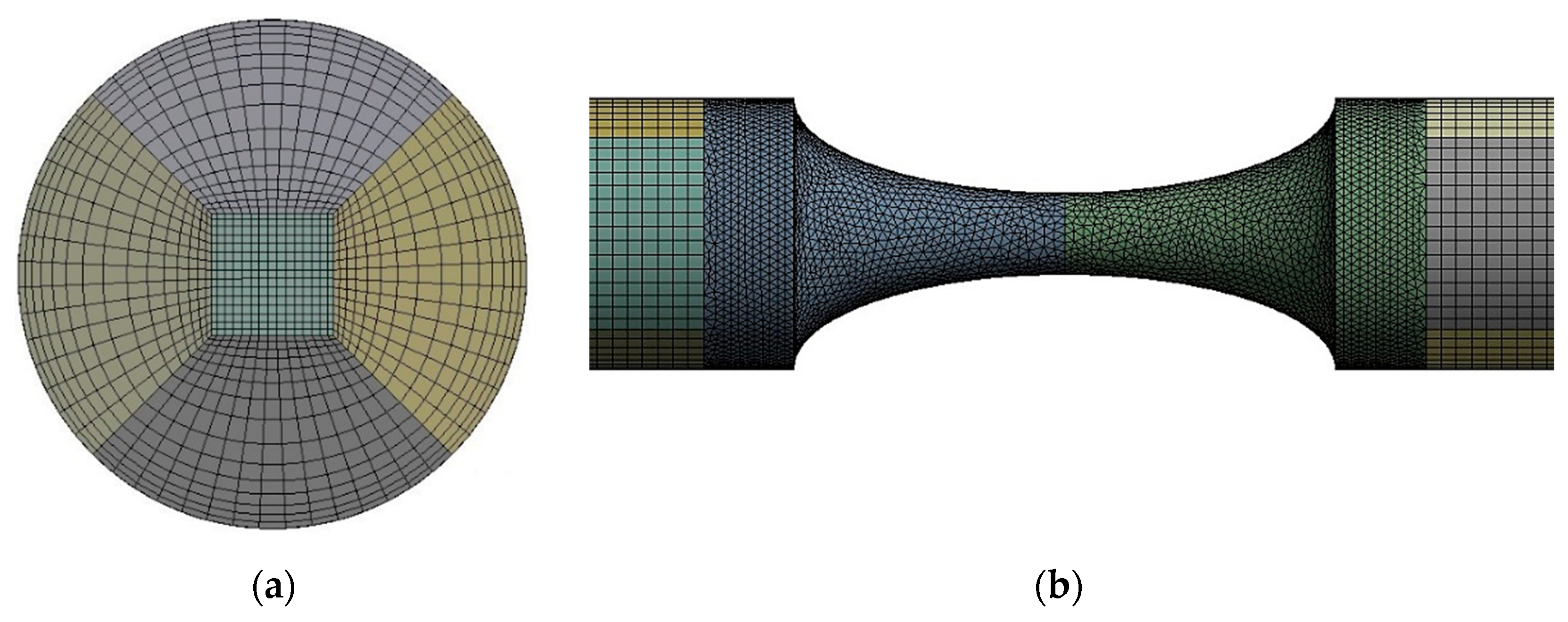
2.4. Numerical Approach
2.5. Image Processing
3. Results and Discussion
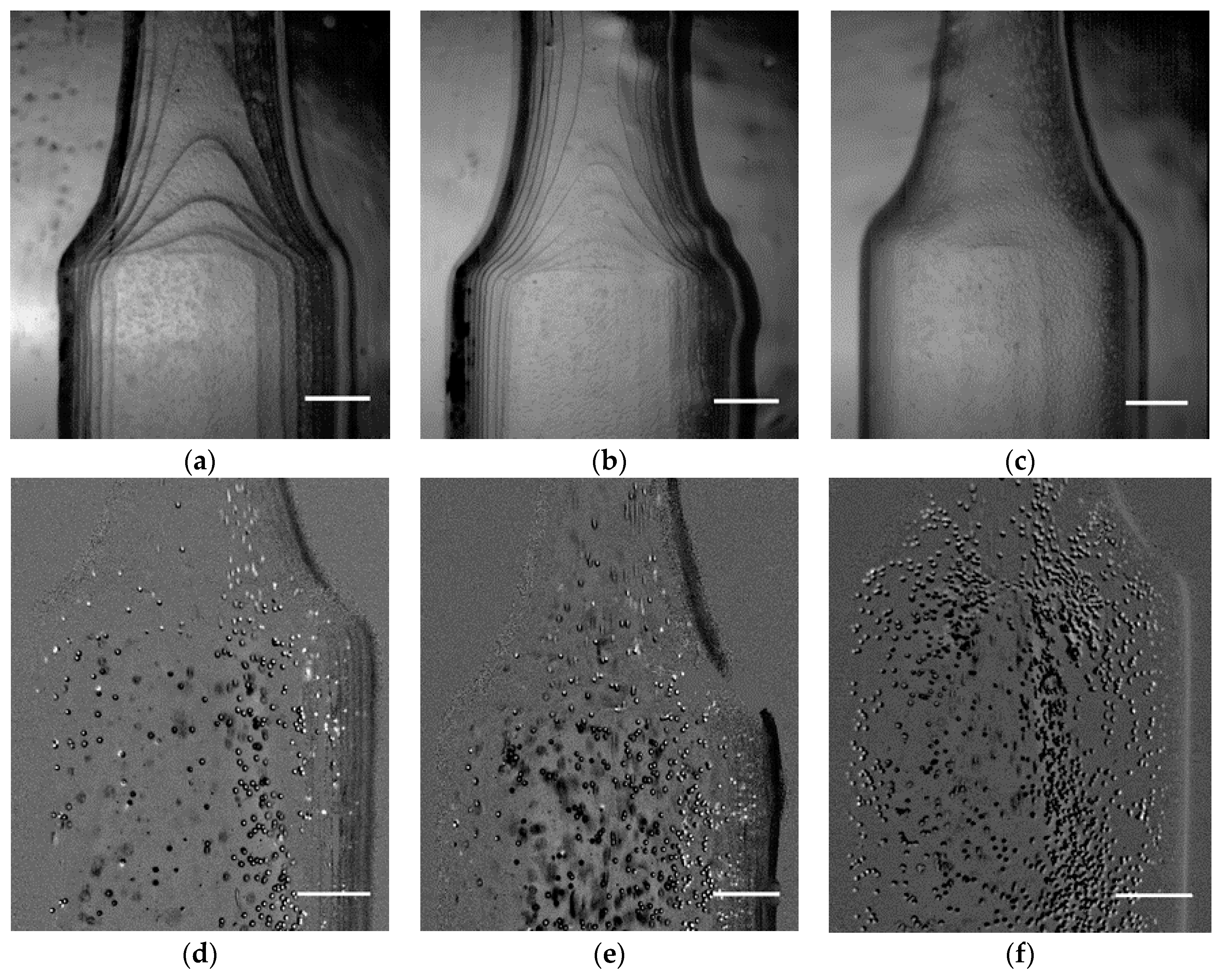
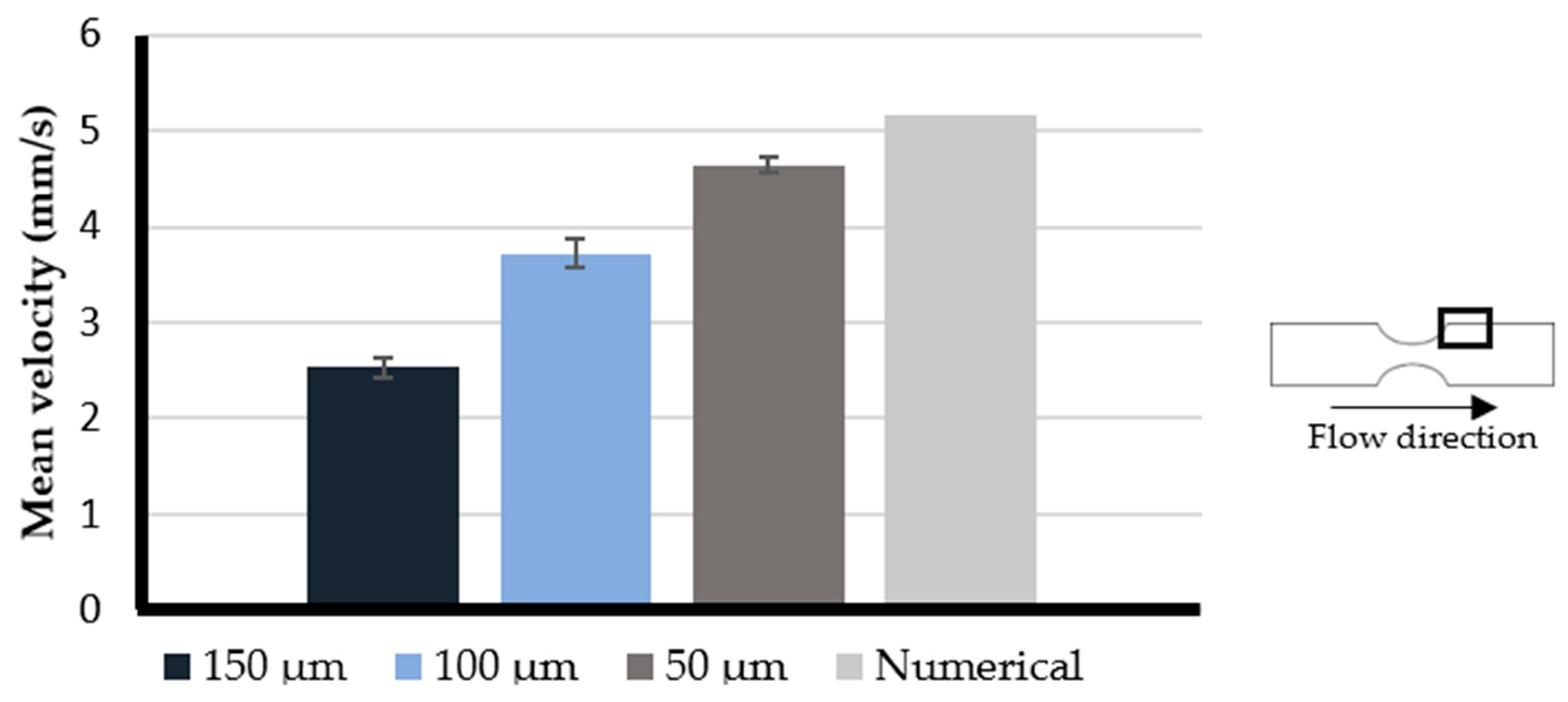
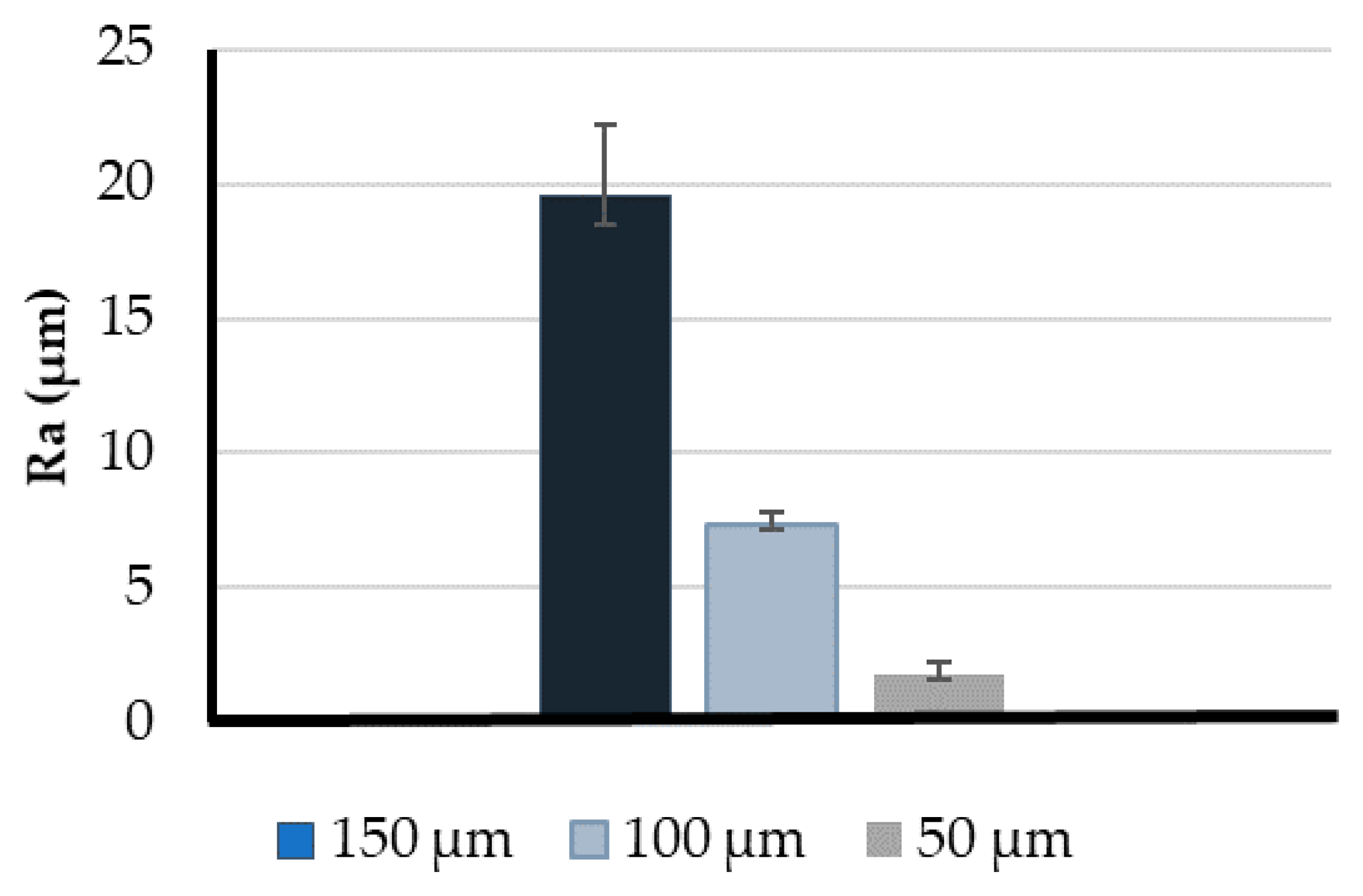
3.1. Printing Resolution Effect
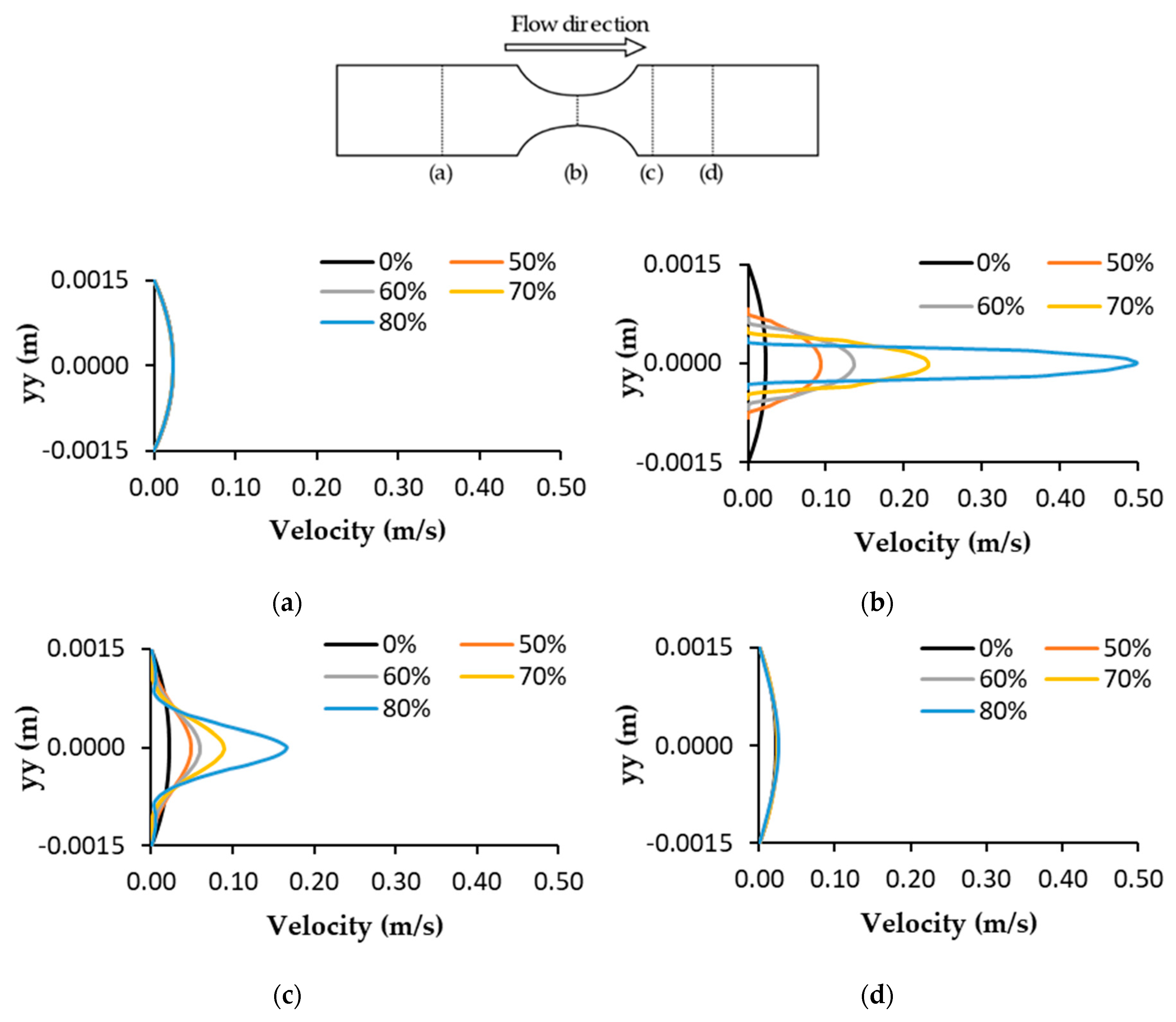
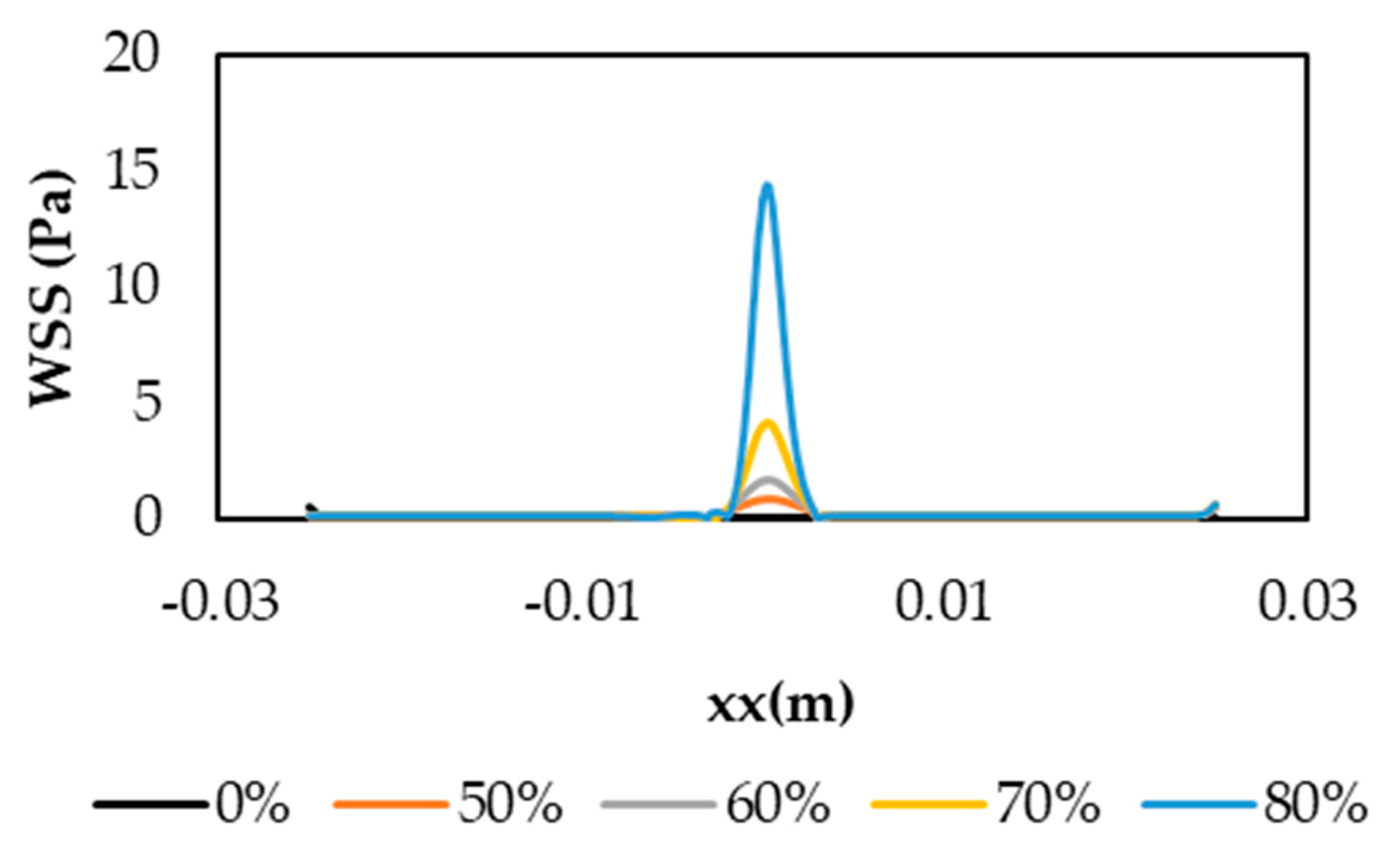
3.2. Velocity Profiles and Wall Shear Stress Evaluation
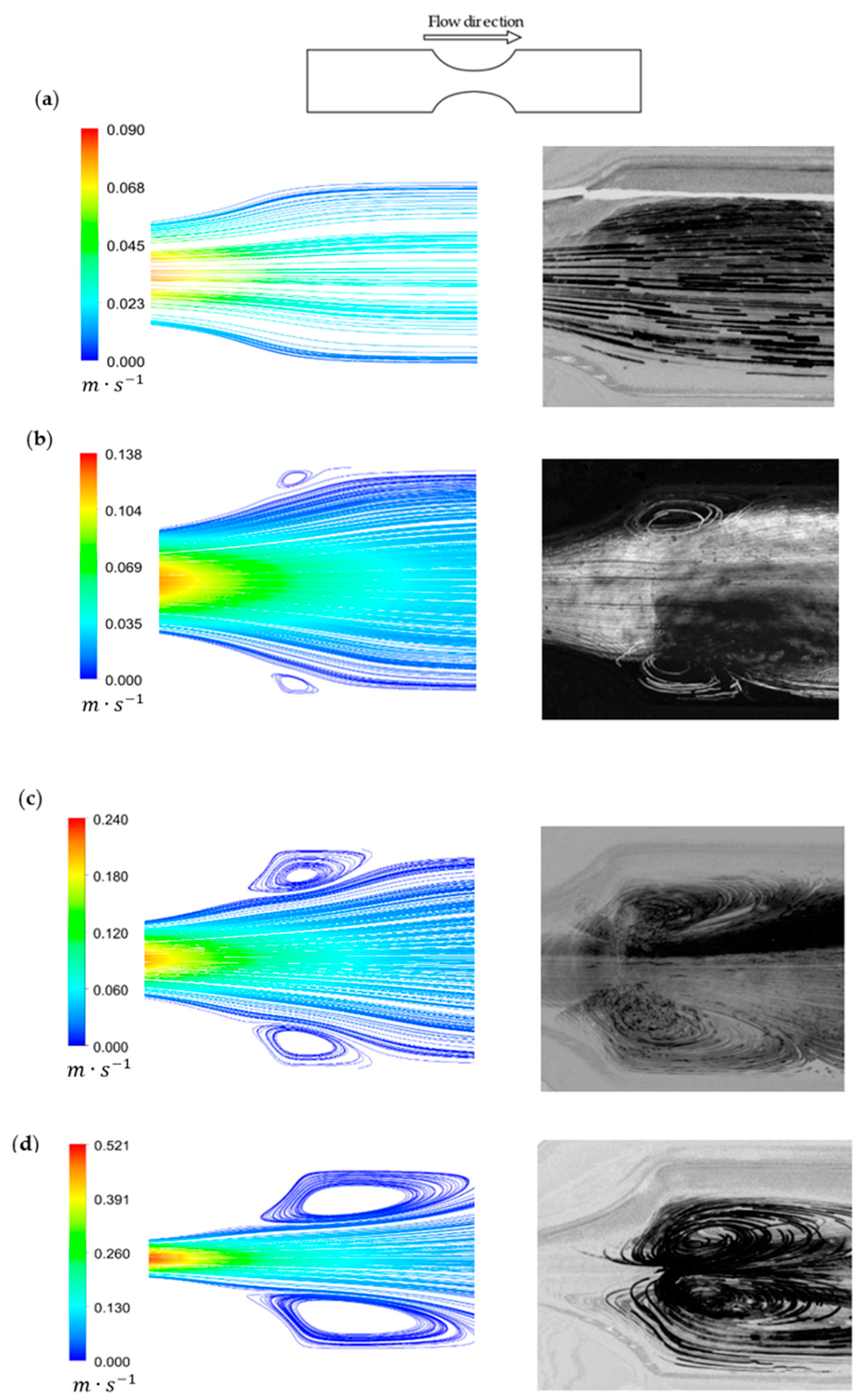
3.3. Velocity Fields and Flow Visualizations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Guan, X. Autophagy: A new target for the treatment of atherosclerosis. Front. Lab. Med. 2018, 2, 68–71. [Google Scholar] [CrossRef]
- Abdolmaleki, F.; Hayat, S.M.G.; Bianconi, V.; Johnston, T.P.; Sahebkar, A. Atherosclerosis and immunity: A perspective. Trends Cardiovasc. Med. 2019, 29, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Queijo, L.; Lima, R. PDMS Anatomical realistic models for hemodynamic studies using rapid prototyping technology. In The International Federation for Medical and Biological Engineering; Springer: Berlin, Germany, 2010; pp. 434–437. [Google Scholar]
- Souza, A.; Ribeir, J.; Lima, R. Manufacturing process of a brain aneurysm biomodel in PDMS using rapid prototyping. In Lecture Notes in Computational Vision and Biomechanics, Proceedings of the Computational Vision and Medical Image Processing, Porto, Portugul, 28 September 2019; Springer: Berlin, Germany, 2019; pp. 671–676. [Google Scholar]
- Doutel, E.; Carneiro, J.; Oliveira, M. Fabrication of 3D milis-scale channels for hemodynamic studies. J. Mech. Med. Biol. 2015, 15, 1550004. [Google Scholar] [CrossRef]
