A Microfluidic Mixer of High Throughput Fabricated in Glass Using Femtosecond Laser Micromachining Combined with Glass Bonding
Abstract
1. Introduction
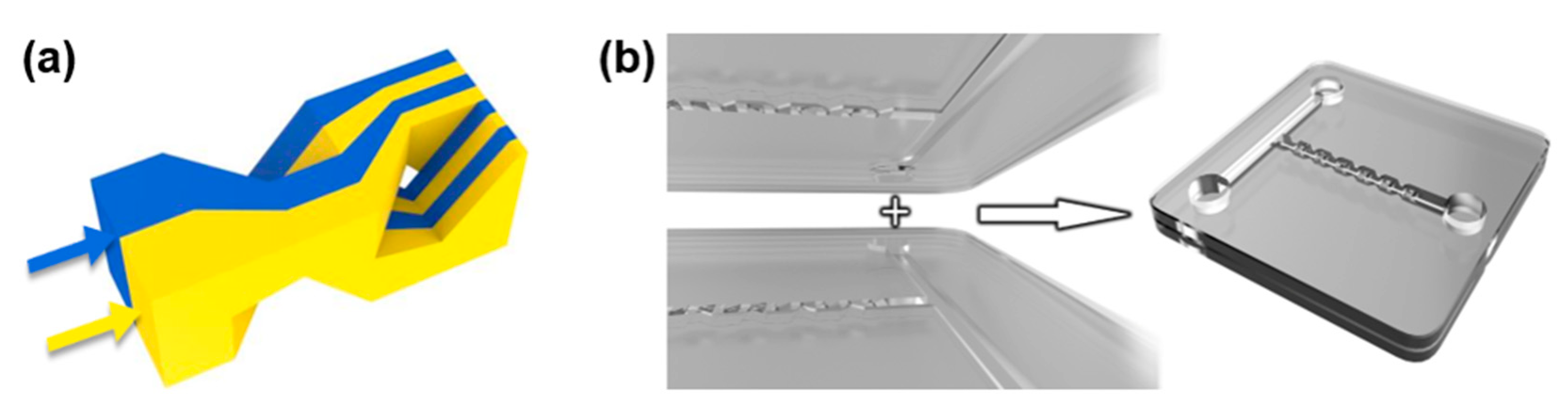
2. Device Design and Numerical Simulations of Mixing Process
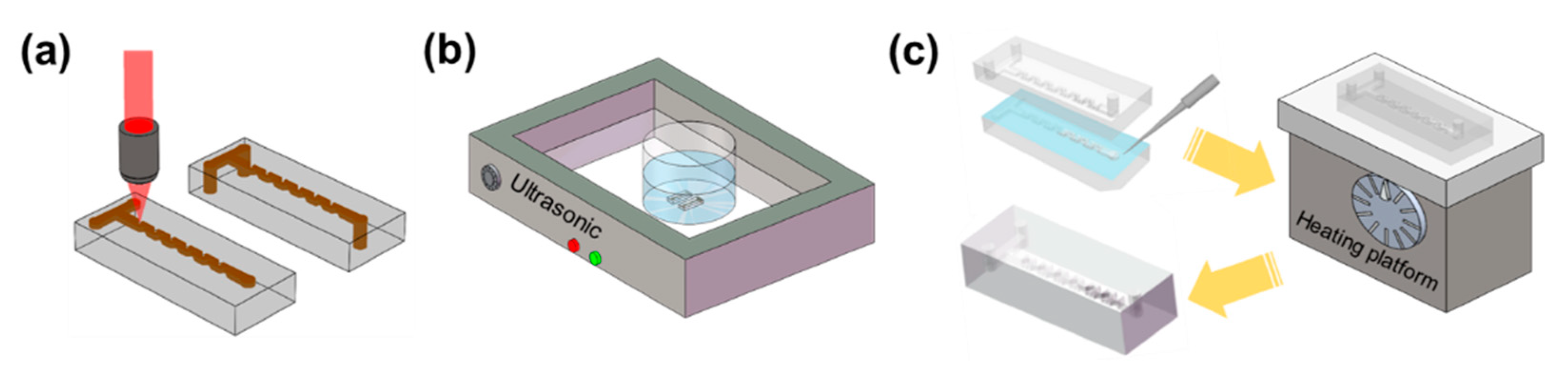
3. Fabrication of the 3D Micromixers
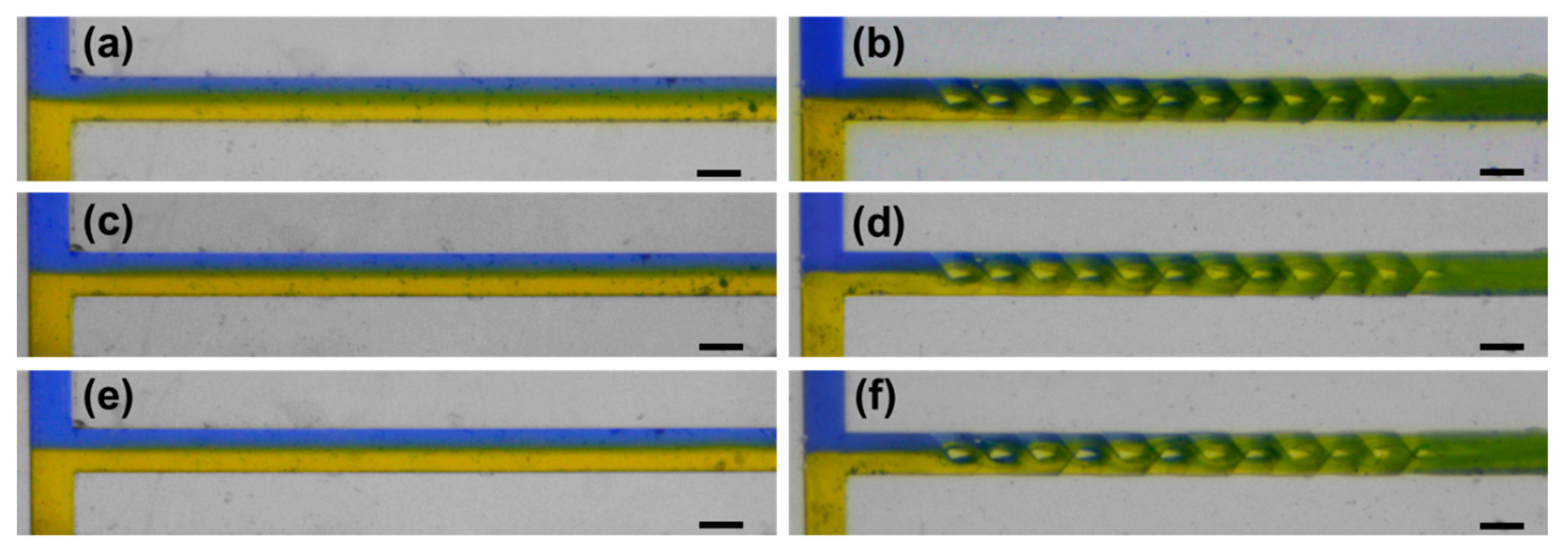
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Losey, M.W.; Schmidt, M.A.; Jensen, K.F. Microfabricated Multiphase Packed-Bed Reactors: Characterization of Mass Transfer and Reactions. Ind. Eng. Chem. Res. 2001, 40, 2555–2562. [Google Scholar] [CrossRef]
- Wu, N.; Wu, S.-Z.; Xu, J.; Niu, L.-G.; Midorikawa, K.; Sugioka, K. Hybrid femtosecond laser microfabrication to achieve true 3D glass/polymer composite biochips with multiscale features and high performance: The concept of ship-in-a-bottle biochip. Laser Photon. Rev. 2014, 8, 458–467. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chang, C.-L.; Wang, Y.-N.; Fu, L.-M. Microfluidic Mixing: A Review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Z.P.; Koh, Y.X.; May, K.T. A microfluidic mixer with self-excited ‘turbulent’ fluid motion for wide viscosity ratio applications. Lab Chip 2010, 10, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Meier, M.; Lyons, S.; Sit, R.V.; Marzluff, W.F.; Quake, S.R.; Chang, H.Y. Systematic reconstruction of RNA functional motifs with high-throughput microfluidics. Nat. Methods 2012, 9, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Gervais, L.; De Rooij, N.; Delamarche, E. Microfluidic Chips for Point-of-Care Immunodiagnostics. Adv. Mater. 2011, 23, H151–H176. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, S.; Shao, T.; Jin, Y.; Cheng, Y. Visualization of micro-scale mixing in miscible liquids using μ-LIF technique and drug nano-particle preparation in T-shaped micro-channels. Chem. Eng. J. 2012, 192, 252–261. [Google Scholar] [CrossRef]
