Cell Lysis Based on an Oscillating Microbubble Array
Abstract
1. Introduction
2. Materials and Methods
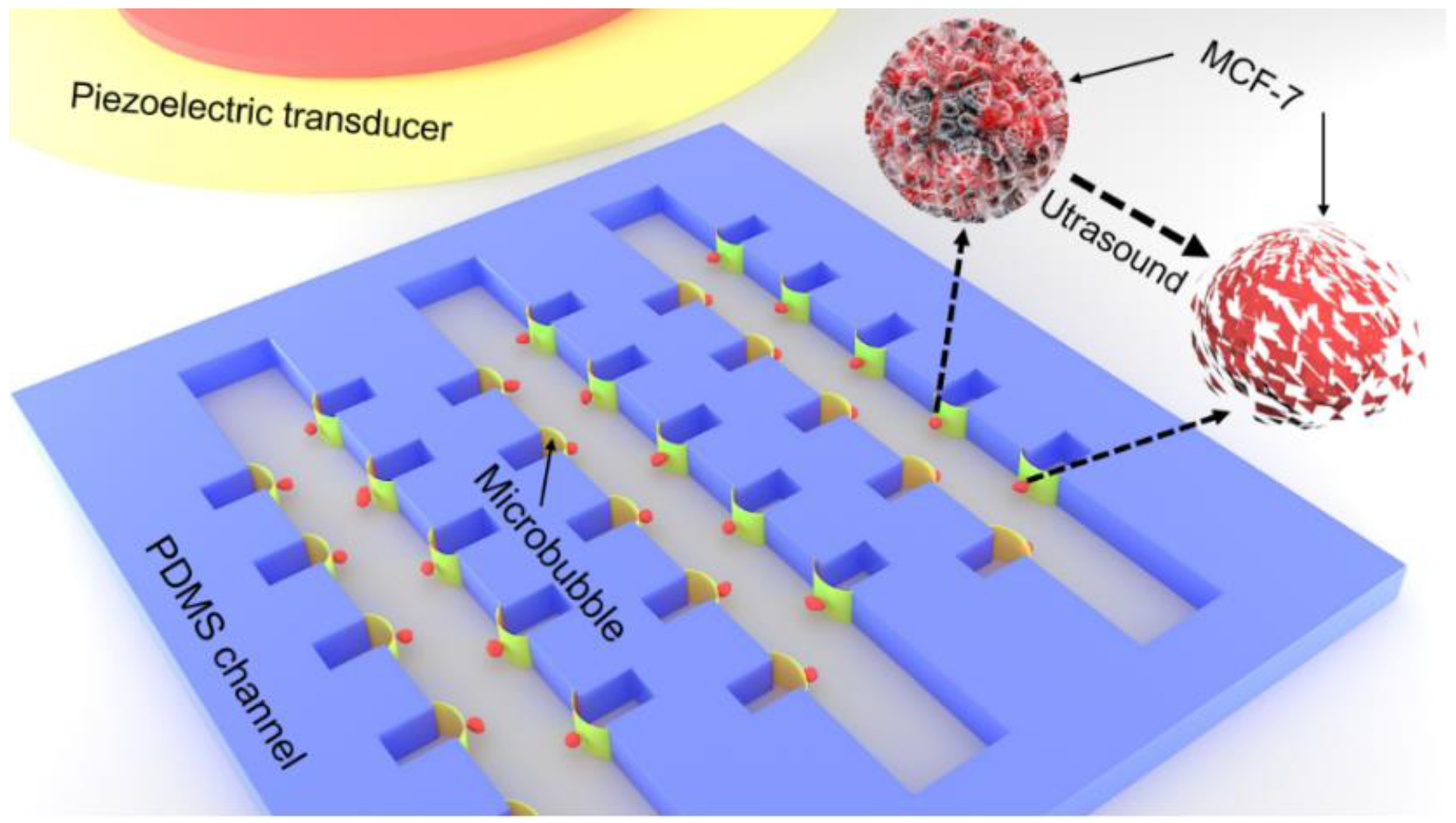
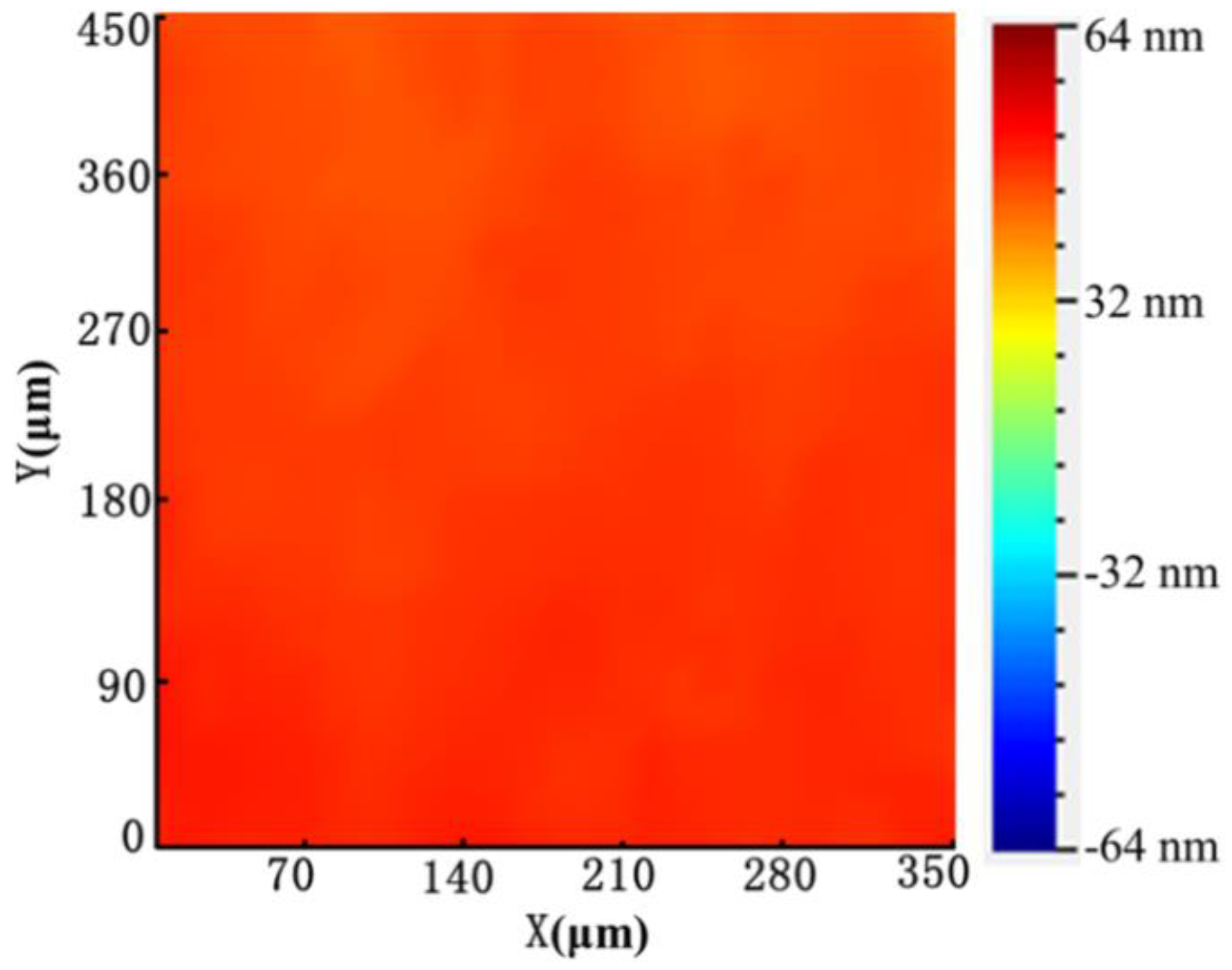
2.1. Fabrication of the Microchip and Acoustic Field
2.2. Measurement of Air Bubble Stable Cavitation and Deformation
2.3. Cell Sample and Particle Preparation
2.4. Cell Lysis in a Microfluidic System
2.5. DNA Gel Electrophoresis Study
2.6. Statistical Analysis
3. Results and Discussion
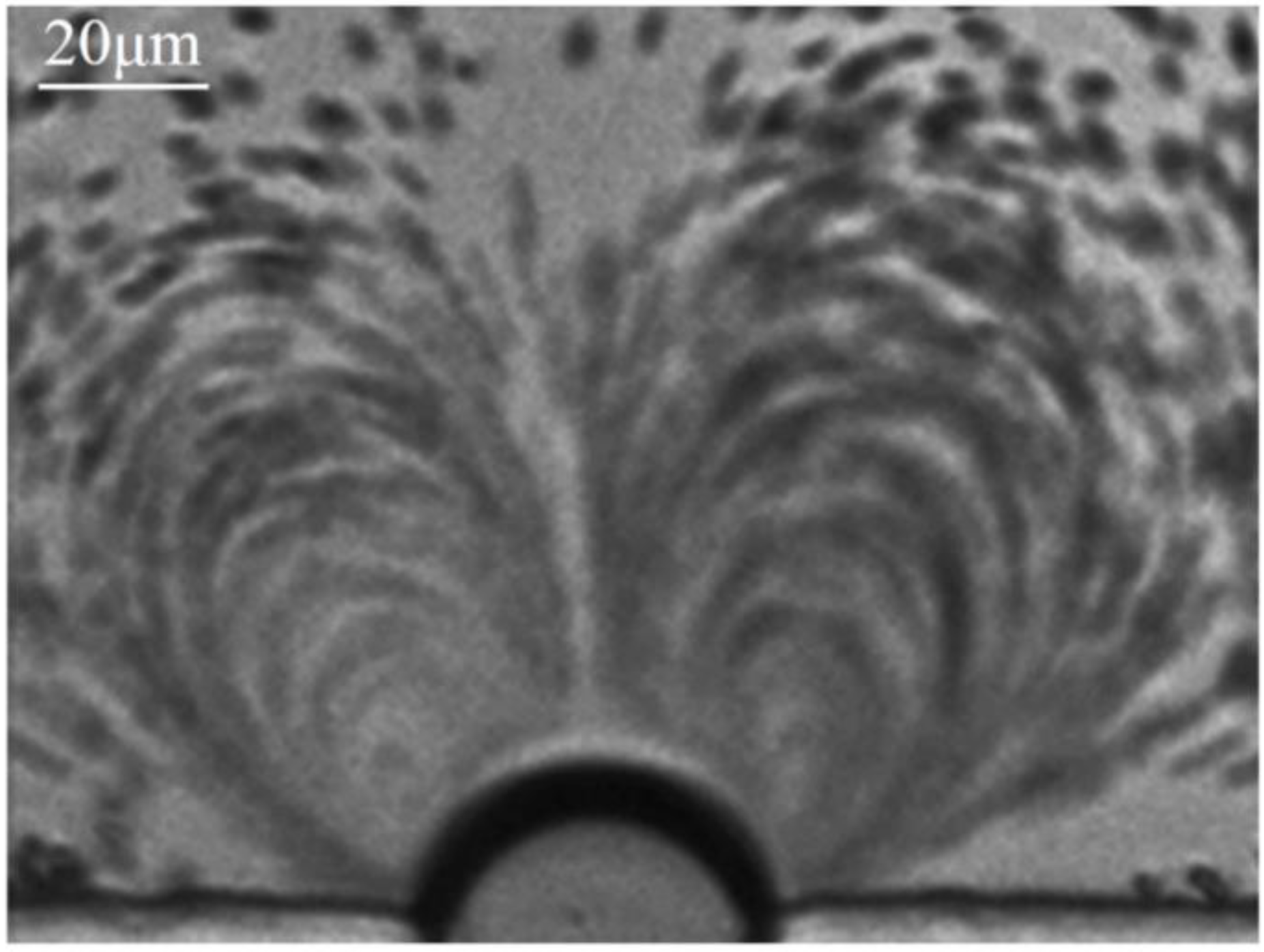
3.1. Microstreaming, Shear Stress and Cell Trapped Induced by Oscillating Microbubble
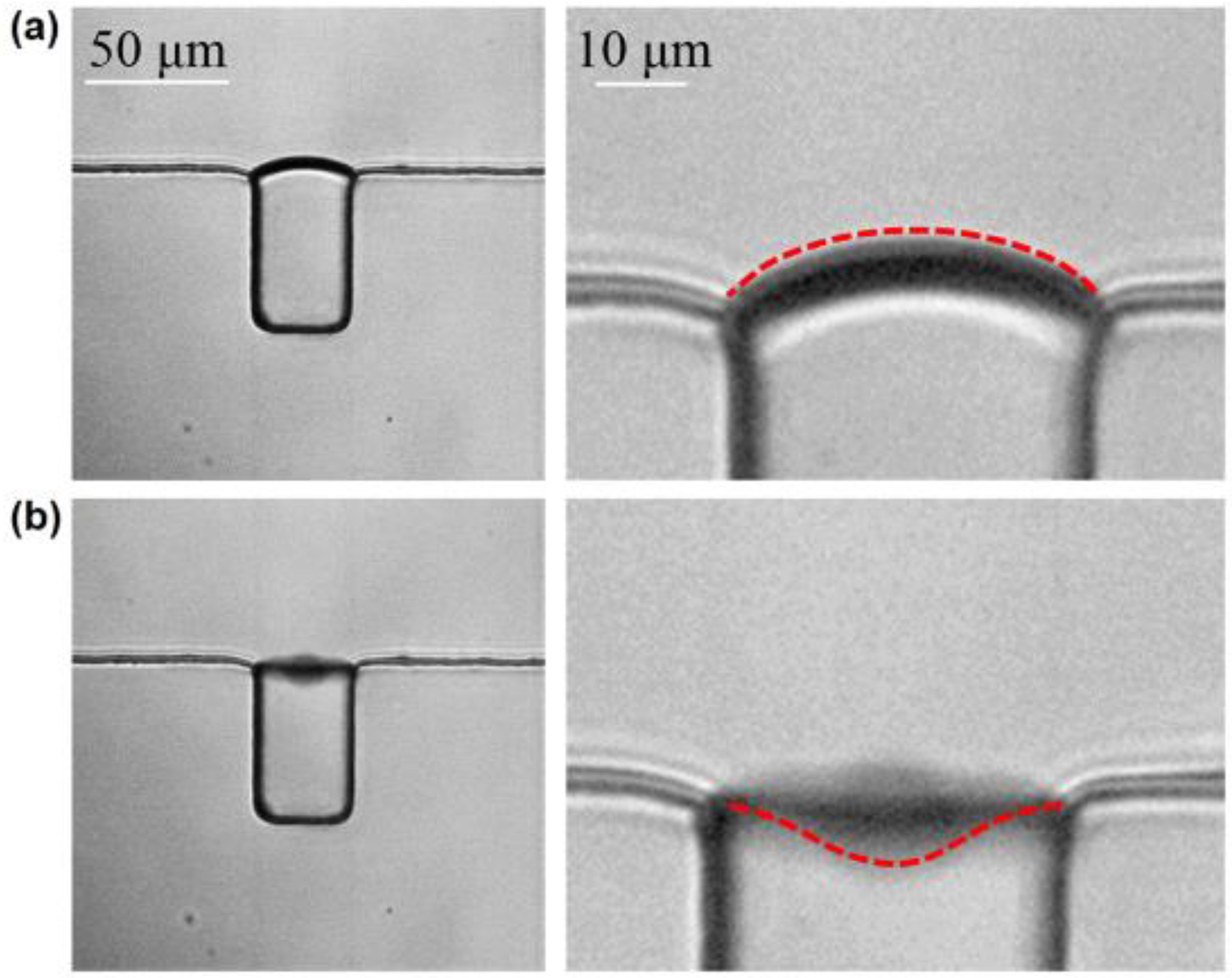
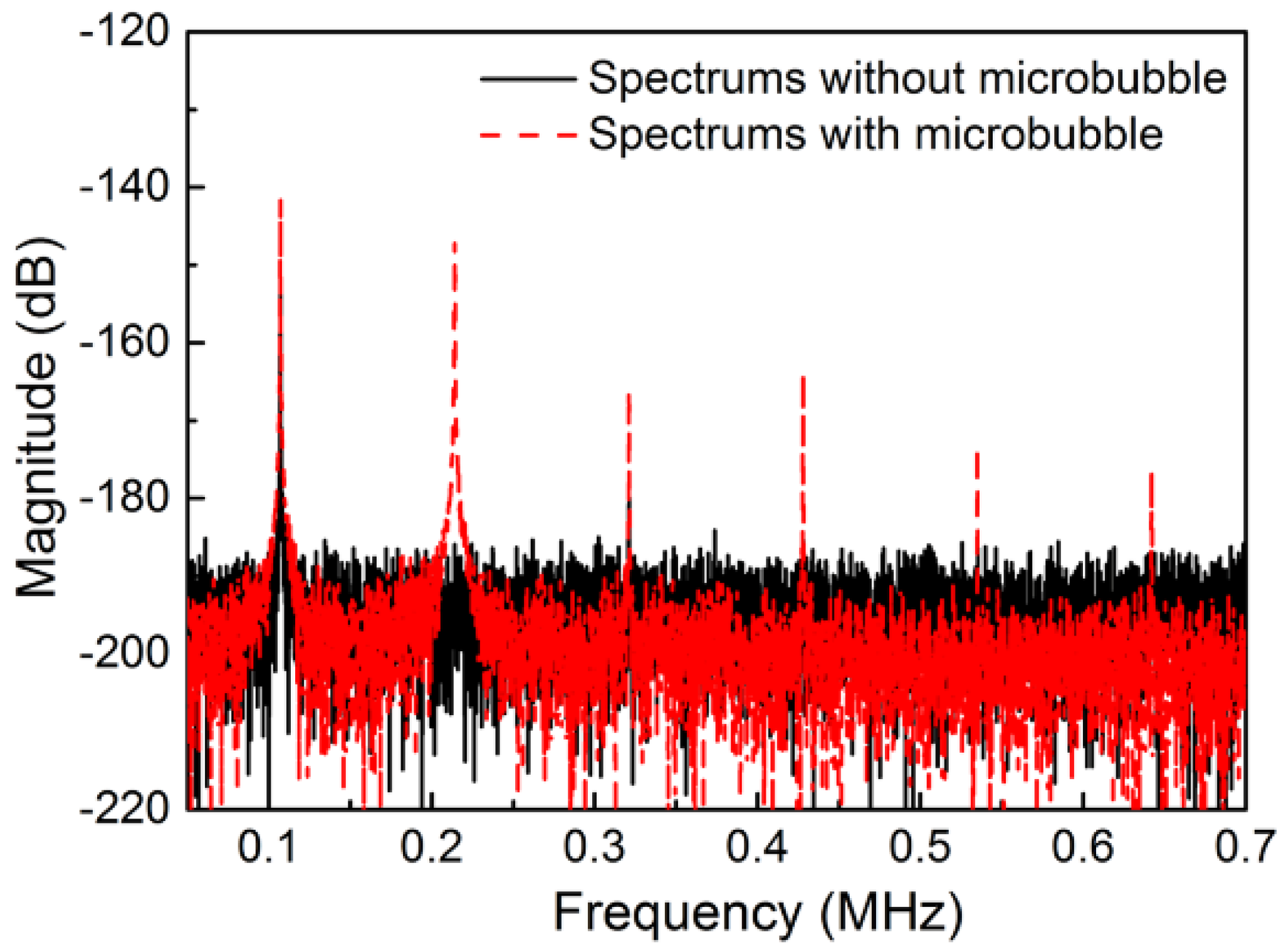
3.2. The Stable Cavitation of Microbubble
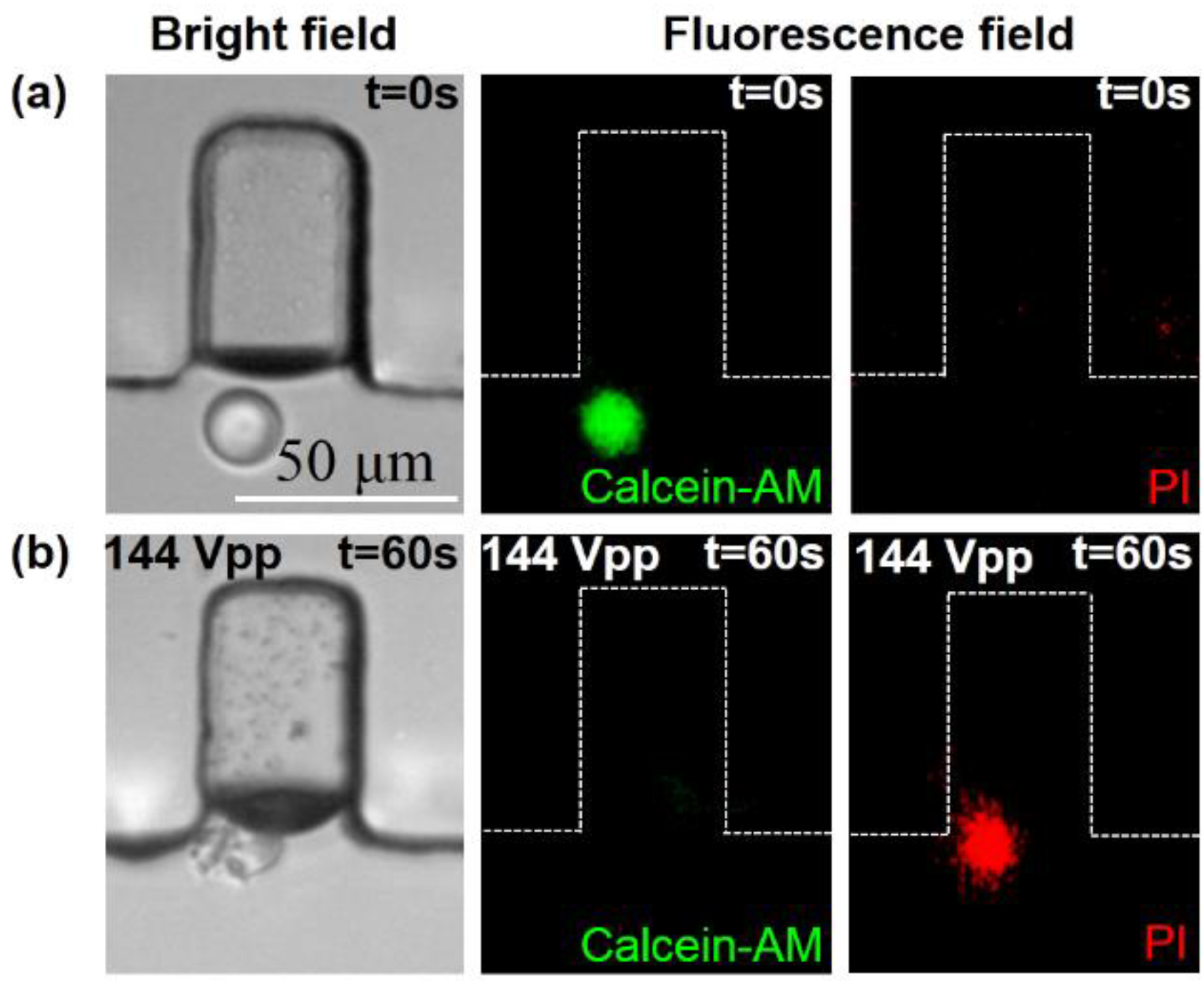
3.3. A Single-Cell Lysis
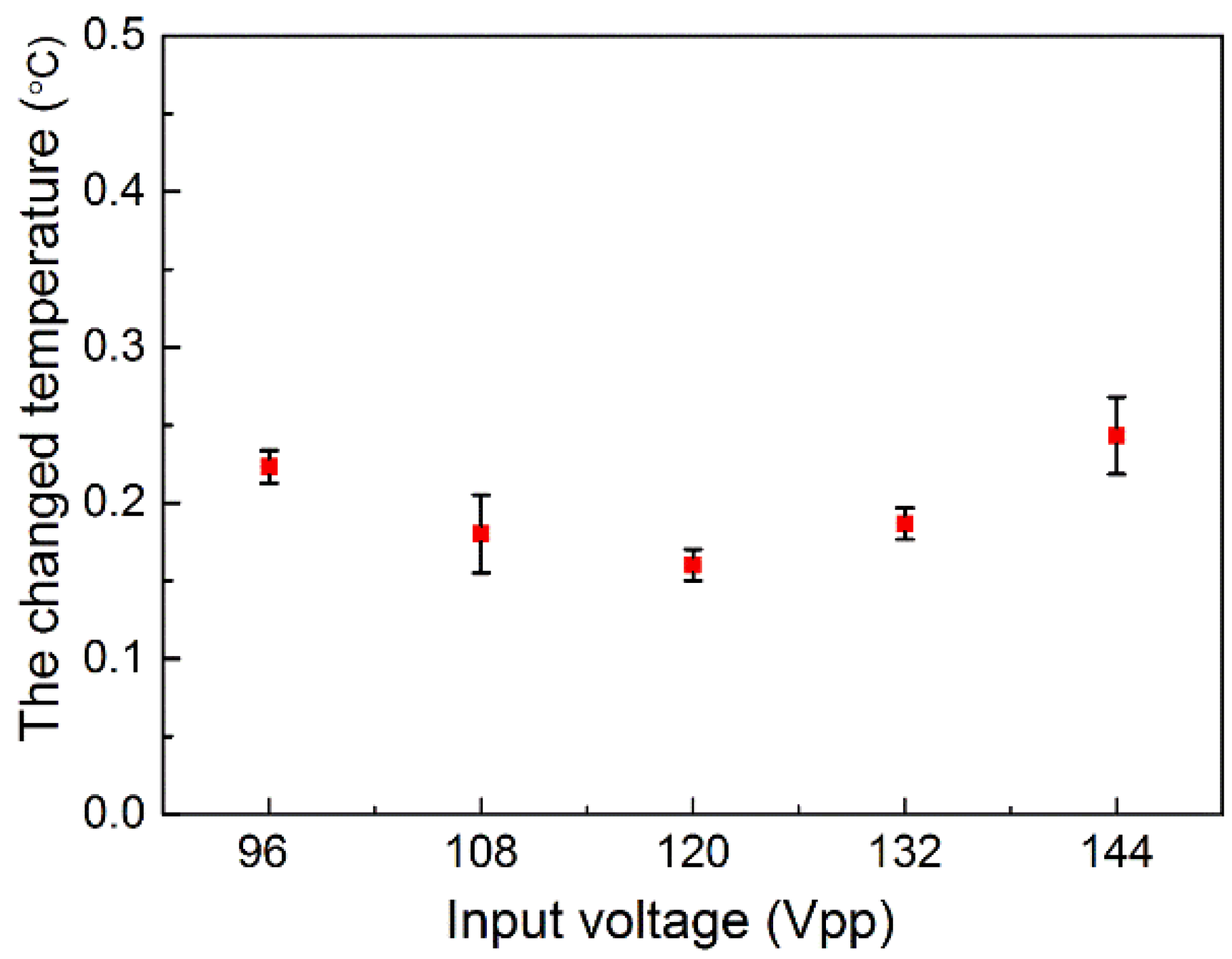
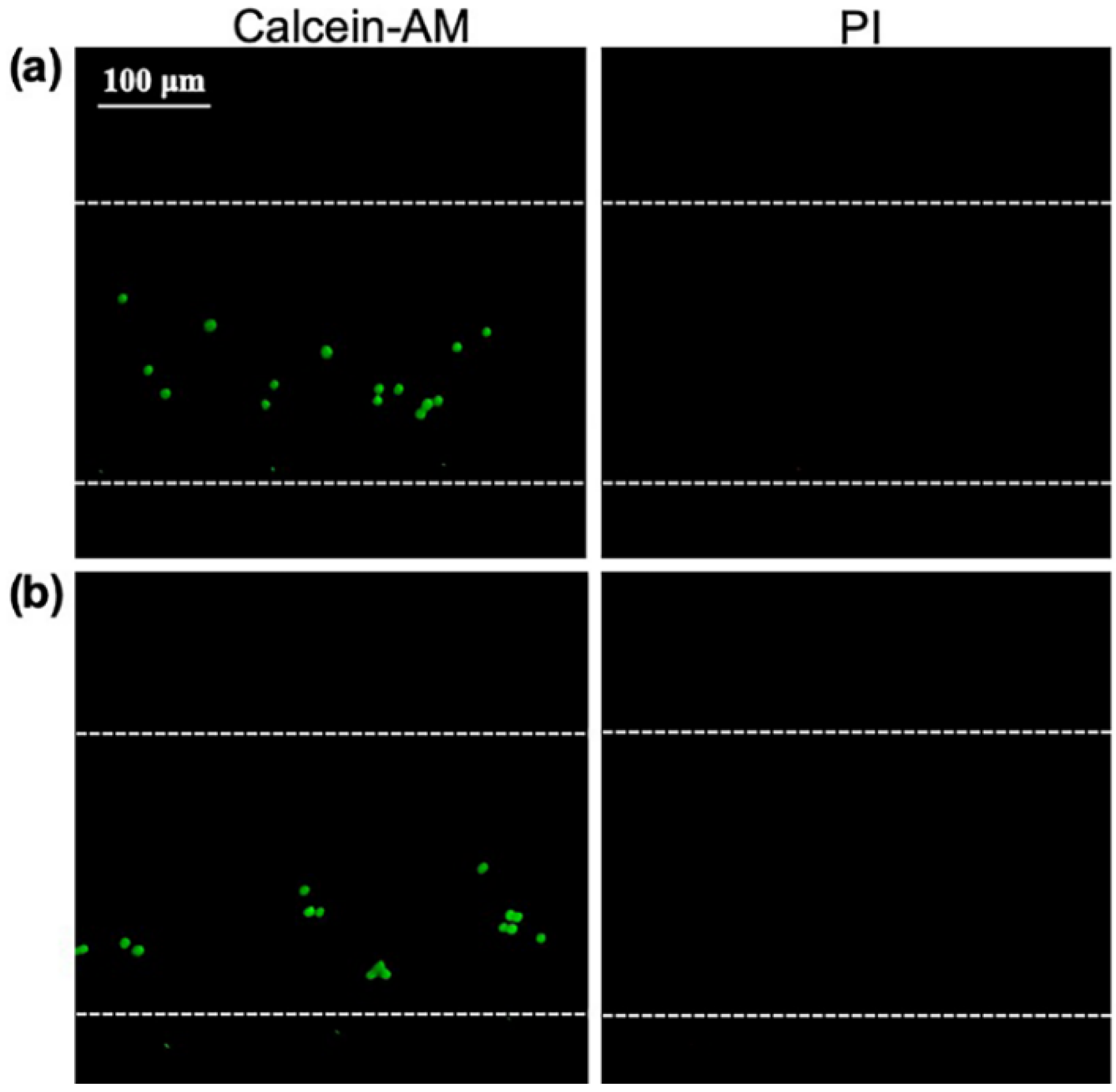
3.4. Cell Lysis Efficiency
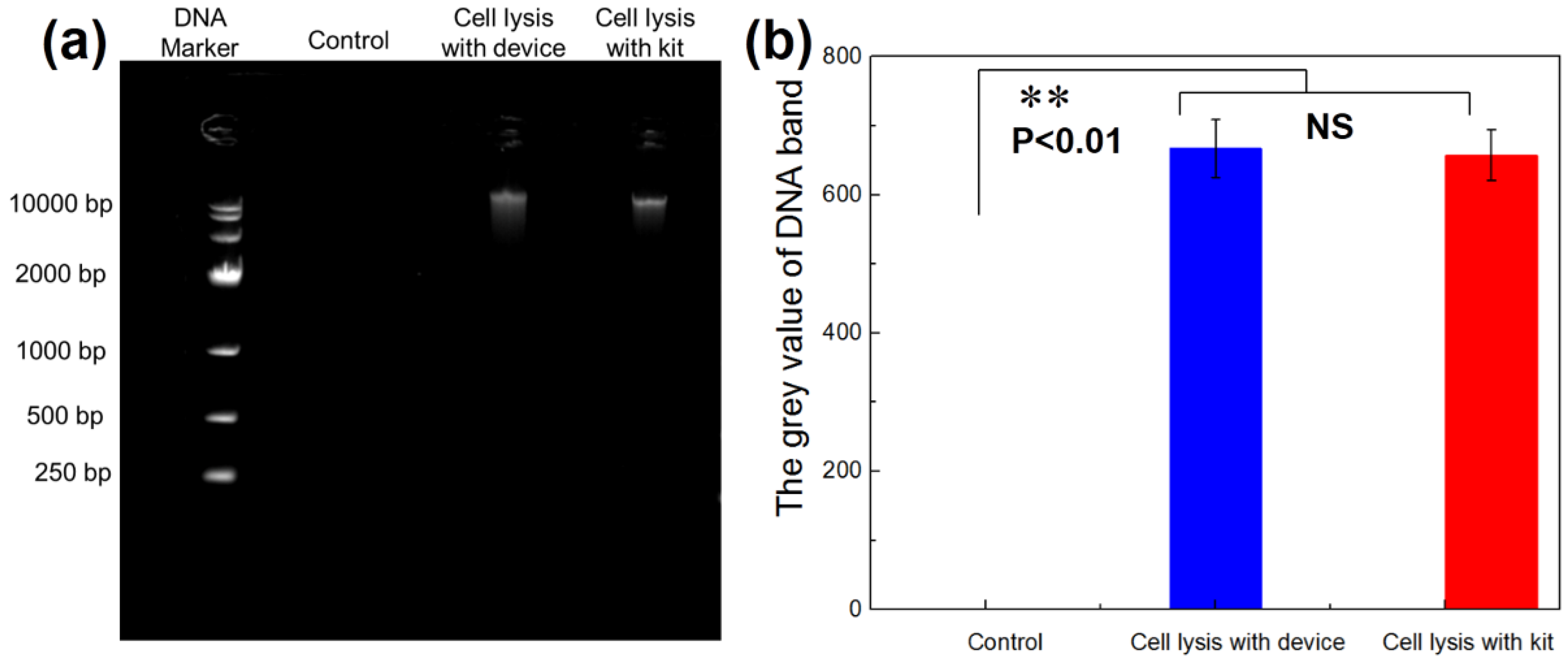
3.5. DNA Agarose-Gel Electrophoresis Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.; Jang, S.H.; Jia, G.Y.; Zoval, J.V.; Da Silva, N.A.; Madou, M.J. Cell lysis on a microfluidic CD (compact disc). Lab Chip 2004, 4, 516. [Google Scholar] [CrossRef]