- Costa, P.F.; Albers, H.J.; Linssen, J.E.A.; Middelkamp, H.T.; Van der Hout, L.; Passier, R.; Van den Berg, A.; Malda, J.; Van der Mee, A.D. Mimicking arterial thrombosis in a 3D-printed microfluidic in vitro vascular model based on computed tomography angiography data. Lab Chip 2017, 17, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.H.; Giddens, D.P. Blood flow in major blood vessels—Modeling and experiments. Ann. Biomed. Eng. 2005, 33, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, W.; Malalasekera, H.K. An Introduction to Computational Fluid Dynamics, The Finite Volume Method, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Fletcher, C.A.J. Computational Techniques for Fluid Dynamics, 2nd ed.; Springer: Berlin, Germany, 1991; Volume 1. [Google Scholar]
- Dokunin, A.V. A modification of the method of differential manometry for registration of the volume velocity of the blood flow. Bull. Exp. Biol. Med. 1958, 46, 1414–1417. [Google Scholar] [CrossRef]
- Ku, D.N.; Giddens, D.P.; Zarins, C.K.; Glagov, S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low and oscillating shear stress. Arterioscler. Thromb. Vasc. Biol. 1985, 5, 293–302. [Google Scholar] [CrossRef]
- Young, D.F. Effect of a time-dependent stenosis on flow through a tube. J. Manuf. Sci. Eng. Trans. ASME 1968, 90, 248–254. [Google Scholar] [CrossRef]
- Lee, J.; Fung, Y. Flow in Locally Constricted Tubes At Low Reynolds Numbers. J. Appl. Mech. 1970. [Google Scholar] [CrossRef]
- Kefayati, S.; Holdsworth, D.W.; Poepping, T.L. Turbulence intensity measurements using particle image velocimetry in diseased carotid artery models: Effect of stenosis severity, plaque eccentricity, and ulceration. J. Biomech. 2014, 47, 253–263. [Google Scholar] [CrossRef]
- Pielhop, K.; Klaas, M.; Schröder, W. Analysis of the unsteady flow in an elastic stenotic vessel. Eur. J. Mech. B/Fluids 2012, 35, 102–110. [Google Scholar] [CrossRef]
