Free-Form and Deformable Energy Storage as a Forerunner to Next-Generation Smart Electronics
Abstract
1. Introduction
2. Bendable and Foldable Batteries
2.1. Strategies for Achieving Bendability and Foldability: Materials
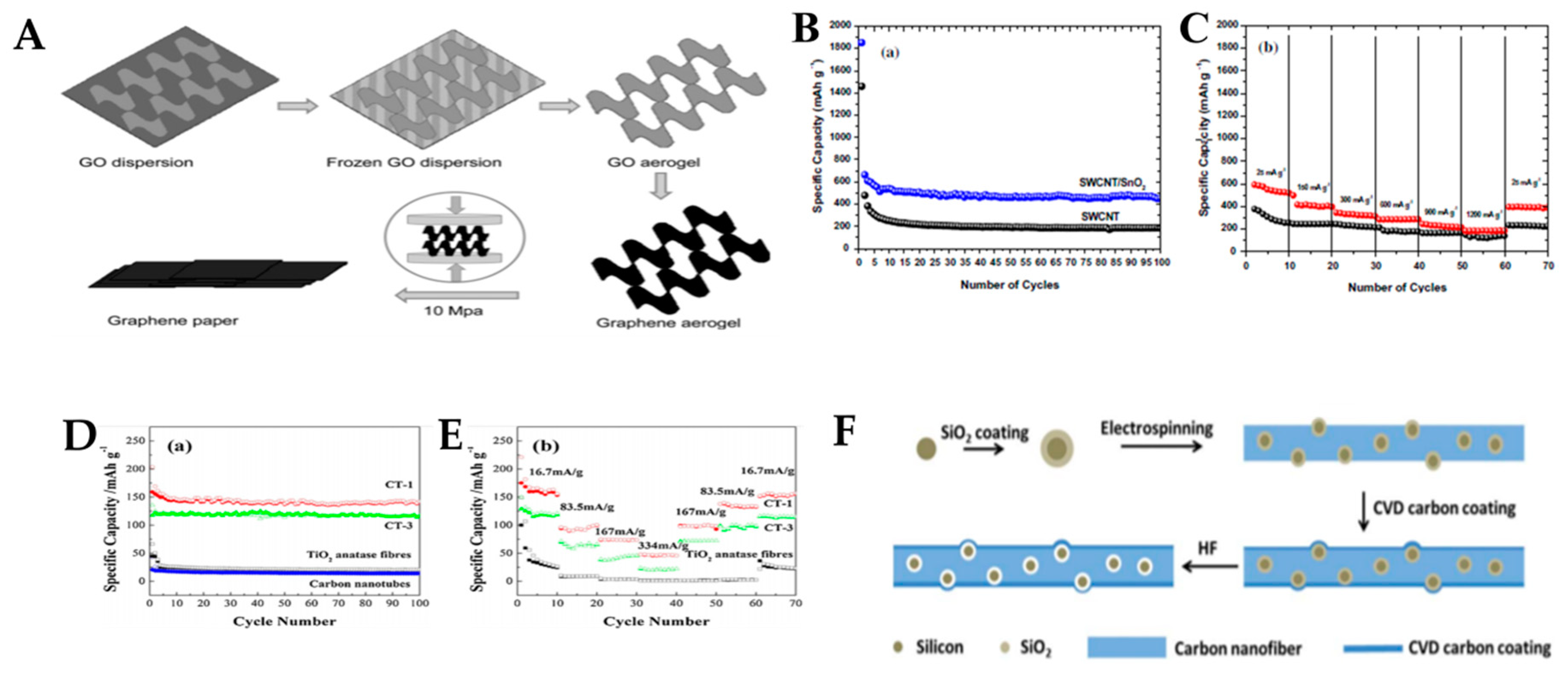
2.1.1. Graphene
2.1.2. Carbon Nanotube (CNT)
2.1.3. Carbon Nanofibers (CNFs)
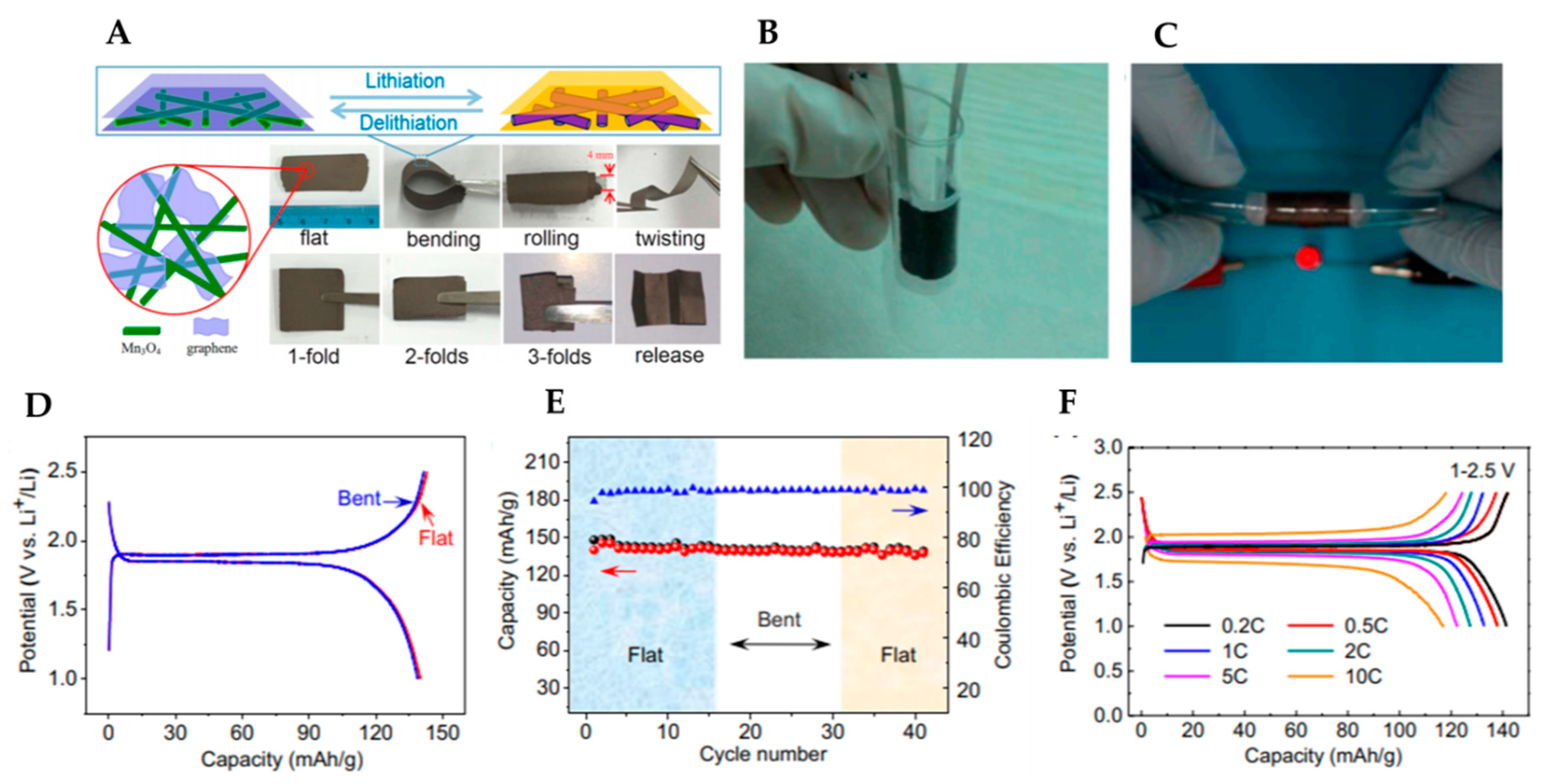
2.1.4. The Other Materials
2.2. Strategies for Achieving Bendability and Foldability: Designs
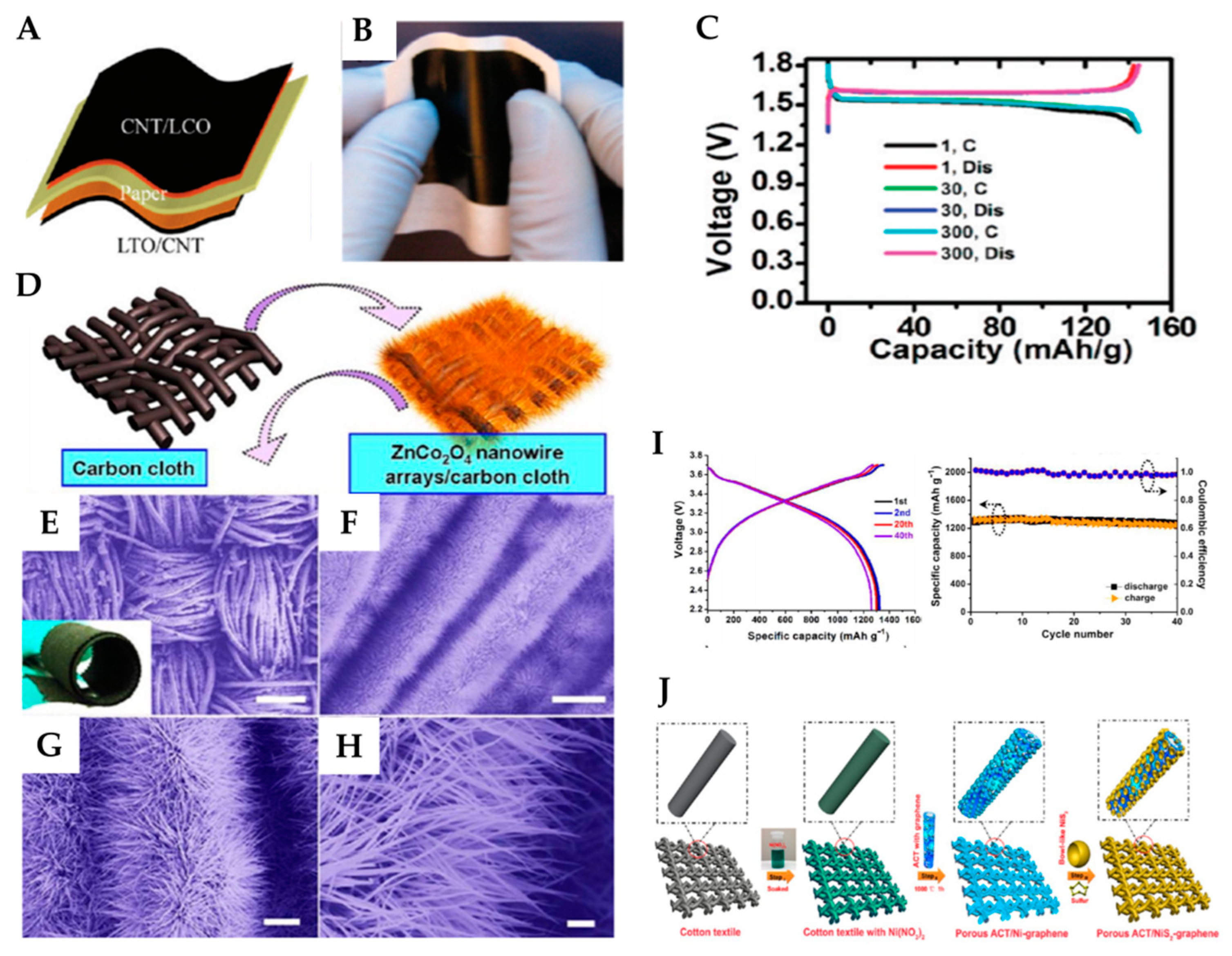
2.2.1. Paper Configuration
2.2.2. Textile Configuration
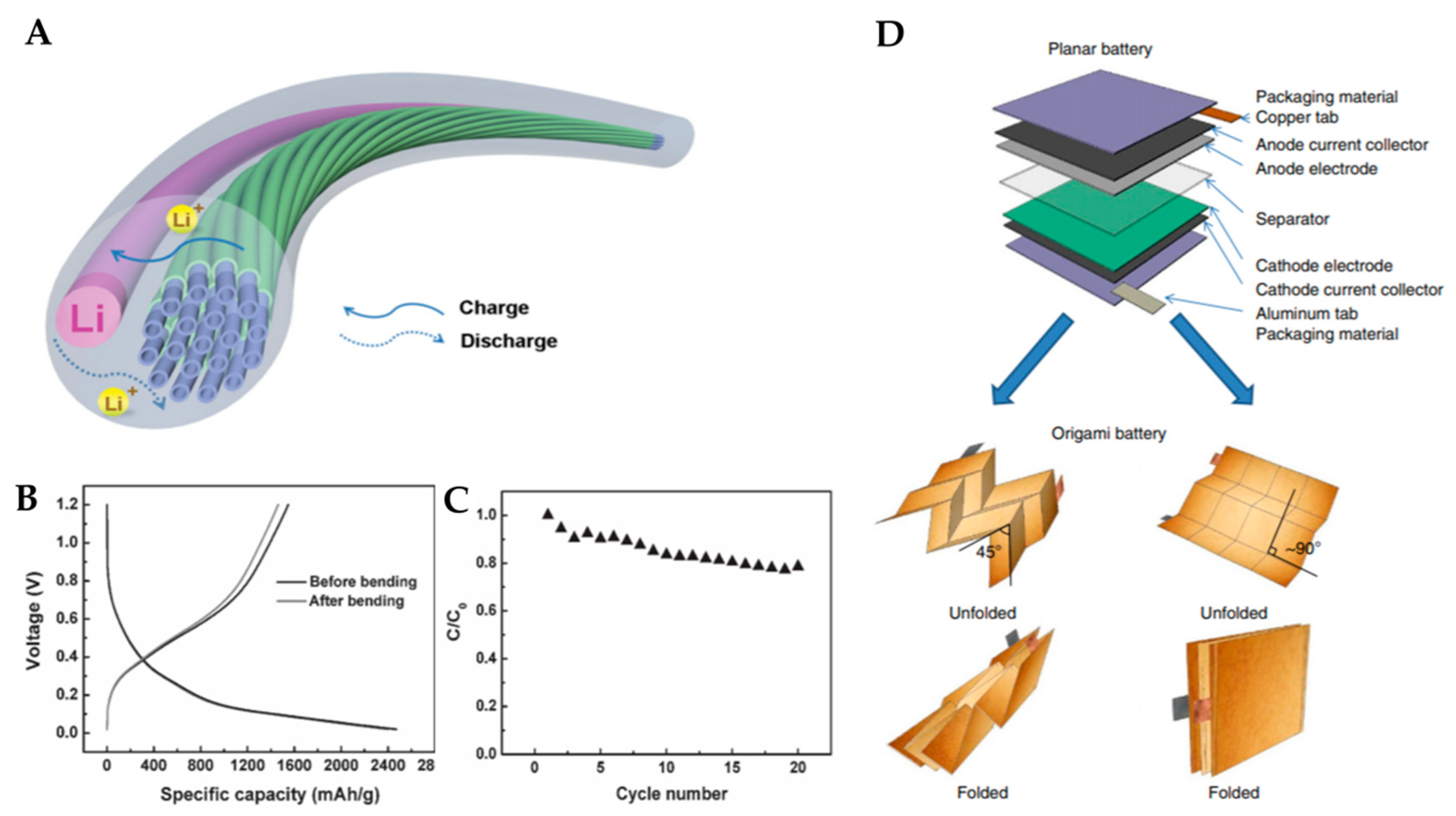
2.2.3. Wire-Shaped Configuration
2.2.4. Origami Configuration
3. Stretchable Batteries
3.1. Stretchable Strategies: Materials
3.1.1. Polydimethylsiloxane (PDMS)
3.1.2. Polyurethane (PU)
3.1.3. The Other Materials
3.2. Stretchable Strategies: Designs
3.2.1. Wavy-Shape Configuration
3.2.2. Kirigami and Origami Configuration
3.2.3. Serpentine Bridge-Island Configuration
3.2.4. Wire and Textile Configuration
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, W.; Song, M.-S.; Kong, B.; Cui, Y. Flexible and stretchable energy storage: Recent advances and future perspectives. Adv. Mater. 2017, 29, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef] [PubMed]
- Nishide, H.; Oyaizu, K. Materials science. Toward flexible batteries. Science 2008, 319, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Nathan, A.; Ahnood, A.; Cole, M.T.; Lee, S.; Suzuki, Y.; Hiralal, P.; Bonaccorso, F.; Hasan, T.; Garcia-Gancedo, L.; Dyadyusha, A.; et al. Flexible electronics: The next ubiquitous platform. Proc. IEEE 2012, 100, 1486–1517. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Rogers, J.A.; Bao, Z.; Baldwin, K.; Dodabalapur, A.; Crone, B.; Raju, V.R.; Kuck, V.; Katz, H.; Amundson, K.; Ewing, J.; et al. Paper-like electronic displays: Large-area rubber-stamped plastic sheets of electronics and microencapsulated electrophoretic inks. Proc. Natl. Acad. Sci. USA 2001, 98, 4835–4840. [Google Scholar] [CrossRef]
- Gelinck, G.H.; Huitema, H.E.A.; van Veenendaal, E.; Cantatore, E.; Schrijnemakers, L.; van der Putten, J.B.P.H.; Geuns, T.C.T.; Beenhakkers, M.; Giesbers, J.B.; Huisman, B.-H.; et al. Flexible active-matrix displays and shift registers based on solution-processed organic transistors. Nat. Mater. 2004, 3, 106–110. [Google Scholar] [CrossRef]
- Hu, X.; Ma, M.; Mendes, R.G.; Zeng, M.; Zhang, Q.; Xue, Y.; Zhang, T.; Rümmeli, M.H.; Fu, L. Li-storage performance of binder-free and flexible iron fluoride@graphene cathodes. J. Mater. Chem. A 2015, 3, 23930–23935. [Google Scholar] [CrossRef]
- Zhou, G.; Li, F.; Cheng, H.-M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Jin, Y.; McGinn, P.J. Bulk solid state rechargeable lithium ion battery fabrication with Al-doped Li7La3Zr2O12 electrolyte and Cu0.1V2O5 cathode. Electrochim. Acta 2013, 89, 407–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Fu, H.; Lee, J.; Su, J.; Hwang, K.-C.; Rogers, J.A.; Huang, Y. Buckling in serpentine microstructures and applications in elastomer-supported ultra-stretchable electronics with high areal coverage. Soft Matter 2013, 9, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Strømme, M. Toward flexible polymer and paper-based energy storage devices. Adv. Mater. 2011, 23, 3751–3769. [Google Scholar] [CrossRef]
