Numerical Investigation of Nanostructure Orientation on Electroosmotic Flow
Abstract
:1. Introduction
2. Numerical Simulations
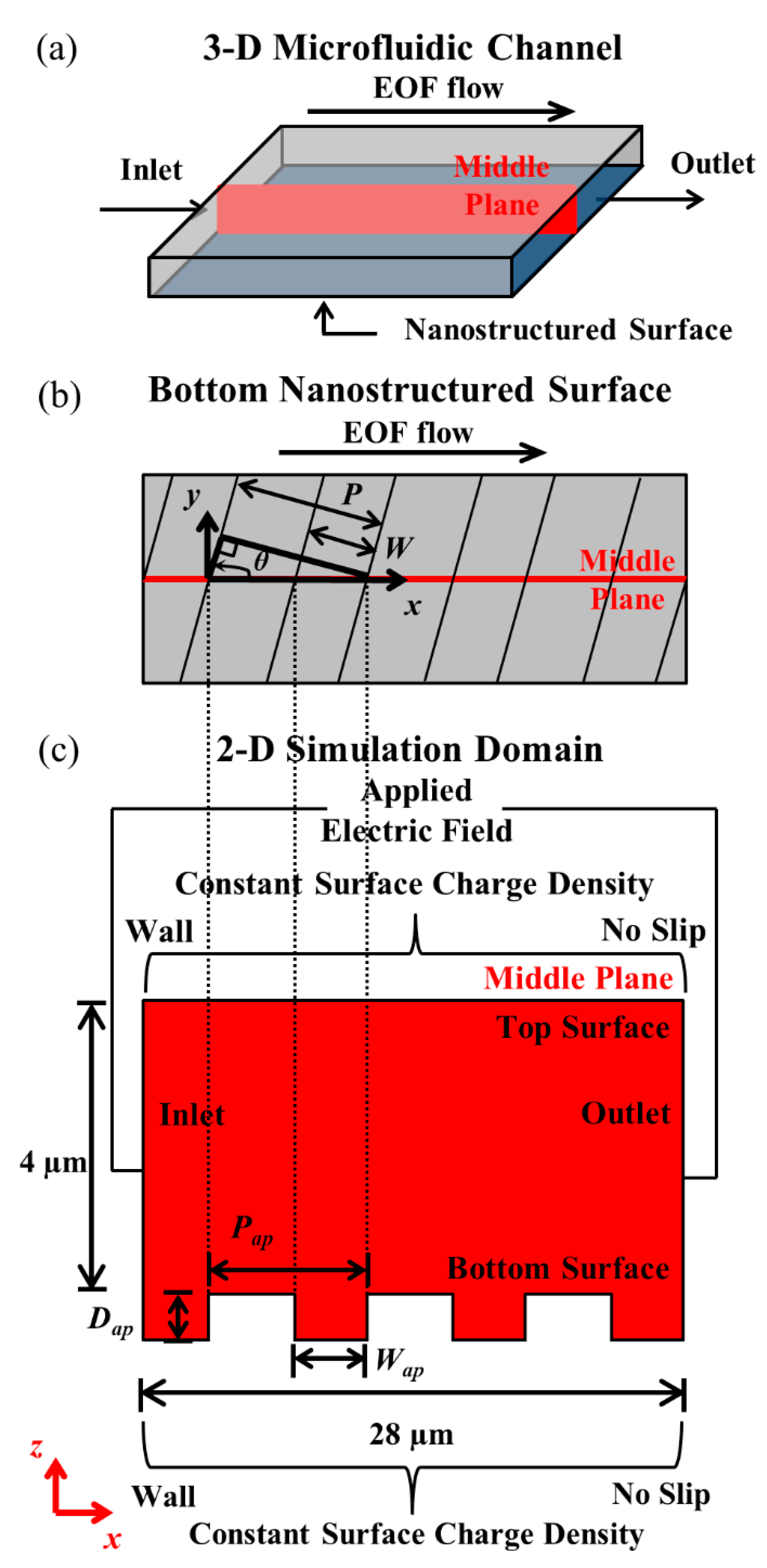
2.1. Simulation Domain
2.2. Boundary Conditions
2.3. Governing Equations for Poisson–Nernst–Planck (PNP) Model
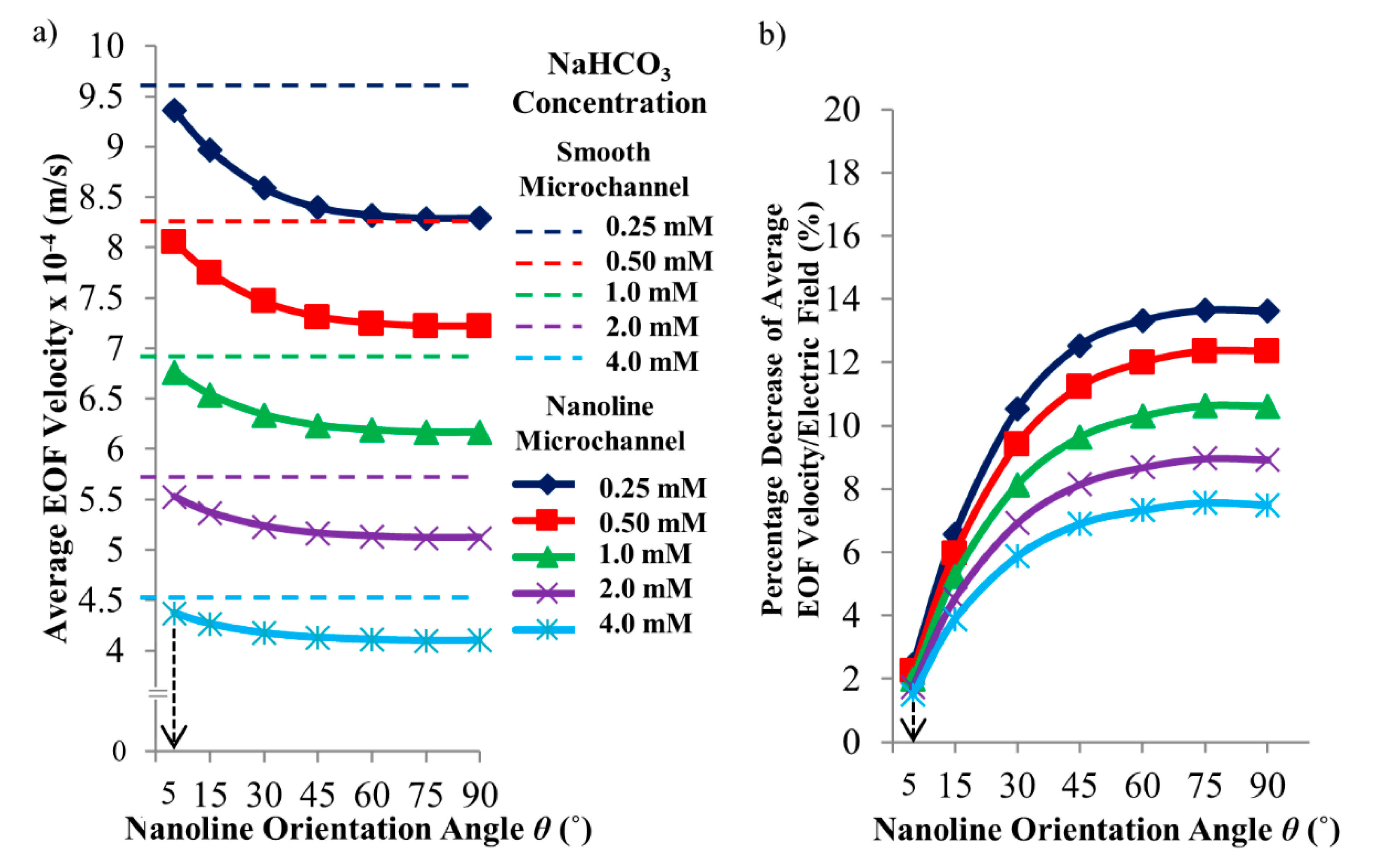
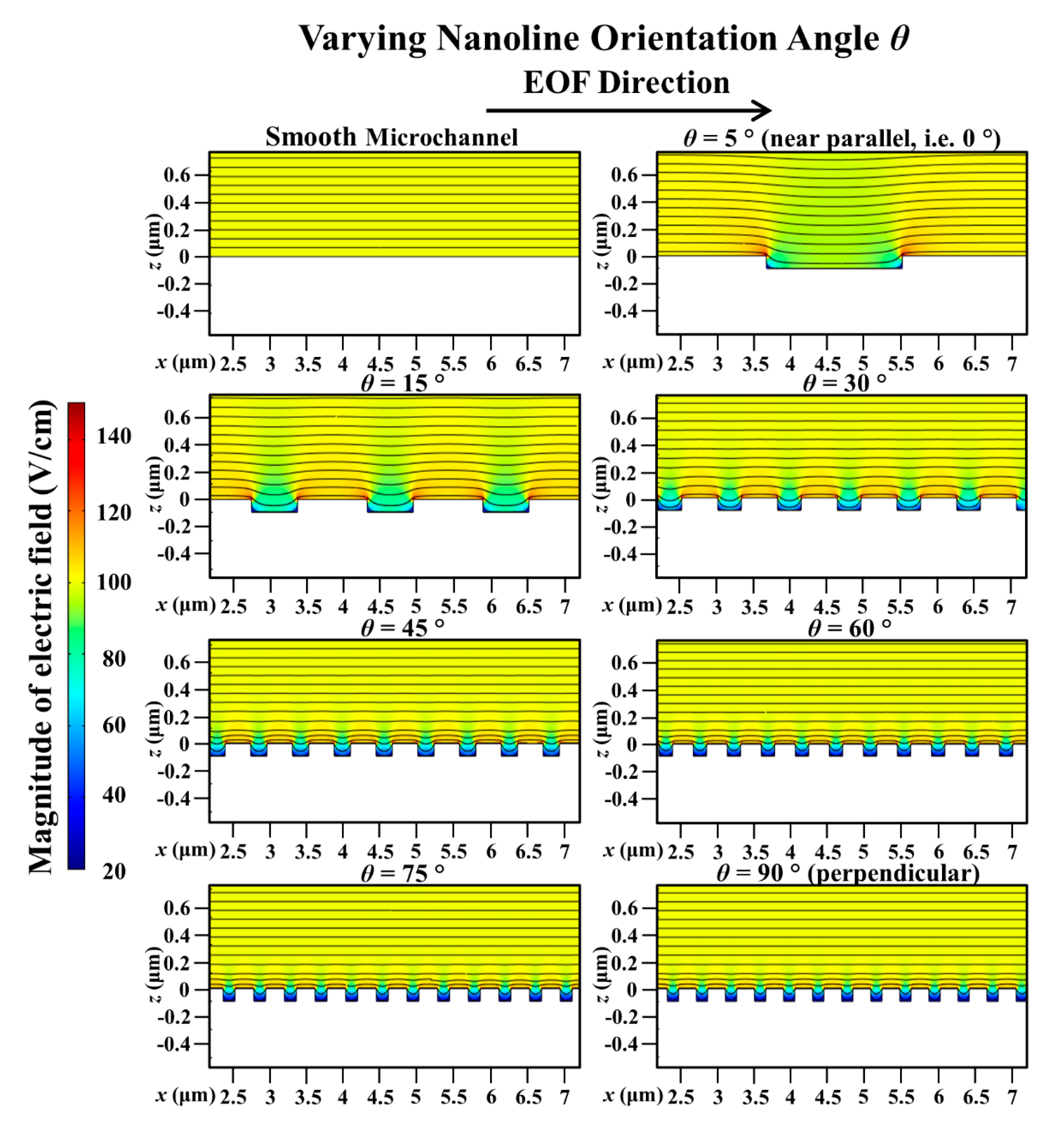
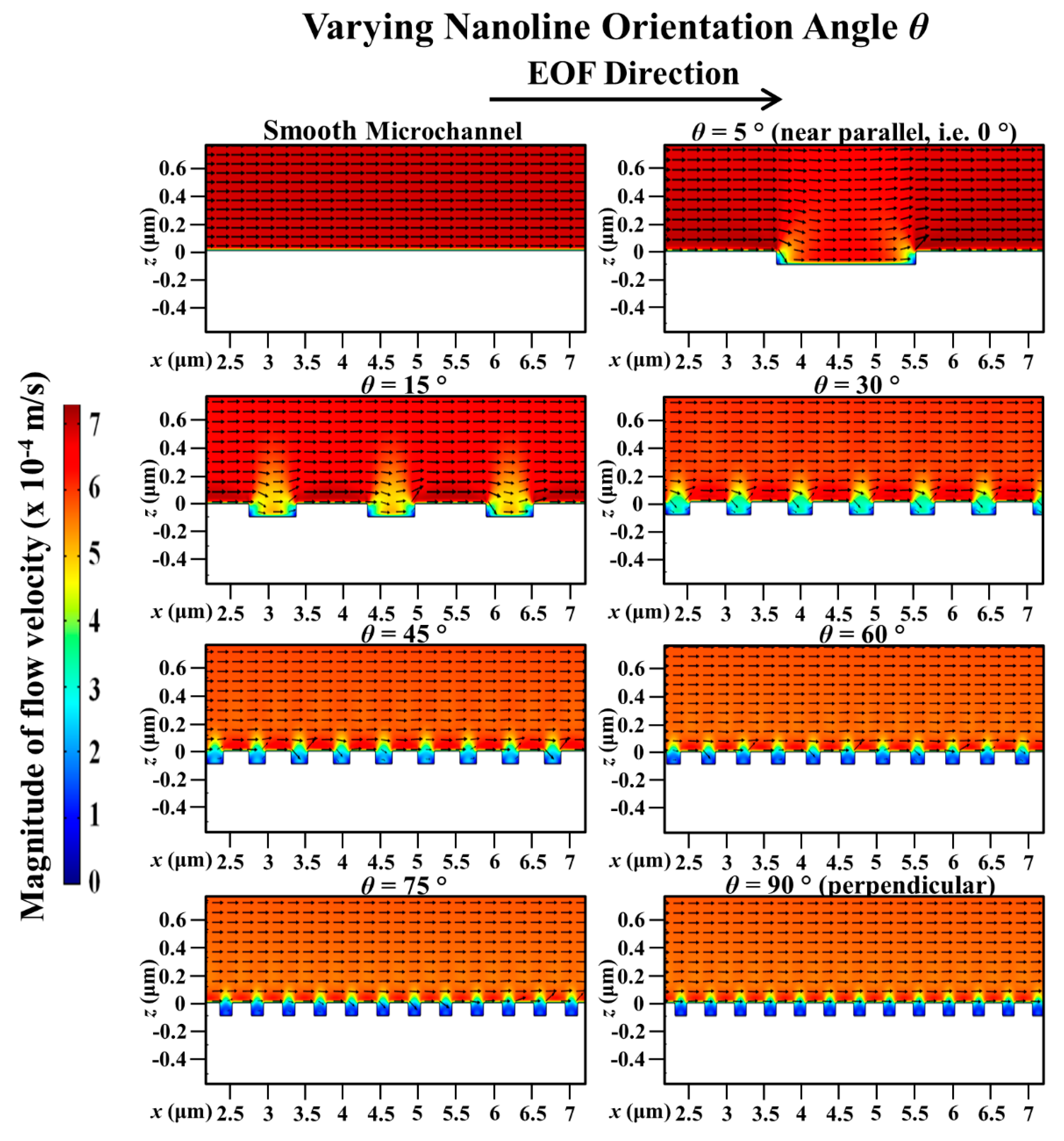
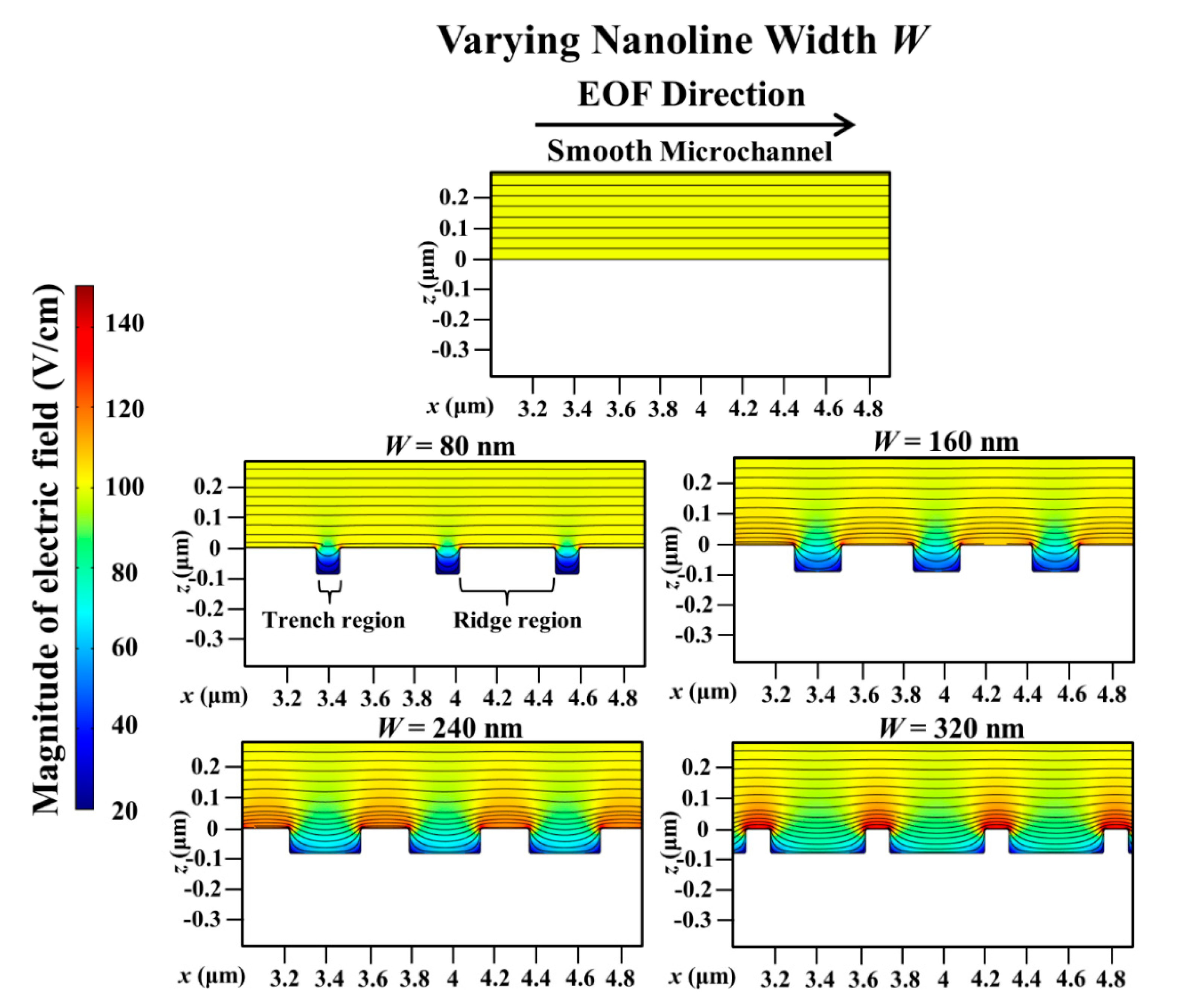
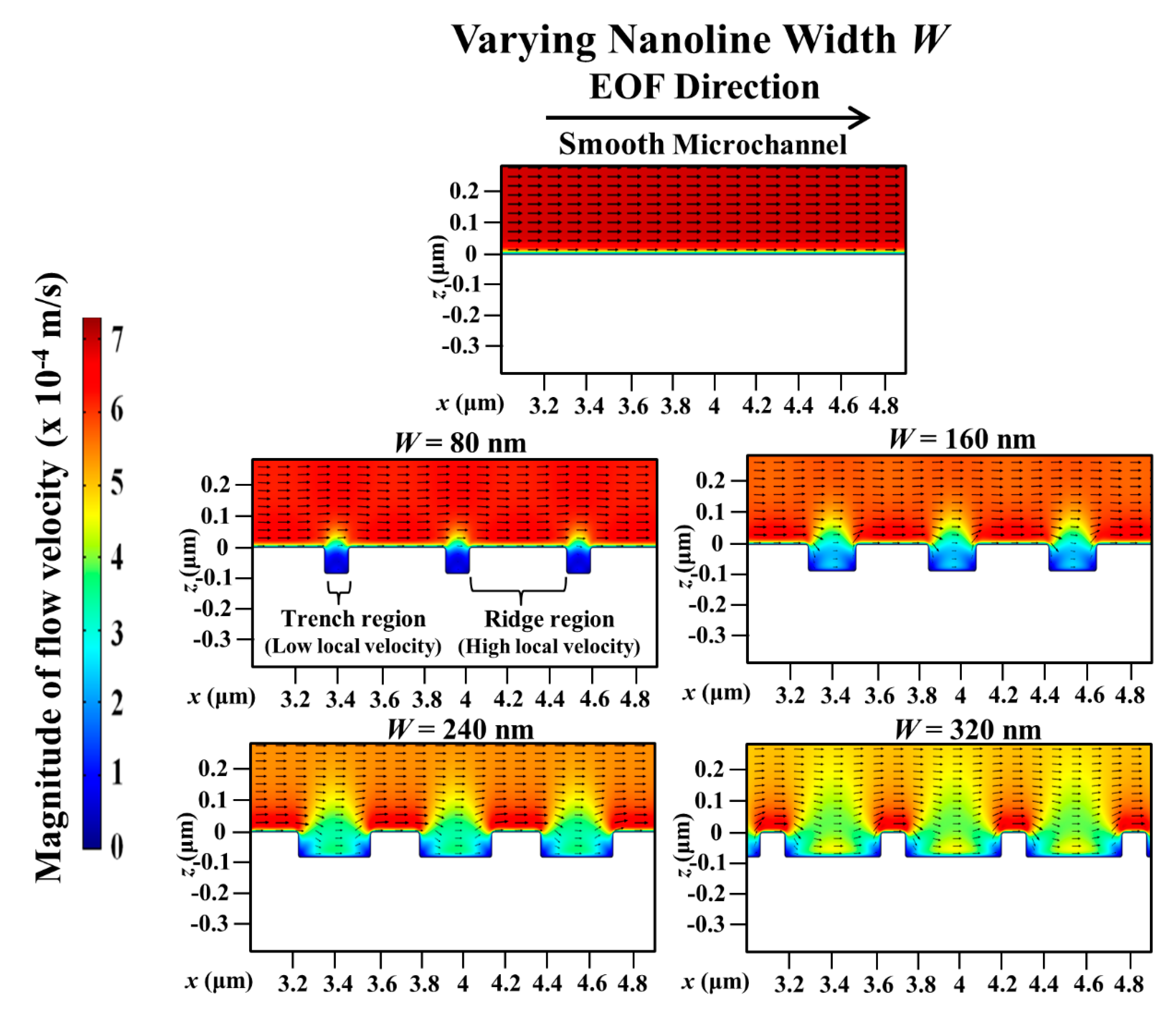
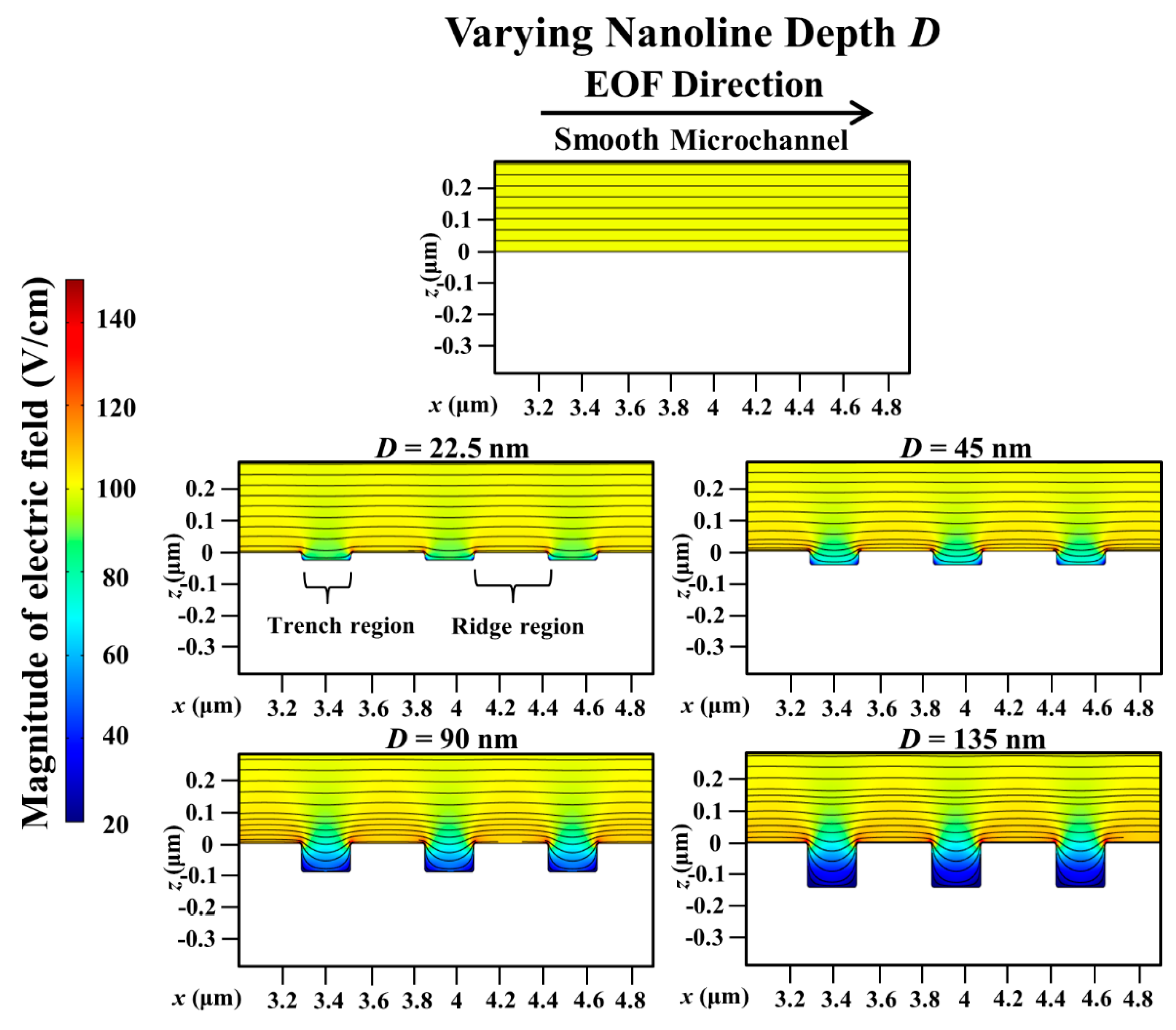
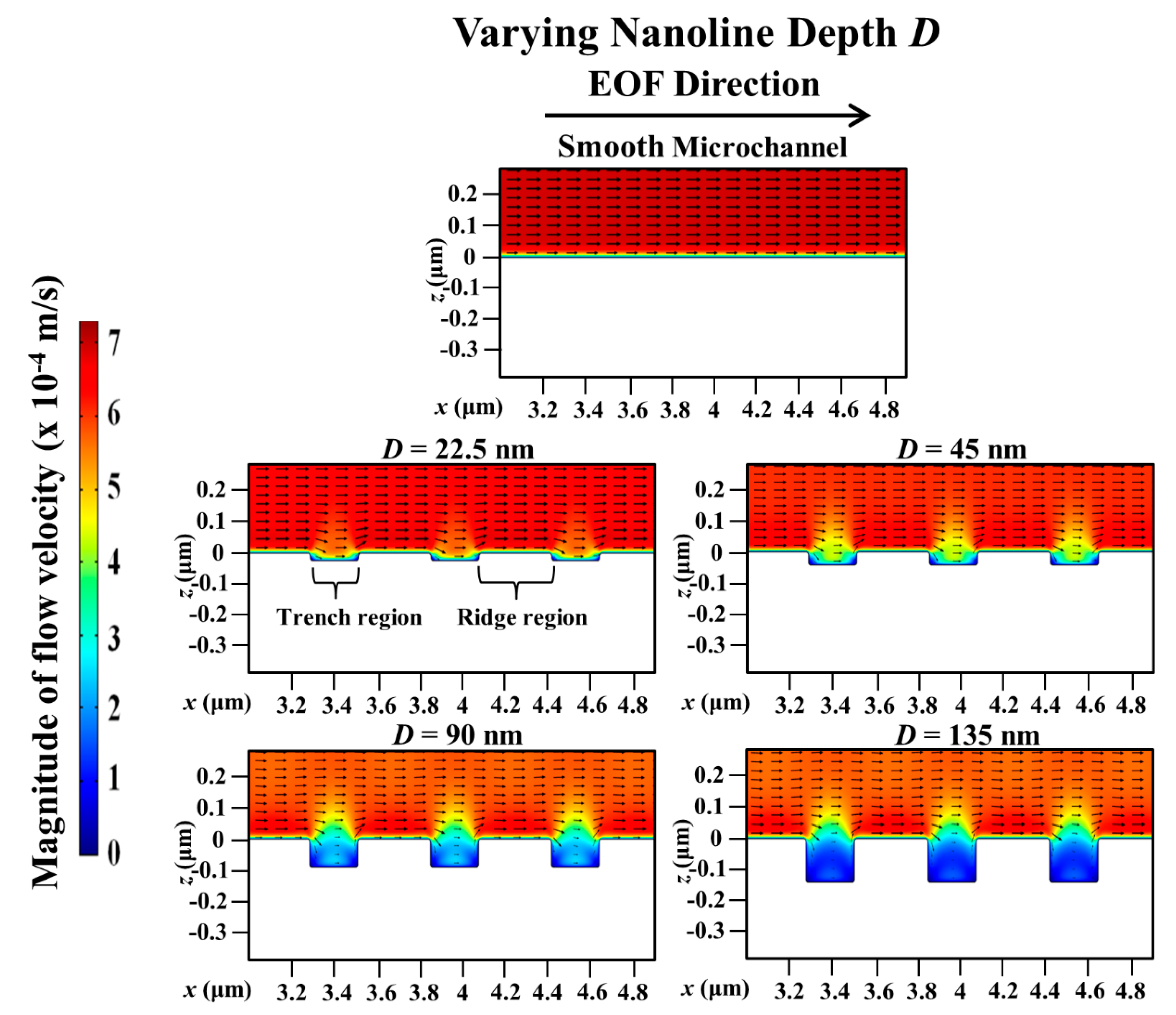
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fu, R.; Wen, D.; Xia, X.; Zhang, W.; Gu, Y. Electrokinetic remediation of chromium (Cr)-contaminated soil with citric acid (CA) and polyaspartic acid (PASP) as electrolytes. Chem. Eng. J. 2017, 316, 601–608. [Google Scholar]
- Kijsamanmith, K.; Vongsavan, N.; Matthews, B. Electroosmosis in human dentine in vitro. Arch. Oral Biol. 2020, 119, 104885. [Google Scholar]
- Borowsky, J.F.; Giordano, B.C.; Lu, Q.; Terray, A.; Collins, G.E. Electroosmotic flow-based pump for liquid chromatography on a planar microchip. Anal. Chem. 2008, 80, 8287–8292. [Google Scholar]
- Berry, J.D.; Davidson, M.R.; Harvie, D.J. A multiphase electrokinetic flow model for electrolytes with liquid/liquid interfaces. J. Comput. Phys. 2013, 251, 209–222. [Google Scholar]
- Ganchenko, G.S.; Demekhin, E.A.; Mayur, M.; Amiroudine, S. Electrokinetic instability of liquid micro-and nanofilms with a mobile charge. Phys. Fluids 2015, 27, 062002. [Google Scholar]
