Fabrication of Infrared-Compatible Nanofluidic Devices for Plasmon-Enhanced Infrared Absorption Spectroscopy
Abstract
1. Introduction
2. Theory of Metal-Insulator-Metal (MIM) Perfect Absorber Metamaterial Structures for Plasmon-Enhanced Infrared Absorption Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Methods
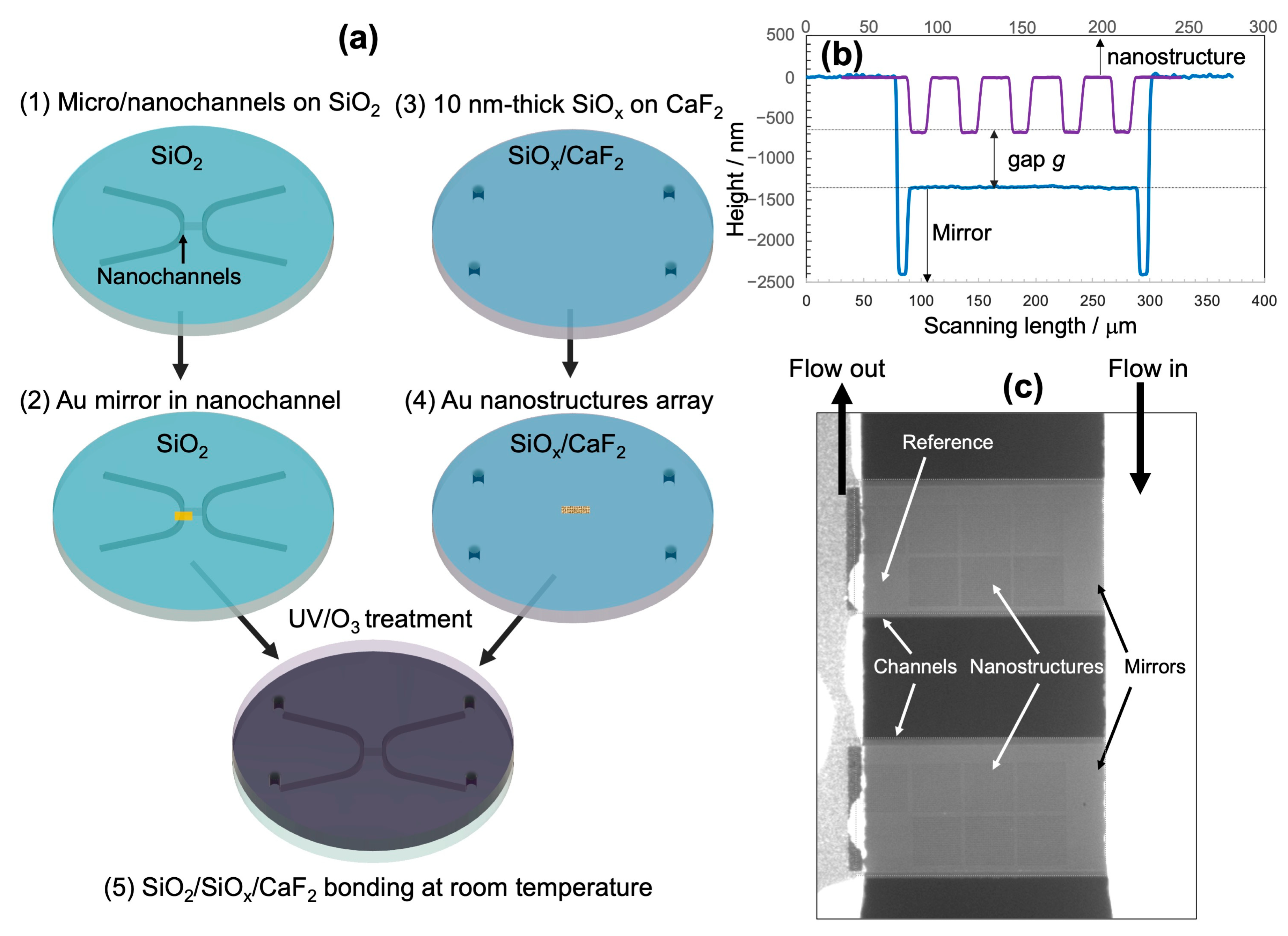
3.2.1. Fabrication of Fluidic Channels on the Bottom of the SiO2 Substrate
3.2.2. Fabrication of Nanostructures on Top of the CaF2 Substrate
3.2.3. Bonding of the Device
3.3. IR Reflectance Measurement
3.4. Numerical Calculation
3.5. Fluidic Operation
4. Results
4.1. Leakage Test
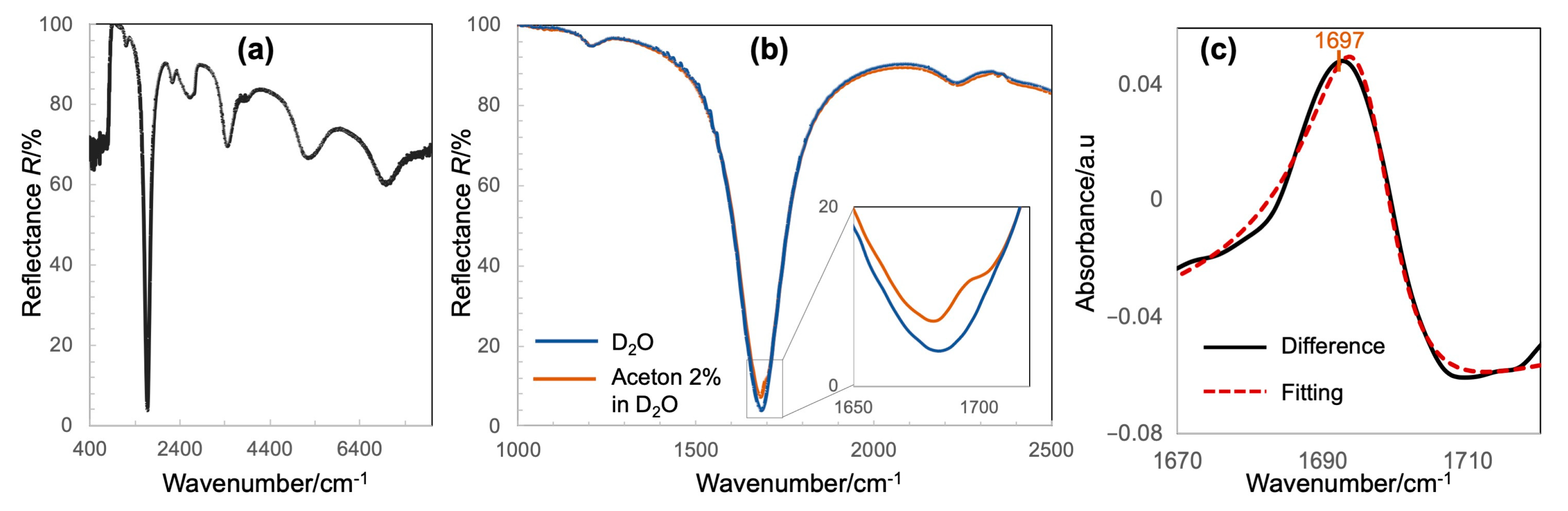
4.2. IR-Compatible Nanofluidic Device Integrated with MIM Metamaterials and Proof-of-Concept of Detection of Molecules
4.3. A Proof-of-Concept of Multi-Band Metal-Insulator-Metal (MIM) Structures towards Multispectral Plasmon-Enhanced Infrared Absorption Spectroscopy
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elvira, K.S.; Solvas, X.C.i.; Wootton, R.C.R.; de Mello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Tsukahara, T.; Mawatari, K.; Kitamori, T. Integrated extended-nano chemical systems on a chip. Chem. Soc. Rev. 2010, 39, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Monat, C.; Domachuk, P.; Eggleton, B.J. Integrated optofluidics: A new river of light. Nat. Photon. 2007, 1, 106–114. [Google Scholar] [CrossRef]
- Aroca, R. Surface Enhanced Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; Ihtesham, R. Fourier transform Infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta. Bioenergy 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Diem, M.; Romeo, M.; Boydston-White, S.; Miljkovic, M.; Matthaus, C. A decade of vibrational micro-spectroscopy of human cells and tissue. Analyst 2004, 129, 880–885. [Google Scholar] [CrossRef]
