Morphology Evolution of Nanoscale-Thick Au/Pd Bimetallic Films on Silicon Carbide Substrate
Abstract
1. Introduction
2. Materials, Experiment Analysis, and Methods
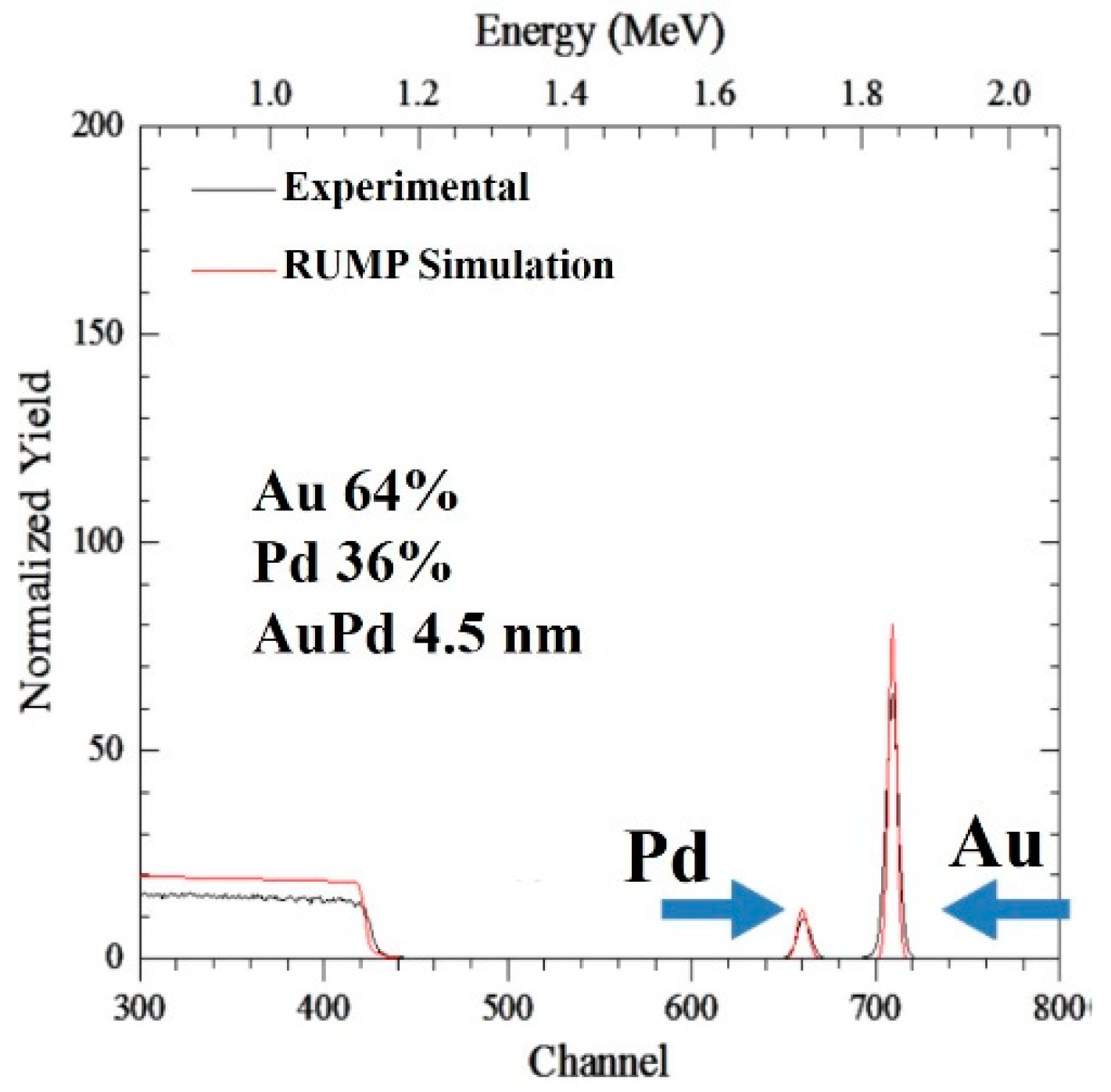
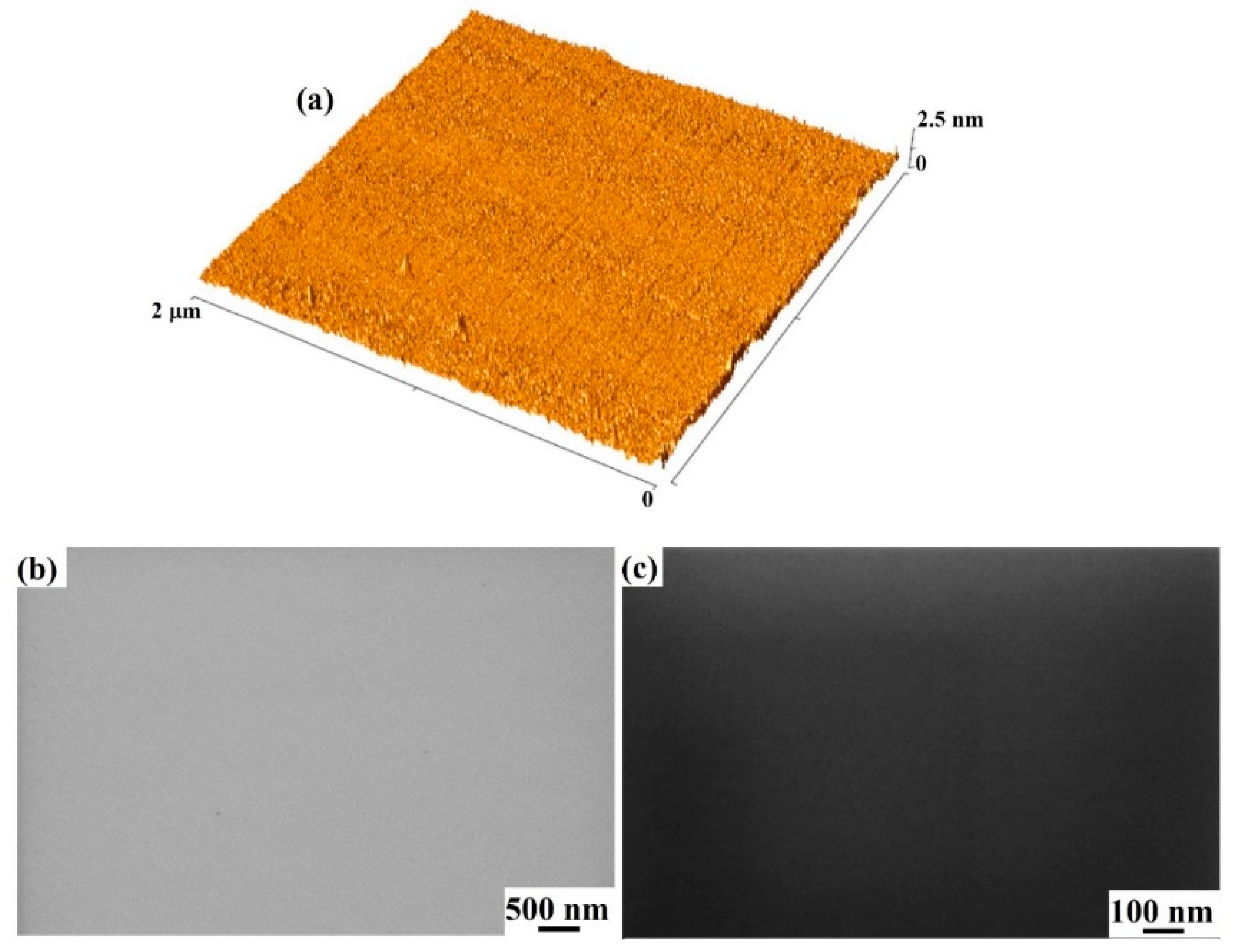
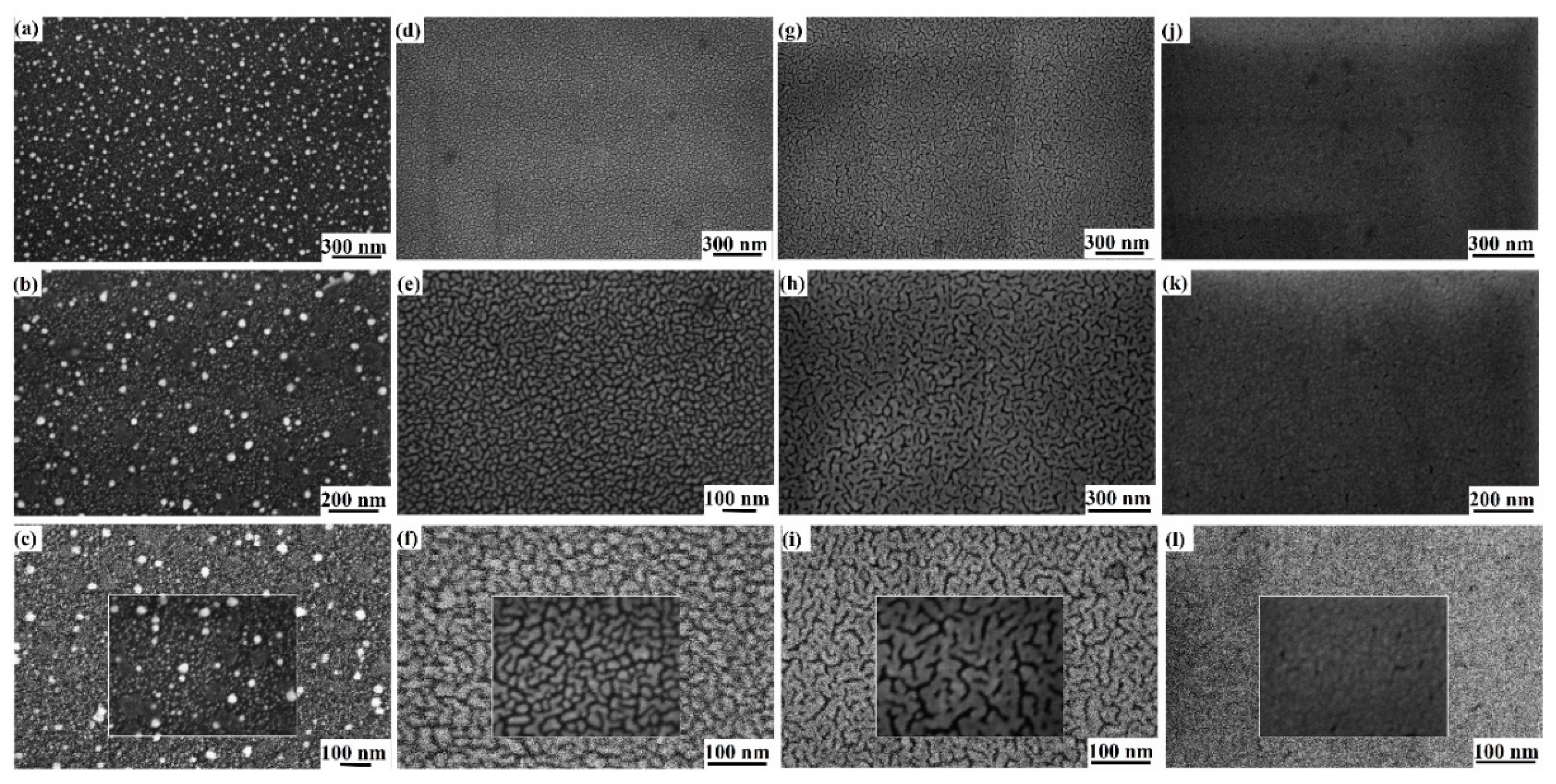
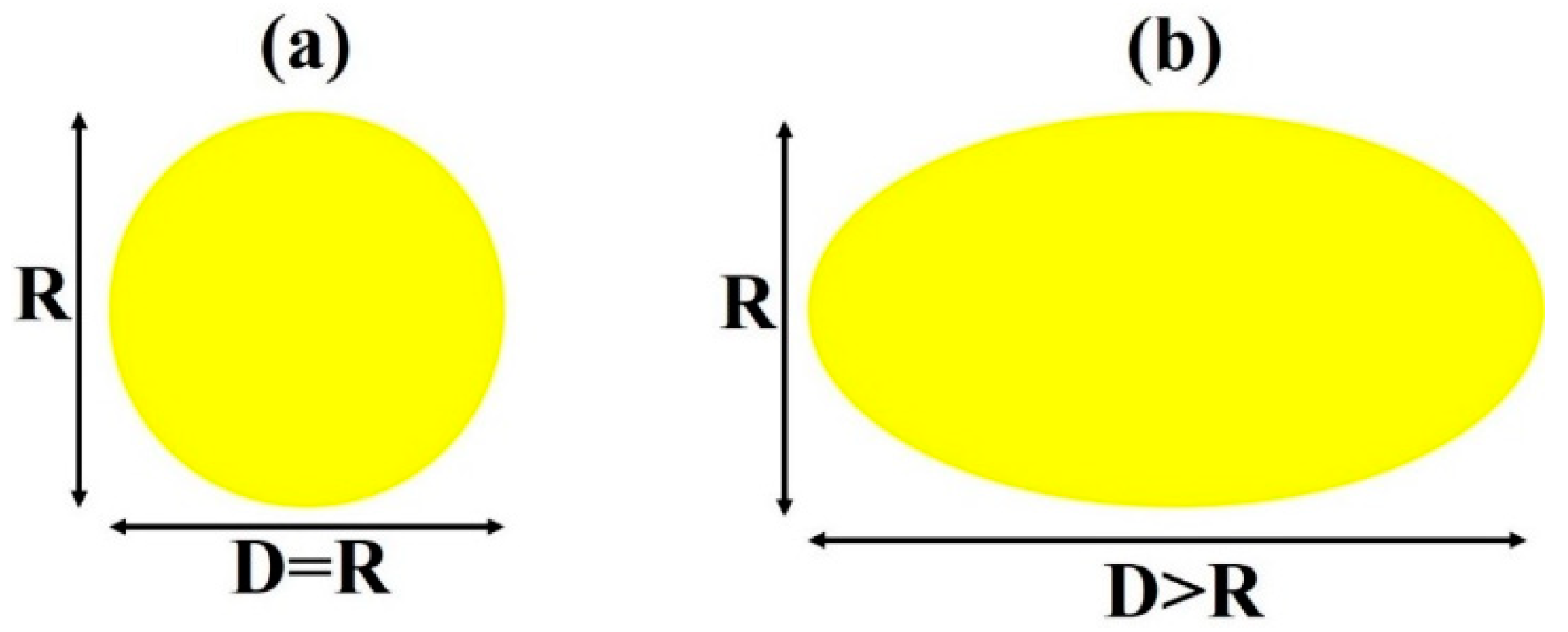
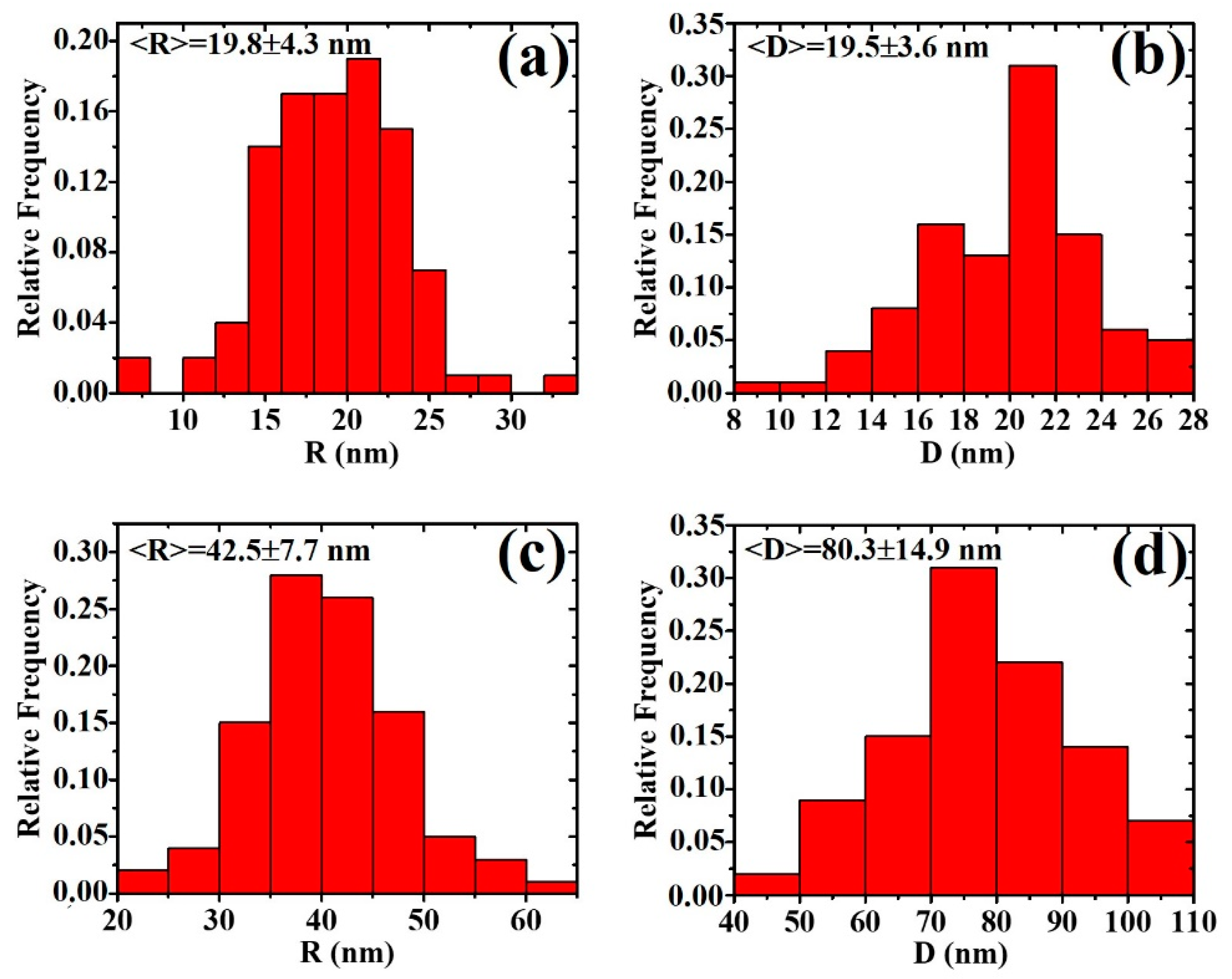
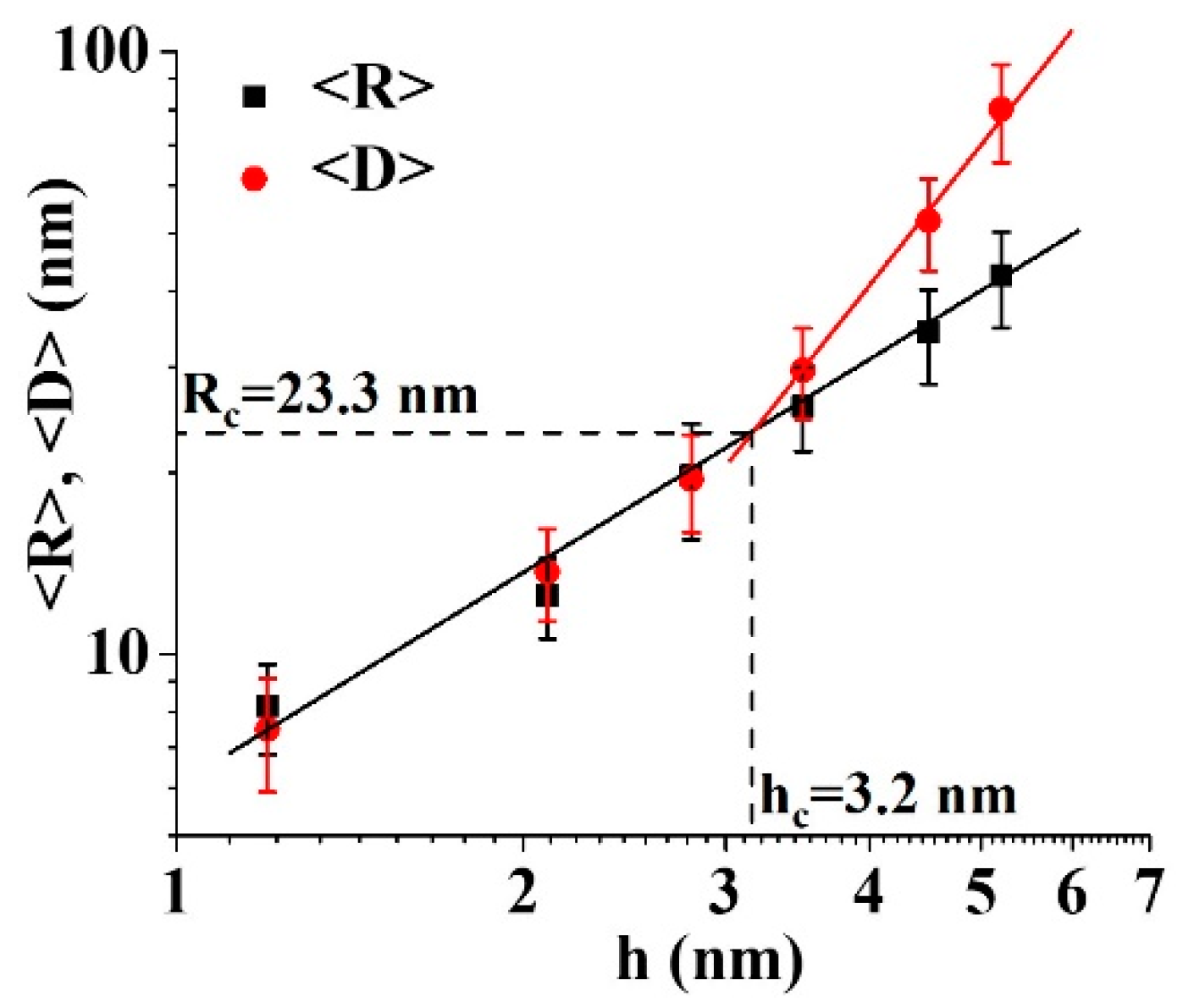
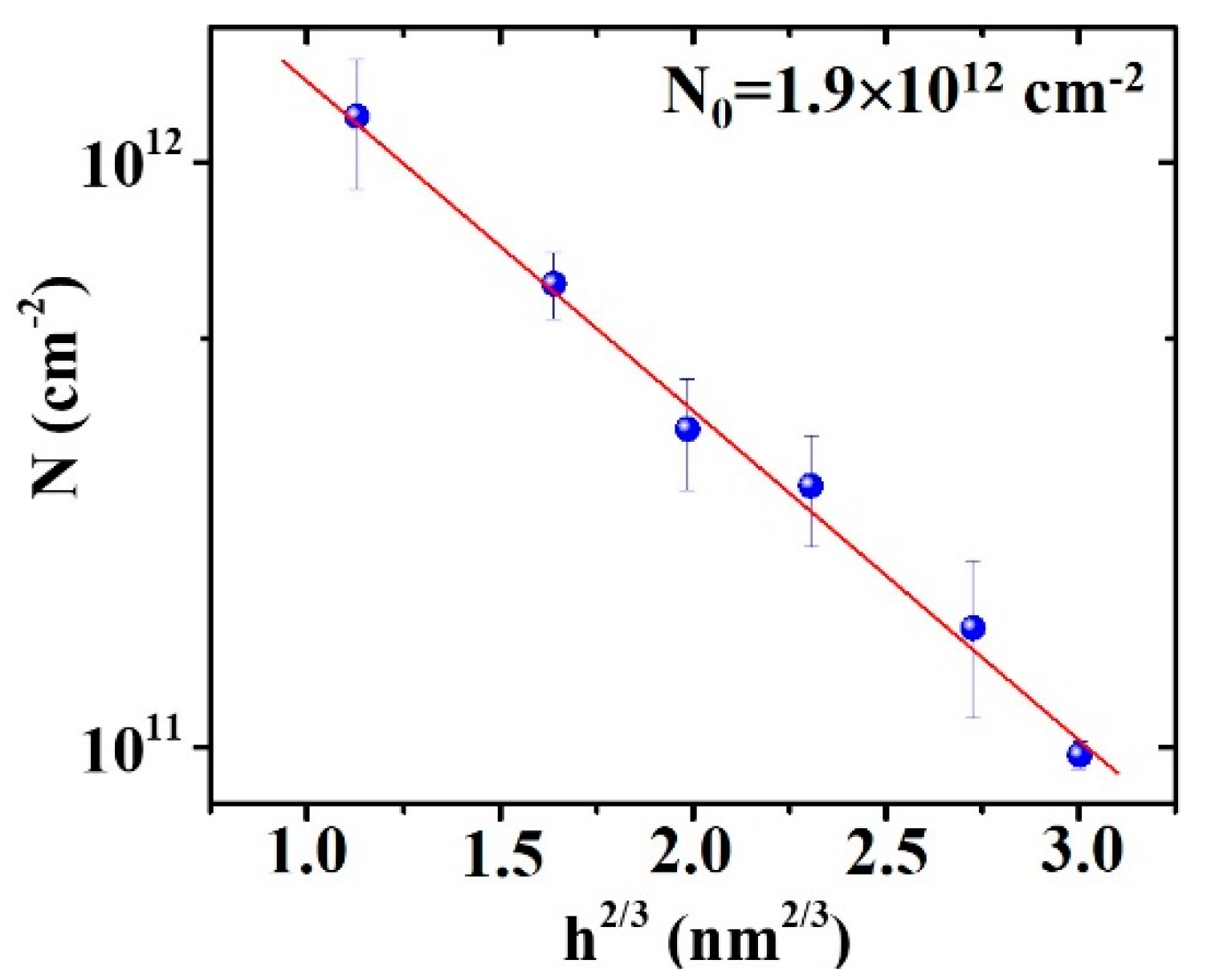
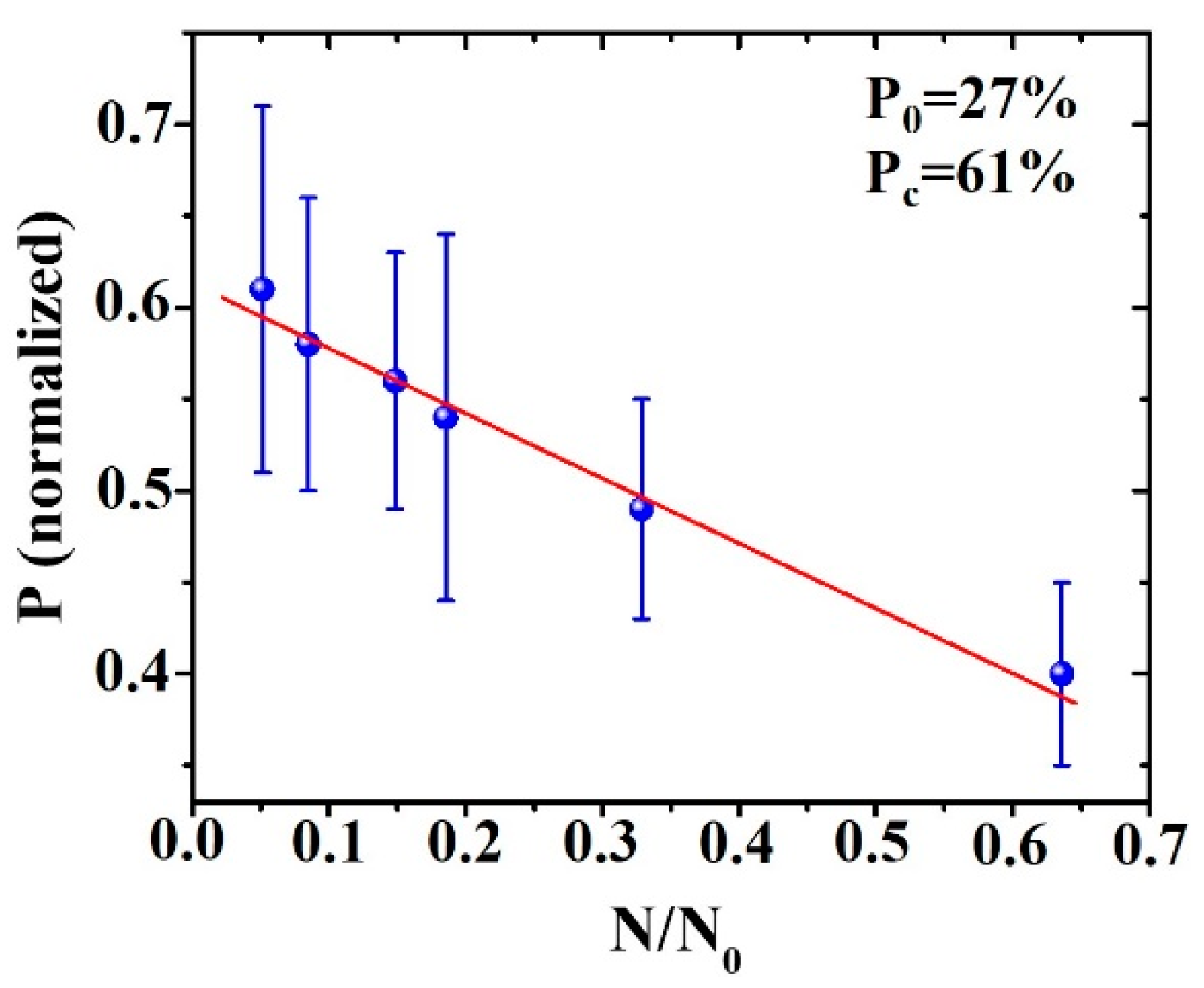
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saddow, S.E.; Agarwal, A. Advances in Silicon Carbide Processing and Applications; Artech House Inc.: Norwood, MA, USA, 2004. [Google Scholar]
- Kimoto, T.; Cooper, J.A. Fundamentals of Silicon Carbide Technology-Growth, Characterization, Devices, and Applications; Wiley: Singapore, 2014. [Google Scholar]
- Shur, M.; Rumyantsev, S.; Levinshtein, M. SiC Materials and Devices, vol. 1 and vol. 2; World Scientific Publishing: Singapore, 2007. [Google Scholar]
- Porter, L.M.; Davis, R.F. A critical review of ohmic and rectifying contacts for silicon carbide. Mater. Sci. Eng. B 1995, 34, 83–105. [Google Scholar] [CrossRef]
- Zekentes, K.; Vasilevskiy, K. Advancing Silicon Carbide Electronics Technology I-Metal Contacts to Silicon Carbide: Physics, Technology, Applications; Materials Research Forum LLC: Millersville, PA, USA, 2018. [Google Scholar]
- Friedrichs, P.; Kimoto, T.; Ley, L.; Pensl, G. Silicon Carbide-Power Devices and Sensors (Vol. 2); Wiley: Weinheim, Germany, 2010. [Google Scholar]
- Kim, C.K.; Lee, J.H.; Choi, S.M.; Noh, I.H.; Kim, H.R.; Cho, N.I.; Hong, C.; Jang, G.E. Pd- and Pt-SiC Schottky diodes for detection of H2 and CH4 at high temperature. Sens. Actuators B 2001, 77, 455–462. [Google Scholar] [CrossRef]
- Khan, S.A.; de Vasconcelos, E.A.; Uchida, H.; Katsube, T. Gas response and modelling of NO-sensitive thin-Pt SiC Schottky diodes. Sens. Actuators B 2003, 92, 181–185. [Google Scholar] [CrossRef]
- Olivier, E.J.; Neethling, J.H. Palladium transport in SiC. Nucl. Eng. Des. 2012, 244, 25–33. [Google Scholar] [CrossRef]
- Yun, S.; Wang, L.; Zhao, C.; Wang, Y.; Ma, T. A new type of loss-cost counter electrode catalyst based on platinum nanoparticles loaded onto silicon carbide (Pt/SiC) for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 4286–4290. [Google Scholar] [CrossRef]
- Njoroge, E.G.; Theron, C.C.; Skuratov, V.A.; Wamwangi, D.; Hlatshwayo, T.T.; Comrie, C.M. Malherbe, J.B.; Interface reactions between Pd thin films and SiC by thermal annealing and SHI irradiation. Nucl. Instr. Meth. Phys. Res. B 2016, 371, 263–267. [Google Scholar] [CrossRef]
- Şennik, E.; Ürdem, Ş.; Erkovan, M.; Kilinç, N. Sputtered platinum thin films for resistive hydrogen sensor application. Mater. Lett. 2016, 177, 104–107. [Google Scholar] [CrossRef]
- Thabethe, T.T.; Nstoane, T.; Biira, S.; Njoroge, E.G.; Hlatshwayo, T.T.; Skuratov, V.A.; Malherbe, J.B. Irradiation effects of swift heavy ions on palladium films deposited on 6H-SiC substrate. Nucl. Instr. Meth. Phys. Res. 2019, 442, 19–23. [Google Scholar] [CrossRef]
- Wright, N.G.; Horsfall, A.B. SiC sensors: A review. J. Phys. D Appl. Phys. 2007, 40, 6345–6354. [Google Scholar] [CrossRef]