- Liu, W.; Lin, D.; Pei, A.; Cui, Y. Stabilizing lithium metal anodes by uniform li-ion flux distribution in nanochannel confinement. J. Am. Chem. Soc. 2016, 138, 15443–15450. [Google Scholar] [CrossRef]
- Yousaf, M.; Shi, H.T.H.; Wang, Y.; Chen, Y.; Ma, Z.; Cao, A.; Naguib, H.E.; Han, R.P.S. Novel pliable electrodes for flexible electrochemical energy storage devices: Recent progress and challenges. Adv. Energy Mater. 2016, 6, 1600490. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Yin, L.-C.; Li, N.; Li, F.; Cheng, H.-M. Oxygen bridges between NiO nanosheets and graphene for improvement of lithium storage. ACS Nano 2012, 6, 3214–3223. [Google Scholar] [CrossRef]
- Gwon, H.; Kim, H.-S.; Lee, K.U.; Seo, D.-H.; Park, Y.C.; Lee, Y.-S.; Ahn, B.T.; Kang, K. Flexible energy storage devices based on graphene paper. Energy Environ. Sci. 2011, 4, 1277–1283. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.; Stoller, M.D.; Zhu, Y.; Ji, H.; Murali, S.; Wu, Y.; Perales, S.; Clevenger, B.; Ruoff, R.S. Highly conductive and porous activated reduced graphene oxide films for high-power supercapacitors. Nano Lett. 2012, 12, 1806–1812. [Google Scholar] [CrossRef]
- Yu, J.; Lu, W.; Pei, S.; Gong, K.; Wang, L.; Meng, L.; Huang, Y.; Smith, J.P.; Booksh, K.S.; Li, Q.; et al. Omnidirectionally stretchable high-performance supercapacitor based on isotropic buckled carbon nanotube films. ACS Nano 2016, 10, 5204–5211. [Google Scholar] [CrossRef]
- Mo, R.; Rooney, D.; Sun, K.; Yang, H.Y. 3D nitrogen-doped graphene foam with encapsulated germanium/nitrogen-doped graphene yolk-shell nanoarchitecture for high-performance flexible Li-ion battery. Nat. Commun. 2017, 8, 13949. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhong, Z.; Wang, D.; Wang, W.U.; Lieber, C.M. High performance silicon nanowire field effect transistors. Nano Lett. 2003, 3, 149–152. [Google Scholar] [CrossRef]
- Liang, W.; Yuhas, B.D.; Yang, P. Magnetotransport in co-doped ZnO nanowires. Nano Lett. 2009, 9, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Huang, F.; Cui, L.; Huang, P.; Yu, Y.; Wang, Y. Hydrothermal synthesis, structural characteristics, and enhanced photocatalysis of SnO2/α-Fe2O3 semiconductor nanoheterostructures. ACS Nano 2010, 4, 681–688. [Google Scholar] [CrossRef]
- Bae, J.; Song, M.K.; Park, Y.J.; Kim, J.M.; Liu, M.; Wang, Z.L. Fiber supercapacitors made of nanowire-fiber hybrid structures for wearable/flexible energy storage. Angew. Chem. Int. Ed. 2011, 50, 1683–1687. [Google Scholar] [CrossRef]
- Hassoun, J.; Lee, K.-S.; Sun, Y.-K.; Scrosati, B. An advanced lithium ion battery based on high performance electrode materials. J. Am. Chem. Soc. 2011, 133, 3139–3143. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible energy-storage devices: Design consideration and recent progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef]