- Griffith, M.D.; Leweke, T.; Thompson, M.C.; Hourigan, K. Pulsatile flow in stenotic geometries: Flow behaviour and stability. J. Fluid Mech. 2009, 622, 291–320. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Xia, Y.; Wu, W.; Xiong, H. Impact of spatial characteristics in the left stenotic coronary artery on the hemodynamics and visualization of 3D replica models. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Doutel, E.; Viriato, N.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Geometrical effects in the hemodynamics of stenotic and non-stenotic left coronary arteries—Numerical and in vitro approaches. Int. J. Numer. Method Biomed. Eng. 2019, 35, e3207. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Teixeira, S.; Lobarinhas, P. Computational fluid dynamics simulations: An approach to evaluate cardiovascular dysfunction. In Modelling, Simulation and Optimization, 1st ed.; Rey, G.R., Muneta, L.M., Eds.; IntechOpen: Rijeka, Croatia, 2010; pp. 26–46. [Google Scholar]
- Kefayati, S.; Milner, J.S.; Holdsworth, D.W.; Poepping, T.L. In vitro shear stress measurements using particle image velocimetry in a family of carotid artery models: Effect of stenosis severity, plaque eccentricity, and ulceration. PLoS ONE 2014, 9, e98209. [Google Scholar] [CrossRef]
- Kefayati, S.; Poepping, T.L. Transitional flow analysis in the carotid artery bifurcation by proper orthogonal decomposition and particle image velocimetry. Med. Eng. Phys. 2013, 35, 898–909. [Google Scholar] [CrossRef]
- DiCarlo, A.L.; Holdsworth, D.W.; Poepping, T.L. Study of the effect of stenosis severity and non-Newtonian viscosity on multidirectional wall shear stress and flow disturbances in the carotid artery using particle image velocimetry. Med. Eng. Phys. 2018, 65, 8–23. [Google Scholar] [CrossRef]
- Dicarlo, A.L.; Poepping, T. Investigation of flow and turbulence in carotid artery models of varying compliance using particle image velocimetry. In World Congress on Medical Physics and Biomedical Engineering, Proceedings of the IFMBE, Toronto, Canada, 7–12 June 2015; Springer International Publishing: Basel, Switzerland, 2015; pp. 1743–1744. [Google Scholar]
- Stepniak, K.; Ursani, A.; Paul, N.; Naguib, H. Novel 3D printing technology for CT phantom coronary arteries with high geometrical accuracy for biomedical imaging applications. Bioprinting 2020, 18, e00074. [Google Scholar] [CrossRef]
- Tsao, C.W. Polymer microfluidics: Simple, low-cost fabrication process bridging academic lab research to commercialized production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, H.; Lan, H.; Liu, T.; Liu, X.; Yu, H. 3D printing of artificial blood vessel: Study on multi-parameter optimization design for vascular molding effect in alginate and gelatin. Micromachines 2017, 8, 237. [Google Scholar] [CrossRef]
- Faria, C.L.; Pinho, D.; Santos, J.; Gonçalves, L.M. Low cost 3D printed biomodels for biofluid mechanics applications. J. Mech. Eng. Biomech. 2018, 3, 1–7. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Pinho, D.; Bento, D.; Lima, R.; Ribeiro, J. Wall expansion assessment of an intracranial aneurysm model by a 3D Digital Image Correlation System. Measurement 2016, 88, 262–270. [Google Scholar] [CrossRef]
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, M.; Minas, G.; Lima, R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines 2019, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Aycock, K.I.; Hariharan, P.; Craven, B.A. Particle image velocimetry measurements in an anatomical vascular model fabricated using inkjet 3D printing. Exp. Fluids. 2017, 5, 81–88. [Google Scholar] [CrossRef]
