Laser-Induced Microgrooves Improve the Mechanical Responses of Cemented Implant Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Design
2.3. Sample Preparation
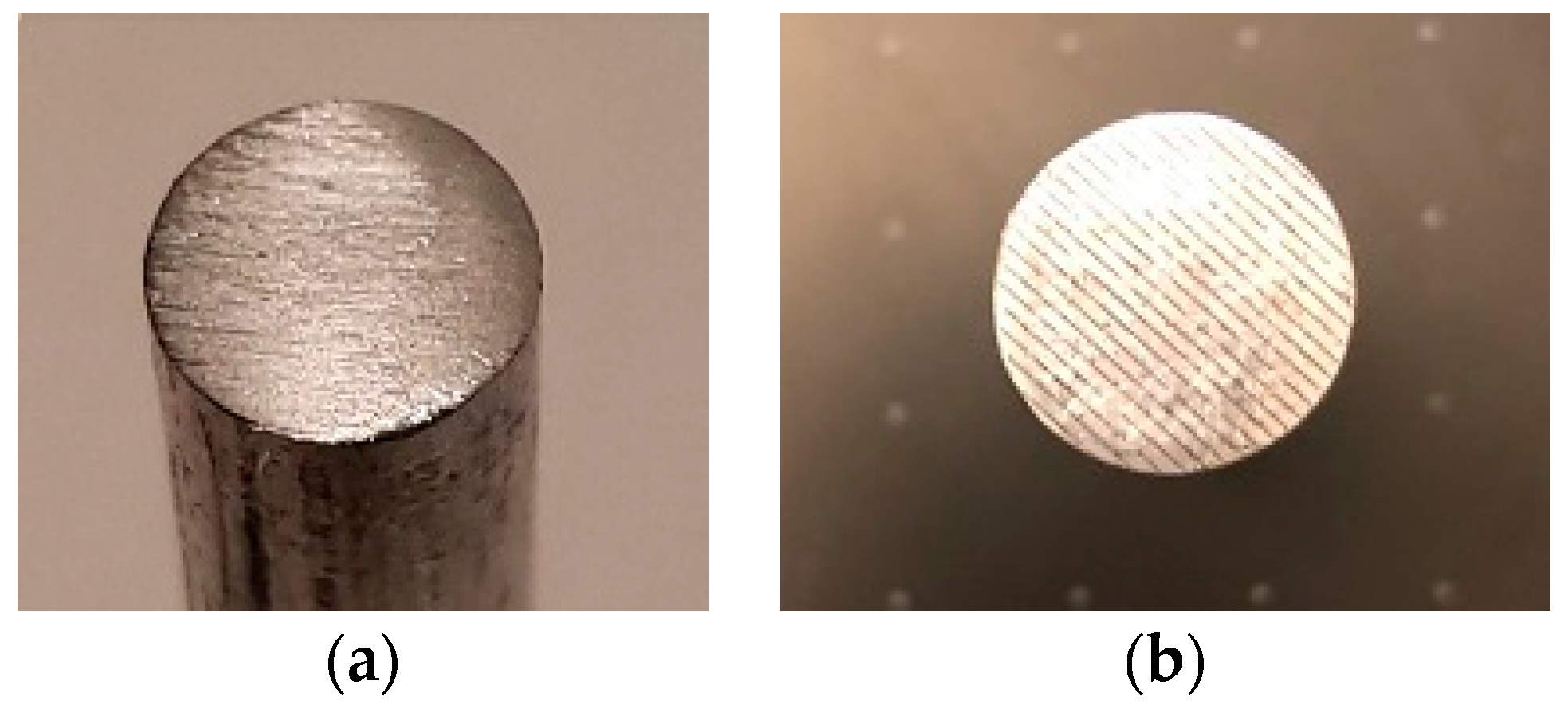
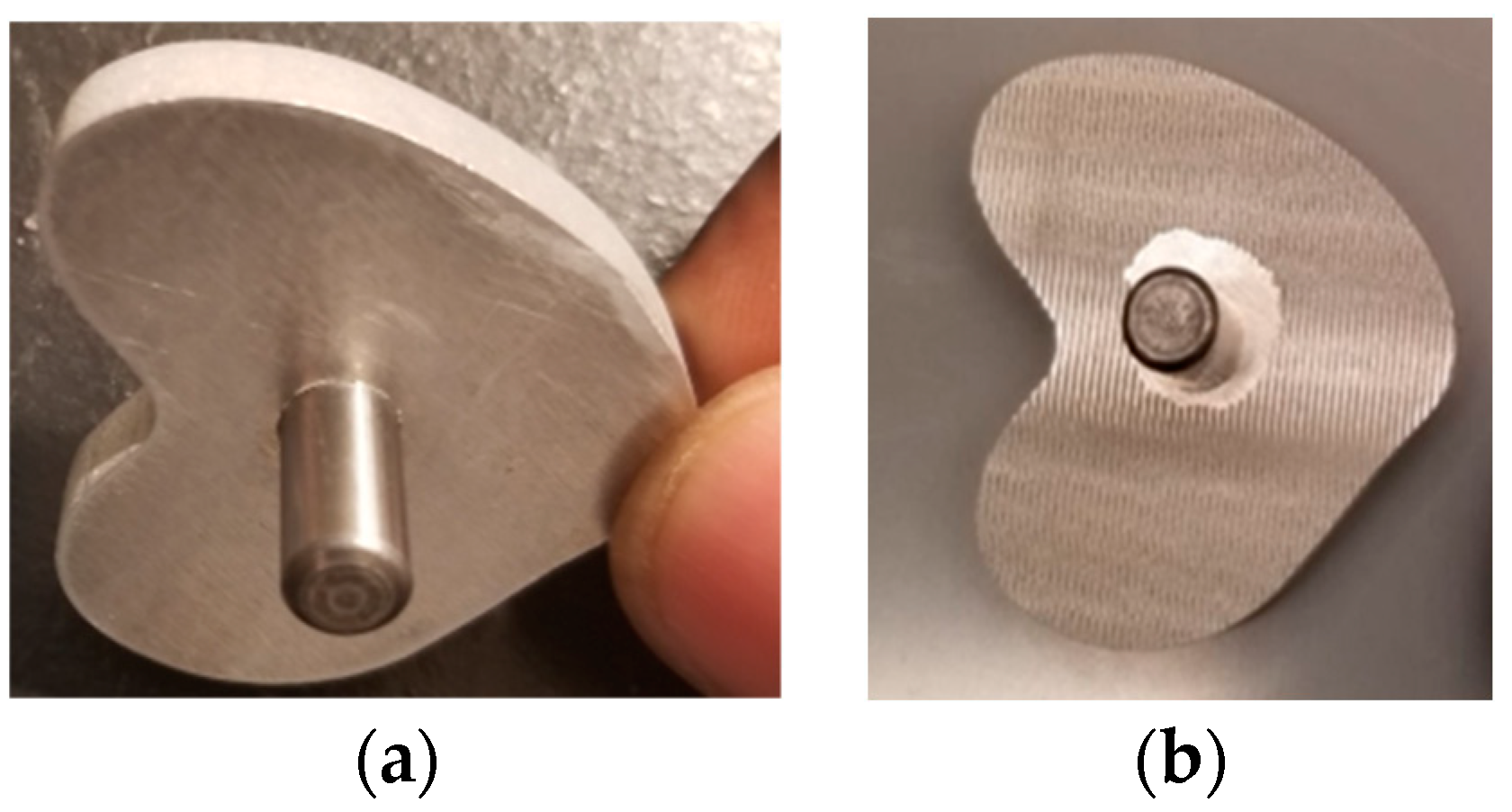
2.3.1. Cemented Rod Implants
2.3.2. Cemented Tibial Tray Implant
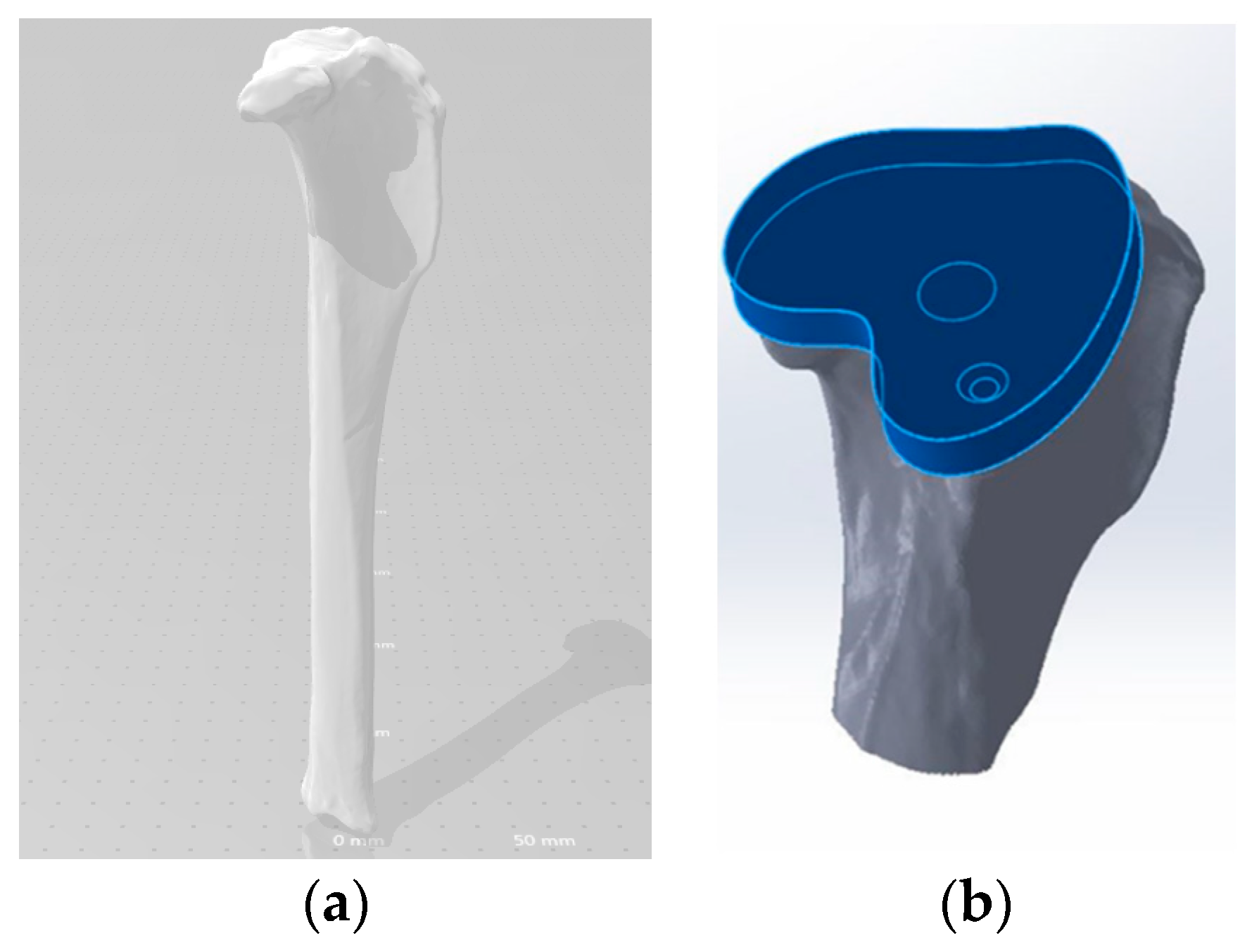
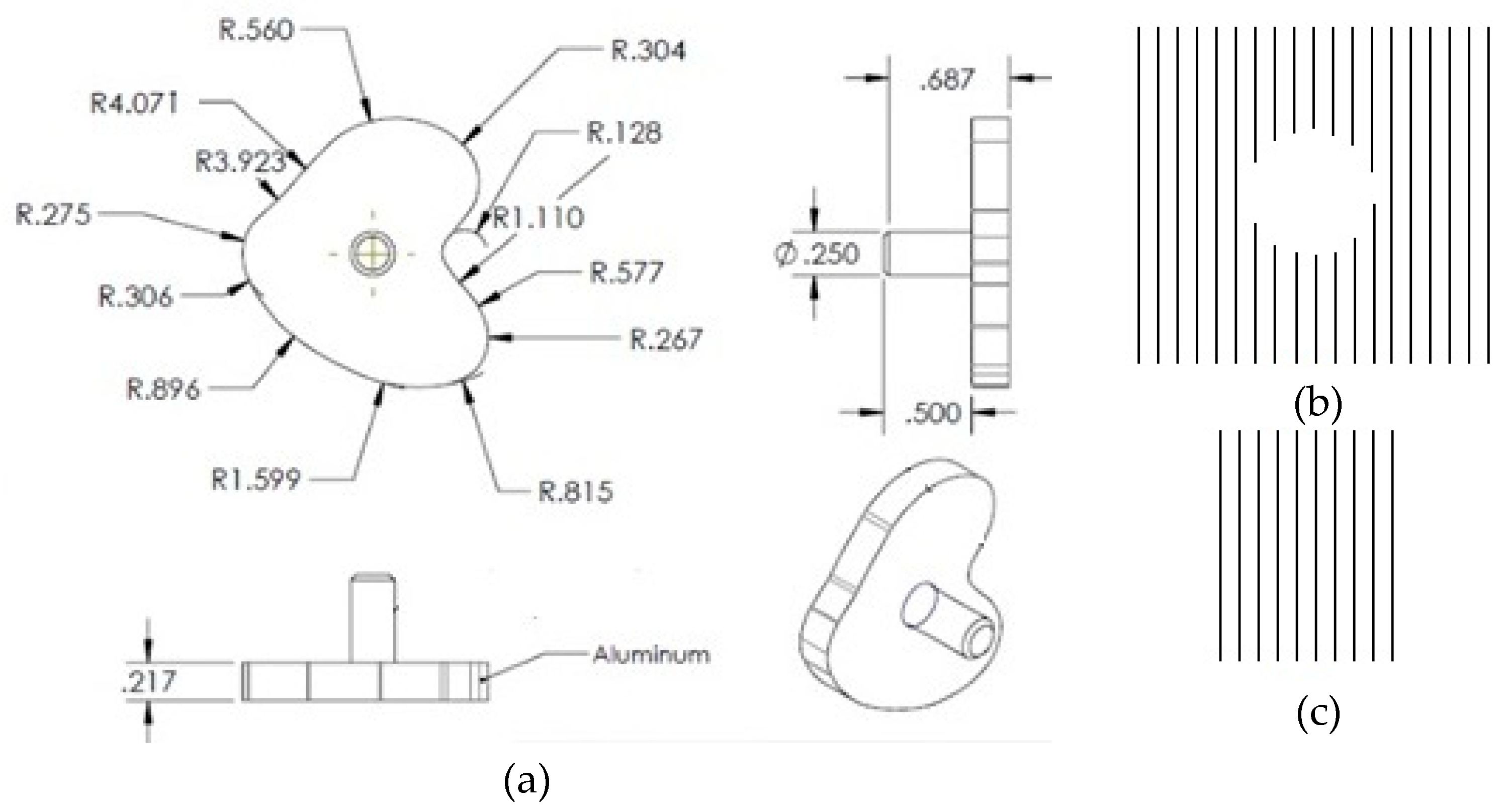
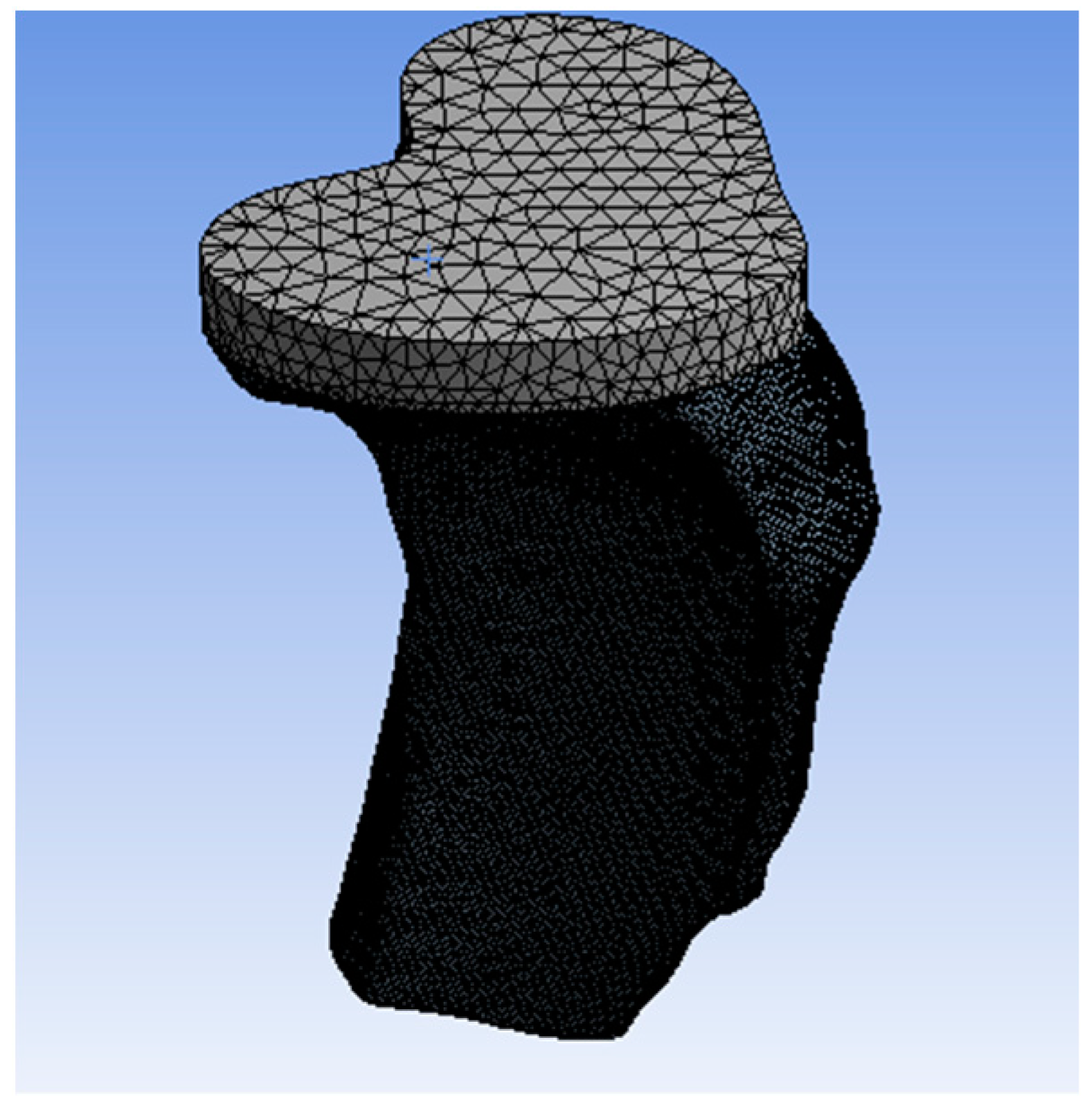
Geometry and Modeling
Tibia Tray Manufacture
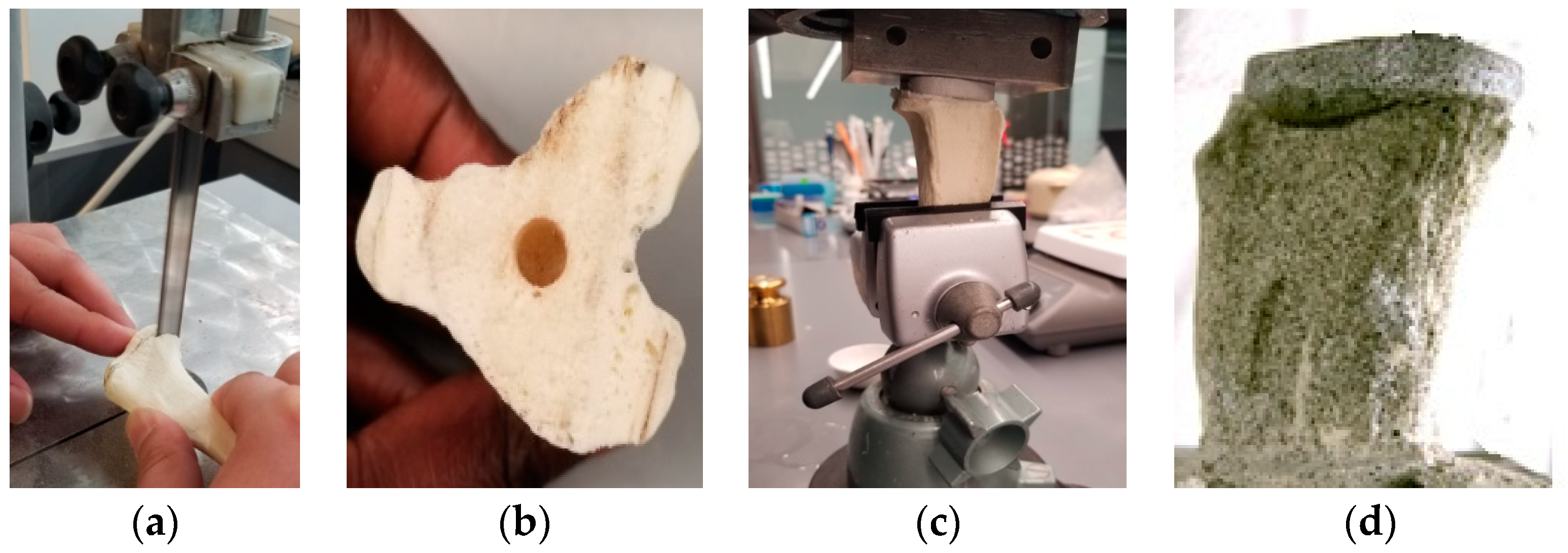
Cemented Tibia Tray/Bone Sample Preparation
2.4. Experiments and Analysis
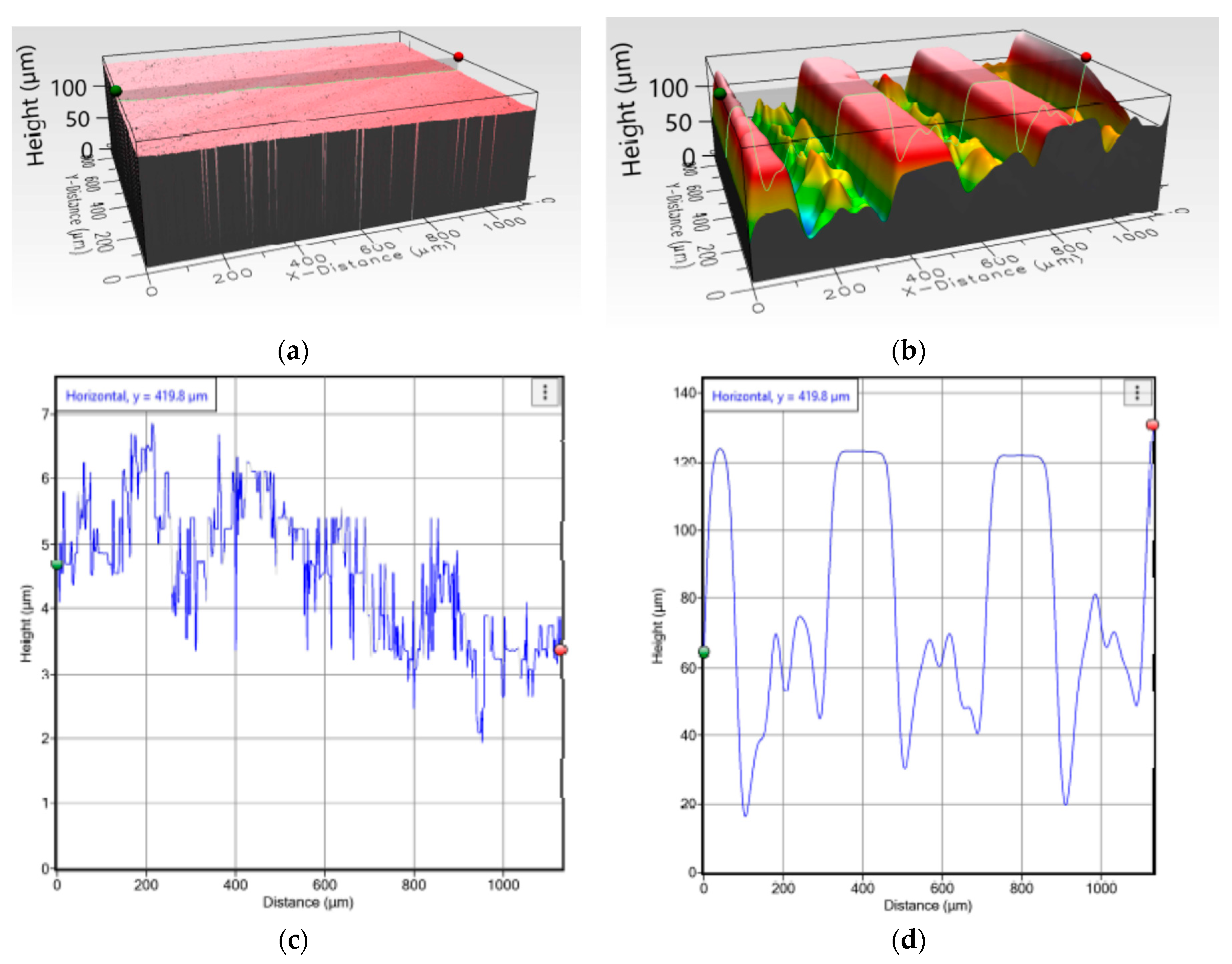
2.4.1. Topographical Characteristics
2.4.2. Mechanical Characteristics
