An Approach to Ring Resonator Biosensing Assisted by Dielectrophoresis: Design, Simulation and Fabrication
Abstract
1. Introduction
2. Materials and Methods
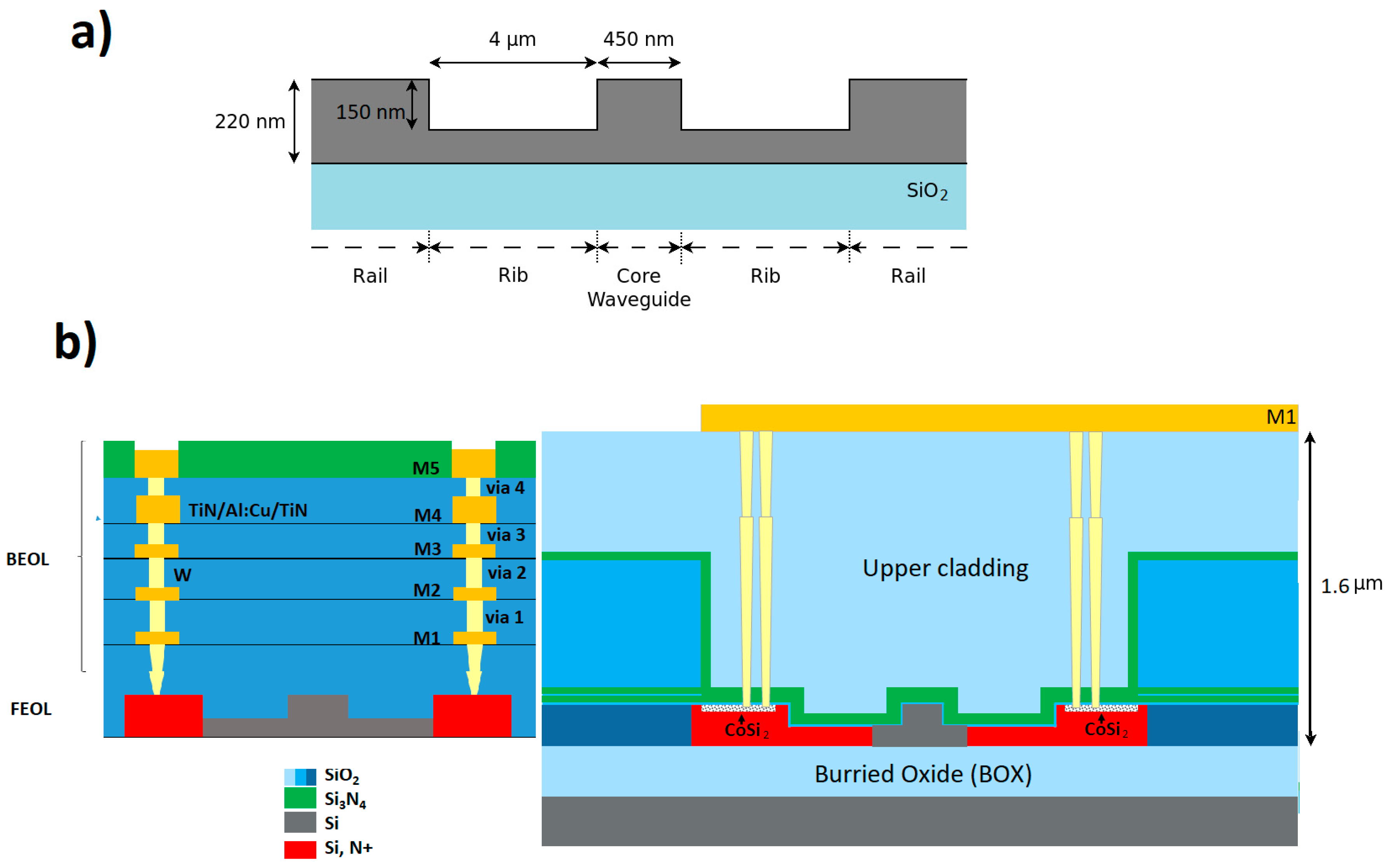
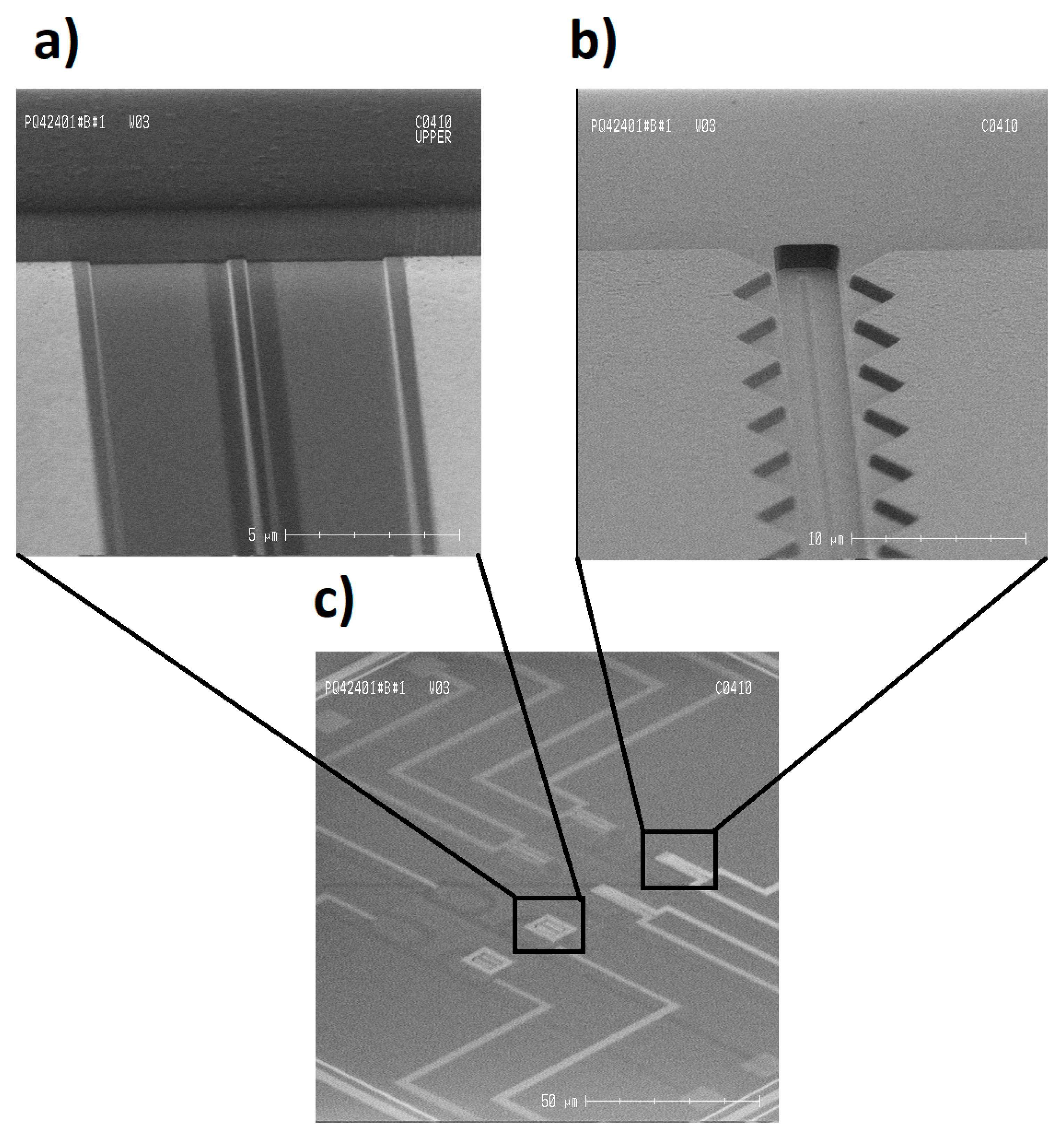
2.1. Fabrication
2.2. Simulation by the Finite Element Method (FEM)
3. Results
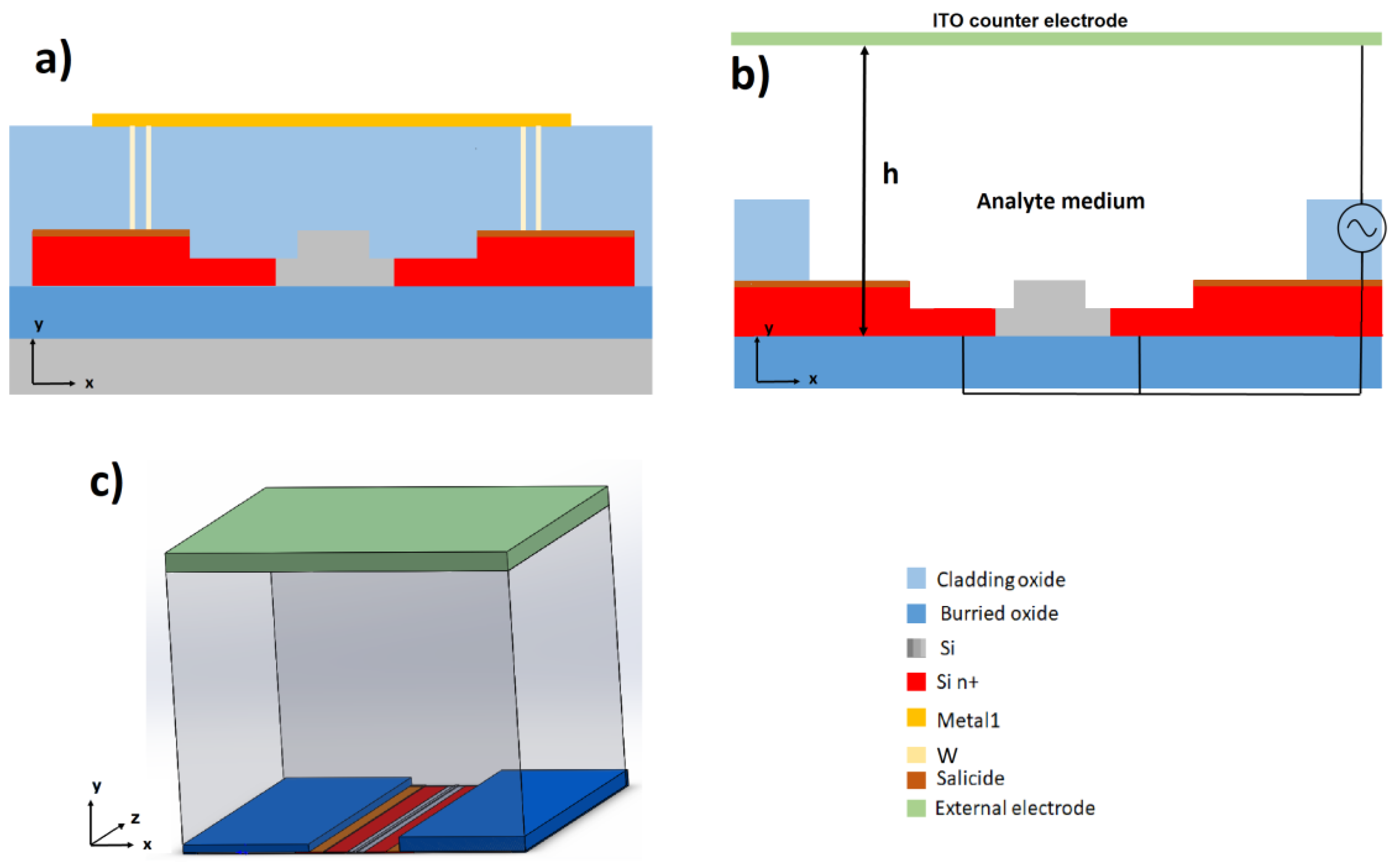
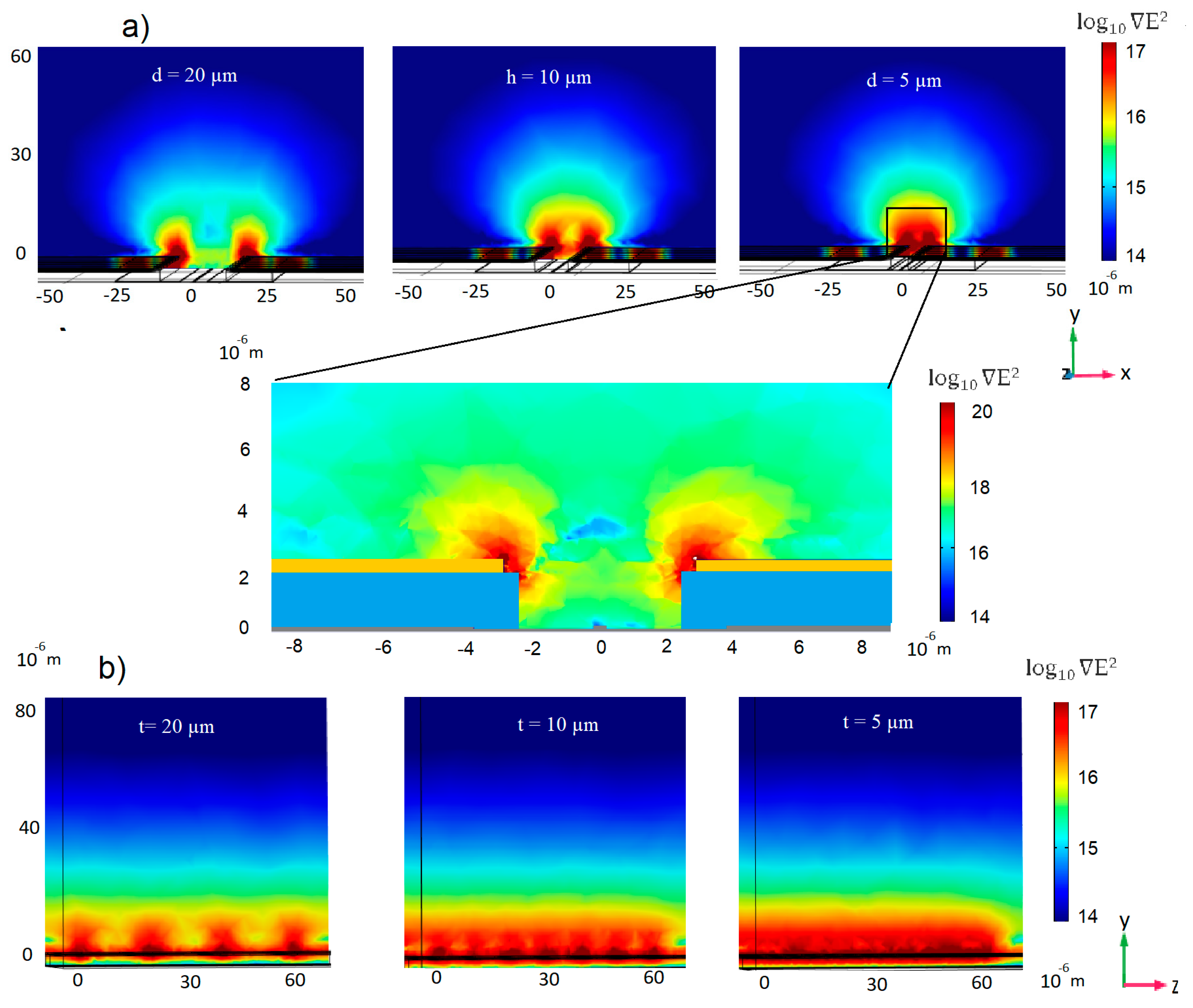
3.1. Electrode Design and Simulation of the ∇E2 Distribution