- Li, N.; Chen, Z.; Ren, W.; Li, F.; Cheng, H.-M. Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates. Proc. Natl. Acad. Sci. USA 2012, 109, 17360–17365. [Google Scholar] [CrossRef]
- Liu, F.; Song, S.; Xue, D.; Zhang, H. Folded structured graphene paper for high performance electrode materials. Adv. Mater. 2012, 24, 1089–1094. [Google Scholar] [CrossRef]
- Fu, K.K.; Cheng, J.; Li, T.; Hu, L. Flexible batteries: From mechanics to devices. ACS Energy Lett. 2016, 1, 1065–1079. [Google Scholar] [CrossRef]
- Noerochim, L.; Wang, J.-Z.; Chou, S.-L.; Wexler, D.; Liu, H.-K. Free-standing single-walled carbon nanotube/SnO2 anode paper for flexible lithium-ion batteries. Carbon 2012, 50, 1289–1297. [Google Scholar] [CrossRef]
- Zhang, P.; Qiu, J.; Zheng, Z.; Liu, G.; Ling, M.; Martens, W.; Wang, H.; Zhao, H.; Zhang, S. Free-standing and bendable carbon nanotubes/TiO2 nanofibres composite electrodes for flexible lithium ion batteries. Electrochim. Acta 2013, 104, 41–47. [Google Scholar] [CrossRef][Green Version]
- Bavykin, D.V.; Parmon, V.N.; Lapkin, A.A.; Walsh, F.C. The effect of hydrothermal conditions on the mesoporous structure of TiO2 nanotubes. J. Mater. Chem. 2004, 14, 3370–3377. [Google Scholar] [CrossRef]
- Wang, Y.; Su, X.; Lu, S. Shape-controlled synthesis of TiO2 hollow structures and their application in lithium batteries. J. Mater. Chem. 2012, 22, 1969–1976. [Google Scholar] [CrossRef]
- Fu, K.; Lu, Y.; Dirican, M.; Chen, C.; Yanilmaz, M.; Shi, Q.; Bradford, P.D.; Zhang, X. Chamber-confined silicon–carbon nanofiber composites for prolonged cycling life of Li-ion batteries. Nanoscale 2014, 6, 7489–7495. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xue, L.; Yildiz, O.; Li, S.; Lee, H.; Li, Y.; Xu, G.; Zhou, L.; Bradford, P.D.; Zhang, X. Effect of CVD carbon coatings on Si@CNF composite as anode for lithium-ion batteries. Nano Energy 2013, 2, 976–986. [Google Scholar] [CrossRef]
- Bonino, C.A.; Ji, L.; Lin, Z.; Toprakci, O.; Zhang, X.; Khan, S.A. Electrospun carbon-tin oxide composite nanofibers for use as lithium ion battery anodes. ACS Appl. Mater. Interfaces 2011, 3, 2534–2542. [Google Scholar] [CrossRef]
- Das, T.K.; Prusty, S. Review on conducting polymers and their applications. Polym. Plast. Technol. Eng. 2012, 51, 1487–1500. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Xiao, P.; Bu, F.; Yang, G.; Zhang, Y.; Xu, Y. Integration of graphene, nano sulfur, and conducting polymer into compact, flexible lithium-sulfur battery cathodes with ultrahigh volumetric capacity and superior cycling stability for foldable devices. Adv. Mater. 2017, 29, 1703324. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, X.; Lv, R.; Na, B.; Ouyang, J.; Liu, H. Nanostructured polyaniline-cellulose papers for solid-state flexible aqueous zn-ion battery. ACS Sustain. Chem. Eng. 2018, 6, 8697–8703. [Google Scholar] [CrossRef]
- Hu, L.; Choi, J.W.; Yang, Y.; Jeong, S.; La Mantia, F.; Cui, L.-F.; Cui, Y. Highly conductive paper for energy-storage devices. Proc. Natl. Acad. Sci. USA 2009, 106, 21490–21494. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.; Kim, G.-P.; Park, S.; Han, J.W.; Yi, J. All-solid-state, origami-type foldable supercapacitor chips with integrated series circuit analogues. Energy Environ. Sci. 2014, 7, 1095–1102. [Google Scholar] [CrossRef]