- Jewkes, R.; Burton, H.E.; Espino, D.M. Towards additive manufacture of functional, spline-based morphometric models of healthy and diseased coronary arteries: In vitro proof-of-concept using a porcine template. J. Funct. Biomater. 2018, 9, 15. [Google Scholar] [CrossRef]
- Kalaskar, D.M. 3D Printing in Medicine, 1st ed.; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- Peltola, S.; Grijpam, D. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef]
- Kumar, L.J.; Pandey, P.M.; Wimpenny, D.I. 3D Printing and Additive Manufacturing Technologies; Springer: Berlin, Germany, 2019. [Google Scholar]
- Einav, S.; Berman, H.J.; Fuhro, R.L.; DiGiovanni, P.R.; Fine, S.; Fridman, J.D. Measurement of velocity profiles of red blood cells in the microcirculation by laser Doppler anemometry (LDA). Biorheology 1975, 12, 207–210. [Google Scholar] [CrossRef]
- Ravnic, D.J.; Zhang, Y.Z.; Tsuda, A.; Pratt, J.P.; Huss, H.T.; Mentzer, S.J. Multi-image particle tracking velocimetry of the microcirculation using fluorescent nanoparticles. Microvasc. Res. 2006, 72, 27–33. [Google Scholar] [CrossRef]
- Büsen, M.; Kaufmann, T.A.S.; Neidlin, M.; Steinseifer, U.; Sonntag, S.J. In vitro flow investigations in the aortic arch during cardiopulmonary bypass with stereo-PIV. J. Biomech. 2015, 48, 2005–2011. [Google Scholar] [CrossRef]
- Pinho, D.; Rodrigues, R.O.; Faustino, V.; Yaginuma, T.; Exposto, J.; Lima, R. Red blood cells radial dispersion in blood flowing through microchannels: The role of temperature. J. Biomech. 2016, 49, 2293–2298. [Google Scholar] [CrossRef]
- Lima, R.; Wada, S.; Tanaka, S.; Takeda, M.; Ishikawa, T.; Tsubota, K.I.; Imai, Y.; Yamaguchi, T. In vitro blood flow in a rectangular PDMS microchannel: Experimental observations using a confocal micro-PIV system. Biomed. Microdevices 2008, 10, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-F. Coronary Artery Diameter is Inversely Associated with the Severity of Coronary Lesions in Patients Undergoing Coronary Angiography. Cell. Physiol. Biochem. 2017, 43, 1247–1257. [Google Scholar] [CrossRef]