- Schilling, E.A.; Kamholz, A.E.; Yager, P. Cell lysis and protein extraction in a microfluidic device with detection by a fluorogenic enzyme assay. Anal. Chim. 2002, 74, 1798. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Johnson, M.; Hill, P.; Gale, B.K. Microfluidic sample preparation: Cell lysis and nucleic acid purification. Integr. Biol. 2009, 1, 574. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, D.; Liu, C.; Li, H.; Chen, J. Continuous flow microfluidic device for cell separation, cell lysis and DNA purification. Anal. Chim. Acta 2007, 584, 237. [Google Scholar] [CrossRef]
- Marentis, T.C.; Kusler, B.; Yaralioglu, G.G.; Liu, S.; Haeggstrom, E.O.; Khuri-Yakub, B.T. Microfluidic sonicator for real-time disruption of eukaryotic cells and bacterial spores for DNA analysis. Ultrasound Med. Biol. 2005, 31, 1265. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.; Asenjo, J. Enzymatic lysis and disruption of microbial cells. Trends Biotechnol. 1987, 5, 273. [Google Scholar] [CrossRef]
- Salazar, O.; Asenjo, J.A. Enzymatic lysis of microbial cells. Biotechnol. Lett. 2007, 29, 985. [Google Scholar] [CrossRef] [PubMed]
- Feliciello IChinali, G. A modified alkaline lysis method for the preparation of highly purified plasmid DNA from Escherichia coli. Anal. Biochem. 1993, 212, 394. [Google Scholar] [CrossRef]