- Nimafar, M.; Viktorov, V.; Martinelli, M. Experimental comparative mixing performance of passive micromixers with H-shaped sub-channels. Chem. Eng. Sci. 2012, 76, 37–44. [Google Scholar] [CrossRef]
- Cortes-Quiroz, C.A.; Azarbadegan, A.; Zangeneh, M.; Goto, A. Analysis and multi-criteria design optimization of geometric characteristics of grooved micromixer. Chem. Eng. J. 2010, 160, 852–864. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Z.; Yim, C.; Lin, M.; Cao, X. Evaluation of Floor-grooved Micromixers using Concentration-channel Length Profiles. Micromachines 2010, 1, 19–33. [Google Scholar] [CrossRef]
- Therriault, D.; White, S.; Lewis, J.A. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat. Mater. 2003, 2, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.A.; Johnson, B.N.; Brahmasandra, S.N.; Handique, K.; Webster, J.R.; Krishnan, M.; Sammarco, T.S.; Man, P.M.; Jones, D.; Heldsinger, D.; et al. An Integrated Nanoliter DNA Analysis Device. Science 1998, 282, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Carrière, P. On a three-dimensional implementation of the baker’s transformation. Phys. Fluids 2007, 19, 118110. [Google Scholar] [CrossRef]
- Liao, Y.; Song, J.; Li, E.; Luo, Y.; Shen, Y.; Chen, D.; Cheng, Y.; Xu, Z.; Sugioka, K.; Midorikawa, K. Rapid prototyping of three-dimensional microfluidic mixers in glass by femtosecond laser direct writing. Lab Chip 2012, 12, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Osellame, R.; Cerullo, G.; Ramponi, R. Femtosecond Laser Micromachining: Photonic and Microfluidic Devices in Transparent Materials; Springer: Berlin, Germany, 2012; Volume 123. [Google Scholar]