3.1.1. 3D Electrode Geometry
3.1.2. Coplanar Electrode Pair Configuration
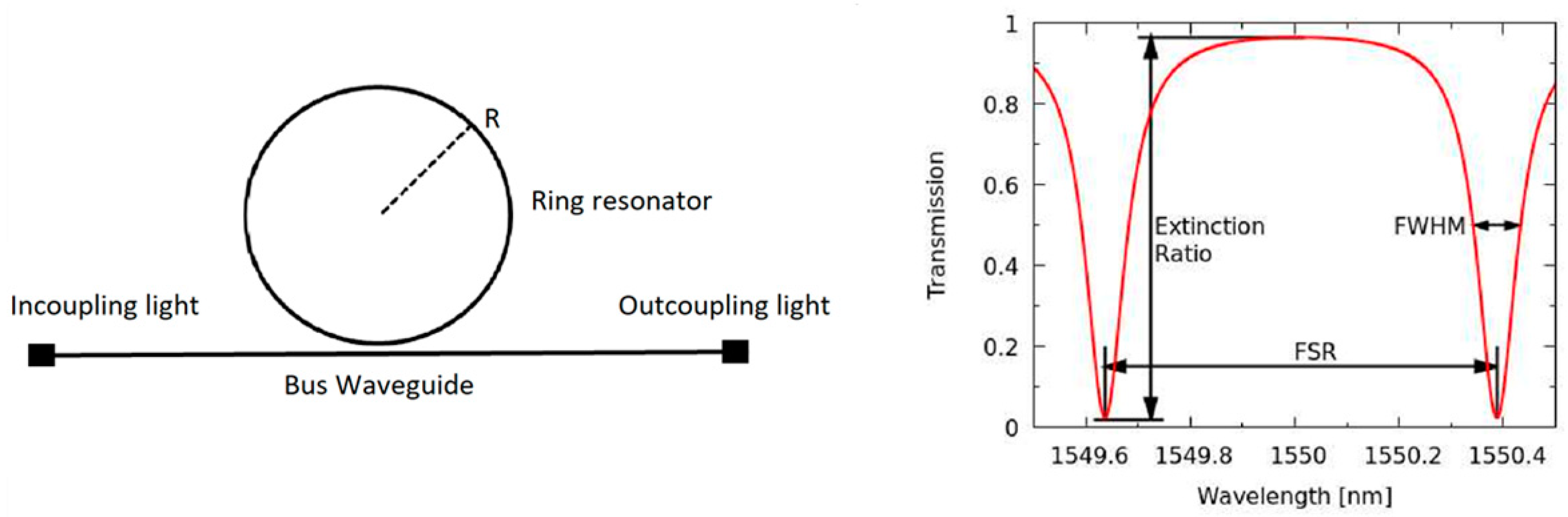
3.2. Ring Resonator Design
3.3. Fabrication and Optical Characteristics
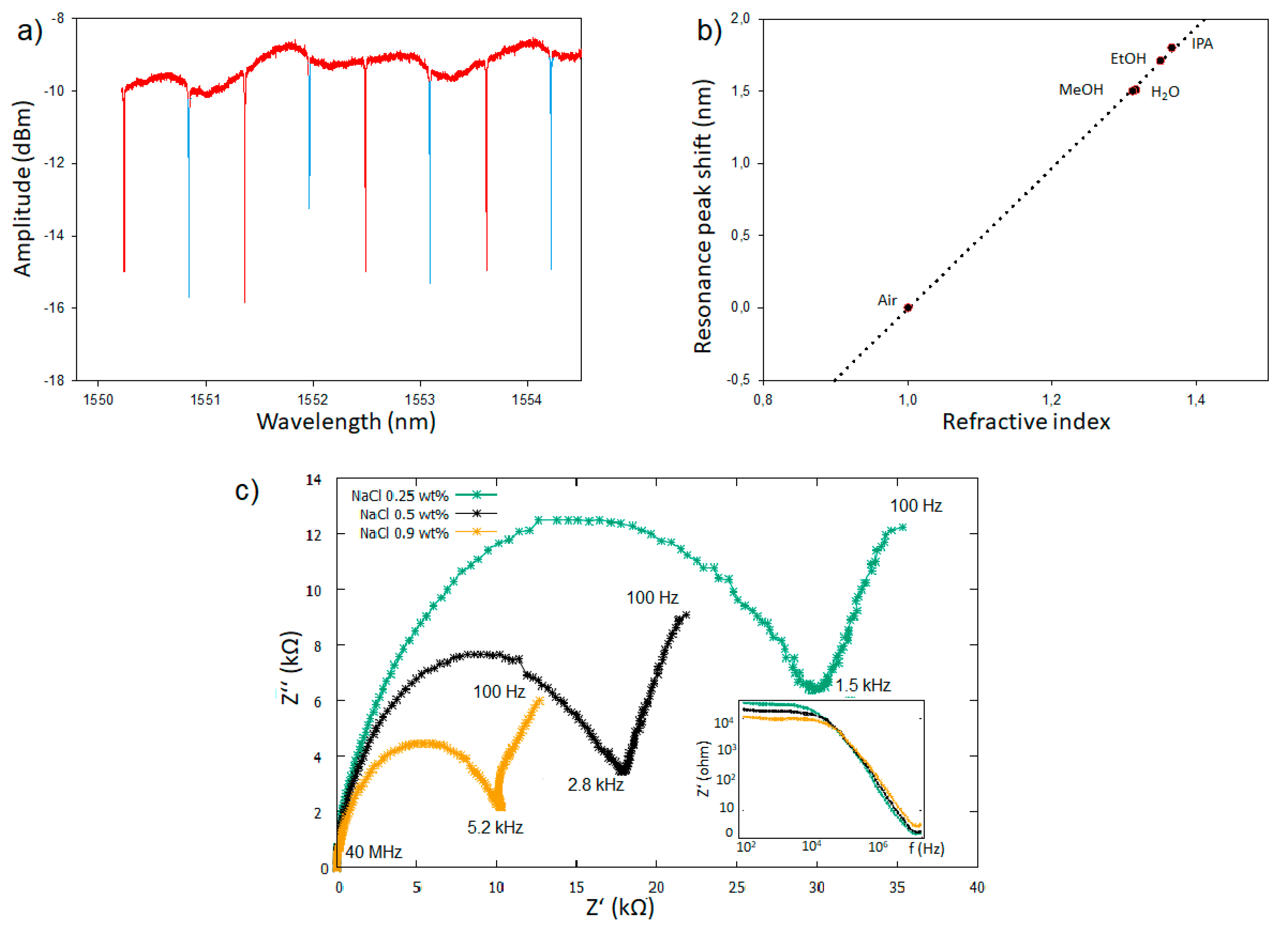
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sarkaleh, A.K.; Lahijani, B.V.; Saberkari, H.; Esmaeeli, A. Optical Ring Resonators: A Platform for Biological Sensing Applications. J. Med. Signals Sens. 2017, 7, 185–191. [Google Scholar] [CrossRef]