- Sharma, R.; Dill, B.D.; Chourey, K.; Shah, M.; VerBerkmoes, N.C.; Hettich, R.L. Coupling a detergent lysis/cleanup methodology with intact protein fractionation for enhanced proteome characterization. J. Proteome Res. 2012, 11, 6008. [Google Scholar] [CrossRef]
- Tamura, K.; Aotsuka, T. Rapid isolation method of animal mitochondrial DNA by the alkaline lysis procedure. Biochem. Genet. 1988, 26, 815. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, L.-A.; Chen, S.-J.; Chang, M.-C.; Chen, T.-L. A modified osmotic shock for periplasmic release of a recombinant creatinase from Escherichia coli. Biochem. Eng. J. 2004, 19, 211. [Google Scholar] [CrossRef]
- Byreddy, A.; Gupta, A.; Barrow, C.; Puri, M. Comparison of cell disruption methods for improving lipid extraction from thraustochytrid strains. Mar. Drugs 2015, 13, 5111–5127. [Google Scholar] [CrossRef]
- Shehadul Islam, M.; Aryasomayajula, A.; Selvaganapathy, P. A Review on Macroscale and Microscale Cell Lysis Methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Brown, R.B.; Audet, J. Current techniques for single-cell lysis. J. R. Soc. Interface 2008, 5, S131. [Google Scholar] [CrossRef]
- Meacle, F.; Lander, R.; Ayazi Shamlou, P.; Titchener-Hooker, N. Impact of engineering flow conditions on plasmid DNA yield and purity in chemical cell lysis operations. Biotechnol. Bioeng. 2004, 87, 293. [Google Scholar] [CrossRef]
- Johnson, B.H.; Hecht, M.H. Recombinant proteins can be isolated from E. coli cells by repeated cycles of freezing and thawing. Biol. Technol. 1994, 12, 1357. [Google Scholar] [CrossRef]
- Han, F.T.; Wang, Y.; Sims, C.E.; Bachman, M.; Chang, R.S.; Li, G.P.; Allbritton, N.L. Fast electrical lysis of cells for capillary electrophoresis. Anal. Chim. 2003, 75, 3688. [Google Scholar] [CrossRef]
- Gaitan, D.F. Sonoluminescence and bubble dynamics for a single, stable, cavitation bubble. J. Acoust. Soc. Am. 1992, 91, 3166. [Google Scholar] [CrossRef]
- Petit, B.; Bohren, Y.; Gaud, E.; Bussat, P.; Arditi, M.; Yan, F.; Tranquart, F.; Allemann, E. Sonothrombolysis: The contribution of stable and inertial cavitation to clot lysis. Ultrasound Med. Biol. 2015, 41, 1402. [Google Scholar] [CrossRef]
- Meng, L.; Liu, X.; Wang, Y.; Zhang, W.; Zhou, W.; Cai, F.; Li, F.; Wu, J.; Xu, L.; Niu, L.; et al. Sonoporation of Cells by a Parallel Stable Cavitation Microbubble Array. Adv. Sci. 2019, 6, 1900557. [Google Scholar] [CrossRef]