- Weng, X.; Jiang, H.; Chon, C.H.; Chen, S.; Cao, H.; Li, D. An RNA–DNA hybridization assay chip with electrokinetically controlled oil droplet valves for sequential microfluidic operations. J. Biotechnol. 2011, 155, 330–337. [Google Scholar]
- Weng, X.; Jiang, H.; Li, D. Electrokinetically-controlled RNA-DNA hybridization assay for foodborne pathogens. Microchim. Acta 2012, 178, 381–387. [Google Scholar]
- Miskon, A.; Ibrahim, N.; Tawil, S.N.M. A model of Defence Mechanical Drug Delivery System (DmechD) and the drug release efficacy. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 8–10 December 2014; pp. 22–26. [Google Scholar]
- Villarruel Mendoza, L.A.; Scilletta, N.A.; Bellino, M.G.; Desimone, M.F.; Catalano, P.N. Recent advances in micro-electro-mechanical devices for controlled drug release applications. Front. Bioeng. Biotechnol. 2020, 8, 827. [Google Scholar]
- Wu, Z.; Sweeney, L.B.; Ayoob, J.C.; Chak, K.; Andreone, B.J.; Ohyama, T.; Kerr, R.; Luo, L.; Zlatic, M.; Kolodkin, A.L. A combinatorial semaphorin code instructs the initial steps of sensory circuit assembly in the Drosophila CNS. Neuron 2011, 70, 281–298. [Google Scholar]
- Kashid, M.N.; Harshe, Y.M.; Agar, D.W. Liquid−liquid slug flow in a capillary: An alternative to suspended drop or film contactors. Ind. Eng. Chem. Res. 2007, 46, 8420–8430. [Google Scholar]
- Lim, A.E.; Lim, C.Y.; Lam, Y.C.; Lim, Y.H. Effect of microchannel junction angle on two-phase liquid-gas Taylor flow. Chem. Eng. Sci. 2019, 202, 417–428. [Google Scholar]