- Birkholz, M.; Mai, A.; Wenger, C.; Meliani, C.; Scholz, R. Technology modules from micro- and nano-electronics for the life sciences. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 8, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Lummerstorfer, T.; Hoffmann, H. Click Chemistry on Surfaces: 1,3-Dipolar Cycloaddition Reactions of Azide-Terminated Monolayers on Silica. J. Phys. Chem. B 2004, 108, 3963–3966. [Google Scholar] [CrossRef]
- Henriksson, A.; Nishiori, D.; Maeda, H.; Miyachi, M.; Yamanoi, Y.; Nishihara, H. Attachment chemistry of aromatic compounds on a Silicon(100) surface. Surf. Sci. 2018, 669, 140–144. [Google Scholar] [CrossRef]
- Henriksson, A.; Hoffmann, H. Click Coupling Reactions on Flat and Nanostructured Hydrogen-Passivated Silicon Surfaces. Phys. Status Solidi 2018, 216, 1800683. [Google Scholar] [CrossRef]
- Wang, J.; Sanchez, M.M.; Yin, Y.; Herzer, R.; Ma, L.; Schmidt, O.G. Silicon-Based Integrated Label-Free Optofluidic Biosensors: Latest Advances and Roadmap. Adv. Mater. Technol. 2020, 5, 1901138. [Google Scholar] [CrossRef]
- Bogaerts, W.; De Heyn, P.; Van Vaerenbergh, T.; De Vos, K.; Kumar Selvaraja, S.; Claes, T.; Dumon, P.; Bienstman, P.; Van Thourhout, D.; Baets, R. Silicon microring resonators. Laser Photon. Rev. 2012, 6, 47–73. [Google Scholar] [CrossRef]
- Jäger, M. Spatially Resolved Refractive Index Detection Based on SOI Ring Resonators. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2016. [Google Scholar]
- Steglich, P.; Hülsemann, M.; Dietzel, B.; Mai, A. Optical Biosensors Based on Silicon-On-Insulator Ring Resonators: A Review. Molecules 2019, 24, 519. [Google Scholar] [CrossRef]
- Claes, T.; Bogaerts, W.; Bienstman, P. Vernier-cascade silicon photonic label-free biosensor with very large sensitivity and low-cost interrogation. