Designing Splicing Digital Microfluidics Chips Based on Polytetrafluoroethylene Membrane
Abstract
1. Introduction
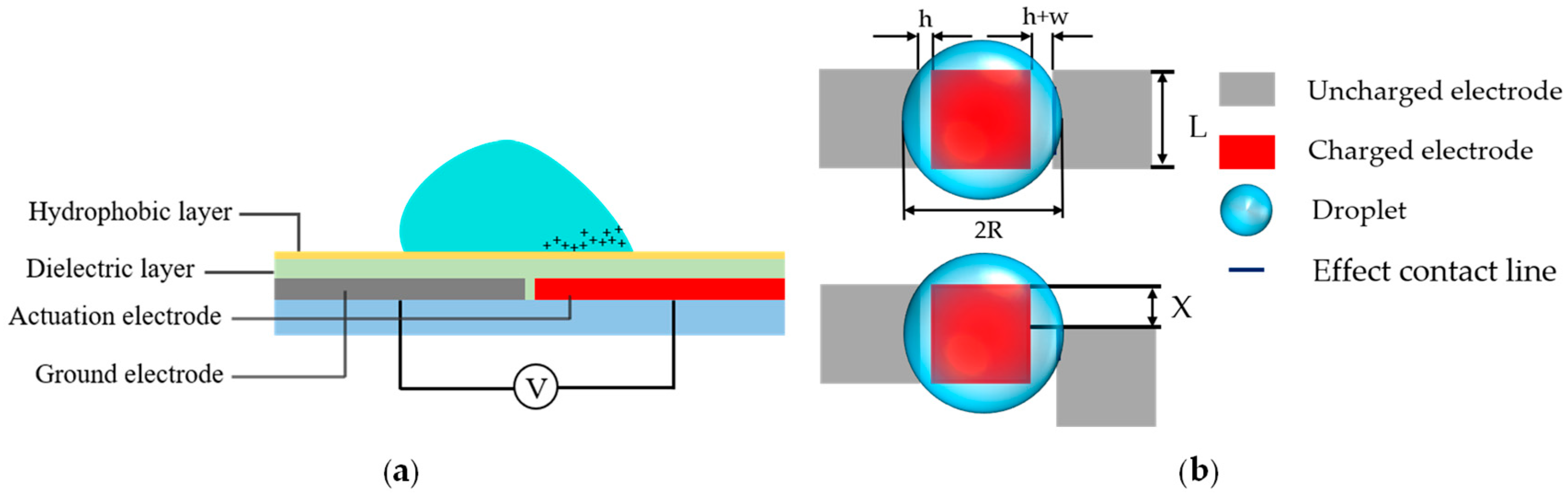
2. Principle
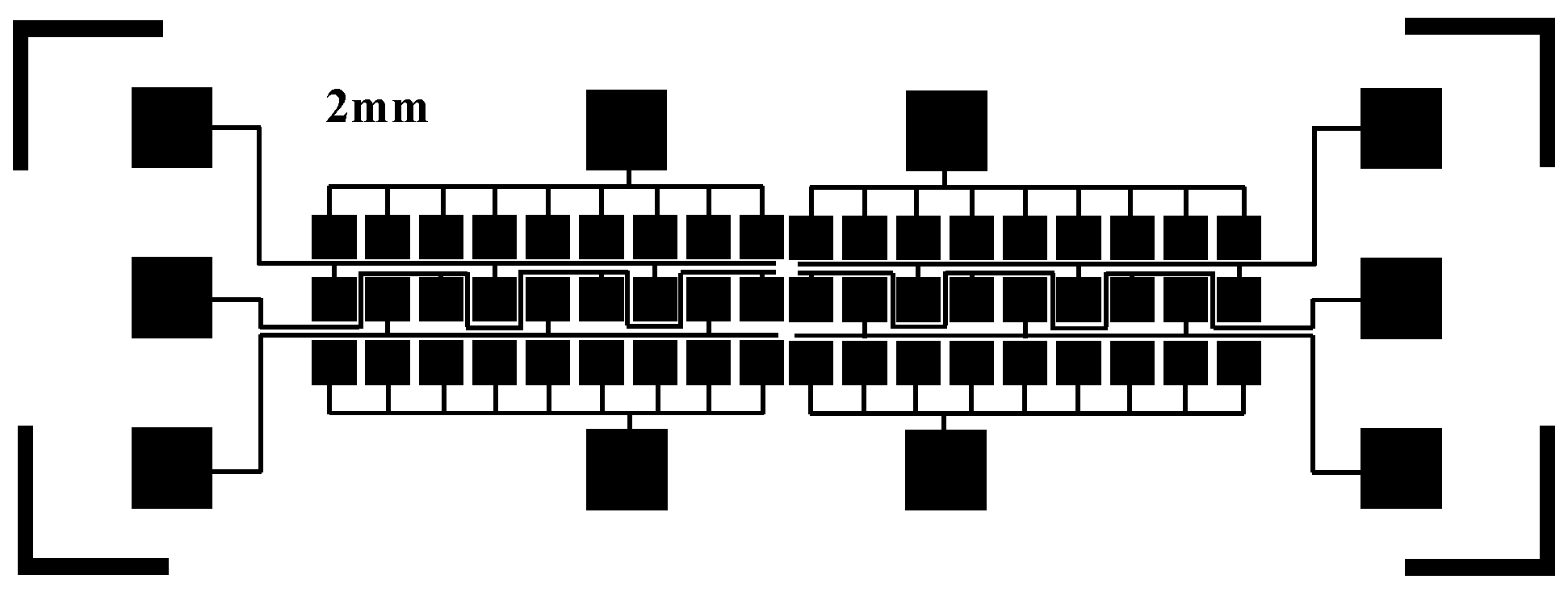
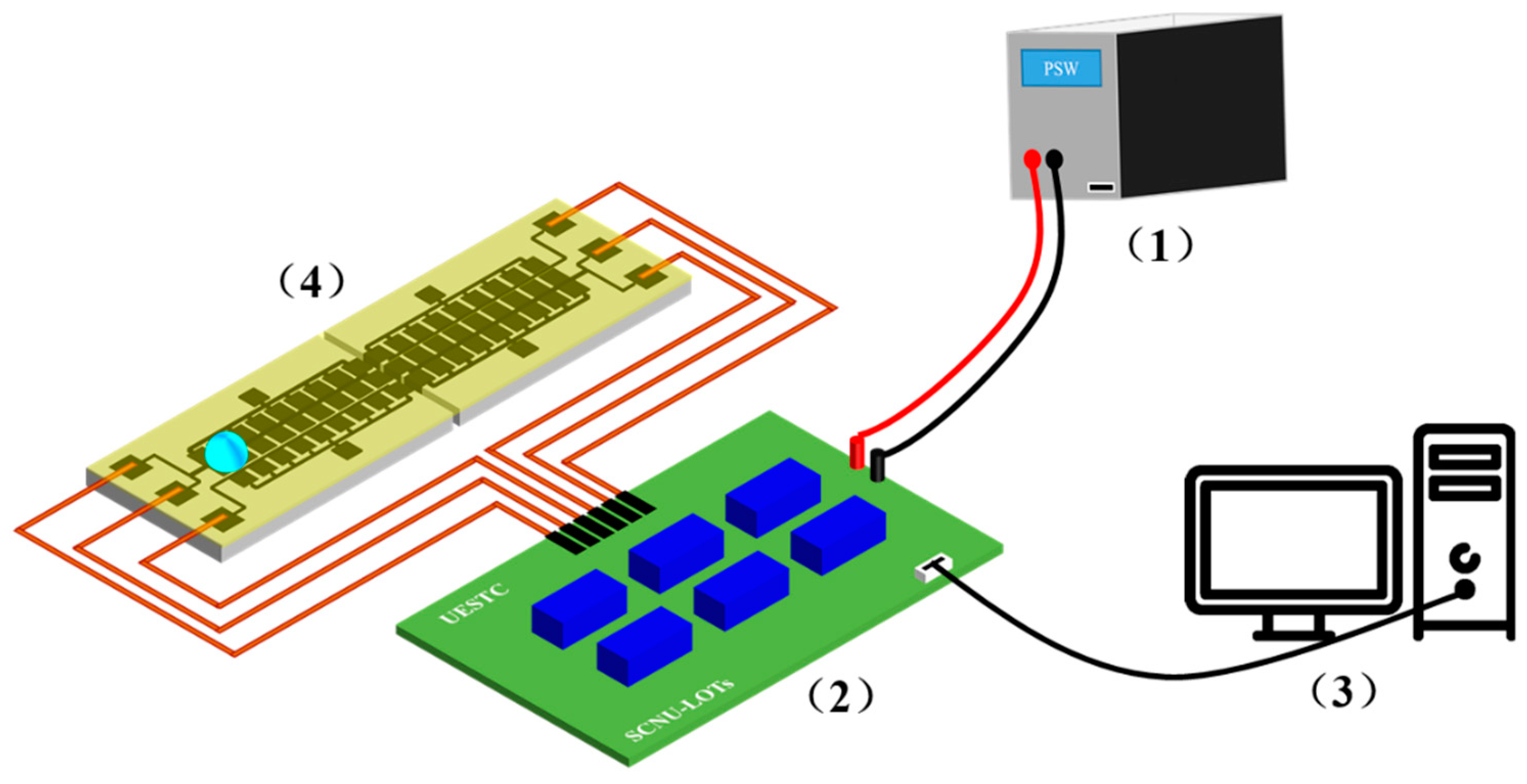
3. Fabrication of the Digital Microfluidics (DMF) System
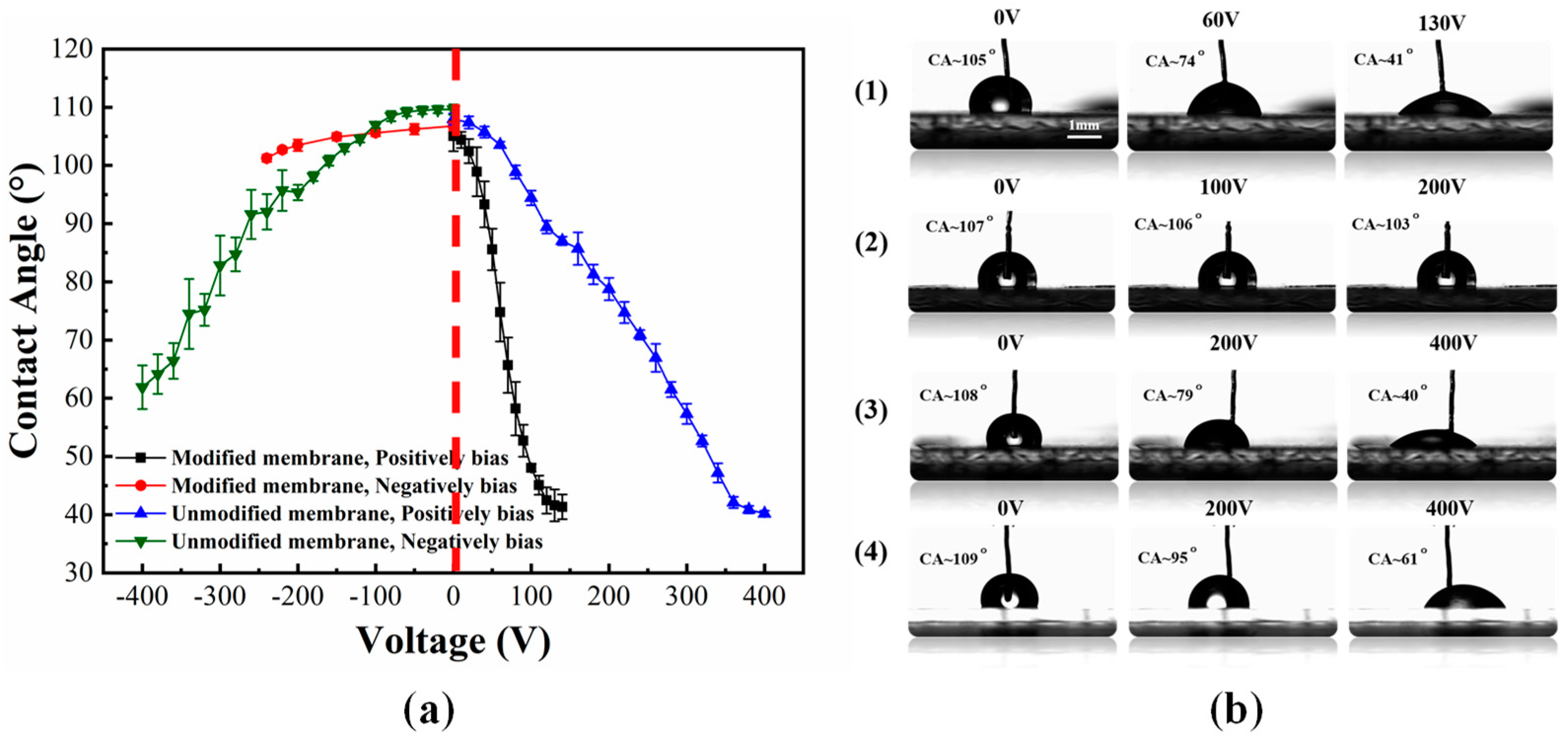
4. Results and Discussions
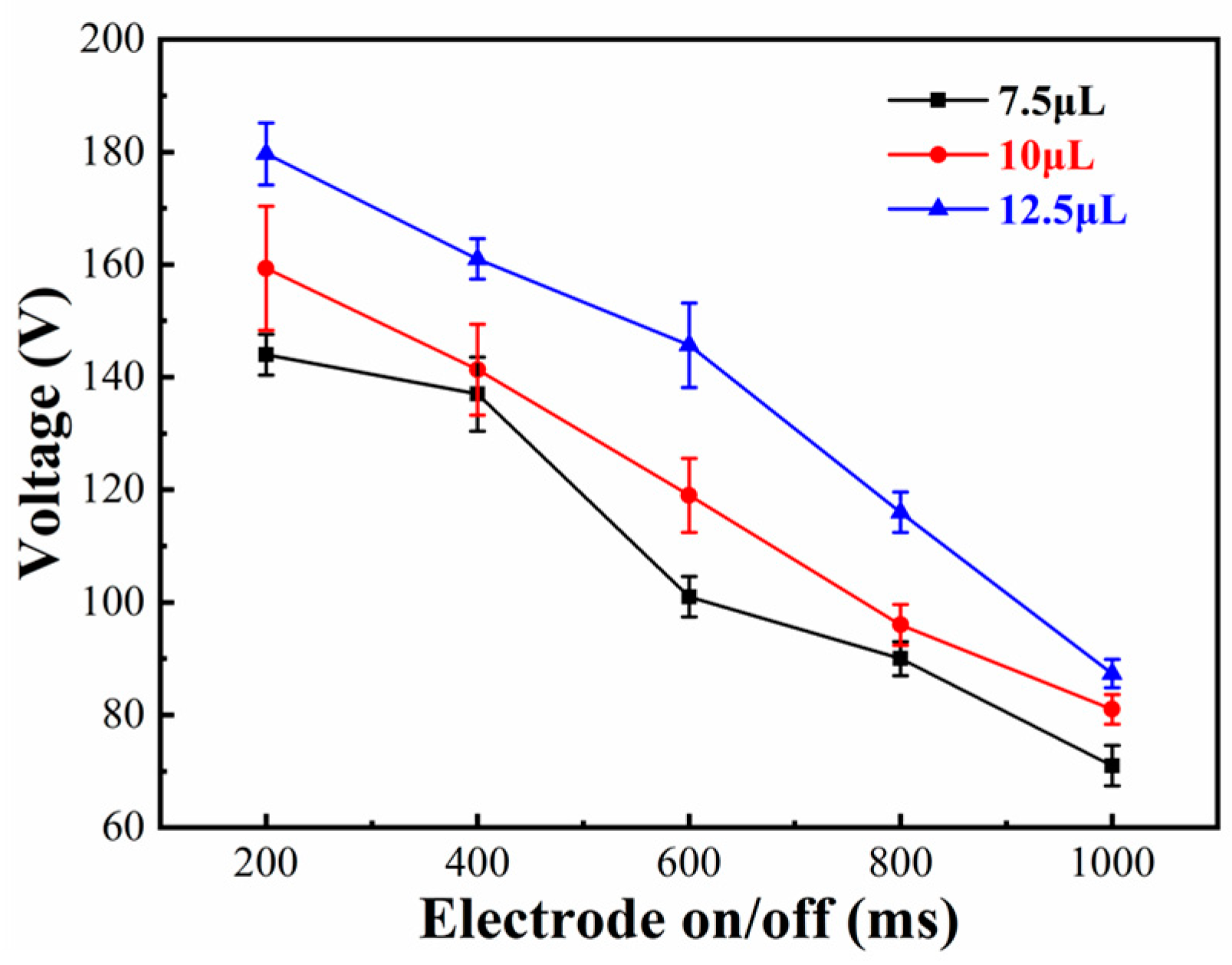
4.1. Effect of the Electrode On/Off Time
4.2. Effect of Different Splicing Solutions
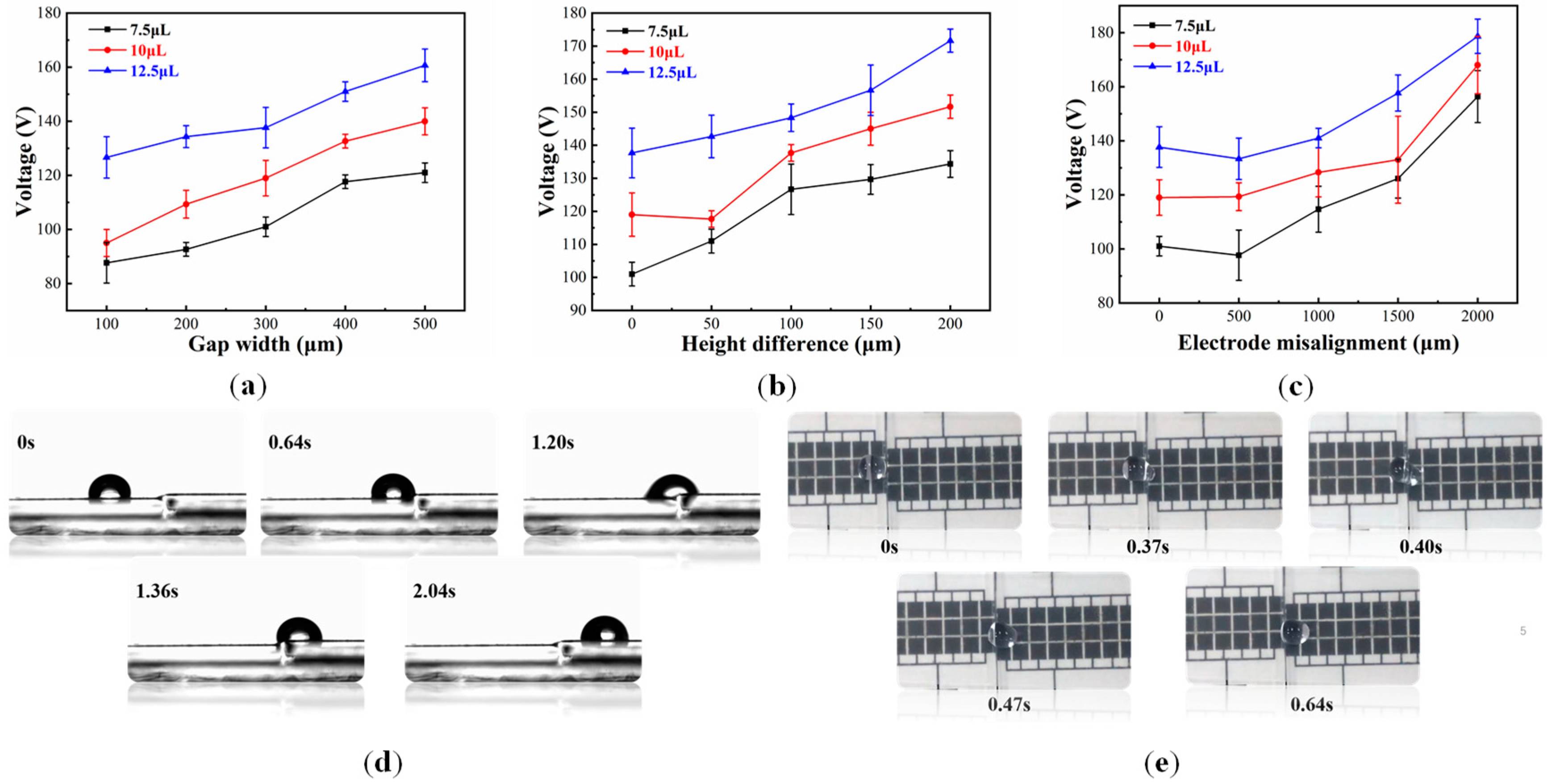
4.2.1. Effect of the Splicing Gap Width
4.2.2. Effect of Height Difference of Splicing Gaps
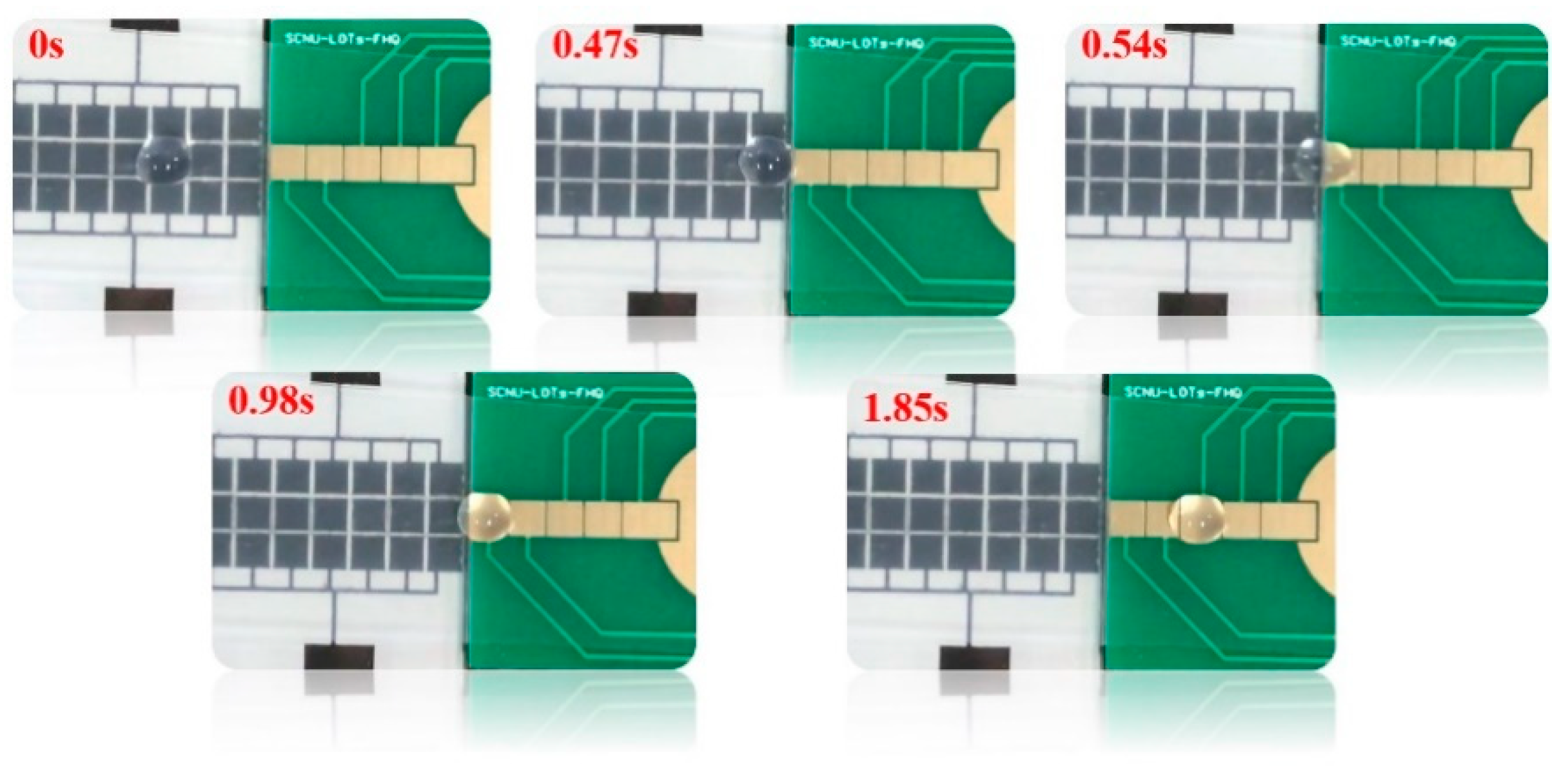
4.2.3. Effect of Electrode Misalignment of Splicing Chips
4.3. Effect of Different Aqueous Solutions of the Droplet
4.4. Across Performance Testing of Different Material Substrates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Manz, A.; Fettinger, J.C.; Verpoorte, E.; Ludi, H.; Widmer, H.M.; Harrison, D.J. Micromachining of monocrystalline silicon and glass for chemical-analysis systems—A look into next century technology or just a fashionable craze. TRAC-Trends Anal. Chem. 1991, 10, 144–149. [Google Scholar] [CrossRef]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Annu. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Ng, A.H.; Fobel, R.; Wheeler, A.R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.H.C.; Li, B.B.; Chamberlain, M.D.; Wheeler, A.R. Digital microfluidic cell culture. Annu. Rev. Biomed. Eng. 2015, 17, 91–112. [Google Scholar] [CrossRef]
- Mori, E.; Oohashi, T.; Imai, H.; Mawatari, K.; Kitamori, T. A sensitive and ripid assay of BNP in patient blood by micro-ELISA. Anal. Methods 2017, 9, 2830–2834. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Loung, J.H.T. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef]
- Fair, R.B. Digital microfluidics: Is a true lab-on-a-chip possible? Microfluid. Nanofluid. 2007, 3, 245–281. [Google Scholar] [CrossRef]
- Cho, S.K.; Moon, H.J.; Kim, C.J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar] [CrossRef]
- Guan, Y.; Tu, J.; Li, B.; Fu, J.; Zhu, M.; Chen, X.; Zhou, C. Stripped electrode based electrowetting-on-dielectric digital microfluidics for precise and controllable parallel microdrop generation. Langmuir 2020, 36, 9540–9550. [Google Scholar] [CrossRef]
- Min, X.; Woo, S.K. Beyond high voltage in the digital microfluidic devices for an integrated portable sensing system. Microfluid. Nanofluid. 2019, 23, 11. [Google Scholar] [CrossRef]
- Nelson, W.C.; Kim, C.J. Droplet actuation by electrowetting-on-dielectric (ewod): A review. J. Adhes. Sci. Technol. 2012, 26, 1747–1771. [Google Scholar] [CrossRef]