- Sebastian, B.; Dittrich, P.S. Microfluidics to Mimic Blood Flow in Health and Disease. Annu. Rev. Fluid Mech. 2018, 50, 483–504. [Google Scholar] [CrossRef]
- Kabir, M.A.; Alam, M.F.; Uddin, M.A. A numerical study on the effects of reynolds number on blood flow with spiral velocity through regular arterial stenosis. Chiang Mai J. Sci. 2018, 45, 2515–2527. [Google Scholar]
- Rabby, M.G.; Razzak, A.; Molla, M. Pulsatile non-Newtonian blood flow through a model of arterial stenosis. Procedia Eng. 2013, 56, 225–231. [Google Scholar] [CrossRef]
- Buradi, A.; Mahalingam, A. Effect of stenosis severity on wall shear stress based hemodynamic descriptors using multiphase mixture theory. J. Appl. Fluid Mech. 2018, 11, 1497–1509. [Google Scholar] [CrossRef]
- Li, M.X.; Beech-Brandt, J.J.; John, L.R. Numerical analysis of pulsatile blood flow and vessel wall mechanics in different degrees of stenoses. J. Biomech. 2007, 40, 3715–3724. [Google Scholar] [CrossRef]
- Zipes, D.; Libby, P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 11th ed.; Elsevier: New York, NY, USA, 2018. [Google Scholar]
- Calejo, J.; Pinho, D.; Galindo-Rosales, F.; Lima, R.; Campo-Deaño, L. Particulate Blood Analogues Reproducing the Erythrocytes Cell-Free Layer in a Microfluidic Device Containing a Hyperbolic Contraction. Micromachines 2016, 7, 4. [Google Scholar] [CrossRef]
- Pinho, D.; Muñoz-Sánchez, B.N.; Anes, C.F.; Vega, E.J.; Lima, R. Flexible PDMS microparticles to mimic RBCs in blood particulate analogue fluids. Mech. Res. Commun. 2019, 100, 18–20. [Google Scholar] [CrossRef]