Cemented Rod Implants under Static Tension
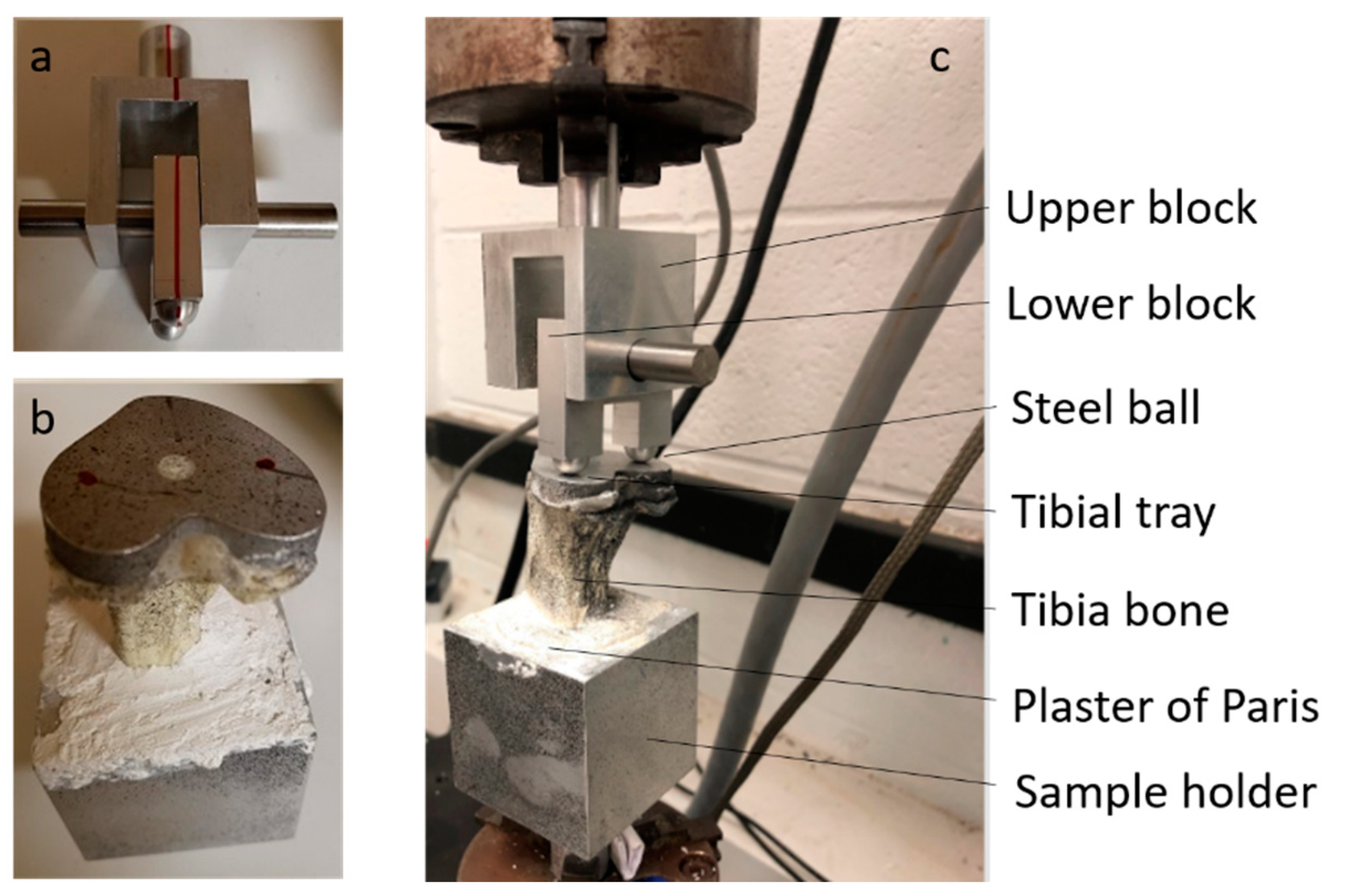
Cemented Tibial Tray Implants with Bone
2.4.3. Statistical Analysis
3. Results
3.1. Topographyical Chracteristics
3.2. Mechanical Chracteristics
3.2.1. Cemented Rod Implants
3.2.2. Cemented TT Implant with Bone
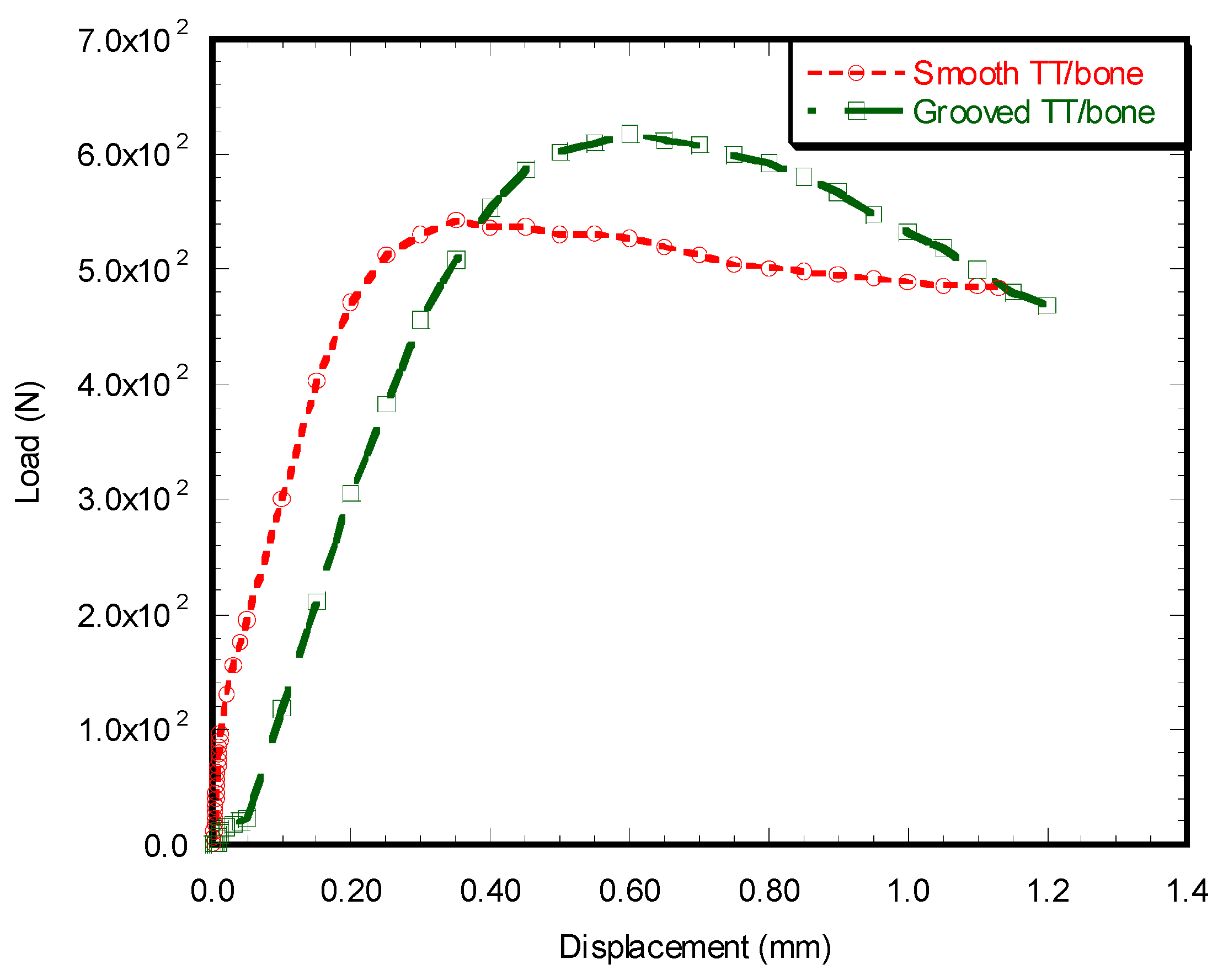
Load vs. Displacement Characteristics
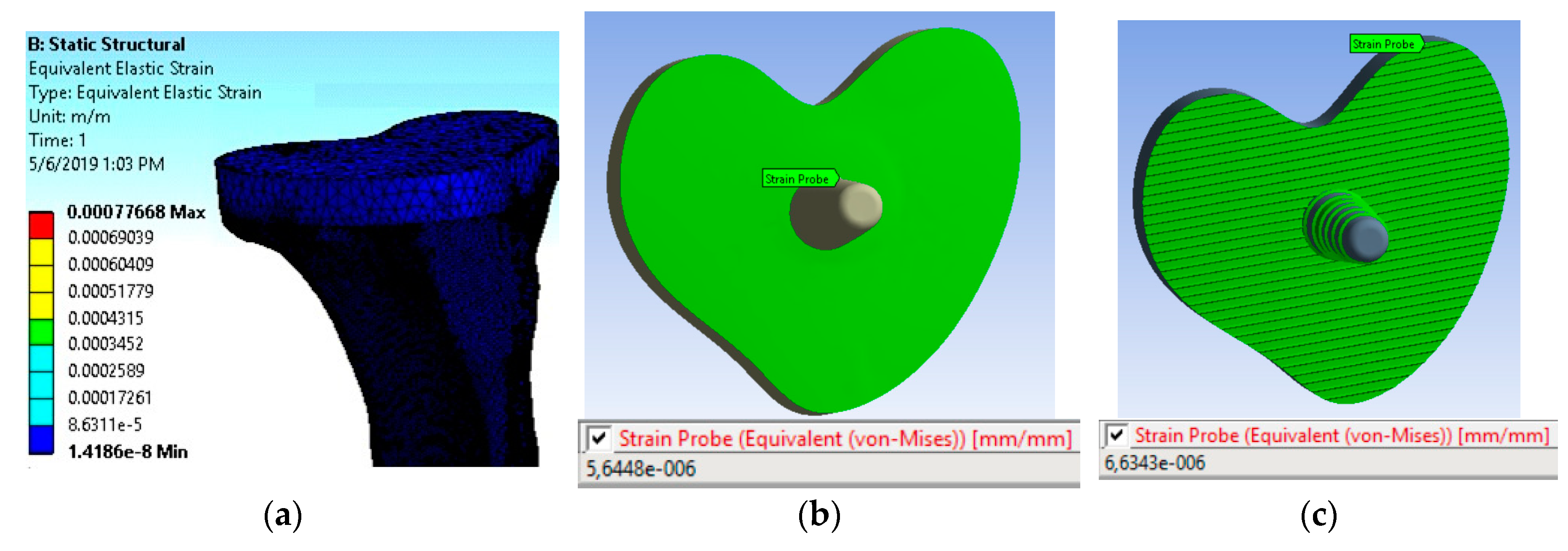
Microstrain Analysis around the TT–bone Interface
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lennon, A.B.; McCormack, B.A.O.; Prendergast, P.J. The relationship between cement fatigue damage and implant surface finish in proximal femoral prostheses. Med. Eng. Phys. 2003, 25, 833–841. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Marsich, E.; Donati, I.; Foulc, M.P.; Moritz, N.; Aro, H.T.; Paoletti, S. Polysaccharide-coated thermosets for orthopedic applications: From material characterization to in vivo tests. Biomacromolecules 2012, 13, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.S.; Restrepo, C.; Sharkey, P.F. Global Perspectives on Arthroplasty of Hip and Knee Joints. Orthop. Clin. N. Am. 2020, 51, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Berce, C.; Lucaciu, D.; Cosma, D. Current methods of preventing aseptic loosening and improving osseointegration of titanium implants in cementless total hip arthroplasty: A review. J. Int. Med. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Van Lenthe, G.H.; de Waal Malefijt, M.C.; Huiskes, R. Stress shielding after total knee replacement may cause bone resorption in the distal femur. J. Bone Jt. Surg. Br. Vol. 1997, 79, 117–122. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Im, B.J.; Lee, S.W.; Oh, N.; Hyun Lee, M.; HoKang, J.; Richard, L.; Lee, S.C.; Ahn, S.J.; Park, J.S. Texture direction of combined microgrooves and submicroscale topographies of titanium substrata influence adhesion, proliferation, and differentiation in human primary cells. Arch. Oral Biol. 2012, 57, 898–905. [Google Scholar] [CrossRef]
- Khandaker, M.; Riahinezhad, S.; Inventors. Method and Apparatus to Coat a Metal Implant with an Extracellular Matrix Made with Electrospun Fiber. U.S. Patent 9,809,906, 19 February 2019. [Google Scholar]
