Design and Electrochemical Characterization of Spiral Electrochemical Notification Coupled Electrode (SENCE) Platform for Biosensing Application
Abstract
1. Introduction
2. Materials and Methods
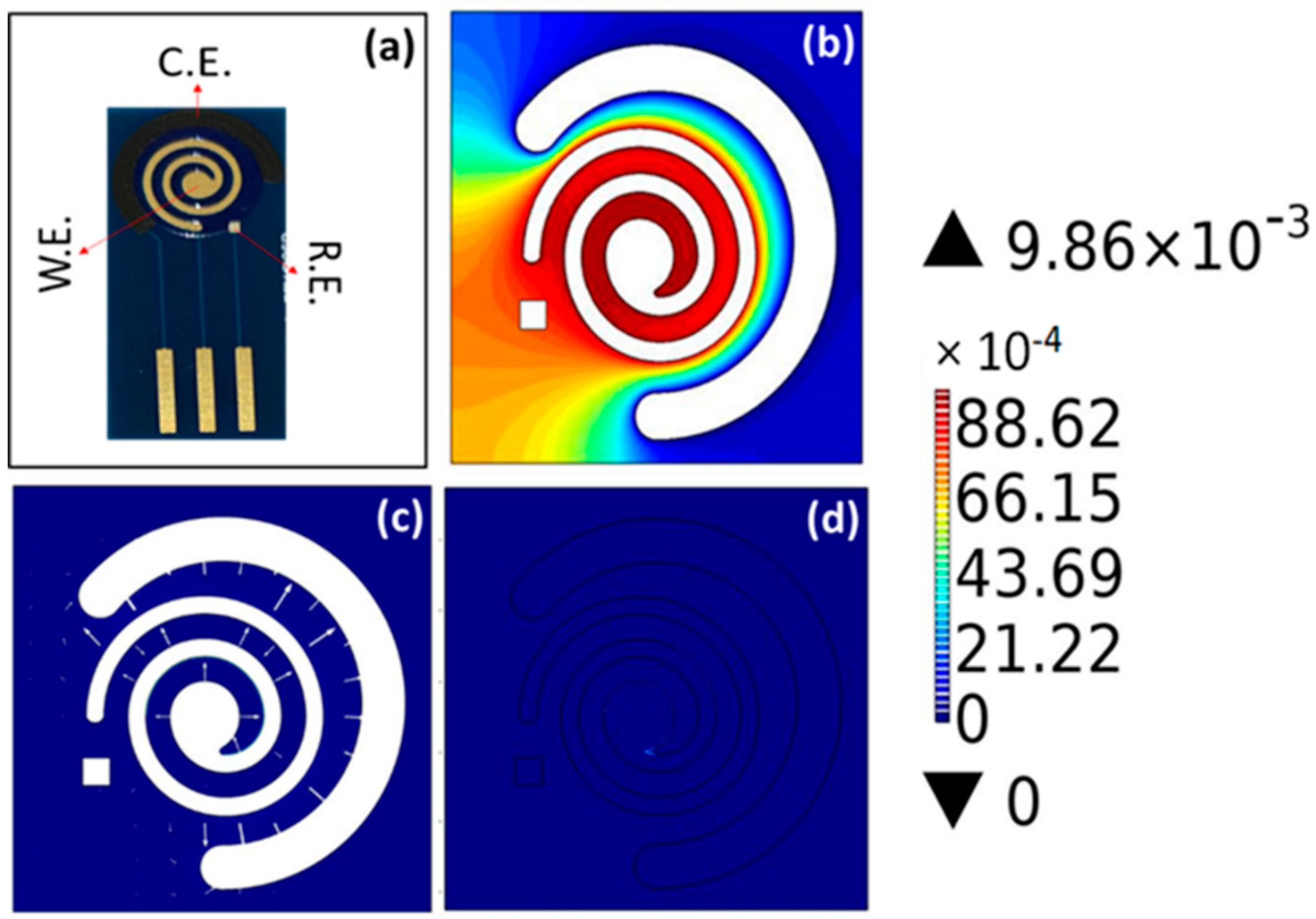
2.1. COMSOL
2.2. Design of Sensor
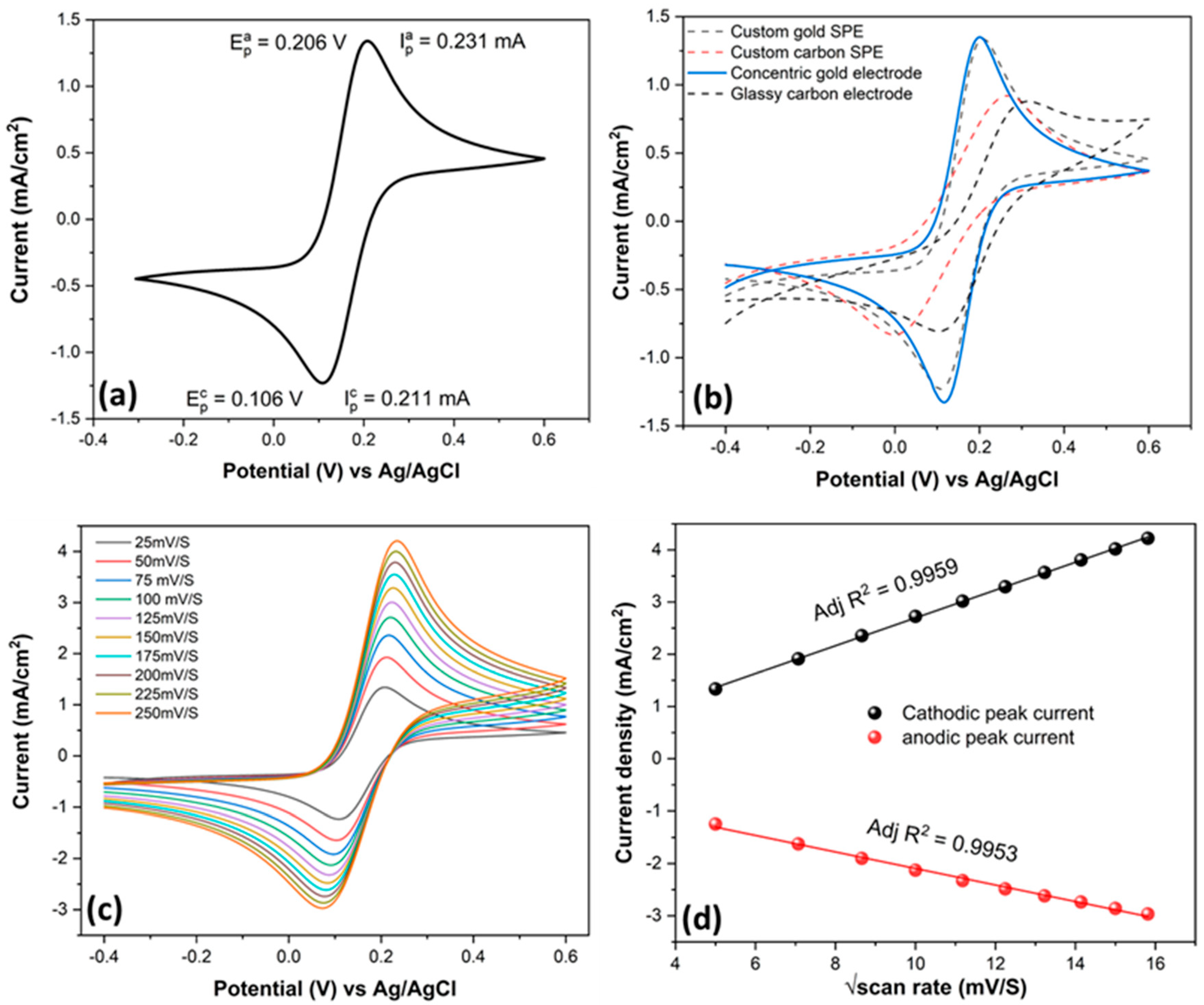
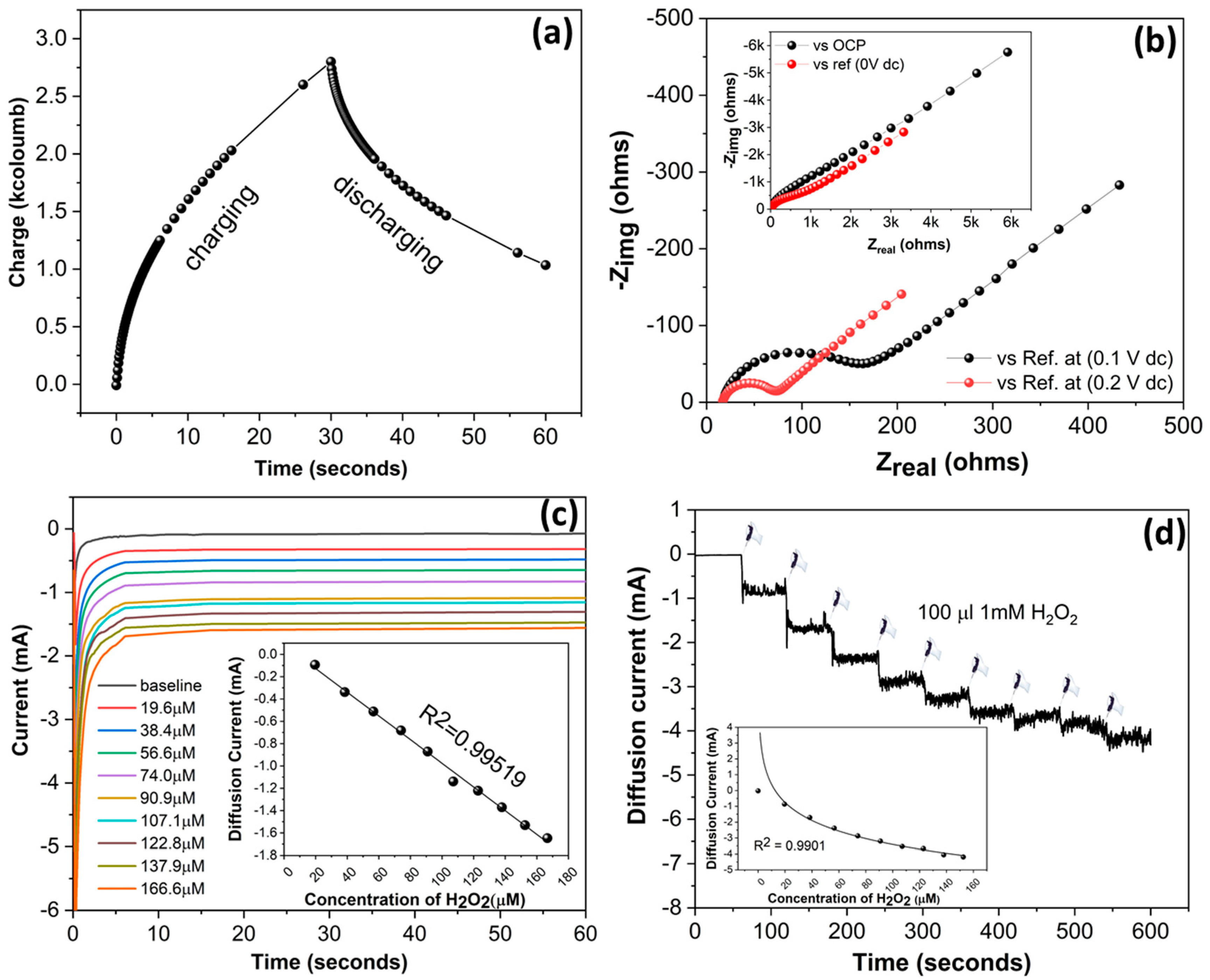
2.3. Electrochemical Methods
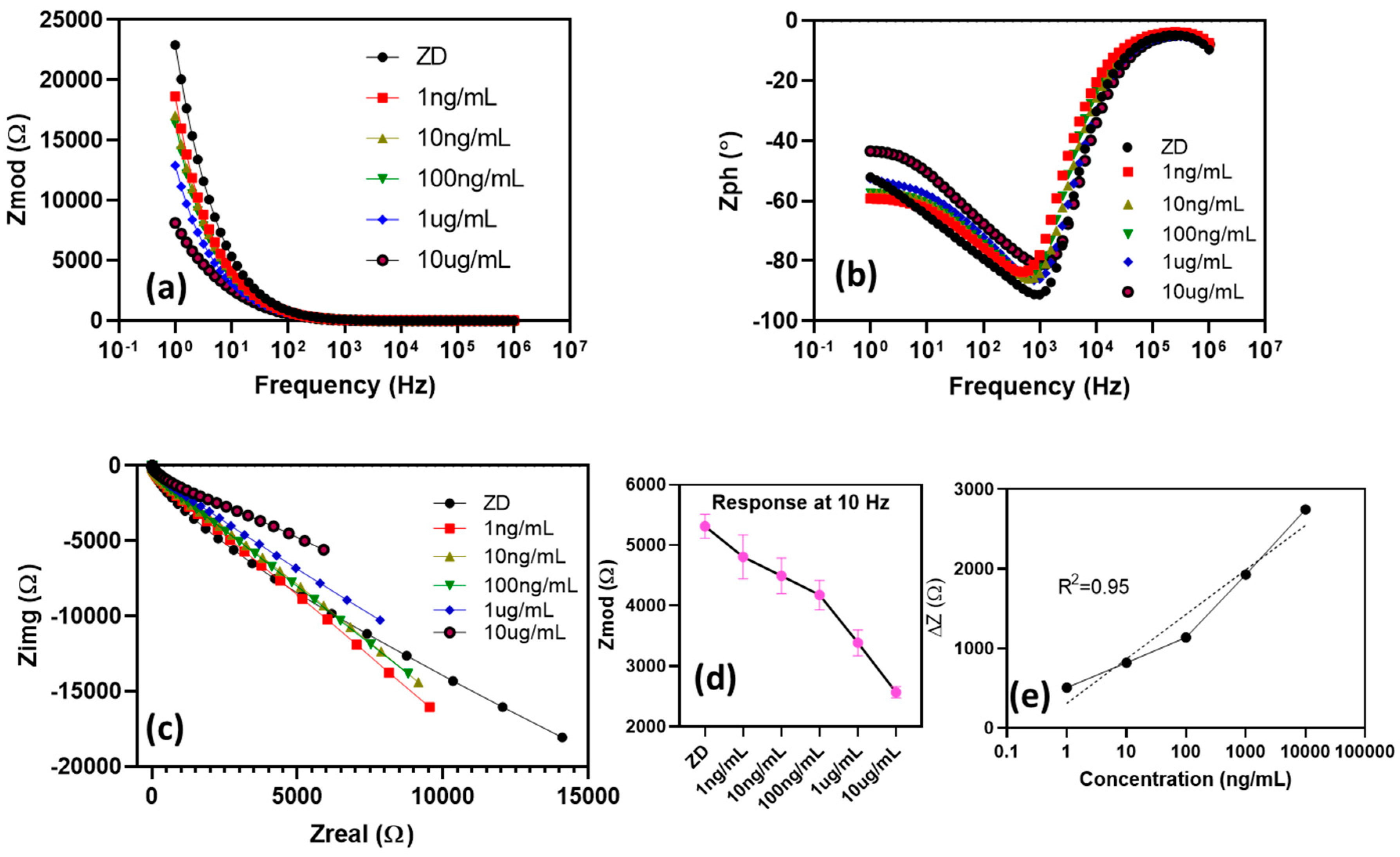
2.4. Immunoassay Functionalization
3. Results and Discussion
3.1. COMSOL Results
3.2. Electrochemical Characterization of SENCE System
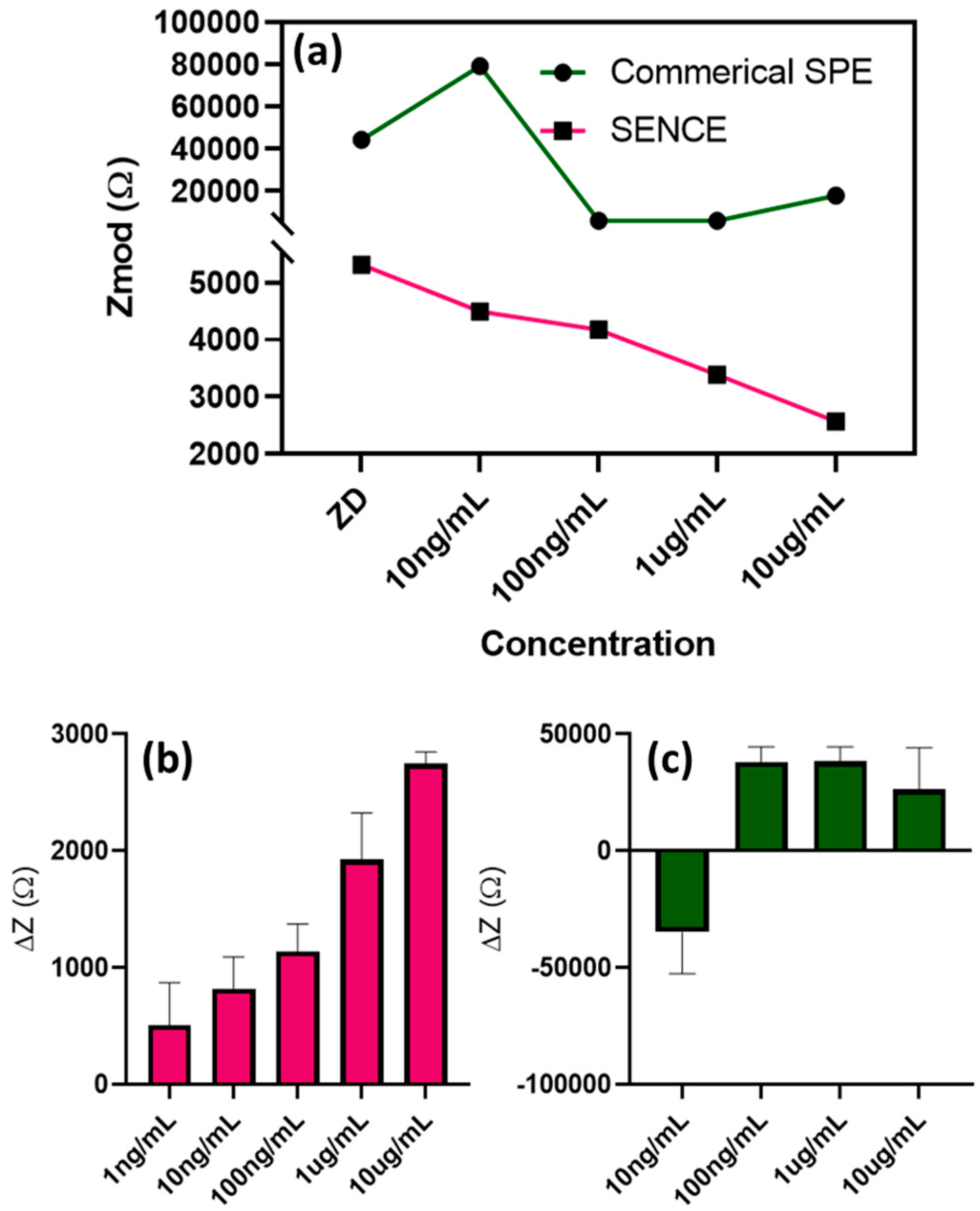
3.3. Electrochemical Characterization of the Immunoassay Using Electrochemical Impedance Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Shivpuri, S.; Gallo, L.C.; Crouse, J.R.; Allison, M.A. The association between chronic stress type and C-Reactive protein in the multi-ethnic study of atherosclerosis: Does gender make a difference? J. Behav. Med. 2012, 35, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Singh, U.; Jialal, I. Human C-Reactive protein and the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 182–189. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Mainous, A.G.; Buchanan, T.A.; Pearson, W.S. C-Reactive protein and glycemic control in adults with diabetes. Diabetes Care 2003, 26, 1535–1539. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Agassandian, M.; Shurin, G.V.; Ma, Y.; Shurin, M.R. C-Reactive protein and lung diseases. Int. J. Biochem. Cell Biol. 2014, 53, 77–88. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Ledue, T.B.; Weiner, D.L.; Sipe, J.D.; Poulin, S.E.; Collins, M.F.; Rifai, N. Analytical evaluation of particle-Enhanced immunonephelometric assays for C-Reactive protein, serum amyloid A and mannose-Binding protein in human serum. Ann. Clin. Biochem. 1998, 35, 745–753. [Google Scholar] [CrossRef]
- Dupuy, A.M.; Badiou, S.; Descomps, B.; Cristol, J.P. Immunoturbidimetric determination of C-Reactive protein (CRP) and high-sensitivity CRP on heparin plasma. Comparison with serum determination. Clin. Chem. Lab. Med. 2003, 41, 948–949. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.Y.; Li, W.; Shen, Z.Y.; Wang, Y.-D.; Ji, S.R.; Wu, Y. An ELISA assay for quantifying monomeric C-Reactive protein in plasma. Front. Immunol. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Kim, C.H.; Ahn, J.H.; Kim, J.Y.; Choi, J.M.; Lim, K.C.; Jung Park, T.; Su Heo, N.; Gu Lee, H.; Kim, J.W.; Choi, Y.K. CRP detection from serum for chip-based point-Of-Care testing system. Biosens. Bioelectron. 2013, 41, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Maigler, M.V.; Holgado, M.; Laguna, M.F.; Sanza, F.J.; Santamaria, B.; Lavin, A.; Espinosa, R.L. A New Device Based on Interferometric Optical Detection Method for Label-Free Screening of C-Reactive Protein. IEEE Trans. Instrum. Meas. 2018, 68, 1–7. [Google Scholar] [CrossRef]
- Pohanka, M. Piezoelectric immunosensor for the determination of C-Reactive protein. Int. J. Electrochem. Sci. 2019, 14, 8470–8478. [Google Scholar] [CrossRef]
- Salvo, P.; Dini, V.; Kirchhain, A.; Janowska, A.; Oranges, T.; Chiricozzi, A.; Lomonaco, T.; Di Francesco, F.; Romanelli, M. Sensors and biosensors for C-reactive protein, temperature and pH, and their applications for monitoring wound healing: A review. Sensors 2017, 17, 2952. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Selvam, A.P.; Reddy, R.K.; Love, A. Silicon nanosensor for diagnosis of Cardiovascular proteomic markers. J. Lab. Autom. 2013, 18, 143–151. [Google Scholar] [CrossRef]