- Casals, O.; Barcones, B.; Romano-Rodríguez, A.; Serre, C.; Pérez-Rodríguez, A.; Morante, J.R.; Godignon, P.; Montserrat, J.; Millán, J. Characterisation and stabilisation of Pt/TaSiX/SiO2/SiC gas sensor. Sens. Actuators B Chem. 2005, 109, 119–127. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Island-to-percolation transition during the room-temprature growth of sputtered nanoscale Pd films on hexagonal SiC. J. Appl. Phys. 2010, 107, 074301. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G.; Giannazzo, F.; Roccaforte, F.; Raineri, V. Size-dependent Schottky barrier height in self-assembled gold nanoparticles. Appl. Phys. Lett. 2006, 89, 243113. [Google Scholar] [CrossRef]
- Ruffino, F.; Canino, A.; Grimaldi, M.G.; Giannazzo, F.; Bongiorno, C.; Roccaforte, F.; Raineri, V. Self-organization of gold nanoclusters on hexagonal SiC and SiO2 surfaces. J. Appl. Phys. 2007, 101, 064306. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Formation of patterned arrays of Au nanoparticles on SiC surface by template confined dewetting of normal and oblique deposited nanoscale films. Thin Solid Films 2013, 536, 99–110. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Dewetting of template-confined Au films on SiC surface: From patterned films to patterned arrays of nanoparticles. Vacuum 2014, 99, 28–37. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, H.; Fan, S.; Deng, S.; Lv, Q.; Lin, J.; Li, C.-P. Label-free electrochemical immunosensor based gold-silicon carbide nanocomposites for sensitive detection of human chorionic gonadotrophin. Biosens. Bioelectron. 2014, 57, 199–206. [Google Scholar] [CrossRef]
- Zheng, G.; Xu, L.; Zou, X.; Liu, Y. Excitation of surface phonon polariton modes in gold gratings with silicon carbide substrate and their potential sensing applications. Appl. Surf. Sci. 2017, 396, 711–716. [Google Scholar] [CrossRef]