- Khandaker, M.; Riahinezhad, S.; Sultana, F.; Morris, T.; Wolf, R.; Vaughan, M. Effect of collagen- polycaprolactone extracellular matrix on the in vitro cytocompatibility and in vivo bone responses of titanium. J. Med. Biol. Eng. 2017, 38, 1–14. [Google Scholar]
- Khandaker, M.; Riahinezhad, S.; Williams, W.R.; Wolf, R. Microgroove and Collagen-poly(epsilon-caprolactone) Nanofiber Mesh Coating Improves the Mechanical Stability and Osseointegration of Titanium Implants. Nanomaterials 2017, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Calderon, M.; Manso-Silván, M.; Rodríguez, A.; Gómez-Aranzadi, M.; García-Ruiz, J.P.; Olaizola, S.M.; Martín-Palma, R.J. Surface micro- and nano-texturing of stainless steel by femtosecond laser for the control of cell migration. Sci. Rep. 2016, 6, 36296. [Google Scholar] [CrossRef] [PubMed]
- García David, S.; Cortijo Martínez, J.A.; Navarro Bermúdez, I. The geometry of the keel determines the behaviour of the tibial tray against torsional forces in total knee replacement. Rev. Española Cirugía Ortopédica Traumatol. 2014, 58, 329–335. [Google Scholar] [CrossRef]
- Ries, M.D.; Rauscher, L.A.; Hoskins, S.; Lott, D.; Richman, J.A.; Lynch, F. Intramedullary pressure and pulmonary function during total knee arthroplasty. Clin. Orthop. Relat. Res. 1998, 356, 154–160. [Google Scholar] [CrossRef]
- Clary, C.; Maletsky, L. 11—Mechanical Testing of Knee Implants. In Mechanical Testing of Orthopaedic Implants; Friis, E., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 207–229. [Google Scholar]
- Moran, M.F.; Bhimji, S.; Racanelli, J.; Piazza, S.J. Computational assessment of constraint in total knee replacement. J. Biomech. 2008, 41, 2013–2020. [Google Scholar] [CrossRef]
- Morrison, J.B. The mechanics of the knee joint in relation to normal walking. J. Biomech. 1970, 3, 51–61. [Google Scholar] [CrossRef]
- Eberl, C. Digital Image Correlation and Tracking. MATLAB Central File Exchange. 2020. Available online: https://www.mathworks.com/matlabcentral/fileexchange/12413-digital-image-correlation-and-tracking (accessed on 27 April 2020).