- Loutherback, K.; Birarda, G.; Chen, L.; Holman, H.-Y.N. Microfluidic approaches to synchrotron radiation-based Fourier transform infrared (SR-FTIR) spectral microscopy of living biosystems. Protein Pept. Lett. 2016, 23, 273–282. [Google Scholar] [CrossRef]
- Vaccari, L.; Birarda, G.; Businaro, L.; Pacor, S.; Grenci, G. Infrared microspectroscopy of live cells in microfluidic devices (MD-IRMS): Toward a powerful label-free cell-based assay. Anal. Chem. 2012, 84, 4768–4775. [Google Scholar] [CrossRef]
- Andrew Chan, K.L.; Gulati, S.; Edel, J.B.; de Mello, A.J.; Kazarian, S.G. Chemical imaging of microfluidic flows using ATR-FTIR spectroscopy. Lab Chip 2009, 9, 2909–2913. [Google Scholar] [CrossRef]
- Adato, R.; Yanik, A.A.; Amsden, J.J.; Kaplan, D.L.; Omenetto, F.J.; Hong, M.K.; Erramilli, S.; Altug, H. Ultra-sensitive vibrational spectroscopy of protein monolayers with plasmonic nanoantenna arrays. Proc. Natl. Acad. Sci. USA 2009, 106, 19227–19232. [Google Scholar] [CrossRef]
- Bomers, M.; Charlot, B.; Barho, F.; Chanuel, A.; Mezy, A.; Cerutti, L.; Gonzalez-Posada, F.; Taliercio, T. Microfluidic surface-enhanced infrared spectroscopy with semiconductor plasmonics for the fingerprint region. React. Chem. Eng. 2020, 5, 124–135. [Google Scholar] [CrossRef]
- Adato, R.; Altug, H. In-situ ultra-sensitive infrared absorption spectroscopy of biomolecule interactions in real time with plasmonic nanoantennas. Nat. Commun. 2013, 4, 2154. [Google Scholar] [CrossRef]
- Rodrigo, D.; Tittl, A.; Ait-Bouziad, N.; John-Herpin, A.; Limaj, O.; Kelly, C.; Yoo, D.; Wittenberg, N.J.; Oh, S.; Lashuel, H.A.; et al. Resolving molecule-specific information in dynamic lipid membrane processes with multi-resonant infrared metasurfaces. Nat. Commun. 2018, 9, 2160. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.T.; Morikawa, K.; Shimizu, H. Advances in label-free detections for nanofluidic analytical devices. Micromachines 2020, 11, 885. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photon. 2011, 5, 591–597. [Google Scholar] [CrossRef]