- Zhang, L.C.; Sun, X.; Hu, Z.; Yuan, C.C.; Chen, C.H. Rice paper as a separator membrane in lithium-ion batteries. J. Power Sources 2012, 204, 149–154. [Google Scholar] [CrossRef]
- Pushparaj, V.L.; Shaijumon, M.M.; Kumar, A.; Murugesan, S.; Ci, L.; Vajtai, R.; Linhardt, R.J.; Nalamasu, O.; Ajayan, P.M. Flexible energy storage devices based on nanocomposite paper. Proc. Natl. Acad. Sci. USA 2007, 104, 13574–13577. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; La Mantia, F.; Yang, Y.; Cui, Y. Thin, flexible secondary li-ion paper batteries. ACS Nano 2010, 4, 5843–5848. [Google Scholar] [CrossRef]
- Gao, Z.; Song, N.; Zhang, Y.; Li, X. Cotton-textile-enabled, flexible lithium-ion batteries with enhanced capacity and extended lifespan. Nano Lett. 2015, 15, 8194–8203. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Wang, X.; Chen, G.; Chen, D.; Zhou, C.; Shen, G. Hierarchical three-dimensional ZnCo2O4 nanowire arrays/carbon cloth anodes for a novel class of high-performance flexible lithium-ion batteries. Nano Lett. 2012, 12, 3005–3011. [Google Scholar] [CrossRef]
- Hu, L.; La Mantia, F.; Wu, H.; Xie, X.; McDonough, J.; Pasta, M.; Cui, Y. Lithium-Ion Textile Batteries with Large Areal Mass Loading. Adv. Energy Mater. 2011, 1, 1012–1017. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Cheng, X.; Weng, W.; Ren, J.; Fang, X.; Jiang, Y.; Chen, P.; Zhang, Z.; Wang, Y.; et al. Realizing both high energy and high power densities by twisting three carbon-nanotube-based hybrid fibers. Angew. Chem. Int. Ed. 2015, 54, 11177–11182. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Weng, W.; Ren, J.; Qiu, L.; Zhang, Z.; Chen, P.; Chen, X.; Deng, J.; Wang, Y.; Peng, H. Twisted aligned carbon nanotube/silicon composite fiber anode for flexible wire-shaped lithium-ion battery. Adv. Mater. 2014, 26, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ma, T.; Tang, R.; Cheng, Q.; Wang, X.; Krishnaraju, D.; Panat, R.; Chan, C.K.; Yu, H.; Jiang, H. Origami lithium-ion batteries. Nat. Commun. 2014, 5, 3140. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, Y.; Lai, W.-Y.; Huang, W. Stretchable thin-film electrodes for flexible electronics with high deformability and stretchability. Adv. Mater. 2015, 27, 3349–3376. [Google Scholar] [CrossRef]
- Yan, C.; Lee, P.S. Stretchable energy storage and conversion devices. Small 2014, 10, 3443–3460. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603. [Google Scholar] [CrossRef]
- Chen, D.; Lou, Z.; Jiang, K.; Shen, G. Device configurations and future prospects of flexible/stretchable lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1805596. [Google Scholar] [CrossRef]
- Zhao, J.; Chi, Z.; Yang, Z.; Chen, X.; Arnold, M.S.; Zhang, Y.; Xu, J.; Chia, Z.; Aldreda, M.P. Recent developments of truly stretchable thin film electronic and optoelectronic devices. Nanoscale 2017, 5764–5792. [Google Scholar] [CrossRef]
- Lacour, S.P.; Wagner, S.; Huang, Z.; Suo, Z. Stretchable gold conductors on elastomeric substrates. Appl. Phys. Lett. 2003, 82, 2404–2406. [Google Scholar] [CrossRef]
- Lacour, S.P.; Chan, D.; Wagner, S.; Li, T.; Suo, Z. Mechanisms of reversible stretchability of thin metal films on elastomeric substrates. Appl. Phys. Lett. 2006, 88, 204103. [Google Scholar] [CrossRef]
- Lacour, S.P.; Jones, J.; Wagner, S.; Teng, L.; Zhigang, S. Stretchable interconnects for elastic electronic surfaces. Proc. IEEE 2005, 93, 1459–1467. [Google Scholar] [CrossRef]
- Khang, D.-Y.; Jiang, H.; Huang, Y.; Rogers, J.A. A Stretchable form of single-crystal silicon for high-performance electronics on rubber substrates. Science 2006, 311, 208–212. [Google Scholar] [CrossRef] [PubMed]
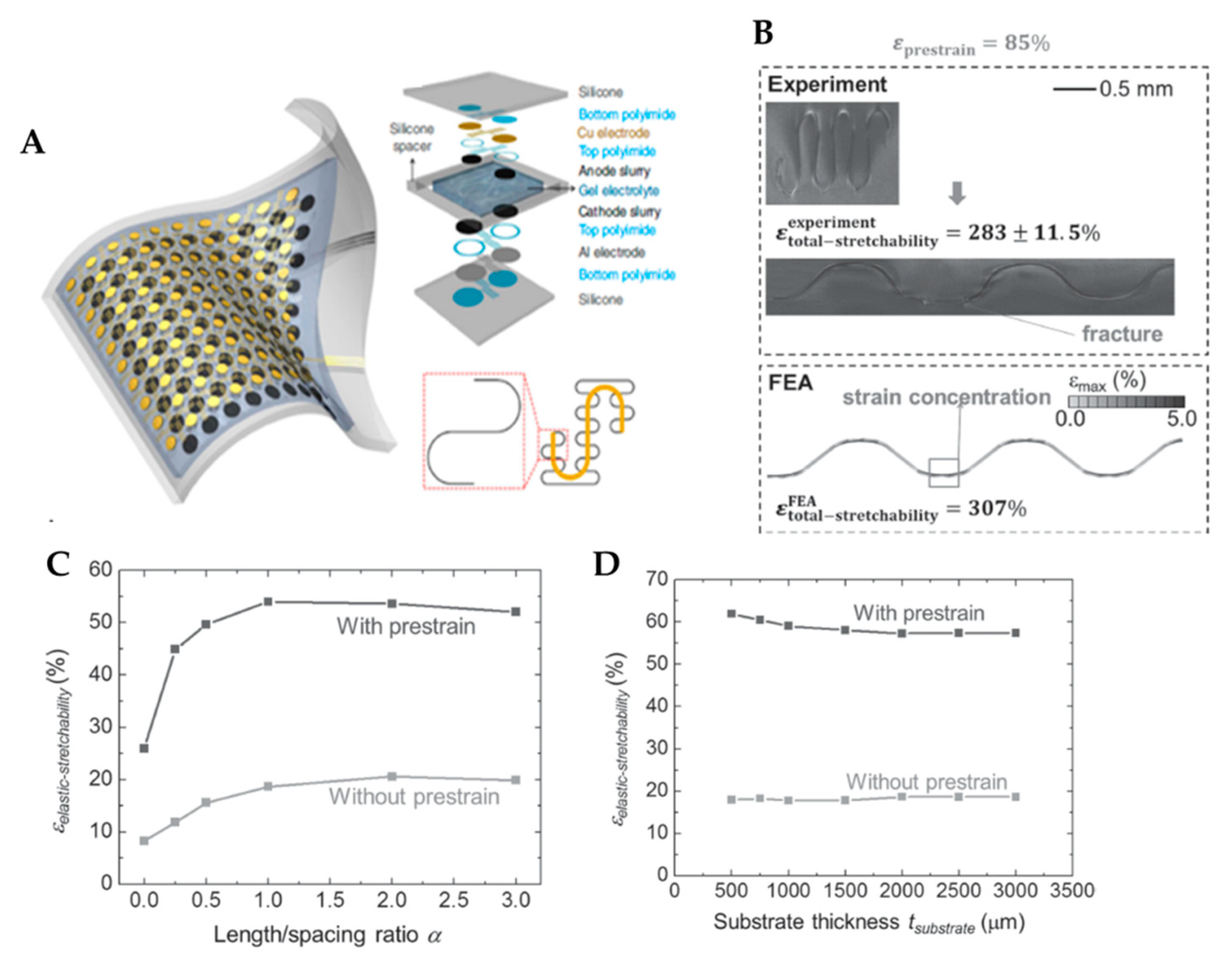
- Xu, S.; Zhang, Y.; Cho, J.; Lee, J.; Huang, X.; Jia, L.; Fan, J.A.; Su, Y.; Su, J.; Zhang, H.; et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Wang, S.; Oh, J.Y.; Xu, J.; Tran, H.; Bao, Z. Skin-inspired electronics: An emerging paradigm. Acc. Chem. Res. 2018, 51, 1033–1045. [Google Scholar] [CrossRef]
- Morent, R.; Geyter, N.D.; Axisa, F.; Smet, N.D.; Gengembre, L.; Leersnyder, E.D.; Leys, C.; Vanfleteren, J.; Rymarczyk-Machal, M.; Schacht, E.; et al. Adhesion enhancement by a dielectric barrier discharge of PDMS used for flexible and stretchable electronics. J. Phys. D Appl. Phys. 2007, 40, 7392–7401. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, W.; Yue, Z.; Too, C.O.; Wallace, G.G. Buckled, stretchable polypyrrole electrodes for battery applications. Adv. Mater. 2011, 23, 3580–3584. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Z.; Zhou, G.; Sun, Y.; Lee, H.R.; Liu, C.; Yao, H.; Bao, Z.; Cui, Y. 3D porous sponge-inspired electrode for stretchable lithium-ion batteries. Adv. Mater. 2016, 28, 3578–3583. [Google Scholar] [CrossRef]
- Yu, C.; Masarapu, C.; Rong, J.; Wei, B.; Jiang, H. Stretchable supercapacitors based on buckled single-walled carbon nanotube macrofilms. Adv. Mater. 2009, 21, 4793–4797. [Google Scholar] [CrossRef]
- Mamunya, Y.; Boudenne, A.; Lebovka, N.; Ibos, L.; Candau, Y.; Lisunova, M. Electrical and thermophysical behaviour of PVC-MWCNT nanocomposites. Compos. Sci. Technol. 2008, 68, 1981. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kim, J.-S.; Noh, J.; Lee, I.; Kim, H.J.; Choi, S.; Seo, J.; Jeon, S.; Kim, T.-S.; Lee, J.-Y.; et al. Wearable textile battery rechargeable by solar energy. Nano Lett. 2013, 13, 5753–5761. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zhu, J.; Yeom, B.; Di Prima, M.; Su, X.; Kim, J.-G.; Yoo, S.J.; Uher, C.; Kotov, N.A. Stretchable nanoparticle conductors with self-organized conductive pathways. Nature 2013, 500, 59. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Urban, M.W. Self-repairing oxetane-substituted chitosan polyurethane networks. Science 2009, 323, 1458. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Banno, K.; Maruo, T.; Nozu, R. New design for a safe lithium-ion gel polymer battery. J. Power Sources 2005, 152, 264–271. [Google Scholar] [CrossRef]
- Pan, B.; Qian, K.; Xie, H.; Asundi, A. Two-dimensional digital image correlation for in-plane displacement and strain measurement: A review. Meas. Sci. Technol. 2009, 20, 062001. [Google Scholar] [CrossRef]
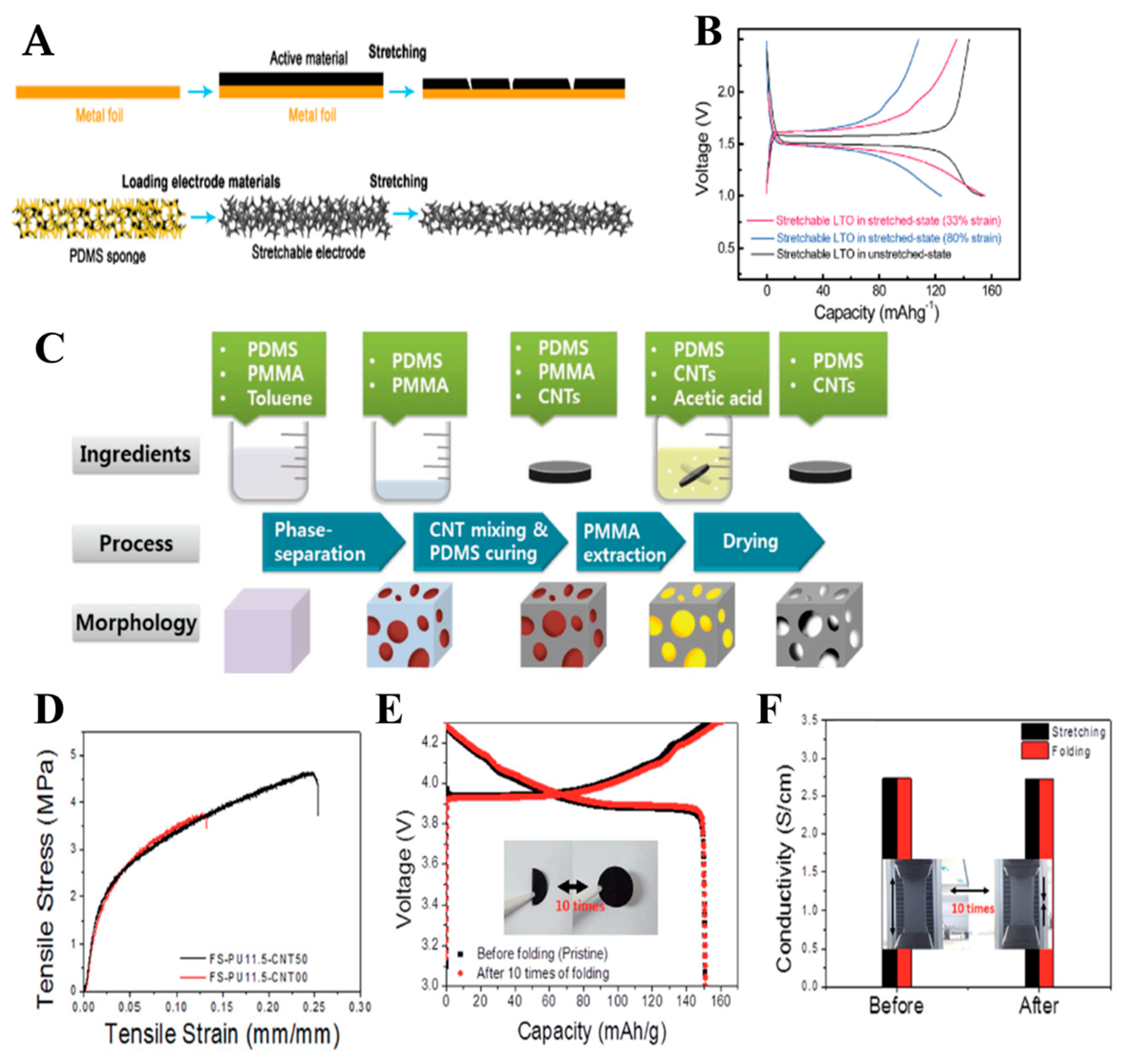
- Lee, H.; Yoo, J.-K.; Park, J.-H.; Kim, J.H.; Kang, K.; Jung, Y.S. A stretchable polymer–carbon nanotube composite electrode for flexible lithium-ion batteries: Porosity engineering by controlled phase separation. Adv. Energy Mater. 2012, 2, 976–982. [Google Scholar] [CrossRef]
- Wu, W. Stretchable electronics: Functional materials, fabrication strategies and applications. Sci. Technol. Adv. Mater. 2019, 20, 187–224. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Pan, J.; Peng, H. Stretchable lithium-air batteries for wearable electronics. J. Mater. Chem. A 2016, 4, 13419–13424. [Google Scholar] [CrossRef]
- Cao, C.; Chu, Y.; Zhou, Y.; Zhang, C.; Qu, S. Recent advances in stretchable supercapacitors enabled by low-dimensional nanomaterials. Small 2018, 14, 1–26. [Google Scholar] [CrossRef]
- Wang, S.; Liu, N.; Su, J.; Li, L.; Long, F.; Zou, Z.; Jiang, X.; Gao, Y. Highly stretchable and self-healable supercapacitor with reduced graphene oxide based fiber springs. ACS Nano 2017, 11, 2066. [Google Scholar] [CrossRef]
- Xu, J.; Chen, J.; Zhang, M.; Hong, J.-D.; Shi, G. Highly conductive stretchable electrodes prepared by in situ reduction of wavy graphene oxide films coated on elastic tapes. Adv. Electron. Mater. 2016, 2, 1600022. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Z.; Liu, Y.; Leow, W.R.; Zhu, B.; Yang, H.; Yu, J.; Wang, W.; Wang, H.; Yin, S.; et al. Suspended wavy graphene microribbons for highly stretchable microsupercapacitors. Adv. Mater. 2015, 27, 5559–5566. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Choi, W.M.; Jiang, H.; Huang, Y.Y.; Rogers, J.A. Controlled buckling of semiconductor nanoribbons for stretchable electronics. Nat. Nanotechnol. 2006, 1, 201. [Google Scholar] [CrossRef] [PubMed]
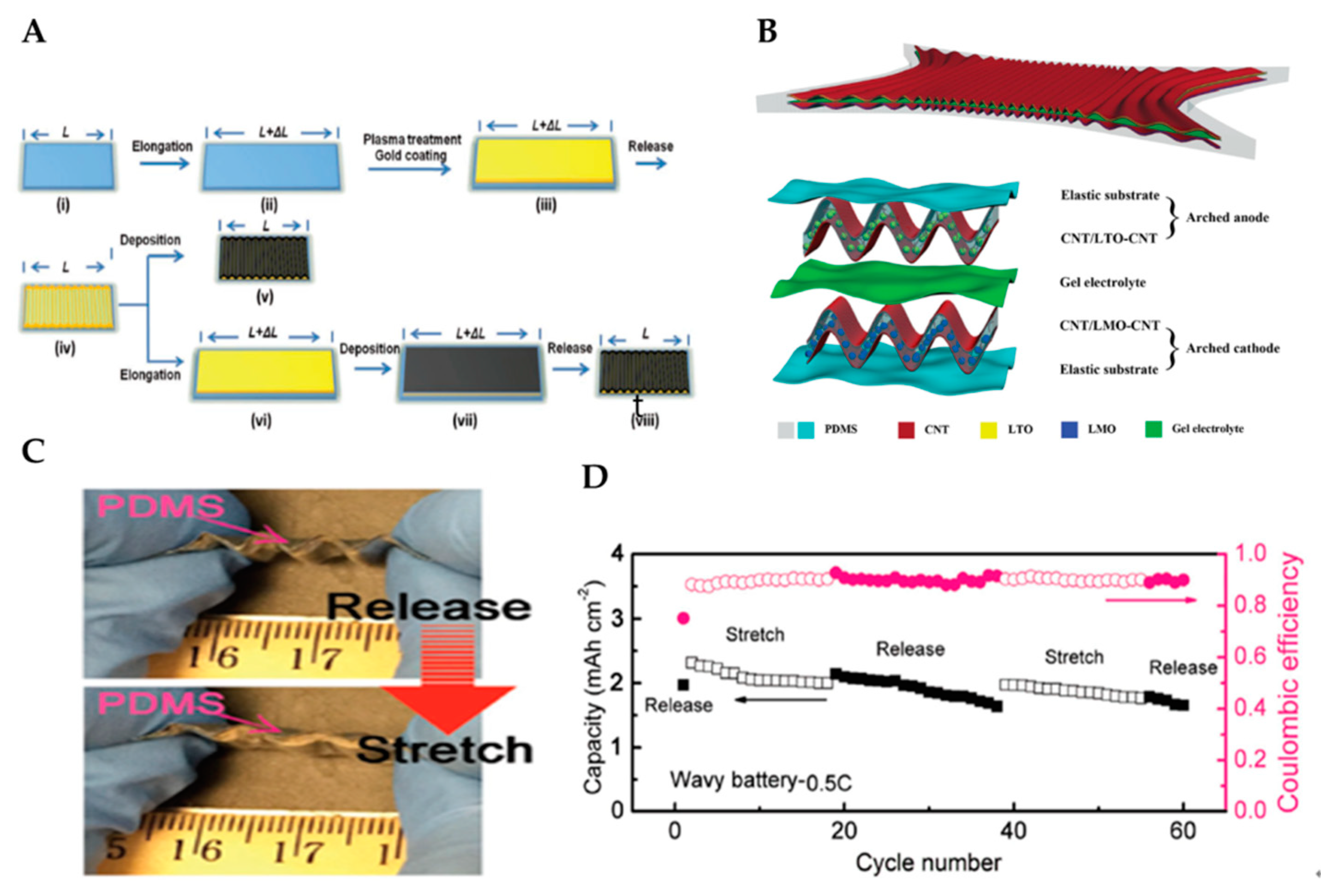
- Weng, W.; Sun, Q.; Zhang, Y.; He, S.; Wu, Q.; Deng, J.; Fang, X.; Guan, G.; Ren, J.; Peng, H. A gum-like lithium-ion battery based on a novel arched structure. Adv. Mater. 2015, 27, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kang, H.; Gwon, S.H.; Choi, G.M.; Lim, S.; Sun, J.-Y.; Joo, Y.-C. A Strain-insensitive stretchable electronic conductor: PEDOT:PSS/acrylamide organogels. Adv. Mater. 2016, 28, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wu, H.; Mao, L.; Yuan, H.; Ahmad, A.; Wei, Z. Combining electrode flexibility and wave-like device architecture for highly flexible li-ion batteries. Adv. Mater. Technol. 2017, 2, 1700032. [Google Scholar] [CrossRef]
- Liu, L.; Ye, D.; Yu, Y.; Liu, L.; Wu, Y. Carbon-based flexible micro-supercapacitor fabrication via mask-free ambient micro-plasma-jet etching. ScienceDirect 2017, 111, 121–127. [Google Scholar] [CrossRef]
- Kim, D.-H.; Xiao, J.; Song, J.; Huang, Y.; Rogers, J.A. Stretchable, curvilinear electronics based on inorganic materials. Adv. Mater. 2010, 22, 2108–2124. [Google Scholar] [CrossRef]
- Khang, D.-Y.; Rogers, J.A.; Lee, H.H. Mechanical buckling: Mechanics, metrology, and stretchable electronics. Adv. Funct. Mater. 2009, 19, 1526–1536. [Google Scholar] [CrossRef]
- Gu, T.; Cao, Z.; Wei, B. All-manganese-based binder-free stretchable lithium-ion batteries. Adv. Energy Mater. 2017, 7, 1700369. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Chen, Z.; Liu, K.; Zhou, G.; Sun, Y.; Song, M.-S.; Bao, Z.; Cui, Y. Stretchable lithium-ion batteries enabled by device-scaled wavy structure and elastic-sticky separator. Adv. Energy Mater. 2017, 7, 1701076. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Yang, H.; Sun, Y.; Hu, C.; Huang, Y. Flexible asymmetric micro-supercapacitors based on bi2o3and mno2nanoflowers: Larger areal mass promises higher energy density. Adv. Energy Mater. 2015, 5, 1401882. [Google Scholar] [CrossRef]
- Song, Z.; Wang, X.; Lv, C.; An, Y.; Liang, M.; Ma, T.; He, D.; Zheng, Y.-J.; Huang, S.-Q.; Yu, H.; et al. Kirigami-based stretchable lithium-ion batteries. Sci. Rep. 2015, 5, 10988. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Wang, X.; Zhang, Y.; Yu, X.; Choi, D.; Zheng, N.; Kim, D.S.; Huang, Y.; Zhang, Y.; Rogers, J.A. Assembly of advanced materials into 3D functional structures by methods inspired by origami and kirigami: A review. Adv. Mater. Interfaces 2018, 5, 1800284. [Google Scholar] [CrossRef]
- Ohishi, T.; Kawada, H.; Yoshida, T.; Ohwada, T. Development of a high-performance flexible substrate for flexible electronics: Joining TAC films and an ultra-thin glassby using TEOS-DAC synthesized by the Sol-Gel method. Adv. Mater. 2010, 22, 2228–2246. [Google Scholar]
- Sekitani, T.; Zschieschang, U.; Klauk, H.; Someya, T. Flexible organic transistors and circuits with extreme bending stability. Nat. Mater. 2010, 9, 1015–1022. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lu, N.; Huang, Y.; Rogers, J.A. Materials for stretchable electronics in bioinspired and biointegrated devices. MRS Bull. 2012, 37, 226–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, X.; Fan, J.A.; Xu, S.; Song, Y.M.; Choi, K.-J.; Yeo, W.-H.; Lee, W.; Nazaar, S.N.; et al. Experimental and theoretical studies of serpentine microstructures bonded to prestrained elastomers for stretchable electronics. Adv. Funct. Mater. 2014, 24, 2028–2037. [Google Scholar] [CrossRef]
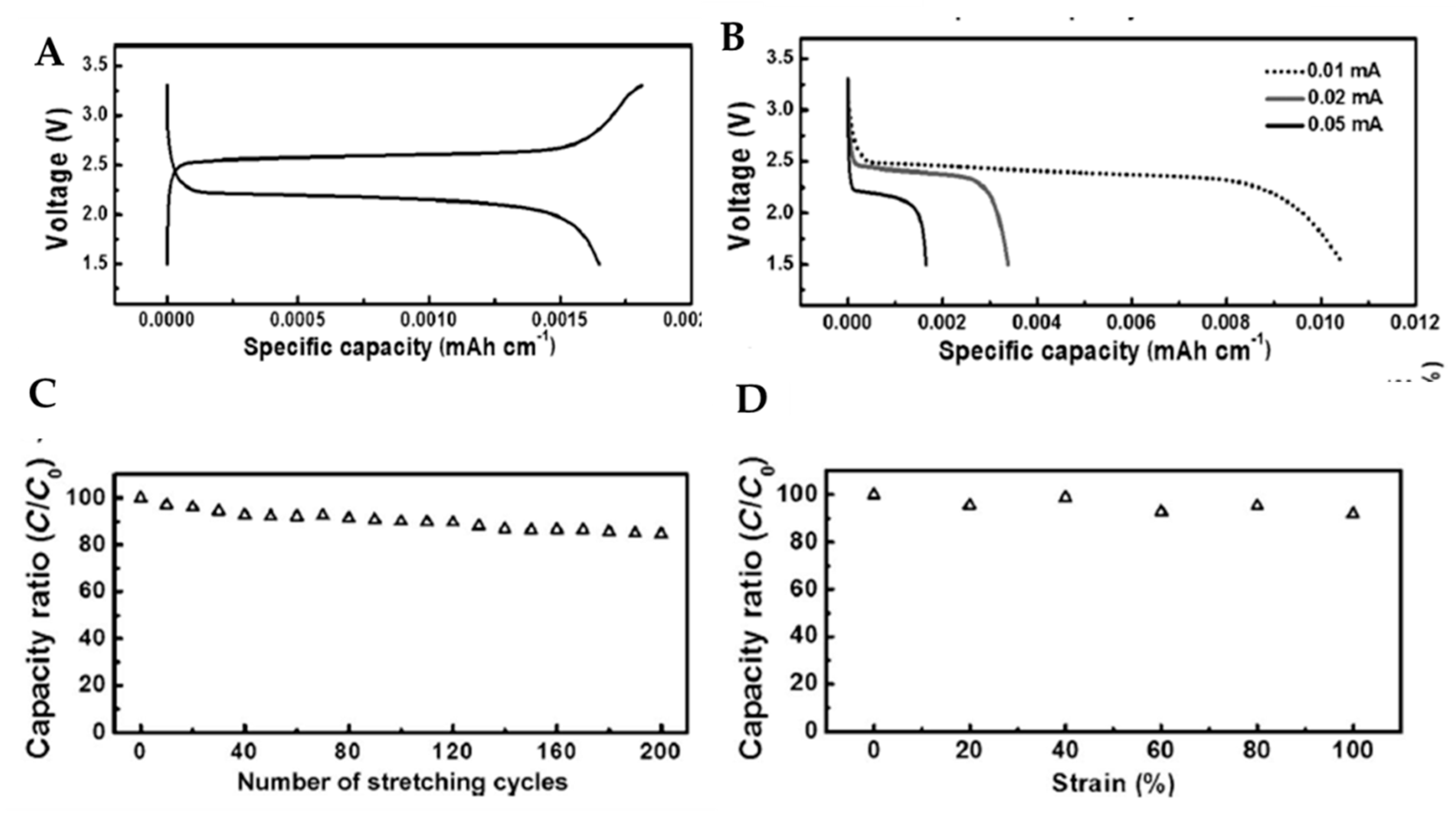
- Ren, J.; Zhang, Y.; Bai, W.; Chen, X.; Zhang, Z.; Fang, X.; Weng, W.; Wang, Y.; Peng, H. Elastic and wearable wire-shaped lithium-ion battery with high electrochemical performance. Angew. Chem. Int. Ed. 2014, 53, 7864–7869. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, S.; Baik, H.-K.; Jeong, U. Conducting polymer dough for deformable electronics. Adv. Mater. 2016, 28, 4455–4461. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Liu, H.; Wang, Z.; Ling, H.; Wang, C.; Ni, J.; Çelebi-Saltik, B.; Wang, X.; Meng, X.; et al. Room-temperature-formed PEDOT:PSS hydrogels enable injectable, soft, and healable organic bioelectronics. Adv. Mater. 2020, 32, 1904752. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Nishihara, M.; Kanehara, T.; Amamoto, Y.; Takahara, A.; Otsuka, H. Self-healing of chemical gels cross-linked by diarylbibenzofuranone-based trigger-free dynamic covalent bonds at room temperature. Angew. Chem. Int. Ed. 2012, 124, 1164–1168. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618. [Google Scholar] [CrossRef]