- Zhigal’skii, G.P.; Jones, B.K. The Physical Properties of Thin Metal Films; Taylor and Francis: New York, NY, USA, 2003. [Google Scholar]
- Barmak, K.; Coffey, K. Metallic Films for Electronic, Optical and Magnetic Applications-Structure, Processing and Properties; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Johnston, R.L.; Wilcoxon, J.P. Metal Nanoparticles and Nanoalloys; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Xiong, Y.; Lu, X. Metallic Nanostructures-From Controlled Synthesis to Applications; Springer: New York, NY, USA, 2014. [Google Scholar]
- Ruffino, F.; Crupi, I.; Simone, F.; Grimaldi, M.G. Formation and evolution of self-organized Au nanorings on indium-tin-oxide surface. Appl. Phys. Lett. 2011, 98, 023101. [Google Scholar] [CrossRef]
- Sanzone, G.; Zimbone, M.; Cacciato, G.; Ruffino, F.; Carles, R.; Privitera, V.; Grimaldi, M.G. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlatt. Microstruct. 2018, 123, 394–402. [Google Scholar] [CrossRef]
- Gentile, A.; Ruffino, F.; Grimaldi, M.G. Complex-morphology metal-based nanostructures: Fabrication, characterization, and applications. Nanomaterials 2016, 6, 110. [Google Scholar] [CrossRef]
- Ruffino, F.; Pugliara, A.; Carria, E.; Romano, L.; Bongiorno, C.; Fisicaro, G.; La Magna, A.; Spinella, C.; Grimaldi, M.G. Towards a laser fluence dependent nanostructuring of thin Au films on Si by nanosecond laser irradiation. Appl. Surf. Sci. 2012, 258, 9128–9137. [Google Scholar] [CrossRef]
- Kim, K.-S.; Chung, G.-S. Characterization of porous cubic silicon carbide deposited with Pd and Pt nanoparticles as a hydrogen sensor. Sens. Actuators B 2011, 157, 482–487. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Lin, Y.; Xu, G.; Yang, C.; Tong, Y.; Guo, L.; Chen, G. A new metal electrocatalyst supported matrix: Palladium particles supported silicon carbide nanoparticles and its application for alcohol electrooxidation. Electrochim. Acta 2012, 85, 644–649. [Google Scholar] [CrossRef]
- Zhang, Y.-W. Bimetallic Nanostructures: Shape-controlled Synthesis for Catalysis, Plasmonics, and Sensing Applications; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Yamauchi, M.; Kobayashi, H.; Kitagawa, H. Hydrogen storage mediated by Pd and Pt nanoparticles. ChemPhysChem 2009, 10, 2566–2576. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Yi, S.-I.; Ma, L.; Chen, Y.; Dai, W.; Sinyukov, A.M.; Liang, H. Morphology dependent catalysis and enhanced Raman scattering (SERS) studies using Pd nanostructures in DNA, CTAB, and PVA scaffolds. Dalton Trans. 2017, 46, 9678–9691. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jia, H.; Liu, Z.; Gao, P.; Zhao, J.; Luo, Z. Understanding of strain effects in the electrochemical reduction of CO2: Using Pd nanostructures as an ideal platform. Angew. Chem. 2017, 56, 3594–3598. [Google Scholar] [CrossRef]
- Ahmed, H.B.; Emam, H.E. Synergistic catalysis of monometallic (Ag, Au, Pd) and bimetallic (Ag–Au, Au–Pd) versus trimetallic (Ag–Au–Pd) nanostructures effloresced via analogical techniques. J. Mol. Liq. 2019, 287, 110975. [Google Scholar] [CrossRef]
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles: Novel materials for chemical and physical applications. New. J. Chem. 1998, 1998, 1179–1201. [Google Scholar] [CrossRef]