- Bolivar, J.M.; Nidetzky, B. Multiphase biotransformations in microstructured reactors: Opportunities for biocatalytic process intensification and smart flow processing. Green Process. Synth. 2013, 2, 541–559. [Google Scholar]
- Dahl, K.; Schou, M.; Ulin, J.; Sjoberg, C.-O.; Farde, L.; Halldin, C. 11C-carbonylation reactions using gas-liquid segmented microfluidics. RSC Adv. 2015, 5, 88886–88889. [Google Scholar]
- Li, J.; Zrazhevskiy, P.; Gao, X. Eliminating size-associated diffusion constraints for rapid on-surface bioassays with nanoparticle probes. Small 2016, 12, 1035–1043. [Google Scholar]
- Maturos, T.; Pogfay, T.; Rodaree, K.; Chaotheing, S.; Jomphoak, A.; Wisitsoraat, A.; Suwanakitti, N.; Wongsombat, C.; Jaruwongrungsee, K.; Shaw, P.; et al. Enhancement of DNA hybridization under acoustic streaming with three-piezoelectric-transducer system. Lab Chip 2012, 12, 133–138. [Google Scholar]
- Xiong, Q.; Lim, A.E.; Lim, Y.; Lam, Y.C.; Duan, H. Dynamic magnetic nanomixers for improved microarray assays by eliminating diffusion limitation. Adv. Healthc. Mater. 2019, 8, 1801022. [Google Scholar]
- Zhang, K.; Ren, Y.; Tao, Y.; Deng, X.; Liu, W.; Jiang, T.; Jiang, H. Efficient particle and droplet manipulation utilizing the combined thermal buoyancy convection and temperature-enhanced rotating induced-charge electroosmotic flow. Anal. Chim. Acta 2020, 1096, 108–119. [Google Scholar]
- Horng, T.L.; Lin, T.C.; Liu, C.; Eisenberg, B. PNP equations with steric effects: A model of ion flow through channels. J. Phys. Chem. B 2012, 116, 11422–11441. [Google Scholar]
- Caplan, D.A.; Subbotina, J.O.; Noskov, S.Y. Molecular mechanism of ion-ion and ion-substrate coupling in the Na+-dependent leucine transporter LeuT. Biophys. J. 2008, 95, 4613–4621. [Google Scholar]
- Hsu, W.L.; Harvie, D.J.; Davidson, M.R.; Dunstan, D.E.; Hwang, J.; Daiguji, H. Viscoelectric effects in nanochannel electrokinetics. J. Phys. Chem. C 2017, 121, 20517–20523. [Google Scholar]
- Yu, F.; Wang, L.; Wang, Y.; Shen, X.; Cheng, Y.; Ma, J. Faradaic reactions in capacitive deionization for desalination and ion separation. J. Mater. Chem. A 2019, 7, 15999–16027. [Google Scholar]
- Lim, C.Y.; Lim, A.E.; Lam, Y.C. Ionic origin of electro-osmotic flow hysteresis. Sci. Rep. 2016, 6, 22329. [Google Scholar]
- Lim, C.Y.; Lim, A.E.; Lam, Y.C. pH change in electroosmotic flow hysteresis. Anal. Chem. 2017, 89, 9394–9399. [Google Scholar]
- Lim, A.E.; Lim, C.Y.; Lam, Y.C. Electroosmotic flow hysteresis for dissimilar ionic solutions. Biomicrofluidics 2015, 9, 024113. [Google Scholar]
- Lim, A.E.; Lim, C.Y.; Lam, Y.C. Electroosmotic flow hysteresis for dissimilar anionic solutions. Anal. Chem. 2016, 88, 8064–8073. [Google Scholar]
- Kouravand, S.; Imani, B.M. Developing a surface roughness model for end-milling of micro-channel. Mach. Sci. Technol. 2014, 18, 299–321. [Google Scholar]
- Snyder, J.C.; Stimpson, C.K.; Thole, K.A.; Mongillo, D.J. Build direction effects on microchannel tolerance and surface roughness. J. Mech. Des. 2015, 137, 111411. [Google Scholar]
- Hu, Y.; Werner, C.; Li, D. Electrokinetic transport through rough microchannels. Anal. Chem. 2003, 75, 5747–5758. [Google Scholar]
- Hu, Y.; Werner, C.; Li, D. Influence of the three-dimensional heterogeneous roughness on electrokinetic transport in microchannels. J. Colloid Interface Sci. 2004, 280, 527–536. [Google Scholar]
- Hu, Y.; Xuan, X.; Werner, C.; Li, D. Electroosmotic flow in microchannels with prismatic elements. Microfluid. Nanofluidics 2007, 3, 151–160. [Google Scholar]
- Kang, S.; Suh, Y.K. Numerical analysis on electroosmotic flows in a microchannel with rectangle-waved surface roughness using the Poisson–Nernst–Planck model. Microfluid. Nanofluidics 2009, 6, 461–477. [Google Scholar]
- Masilamani, K.; Ganguly, S.; Feichtinger, C.; Bartuschat, D.; Rüde, U. Effects of surface roughness and electrokinetic heterogeneity on electroosmotic flow in microchannel. Fluid Dyn. Res. 2015, 47, 035505. [Google Scholar]
- Kim, D.; Darve, E. Molecular dynamics simulation of electro-osmotic flows in rough wall nanochannels. Phys. Rev. E 2006, 73, 051203. [Google Scholar]
- Messinger, R.J.; Squires, T.M. Suppression of electro-osmotic flow by surface roughness. Phys. Rev. Lett. 2010, 105, 144503. [Google Scholar]
- Kaji, N.; Tezuka, Y.; Takamura, Y.; Ueda, M.; Nishimoto, T.; Nakanishi, H.; Horiike, Y.; Baba, Y. Separation of long DNA molecules by quartz nanopillar chips under a direct current electric field. Anal. Chem. 2004, 76, 15–22. [Google Scholar]
- Huang, K.D.; Yang, R.J. Electrokinetic behaviour of overlapped electric double layers in nanofluidic channels. Nanotechnology 2007, 18, 115701. [Google Scholar]
- Wang, M.; Kang, Q.; Ben-Naim, E. Modeling of electrokinetic transport in silica nanofluidic channels. Anal. Chim. Acta 2010, 664, 158–164. [Google Scholar]
- Ma, Y.; Yeh, L.H.; Lin, C.Y.; Mei, L.; Qian, S. pH-regulated ionic conductance in a nanochannel with overlapped electric double layers. Anal. Chem. 2015, 87, 4508–4514. [Google Scholar]
- Koga, Y.; Kuriyama, R.; Sato, Y.; Hishida, K.; Miki, N. Effects of micromachining processes on electro-osmotic flow mobility of glass surfaces. Micromachines 2013, 4, 67–79. [Google Scholar]
- Lim, A.E.; Lim, C.Y.; Lam, Y.C.; Taboryski, R. Electroosmotic flow in microchannel with black silicon nanostructures. Micromachines 2018, 9, 229. [Google Scholar]
- Yasui, T.; Kaji, N.; Mohamadi, M.R.; Okamoto, Y.; Tokeshi, M.; Horiike, Y.; Baba, Y. Electroosmotic flow in microchannels with nanostructures. ACS Nano 2011, 5, 7775–7780. [Google Scholar]
- Lim, A.E.; Lim, C.Y.; Lam, Y.C.; Taboryski, R.; Wang, S.R. Effect of nanostructures orientation on electroosmotic flow in a microfluidic channel. Nanotechnology 2017, 28, 255303. [Google Scholar]
- Kim, J.; Cho, I.; Lee, H.; Kim, S.J. Ion concentration polarization by bifurcated current path. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Rubinstein, I.; Zaltzman, B. Extended space charge in concentration polarization. Adv. Colloid Interface Sci. 2010, 159, 117–129. [Google Scholar]
- Sun, Z.Y.; Gao, Y.T.; Yu, X.; Liu, Y. Formation of vortices in a combined pressure-driven electro-osmotic flow through the insulated sharp tips under finite Debye length effects. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 1–11. [Google Scholar]
- Thamida, S.K.; Chang, H.C. Nonlinear electrokinetic ejection and entrainment due to polarization at nearly insulated wedges. Phys. Fluids 2002, 14, 4315–4328. [Google Scholar]
- Behrens, S.H.; Grier, D.G. The charge of glass and silica surfaces. J. Chem. Phys. 2001, 115, 6716–6721. [Google Scholar]
- Kim, K.; Kwak, H.S.; Song, T.H. A numerical model for simulating electroosmotic micro- and nanochannel flows under non-Boltzmann equilibrium. Fluid Dyn. Res. 2011, 43, 041401. [Google Scholar]
- Newman, J.; Thomas-Alyea, K.E. Electrochemical Systems; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]


| Variable | Condition a | Boundary |
|---|---|---|
| Applied potential φ | φ = 0.28 V | Inlet |
| φ = 0 V | Outlet | |
| −n·σ∇φ = 0 | Smooth and nanostructured surfaces | |
| Electrostatic wall potential ψ | n·∇ψ = 0 | Inlet and outlet |
| n·∇ψ = S/ɛrɛo = (−9.86 × 10−3)/ɛrɛo | Smooth and nanostructured surfaces | |
| Concentrations of ions ci | ci = co(i) exp(-zieψ/kbT) | Inlet and outlet |
| −n·[-Di∇ci – um(i)ci∇(φ + ψ) + vci] = 0 | Smooth and nanostructured surfaces | |
| Flow velocity v and Pressure p | v = 0 | Smooth and nanostructured surfaces |
| p = 0 | Inlet and outlet |
| Parameters | Symbol (Unit) | Value |
|---|---|---|
| Permittivity of free space | ɛo (C·V−1·m−1) | 8.85 × 10−12 |
| Relative permittivity | ɛr | 80 |
| Viscosity of water | µ (kg·m−1·s−1) | 8.90 × 10−4 |
| Density of water | ρ (kg·m−3) | 1000 |
| Faraday constant | F (C·mol−1) | 96485 |
| Gas constant | R (J·mol−1·K−1) | 8.314 |
| Boltzmann constant | kb (m2·kg·s−2·K−1) | 1.381 × 10−23 |
| Temperature | T (K) | 298 |
| Electron charge | e (C) | 1.602 × 10−19 |
| Avogadro constant | Na (mol−1) | 6.022 × 1023 |
| Diffusion coefficient of Na+ | DNa+ (m2·s−1) | 1.334 × 10−9 [50] |
| Diffusion coefficient of HCO3- | DHCO3- (m2·s−1) | 1.105 × 10−9 [50] |
| Ionic mobility of Na+ | um(Na+) (m2·V−1·s−1) | 5.194 × 10−8 |
| Ionic mobility of HCO3- | um(HCO3-) (m2·V−1·s−1) | −7.919 × 10−8 |
| Ionic charge number of Na+ | zNa+ | +1 |
| Ionic charge number of HCO3- | zHCO3- | −1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.E.; Lam, Y.C. Numerical Investigation of Nanostructure Orientation on Electroosmotic Flow. Micromachines 2020, 11, 971. https://doi.org/10.3390/mi11110971
Lim AE, Lam YC. Numerical Investigation of Nanostructure Orientation on Electroosmotic Flow. Micromachines. 2020; 11(11):971. https://doi.org/10.3390/mi11110971
Chicago/Turabian StyleLim, An Eng, and Yee Cheong Lam. 2020. "Numerical Investigation of Nanostructure Orientation on Electroosmotic Flow" Micromachines 11, no. 11: 971. https://doi.org/10.3390/mi11110971
APA StyleLim, A. E., & Lam, Y. C. (2020). Numerical Investigation of Nanostructure Orientation on Electroosmotic Flow. Micromachines, 11(11), 971. https://doi.org/10.3390/mi11110971