- Malic, L.; Brassard, D.; Veres, T.; Tabrizian, M. Integration and detection of biochemical assays in digital microfluidic loc devices. Lab Chip 2010, 10, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Van Grinsven, K.L.; Ashtiani, A.Q.; Jiang, H.R. Flexible electrowetting-on-dielectric microlens array sheet. Micromachines 2020, 10, 464. [Google Scholar] [CrossRef]
- Whitesides, G. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Nguyen, T.; Hilton, J.P.; Lin, Q. Emerging applications of aptamers to micro- and nanoscale biosensing. Microfluid. Nanofluid. 2009, 6, 347. [Google Scholar] [CrossRef]
- Kang, Y.; Li, D. Electrokinetic motion of particles and cells in microchannels. Microfluid. Nanofluid. 2009, 6, 431–460. [Google Scholar] [CrossRef]
- Oh, K.W.; Park, C.S.; Namkoong, K.; Kim, J.; Ock, K.S.; Kim, S.; Kim, Y.A.; Cho, Y.K.; Ko, C. World-to-chip microfluidic interface with built-in valves for multichamber chip-based PCR assays. Lab Chip 2005, 5, 845–850. [Google Scholar] [CrossRef]
- Maeki, M.; Yamazaki, S.; Pawate, A.S.; Ishida, A.; Tani, H.; Yamashita, K.; Sugishima, M.; Watanabe, K.; Tokeshi, M.; Kenis, P.J.A.; et al. A microfluidic-based protein crystallization method in 10 micrometer-sized crystallization space. Crystengcomm 2016, 18, 7722–7727. [Google Scholar] [CrossRef]