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Hydrogels: Preorganized hydrogel: Self-healing properties of supramolecular hydrogels formed by polymerization of host–guest-monomers that contain cyclodextrins and hydrophobic guest groups. Adv. Mater. 2013, 25, 2758. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Wu, S.; Tian, H. A Rapidly self-healing supramolecular polymer hydrogel with photostimulated room-temperature phosphorescence responsiveness. Angew. Chem. Int. Ed. 2014, 126, 14373–14376. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977. [Google Scholar] [CrossRef]
- Wang, C.; Liu, N.; Allen, R.; Tok, J.B.-H.; Wu, Y.; Zhang, F.; Chen, Y.; Bao, Z. A rapid and efficient self-healing thermo-reversible elastomer crosslinked with graphene oxide. Adv. Mater. 2013, 25, 5785–5790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Sun, H.; Dong, X.; Cao, J.; Wang, L.; Xu, Y.; Ren, J.; Hwang, Y.; Son, I.H.; et al. A self-healing aqueous lithium-ion battery. Angew. Chem. Int. Ed. 2016, 55, 14384–14388. [Google Scholar] [CrossRef]
- Zhang, S.; Cicoira, F. Water-enabled healing of conducting polymer films. Adv. Mater. 2017, 29, 1703098. [Google Scholar] [CrossRef]
- Hippauf, F.; Schumm, B.; Doerfler, S.; Althues, H.; Fujiki, S.; Shiratsuchi, T.; Tsujimura, T.; Aihara, Y.; Kaskel, S. Overcoming binder limitations of sheet-type solid-state cathodes using a solvent-free dry-film approach. Energy Storage Mater. 2019, 21, 390–398. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Clem, P.G.; Doyle, B.L.; Kotula, P.G.; Fenton, K.R.; Apblett, C.A. Fast lithium-ion conducting thin-film electrolytes integrated directly on flexible substrates for high-power solid-state batteries. Adv. Mater. 2011, 23, 5663–5667. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.; Park, K.-I.; Lee, S.H.; Suh, M.; Jeon, D.Y.; Choi, J.W.; Kang, K.; Lee, K.J. Bendable inorganic thin-film battery for fully flexible electronic systems. Nano Lett. 2012, 12, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, S.; Kang, J.; Nam, I.; Yi, J. Free-Form and Deformable Energy Storage as a Forerunner to Next-Generation Smart Electronics. Micromachines 2020, 11, 347. https://doi.org/10.3390/mi11040347
Kwak S, Kang J, Nam I, Yi J. Free-Form and Deformable Energy Storage as a Forerunner to Next-Generation Smart Electronics. Micromachines. 2020; 11(4):347. https://doi.org/10.3390/mi11040347
Chicago/Turabian StyleKwak, Soyul, Jihyeon Kang, Inho Nam, and Jongheop Yi. 2020. "Free-Form and Deformable Energy Storage as a Forerunner to Next-Generation Smart Electronics" Micromachines 11, no. 4: 347. https://doi.org/10.3390/mi11040347
APA StyleKwak, S., Kang, J., Nam, I., & Yi, J. (2020). Free-Form and Deformable Energy Storage as a Forerunner to Next-Generation Smart Electronics. Micromachines, 11(4), 347. https://doi.org/10.3390/mi11040347