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Frehill, B.; Crocombe, A.D. Finite element assessment of metaphyseal sleeves in total knee arthroplasty. J. Orthop. 2020, 19, 1–8. [Google Scholar] [CrossRef]
- Completo, A.; Simoes, J.A.; Fonseca, F.; Oliveira, M. The influence of different tibial stem designs in load sharing and stability at the cement-bone interface in revision TKA. Knee 2008, 15, 227–232. [Google Scholar] [CrossRef]
- Janssen, D.; Mann, K.A.; Verdonschot, N. Finite element simulation of cement-bone interface micromechanics: A comparison to experimental results. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2009, 27, 1312–1318. [Google Scholar] [CrossRef]
- Walsh, W.R.; Svehla, M.J.; Russell, J.; Saito, M.; Nakashima, T.; Gillies, R.M.; Bruce, W.; Hori, R. Cemented fixation with PMMA or Bis-GMA resin hydroxyapatite cement: Effect of implant surface roughness. Biomaterials 2004, 25, 4929–4934. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.; Riahinezhad, S.; Sultana, F.; Vaughan, M.; Knight, J.; Morris, T. Peen treatment on a titanium implant: Effect of roughness, osteoblast cell functions, and bonding with bone cement. Int. J. Nanomed. 2016, 11, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Ait Moussa, A.; Fischer, J.; Yadav, R.; Khandaker, M. Minimizing Stress Shielding and Cement Damage in Cemented Femoral Component of a Hip Prosthesis through Computational Design Optimization. Adv. Orthop. 2017, 2017, 8437956. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A. Optimizing the biomechanical compatibility of orthopedic screws for bone fracture fixation. Med. Eng. Phys. 2002, 24, 337–347. [Google Scholar] [CrossRef]
- Haase, K.; Rouhi, G. Prediction of stress shielding around an orthopedic screw: Using stress and strain energy density as mechanical stimuli. Comput. Biol. Med. 2013, 43, 1748–1757. [Google Scholar] [CrossRef]
- Venkatsurya, P.K.C.; Girase, B.; Misra, R.D.K.; Pesacreta, T.C.; Somani, M.C.; Karjalainen, L.P. The interplay between osteoblast functions and the degree of nanoscale roughness induced by grain boundary grooving of nanograined materials. Mater. Sci. Eng. C 2012, 32, 330–340. [Google Scholar] [CrossRef]
- Kashiwazaki, H.; Kishiya, Y.; Matsuda, A. Fabrication of porous chitosan/hydroxyapatite nanocomposites: Their mechanical and biological properties. Bio-Med. Mater. Eng. 2009, 19, 133–140. [Google Scholar] [CrossRef]
- Lamers, E.; Frank Walboomers, X.; Domanski, M. The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition. Biomaterials 2010, 31, 3307–3316. [Google Scholar] [CrossRef]
- Zant, N.P.; Heaton-Adegbile, P.; Hussell, J.G.; Tong, J. In Vitro Fatigue Failure of Cemented Acetabular Replacements: A Hip Simulator Study. J. Biomech. Eng. 2008, 130, 021019. [Google Scholar] [CrossRef]
- Anders, A. Metal plasma immersion ion implantation and deposition: A review. Surf. Coat. Technol. 1997, 93, 158–167. [Google Scholar] [CrossRef]
- Qiao, H.; Zhao, J.; Gao, Y. Experimental investigation of laser peening on TiAl alloy microstructure and properties. Chin. J. Aeronaut. 2015, 28, 609–616. [Google Scholar] [CrossRef]


| Properties | Unit | Bone [20] | Aluminum | Cement (PMMA) [21] |
|---|---|---|---|---|
| Density | kg/m3 | 1550 | 4400 | 1770 |
| Young’s Modulus | N/mm2 | 1 × 105 | 1.06 × 105 | 2.27 × 103 |
| Poisson’s Ratio | 0.45 | 0.33 | 0.46 |
| No of Cycles | Smooth | Grooved |
|---|---|---|
| 11,000 | 0.0034 ± 0.0003 (n = 2) | 0.0046 ± 0.0008 (n = 2) |
| 27,000 | 0.0045 ± 0.0002 (n = 2) | 0.0104 (n = 1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandaker, M.; Ait Moussa, A.; Sama, D.N.; Safavinia, F.; Hazra, S.; Kalay, O.C.; Karpat, F.; Clary, E.; Haleem, A. Laser-Induced Microgrooves Improve the Mechanical Responses of Cemented Implant Systems. Micromachines 2020, 11, 466. https://doi.org/10.3390/mi11050466
Khandaker M, Ait Moussa A, Sama DN, Safavinia F, Hazra S, Kalay OC, Karpat F, Clary E, Haleem A. Laser-Induced Microgrooves Improve the Mechanical Responses of Cemented Implant Systems. Micromachines. 2020; 11(5):466. https://doi.org/10.3390/mi11050466
Chicago/Turabian StyleKhandaker, Morshed, Abdellah Ait Moussa, Desmond Nuyebga Sama, Fereshteh Safavinia, Susmita Hazra, Onur Can Kalay, Fatih Karpat, Erik Clary, and Amgad Haleem. 2020. "Laser-Induced Microgrooves Improve the Mechanical Responses of Cemented Implant Systems" Micromachines 11, no. 5: 466. https://doi.org/10.3390/mi11050466
APA StyleKhandaker, M., Ait Moussa, A., Sama, D. N., Safavinia, F., Hazra, S., Kalay, O. C., Karpat, F., Clary, E., & Haleem, A. (2020). Laser-Induced Microgrooves Improve the Mechanical Responses of Cemented Implant Systems. Micromachines, 11(5), 466. https://doi.org/10.3390/mi11050466