- Hao, C.-H.; Guo, X.-N.; Sankar, M.; Yang, H.; Ma, B.; Zhang, Y.-F.; Tong, X.-L.; Jin, G.-Q.; Guo, X.-Y. Synergistic effect of segregated Pd and Au nanoparticles on semiconducting SiC for efficient photocatalytic hydrogenation of nitroarenes. ACS Appl. Mater. Interfaces 2018, 10, 23029–23036. [Google Scholar] [CrossRef]
- Zetterling, C.-M. Process Technology for Silicon Carbide Devices; INSPEC: London, UK, 2002. [Google Scholar]
- Lundberg, N.; Ötling, M.; Tägtström, P.; Jansson, U. Chemical vapor deposition of tungsten Schottky diodes to 6H-SiC. J. Electrochem. Soc. 1996, 143, 1662–1667. [Google Scholar] [CrossRef]
- Wolborski, M.; Bakowski, M.; Pore, V.; Ritala, M.; Leskelä, M.; Schöner, A.; Hallén, A. Characterization of aluminium and titanium oxides deposited on 4H-SiC by atomic layer deposition. Mater. Sci. Forum 2005, 483–485, 701–704. [Google Scholar] [CrossRef]
- Arakawa, R.; Kawasaki, H. Functionalized nanoparticles and nanostructures surfaces for surface-assisted laser desorption/ionization mass spectrometry. Anal. Sci. 2010, 26, 1229–1240. [Google Scholar] [CrossRef]
- Silina, Y.E.; Volmer, D.A. Nanostructured solid substrates for efficient laser desorption/ionization mass spectrometry (LDI-MS) of low molecular weight compounds. Analyst 2013, 138, 7053–7065. [Google Scholar] [CrossRef]
- Gierz, I.; Suzuki, T.; Lee, D.S.; Krauss, B.; Riedl, C.; Starke, U.; Höchst, H.; Smet, J.H.; Ast, C.R.; Kern, K. Electronic decoupling of an epitaxial graphene monolayer by gold intercalation. Phys. Rev. B 2010, 81, 235408. [Google Scholar] [CrossRef]
- Mishra, N.; Boeckl, J.; Motta, N.; Iacopi, F. Graphene growth on silicon carbide: A review. Phys. Stat. Sol. A 2016, 213, 2277–2289. [Google Scholar] [CrossRef]