- Kirby, B.J. Micro-and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Weisgrab, G.; Ovsianikov, A.; Costa, P. Functional 3D Printing for Microfluidic Chips. Adv. Mater. Technol. 2019, 4, 1900275. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Cheng, Y.; Sugioka, K.; Midorikawa, K.; Masuda, M.; Toyoda, K.; Kawachi, M.; Shihoyama, K. Three-dimensional micro-optical components embedded in photosensitive glass by a femtosecond laser. Opt. Lett. 2003, 28, 1144–1146. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Femtosecond laser processing for optofluidic fabrication. Lab Chip 2012, 12, 3576. [Google Scholar] [CrossRef]
- Hunt, H.C.; Wilkinson, J. Optofluidic integration for microanalysis. Microfluid. Nanofluidics 2007, 4, 53–79. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photon. 2011, 5, 591–597. [Google Scholar] [CrossRef]
- Cheng, Y. Internal Laser Writing of High-Aspect-Ratio Microfluidic Structures in Silicate Glasses for Lab-on-a-Chip Applications. Micromachines 2017, 8, 59. [Google Scholar] [CrossRef]
- Yasui, T.; Omoto, Y.; Osato, K.; Kaji, N.; Suzuki, N.; Naito, T.; Watanabe, M.; Okamoto, Y.; Tokeshi, M.; Shamoto, E.; et al. Microfluidic baker’s transformation device for three-dimensional rapid mixing. Lab Chip 2011, 11, 3356. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chu, W.; Li, W.; Tan, Y.; Liu, F.; Wang, M.; Qi, J.; Lin, J.; Zhang, F.; Wang, Z.; et al. Three-Dimensional Laser Printing of Macro-Scale Glass Objects at a Micro-Scale Resolution. Micromachines 2019, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Lin, Z.; Qi, J.; Wang, P.; Chu, W.; Fang, Z.; Wang, Z.; Chai, Z.; Cheng, Y. Polarization-insensitive space-selective etching in fused silica induced by picosecond laser irradiation. Appl. Surf. Sci. 2019, 485, 188–193. [Google Scholar] [CrossRef]
- Rowan, S.; Twyford, S.; Hough, J.; Gwo, D.-H.; Route, R. Mechanical losses associated with the technique of hydroxide-catalysis bonding of fused silica. Phys. Lett. A 1998, 246, 471–478. [Google Scholar] [CrossRef]
- Elliffe, E.J.; Bogenstahl, J.; Deshpande, A.; Hough, J.; Killow, C.; Reid, S.; Robertson, D.; Rowan, S.; Ward, H.; Cagnoli, G. Hydroxide-catalysis bonding for stable optical systems for space. Class. Quantum Gravity 2005, 22, S257–S267. [Google Scholar] [CrossRef]
- Sun, K.; Suzuki, N.; Li, Z.; Araki, R.; Ueno, K.; Juodkazis, S.; Abe, M.; Noji, S.; Misawa, H. High-fidelity fractionation of ssDNA fragments differing in size by one-base on a spiral-channel electrophoretic chip. Electrophoresis 2009, 30, 4277–4284. [Google Scholar] [CrossRef]


| Channel Width × Height | Maximum Flow Rate | Materials | Processing Method | Ref. |
|---|---|---|---|---|
| 160 µm × 20 µm | ~200 µL/min | PDMS | PDMS molds | [24] |
| 50 µm × 75 µm | ~20 µL/min | Porous glass | Femtosecond laser direct writing and annealing | [14] |
| 500 µm × 500 µm | 6 mL/min | Fused silica | FLISE and bonding | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Li, W.; Chu, W.; Yu, J.; Wu, M.; Liang, Y.; Yin, D.; Wang, P.; Wang, Z.; Wang, M.; et al. A Microfluidic Mixer of High Throughput Fabricated in Glass Using Femtosecond Laser Micromachining Combined with Glass Bonding. Micromachines 2020, 11, 213. https://doi.org/10.3390/mi11020213
Qi J, Li W, Chu W, Yu J, Wu M, Liang Y, Yin D, Wang P, Wang Z, Wang M, et al. A Microfluidic Mixer of High Throughput Fabricated in Glass Using Femtosecond Laser Micromachining Combined with Glass Bonding. Micromachines. 2020; 11(2):213. https://doi.org/10.3390/mi11020213
Chicago/Turabian StyleQi, Jia, Wenbo Li, Wei Chu, Jianping Yu, Miao Wu, Youting Liang, Difeng Yin, Peng Wang, Zhenhua Wang, Min Wang, and et al. 2020. "A Microfluidic Mixer of High Throughput Fabricated in Glass Using Femtosecond Laser Micromachining Combined with Glass Bonding" Micromachines 11, no. 2: 213. https://doi.org/10.3390/mi11020213
APA StyleQi, J., Li, W., Chu, W., Yu, J., Wu, M., Liang, Y., Yin, D., Wang, P., Wang, Z., Wang, M., & Cheng, Y. (2020). A Microfluidic Mixer of High Throughput Fabricated in Glass Using Femtosecond Laser Micromachining Combined with Glass Bonding. Micromachines, 11(2), 213. https://doi.org/10.3390/mi11020213