- Le, H.H.T.; Tanaka, T. Plasmonics-nanofluidics hydrid metamaterial: An ultrasensitive platform for infrared absorption spectroscopy and quantitative measurement of molecules. ACS Nano 2017, 11, 9780–9788. [Google Scholar] [CrossRef]
- Le, H.H.T.; Morita, A.; Mawatari, K.; Kitamori, T.; Tanaka, T. Metamaterials-Enhanced Infrared Spectroscopic Study of Nanoconfined Molecules by Plasmonics-Nanofluidics Hydrid Device. ACS Photonics 2018, 5, 3179–3188. [Google Scholar] [CrossRef]
- Le, H.H.T.; Morita, A.; Tanaka, T. Refractive index of nanoconfined water reveals its anomalous physical properties. Nanoscale Horiz. 2020, 5, 1016–1024. [Google Scholar] [CrossRef]
- Chan, K.L.A.; Niu, X.; de Mello, A.J.; Kazarian, S.G. Rapid prototyping of microfluidic devices for integrating with FT-IR spectroscopic imaging. Lab Chip 2010, 10, 2170–2174. [Google Scholar] [CrossRef]
- Kulka, S.; Kaun, N.; Baena, J.R.; Frank, J.; Svasek, P.; Moss, D.; Vellekoop, M.J.; Lendl, B. Mid-IR synchrotron radiation for molecular specific detection in microchip-based analysis systems. Anal. Bioanal. Chem. 2004, 378, 1735–1740. [Google Scholar] [CrossRef]
- Polshin, E.; Verbruggen, B.; Witters, D.; Sels, B.; DeVos, D.; Nicolaï, B.; Lammertyn, J. Integration of microfluidics and FT-IR microscopy for label-free study of enzyme kinetics. Sens. Actuators B 2014, 196, 175–182. [Google Scholar] [CrossRef]
- Perro, A.; Lebourdon, G.; Henry, S.; Lecomte, S.; Servanta, L.; Marre, S. Combining microfluidics and FT-IR spectroscopy: Towards spatially resolved information on chemical processes. React. Chem. Eng. 2016, 1, 577–594. [Google Scholar] [CrossRef]