- Pinho, D.; Campo-Deaño, L.; Lima, R.; Pinho, F.T. In vitro particulate analogue fluids for experimental studies of rheological and hemorheological behavior of glucose-rich RBC suspensions. Biomicrofluidics 2017, 11, 54105. [Google Scholar] [CrossRef]
- Souza, A.V.; Paiva, M.S.; Pinho, D.; Agujetas, R.; Ferrera, C.; Lima, R.; Puga, H.; Ribeiro, J.E. 3D manufacturing of intracranial aneurysm biomodels for flow visualizations: A low-cost fabrication process. Mech. Res. Commun. 2020. [Google Scholar] [CrossRef]
- Sousa, P.C.; Carneiro, J.; Vaz, R.; Cerejo, A.; Pinho, F.T.; Alves, M.A. Shear viscosity and nonlinear behavior of whole blood under large amplitude oscillatory shear. Biorheology 2013, 50, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Schaefer, C. Rheology of Human Blood Plasma: Viscoelastic Versus Newtonian Behavior. Phys. Rev. Lett. 2013, 110, 078305. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, F.; Gundogdu, M.Y. A critical review on blood flow in large arteries; relevance to blood rheology, viscosity models, and physiologic conditions. Korea Aust. Rheol. J. 2008, 20, 197–211. [Google Scholar]
- Doutel, E.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Experimental and numerical methodology to analyze flows in a coronary bifurcation. Eur. J. Mech. B/Fluids 2018, 67, 341–356. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imageJ. Biophotonics Int. 2004, 11, 36–41. [Google Scholar] [CrossRef]
- Tonietto, L.; Gonzaga, L., Jr. New Method for Evaluating Surface Roughness Parameters Acquired by Laser Scanning. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Alsoufi, M.S.; Elsayed, A.E. Surface Roughness Quality and Dimensional Accuracy—A Comprehensive Analysis of 100% Infill Printed Parts Fabricated by a Personal/Desktop Cost-Effective FDM 3D Printer, Mater. Sci. Appl. 2018, 9, 11–40. [Google Scholar] [CrossRef]