- Meng, L.; Cai, F.; Li, F.; Zhou, W.; Niu, L.; Zheng, H. Acoustic tweezers. J. Phys. D Appl. Phys. 2019, 52, 273001. [Google Scholar] [CrossRef]
- Meng, L.; Cai, F.; Jiang, P.; Deng, Z.; Li, F.; Niu, L.; Chen, Y.; Wu, J.; Zheng, H. On-chip targeted single cell sonoporation with microbubble destruction excited by surface acoustic waves. Appl. Phys. Lett. 2014, 104, 073701. [Google Scholar] [CrossRef]
- Mulvana, H.; Cochran, S.; Hill, M. Ultrasound assisted particle and cell manipulation on-chip. Adv. Drug Deliv. Rev. 2013, 65, 1600. [Google Scholar] [CrossRef]
- Meng, L.; Cai, F.; Zhang, Z.; Niu, L.; Jin, Q.; Yan, F.; Wu, J.; Wang, Z.; Zheng, H. Transportation of single cell and microbubbles by phase-shift introduced to standing leaky surface acoustic waves. Biomicrofluidics 2011, 5, 044104. [Google Scholar] [CrossRef]
- Meng, L.; Deng, Z.; Niu, L.; Li, F.; Yan, F.; Wu, J.; Cai, F.; Zheng, H. A disposable microfluidic device for controlled drug release from thermal-sensitive liposomes by high intensity focused ultrasound. Theranostics 2015, 5, 1203. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.; Liu, X.; Zhang, W.; Cai, F.; Li, F.; Wu, J.; Wang, J.; Wang, Y.; Huang, X.; et al. Selective photothermal ablation of cancer cells by patterned gold nanocages using surface acoustic waves. Lab Chip 2019, 19, 3387–3396. [Google Scholar] [CrossRef]
- Tandiono, T.; Ow, D.S.-W.; Driessen, L.; Chin, C.S.-H.; Klaseboer, E.; Choo, A.B.-H.; Ohl, S.-W.; Ohl, C.-D. Sonolysis of Escherichia coli and Pichia pastoris in microfluidics. Lab Chip 2012, 12, 780. [Google Scholar] [CrossRef]
- Marmottant, P.; Hilgenfeldt, S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003, 423, 153–156. [Google Scholar] [CrossRef]
- Tran-Minh, N.; Hønsvall, B.K.; Karlsen, F. Integrated Microfluidic Device for Cell Lysis in a Continuous Flow Mode. J. Med. Biol. Eng. 2015, 4, 150. [Google Scholar] [CrossRef]
- Meng, L.; Cai, F.; Chen, J.; Niu, L.; Li, Y.; Wu, J.; Zheng, H. Precise and programmable manipulation of microbubbles by two-dimensional standing surface acoustic waves. Appl. Phys. Lett. 2012, 100, 173701. [Google Scholar] [CrossRef]
- Pérez-Díaz, J.L.; Álvarez-Valenzuela, M.A.; García-Prada, J.C. The effect of the partial pressure of water vapor on the surface tension of the liquid water–air interface. J. Colloid Interface Sci. 2012, 381, 180–182. [Google Scholar] [CrossRef]