- Ribet, F.; De Pietro, L.; Roxhed, N.; Stemme, G. Gas diffusion and evaporation control using EWOD actuation of ionic liquid microdroplets for gas sensing applications. Sens. Actuators B: Chem. 2018, 267, 647–654. [Google Scholar] [CrossRef]
- Srinivasan, V.; Vamsee, K.P.; Richard, B.F. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip 2004, 4, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.H.; Choi, K.; Ng, A.H.; Chamberlain, M.D.; Wheeler, A.R. Electrochemiluminescence on digital microfluidics for microrna analysis. Biosens. Bioelectron. 2016, 77, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.J. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Jain, V.; Raj, P.T.; Deshmukh, R.; Patrikar, R. Design, fabrication and characterization of low cost printed circuit board based ewod device for digital microfluidics applications. Microsyst. Technol. 2015, 23, 389–397. [Google Scholar] [CrossRef]
- Yi, Z.C.; Feng, H.Q.; Sun, Z.Z.; Shui, L.L. Design of an open electrowetting on dielectric device based on printed circuit board by using a parafilm m. Front. Phys. 2020, 8, 193. [Google Scholar] [CrossRef]
- Kim, D.Y.; Steckl, A.J. Electrowetting on paper for electronic paper display. ACS Appl. Mater. Interfaces 2010, 2, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, G.; Haapala, M.; Dixon, C.; Wheeler, A.R.; Sikanen, T.M. A digital-to-channel microfluidic interface via inkjet printing of silver and uv curing of thiol-enes. Adv. Mater. Technol. 2020, 5, 2000451. [Google Scholar] [CrossRef]
- Fan, S.K.; Yang, H.P.; Hsu, W.Y. Droplet-on-a-wristband: Chip-to-chip digital microfluidic interfaces between replaceable and flexible electrowetting modules. Lab Chip 2011, 11, 343–347. [Google Scholar] [CrossRef]
- Moazami, E.; Perry, J.M.; Soffer, G.; Husser, M.C.; Shih, S.C.C. Integration of world-to-chip interfaces with digital microfluidics for bacterial transformation and enzymatic assays. Anal. Chem. 2019, 91, 5159–5168. [Google Scholar] [CrossRef]
- Watson, M.W.L.; Jebrail, M.J.; Wheeler, A.R. Multilayer hybrid microfluidics: A digital-to-channel interface for sample processing and separations. Anal. Chem. 2010, 82, 6680–6686. [Google Scholar] [CrossRef] [PubMed]
- Cooney, C.G.; Chen, C.Y.; Emering, M.R.; Nadim, A.; Sterling, J.D. Electrowetting droplet microfluidics on a single planar surface. Microfluid. Nanofluid. 2006, 2, 435–446. [Google Scholar] [CrossRef]
- Sun, D.; Bohringer, K.F. EWOD-aided droplet transport on texture ratchets. Appl. Phys. Lett. 2020, 116, 9. [Google Scholar] [CrossRef]