- Available online: http://www.genplot.com/doc/rump.htm (accessed on 12 April 2020).
- Available online: https://www.ct.imm.cnr.it/?q=articles/characterizations-catania-unit (accessed on 12 April 2020).
- Zinke-Allmang, M.; Feldman, L.C.; Grabov, M.H. Clustering on surfaces. Surf. Sci. Rep. 1992, 16, 377–463. [Google Scholar] [CrossRef]
- Campbell, C.T. Ultrathin metal films and particles on oxide surfaces: Structural, electronic and chemisorptive properties. Surf. Sci. Rep. 1997, 27, 1–111. [Google Scholar] [CrossRef]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys. 1984, 47, 399–459. [Google Scholar] [CrossRef]
- Wang, Z.; Wynblatt, P. The equilibrium form of pure gold crystals. Surf. Sci. 1998, 398, 259–266. [Google Scholar] [CrossRef]
- Yu, X.; Duxbury, P.M.; Jeffers, G.; Dubson, M.A. Coalescence and percolation in thin metal films. Phys. Rev. B 1991, 44, 13163(R)–13166(R). [Google Scholar] [CrossRef]
- Heim, K.R.; Coyle, S.T.; Hembree, G.G.; Venables, J.A.; Scheinfein, M.R. Growth of nanometer-size metallic particles on CaF(111). J. Appl. Phys. 1996, 80, 1161–1170. [Google Scholar] [CrossRef]
- Zhang, L.; Cosandey, F.; Persaud, R.; Madey, T.E. Initial growth and morphology of thin Au films on TiO2(110). Surf. Sci. 1999, 439, 73–85. [Google Scholar] [CrossRef]
- Jeffers, G.; Dubson, M.A.; Duxbury, P.M. Island-to-percolation transition during growth of metal films. J. Appl. Phys. 1994, 75, 5016–5020. [Google Scholar] [CrossRef]
- Duxbury, P.M.; Dubson, M.; Jeffers, G. Substrate inhomogeneity and the growth morphology of thin film. Europhys. Lett. 1994, 26, 601–606. [Google Scholar] [CrossRef]