- Kamakoti, V.; Kinnamon, D.; Choi, K.H.; Jagannath, B.; Prasad, S. Fully electronic urine dipstick probe for combinatorial detection of inflammatory biomarkers. Future Sci. OA 2018, 4. [Google Scholar] [CrossRef]
- Tanak, A.S.; Jagannath, B.; Tamrakar, Y.; Muthukumar, S.; Prasad, S. Non-Faradaic electrochemical impedimetric profiling of procalcitonin and C-Reactive protein as a dual marker biosensor for early sepsis detection. Anal. Chim. Acta X 2019. [Google Scholar] [CrossRef]
- Bhide, A.; Cheeran, S.; Muthukumar, S.; Prasad, S. Enzymatic low volume passive sweat based assays for multi-biomarker detection. Biosensors 2019, 9, 13. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, C.C. Recent Advances on Electrochemical Biosensing Strategies toward Universal Point-Of-Care Systems Angewandte. Angew. Chem. Int. Ed. 2019, 12355–12368. [Google Scholar] [CrossRef]
- Sun, A.C.; Hall, D.A. Point-Of-Care Smartphone-Based Electrochemical Biosensing. Electroanalysis 2019, 31, 2–16. [Google Scholar] [CrossRef]
- Rajesh, D.; Singal, S.; Kotnala, R.K. Single Frequency Impedance Analysis on Reduced Graphene Oxide Screen-Printed Electrode for Biomolecular Detection. Appl. Biochem. Biotechnol. 2017, 183, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.V.; Whited, A.M.; Ragineni, Y.; Barrett, T.W.; King, J.; Solanki, R. 3D nanogap interdigitated electrode array biosensors. Anal. Bioanal. Chem. 2010, 397, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, S.J.; Thilagar, S.H.; Jayasurya, V. Design of Electrochemical Sensor and Determining the Peak Current of Ions in Solution. In Proceedings of the 2019 IEEE International Conference on Intelligent Techniques in Control, Optimization and Signal Processing (INCOS), Tamilnadu, India, 11–13 April 2019; pp. 1–4. [Google Scholar]
- Mathew, M.T.; Ariza, E.; Rocha, L.A.; Fernandes, A.C.; Vaz, F. TiCxOy thin films for decorative applications: Tribocorrosion mechanisms and synergism. Tribol. Int. 2008, 41, 603–615. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Prasad, S. Lancet-Free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuators B Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Ekström, H.; Fontes, E. COMSOL Multiphysics®: Finite element software for electrochemical analysis. A mini-Review. Electrochem. commun. 