- Renaudot, R.; Daunay, B.; Kumemura, K.; Agache, V.; Jalabert, L.; Collard, D.; Fujita, H. Optimized micro devices for liquid-dielectrophoresis (ldep) actuation of conductive solutions. Sens. Actuators B Chem. 2013, 177, 620–626. [Google Scholar] [CrossRef]
- Collins, D.J.; Alan, T.; Helmerson, K.; Neild, A. Surface acoustic waves for on-demand production of picoliter droplets and particle encapsulation. Lab Chip 2013, 13, 3225–3231. [Google Scholar] [CrossRef]
- Jiang, D.Y.; Park, S.Y. Light-driven 3d droplet manipulation on flexible optoelectrowetting devices fabricated by a simple spin-coating method. Lab Chip 2016, 16, 1831–1839. [Google Scholar] [CrossRef]
- Joshi, K.; Velasco, V.; Esfandyarpour, R. A Low-Cost, Disposable and Portable Inkjet-Printed Biochip for the Developing World. Sensors 2020, 20, 3593. [Google Scholar] [CrossRef]
- Mugele, F.; Baret, J.C. Electrowetting: From basics to applications. J. Phys. Condens. Matter. 2005, 17, R705–R774. [Google Scholar] [CrossRef]
- Goog, J.; Kim, C.J. Direct-referencing two-dimensional-array digital microfluidics using multilayer printed circuit board. J. Microelectromech. Syst. 2008, 17, 257–264. [Google Scholar] [CrossRef]