- Kumar, R.; Kuloor, N.R. The formation of bubbles and drops. Adv. Chem. Eng. 1970, 8, 255–368. [Google Scholar]
- Argo, T.F.; Wilson, P.S.; Palan, V. Measurement of the resonance frequency of single bubbles using a laser Doppler vibrometer. J. Acoust. Soc. Am. 2008, 123, El121. [Google Scholar] [CrossRef]
- Habibi, R.; Devendran, C.; Neild, A. Trapping and patterning of large particles and cells in a 1D ultrasonic standing wave. Lab Chip 2017, 17, 3279. [Google Scholar] [CrossRef]
- Le Gac, S.; Zwaan, E.; van den Berg, A.; Ohl, C.-D. Sonoporation of suspension cells with a single cavitation bubble in a microfluidic confinement. Lab Chip 2007, 7, 1666. [Google Scholar] [CrossRef]
- Wu, J.R.; Ross, J.P.; Chiu, J.F. Reparable sonoporation generated by microstreaming. J. Acoust. Soc. Am. 2002, 111, 1460. [Google Scholar] [CrossRef]
- Williams, J.C.; Stonehill, M.A.; Colmenares, K.; Evan, A.P.; Andreoli, S.P.; Cleveland, R.O.; Bailey, M.R.; Crum, L.A.; McAteer, J.A. Effect of macroscopic air bubbles on cell lysis by shock wave lithotripsy in vitro. Ultrasound Med. Biol. 1999, 25, 473. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, P.-H.; Chen, C.; Bachman, H.; Zhao, S.; Yang, S.; Huang, T.J. Cell lysis via acoustically oscillating sharp edges. Lab Chip 2019, 19, 4021. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Farooq, U.; Xuan, W.; Jin, H.; Dong, S.; Luo, J. Ultrafast chemical-free cell lysis by high speed stream collision induced by surface acoustic waves. Appl. Phys. Lett. 2017, 110, 143504. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, J.; Zhang, L.; Huang, X.; Farooq, U.; Pang, N.; Zhou, W.; Qi, L.; Xu, L.; Niu, L.; et al. Cell Lysis Based on an Oscillating Microbubble Array. Micromachines 2020, 11, 288. https://doi.org/10.3390/mi11030288
Liu X, Li J, Zhang L, Huang X, Farooq U, Pang N, Zhou W, Qi L, Xu L, Niu L, et al. Cell Lysis Based on an Oscillating Microbubble Array. Micromachines. 2020; 11(3):288. https://doi.org/10.3390/mi11030288
Chicago/Turabian StyleLiu, Xiufang, Jinyuan Li, Liangyu Zhang, Xiaowei Huang, Umar Farooq, Na Pang, Wei Zhou, Lin Qi, Lisheng Xu, Lili Niu, and et al. 2020. "Cell Lysis Based on an Oscillating Microbubble Array" Micromachines 11, no. 3: 288. https://doi.org/10.3390/mi11030288
APA StyleLiu, X., Li, J., Zhang, L., Huang, X., Farooq, U., Pang, N., Zhou, W., Qi, L., Xu, L., Niu, L., & Meng, L. (2020). Cell Lysis Based on an Oscillating Microbubble Array. Micromachines, 11(3), 288. https://doi.org/10.3390/mi11030288