- Voss, R.F.; Laibowitz, R.B.; Alessandrini, E.I. Fractal (scaling) clusters in thin gold films near the percolation threshold. Phys. Rev. Lett. 1982, 49, 1441–1443. [Google Scholar] [CrossRef]
- Fanfoni, M.; Tomellini, M.; Volpe, M. Coalescence and impingement between islands in thin film growth: Behavior of the island density kinetics. Phys. Rev. B 2001, 64, 075409. [Google Scholar] [CrossRef]
- Vincent, R. A theoretical analysis and computer simulation of the growth of epitaxial films. Proc. R. Soc. Lond. 1971, 321, 53–68. [Google Scholar]
- Briscoe, B.J.; Galvin, K.P. Growth with coalescence during condensation. Phys. Rev. A 1991, 43, 1906–1917. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffino, F.; Censabella, M.; Piccitto, G.; Grimaldi, M.G. Morphology Evolution of Nanoscale-Thick Au/Pd Bimetallic Films on Silicon Carbide Substrate. Micromachines 2020, 11, 410. https://doi.org/10.3390/mi11040410
Ruffino F, Censabella M, Piccitto G, Grimaldi MG. Morphology Evolution of Nanoscale-Thick Au/Pd Bimetallic Films on Silicon Carbide Substrate. Micromachines. 2020; 11(4):410. https://doi.org/10.3390/mi11040410
Chicago/Turabian StyleRuffino, Francesco, Maria Censabella, Giovanni Piccitto, and Maria Grazia Grimaldi. 2020. "Morphology Evolution of Nanoscale-Thick Au/Pd Bimetallic Films on Silicon Carbide Substrate" Micromachines 11, no. 4: 410. https://doi.org/10.3390/mi11040410
APA StyleRuffino, F., Censabella, M., Piccitto, G., & Grimaldi, M. G. (2020). Morphology Evolution of Nanoscale-Thick Au/Pd Bimetallic Films on Silicon Carbide Substrate. Micromachines, 11(4), 410. https://doi.org/10.3390/mi11040410