- Jain, V.D.; Devarasetty, V.; Patrikar, R. Effect of electrode geometry on droplet velocity in open ewod based device for digital microfluidics applications. J. Electrost. 2017, 87, 11–18. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Jin, M.L.; He, T.; Zhou, G.F.; Shui, L.L. Two-layer microstructures fabricated by one-step anisotropic wet etching of si in koh solution. Micromachines 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Mendel, N.; Dey, R.; Baratian, D.; Mugele, F. Contact angle hysteresis and oil film lubrication in electrowetting with two immiscible liquids. Appl. Phys. Lett. 2018, 112, 203703. [Google Scholar] [CrossRef]
- McHale, G.; Bethany, V.O.; Wells, G.G.; Aguilar, R.L. Apparent contact angles on lubricant-impregnated surfaces/slips: From superhydrophobicity to electrowetting. Langmuir 2019, 35, 4197–4204. [Google Scholar] [CrossRef] [PubMed]
- He, X.D.; Qiang, W.B.; Du, C.; Shao, Q.F.; Zhang, X.P.; Deng, Y.Q. Modification of lubricant infused porous surface for low-voltage reversible electrowetting. J. Mater. Chem. A 2017, 5, 19159–19167. [Google Scholar] [CrossRef]
- He, X.D.; Zhang, J.; Zhang, X.; Deng, Y.Q. Droplet manipulation with polarity-dependent low-voltage electrowetting on an open slippery liquid infused porous surface. Soft Matter. 2019, 15, 5211–5219. [Google Scholar] [CrossRef]