2014, 40, 71–74. [Google Scholar] [CrossRef]
- Tanak, A.S.; Muthukumar, S.; Hashim, I.A.; Prasad, S. Rapid electrochemical device for single-Drop point-Of-Use screening of parathyroid hormone. Bioelectron. Med. 2019, 2, 13–27. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef]
- Kamakoti, V.; Shanmugam, N.R.; Tanak, A.S.; Jagannath, B.; Prasad, S. Investigation of molybdenum-Crosslinker interfaces for affinity based electrochemical biosensing applications. Appl. Surf. Sci. 2018, 436, 441–450. [Google Scholar] [CrossRef]
- Daniels, J.S.; Pourmand, N. Label-Free impedance biosensors: Opportunities and challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef]
- O′Rourke, M.; Duffy, N.; de Marco, R.; Potter, I. Electrochemical Impedance Spectroscopy—A Simple Method for the Characterization of Polymer Inclusion Membranes Containing Aliquat 336. Membranes 2011, 1, 132–148. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardesai, A.U.; Dhamu, V.N.; Paul, A.; Muthukumar, S.; Prasad, S. Design and Electrochemical Characterization of Spiral Electrochemical Notification Coupled Electrode (SENCE) Platform for Biosensing Application. Micromachines 2020, 11, 333. https://doi.org/10.3390/mi11030333
Sardesai AU, Dhamu VN, Paul A, Muthukumar S, Prasad S. Design and Electrochemical Characterization of Spiral Electrochemical Notification Coupled Electrode (SENCE) Platform for Biosensing Application. Micromachines. 2020; 11(3):333. https://doi.org/10.3390/mi11030333
Chicago/Turabian StyleSardesai, Abha Umesh, Vikram Narayanan Dhamu, Anirban Paul, Sriram Muthukumar, and Shalini Prasad. 2020. "Design and Electrochemical Characterization of Spiral Electrochemical Notification Coupled Electrode (SENCE) Platform for Biosensing Application" Micromachines 11, no. 3: 333. https://doi.org/10.3390/mi11030333
APA StyleSardesai, A. U., Dhamu, V. N., Paul, A., Muthukumar, S., & Prasad, S. (2020). Design and Electrochemical Characterization of Spiral Electrochemical Notification Coupled Electrode (SENCE) Platform for Biosensing Application. Micromachines, 11(3), 333. https://doi.org/10.3390/mi11030333