- Waite, L.; Fine, J. Applied Biofluid Mechanics, 1st ed.; McGraw-Hill Companies: New York, NY, USA, 2007. [Google Scholar]
- Chaichana, T.; Sun, Z.; Jewkes, J. Hemodynamic impacts of various types of stenosis inthe left coronary artery bifurcation: A patient-specific analysis. Phys. Med. 2013, 29, 447–452. [Google Scholar] [CrossRef]
- Gijsen, F.; Katagiri, Y. Expert recommendations on the assessment of wall shear stress in human coronary arteries: Existing methodologies, technical considerations, and clinical applications. Eur. Heart J. 2019, 40, 3421–3433. [Google Scholar] [CrossRef]

| Stenosis Degree | Reynolds |
|---|---|
| 0% | 23 |
| 50% | 45 |
| 60% | 55 |
| 70% | 71 |
| 80% | 102 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, V.; Rodrigues, N.; Ribeiro, R.; Costa, P.F.; Lima, R.A.; F.C.F. Teixeira, S. 3D Printed Biomodels for Flow Visualization in Stenotic Vessels: An Experimental and Numerical Study. Micromachines 2020, 11, 549. https://doi.org/10.3390/mi11060549
Carvalho V, Rodrigues N, Ribeiro R, Costa PF, Lima RA, F.C.F. Teixeira S. 3D Printed Biomodels for Flow Visualization in Stenotic Vessels: An Experimental and Numerical Study. Micromachines. 2020; 11(6):549. https://doi.org/10.3390/mi11060549
Chicago/Turabian StyleCarvalho, Violeta, Nelson Rodrigues, Ricardo Ribeiro, Pedro F. Costa, Rui A. Lima, and Senhorinha F.C.F. Teixeira. 2020. "3D Printed Biomodels for Flow Visualization in Stenotic Vessels: An Experimental and Numerical Study" Micromachines 11, no. 6: 549. https://doi.org/10.3390/mi11060549
APA StyleCarvalho, V., Rodrigues, N., Ribeiro, R., Costa, P. F., Lima, R. A., & F.C.F. Teixeira, S. (2020). 3D Printed Biomodels for Flow Visualization in Stenotic Vessels: An Experimental and Numerical Study. Micromachines, 11(6), 549. https://doi.org/10.3390/mi11060549