- Lehmkuhl, B.; Noblitt, S.D.; Krummel, A.T.; Henry, C.S. Fabrication of IR-transparent microfluidic devices by anisotropic etching of channels in CaF2. Lab Chip 2015, 15, 4364–4368. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Tanaka, T. Metamaterial absorbers for infrared detection of molecular self-assembled monolayers. Sci. Rep. 2015, 5, 12570. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Adato, R.; Altug, H. Dual-band perfect absorber for multispectral plasmon-enhanced infrared spectroscopy. ACS Nano 2012, 9, 7998–8006. [Google Scholar] [CrossRef] [PubMed]
- Cerjan, B.; Yang, X.; Nordlander, P.; Halas, N.J. Asymmetric aluminum antennas for self-calibrating surface-enhanced infrared absorption spectroscopy. ACS Photonics 2016, 3, 354–360. [Google Scholar] [CrossRef]
- Max, J.-J.; Chapados, C. Infrared spectroscopy of acetone–water liquid mixtures. I. Factor analysis. J. Chem. Phys 2003, 113, 5632–5643. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.H.H.; Matsushita, T.; Ohta, R.; Shimoda, Y.; Matsui, H.; Kitamori, T. Fabrication of Infrared-Compatible Nanofluidic Devices for Plasmon-Enhanced Infrared Absorption Spectroscopy. Micromachines 2020, 11, 1062. https://doi.org/10.3390/mi11121062
Le THH, Matsushita T, Ohta R, Shimoda Y, Matsui H, Kitamori T. Fabrication of Infrared-Compatible Nanofluidic Devices for Plasmon-Enhanced Infrared Absorption Spectroscopy. Micromachines. 2020; 11(12):1062. https://doi.org/10.3390/mi11121062
Chicago/Turabian StyleLe, Thu Hac Huong, Takumi Matsushita, Ryoichi Ohta, Yuta Shimoda, Hiroaki Matsui, and Takehiko Kitamori. 2020. "Fabrication of Infrared-Compatible Nanofluidic Devices for Plasmon-Enhanced Infrared Absorption Spectroscopy" Micromachines 11, no. 12: 1062. https://doi.org/10.3390/mi11121062
APA StyleLe, T. H. H., Matsushita, T., Ohta, R., Shimoda, Y., Matsui, H., & Kitamori, T. (2020). Fabrication of Infrared-Compatible Nanofluidic Devices for Plasmon-Enhanced Infrared Absorption Spectroscopy. Micromachines, 11(12), 1062. https://doi.org/10.3390/mi11121062