- Cao, J.P.; An, Q.; Liu, Z.P.; Jin, M.L.; Yan, Z.B.; Lin, W.J.; Chen, L.; Li, P.F.; Wang, X.; Zhou, G.F.; et al. Electrowetting on liquid-infused membrane for flexible and reliable digital droplet manipulation and application. Sens. Actuators B Chem. 2019, 291, 470–477. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Yi, Z.; Yang, R.; Qin, X.; Shen, S.; Zeng, W.; Shui, L.; Zhou, G.; Zhang, C. Designing Splicing Digital Microfluidics Chips Based on Polytetrafluoroethylene Membrane. Micromachines 2020, 11, 1067. https://doi.org/10.3390/mi11121067
Feng H, Yi Z, Yang R, Qin X, Shen S, Zeng W, Shui L, Zhou G, Zhang C. Designing Splicing Digital Microfluidics Chips Based on Polytetrafluoroethylene Membrane. Micromachines. 2020; 11(12):1067. https://doi.org/10.3390/mi11121067
Chicago/Turabian StyleFeng, Haoqiang, Zichuan Yi, Ruizhi Yang, Xiaofeng Qin, Shitao Shen, Wenjun Zeng, Lingling Shui, Guofu Zhou, and Chongfu Zhang. 2020. "Designing Splicing Digital Microfluidics Chips Based on Polytetrafluoroethylene Membrane" Micromachines 11, no. 12: 1067. https://doi.org/10.3390/mi11121067
APA StyleFeng, H., Yi, Z., Yang, R., Qin, X., Shen, S., Zeng, W., Shui, L., Zhou, G., & Zhang, C. (2020). Designing Splicing Digital Microfluidics Chips Based on Polytetrafluoroethylene Membrane. Micromachines, 11(12), 1067. https://doi.org/10.3390/mi11121067





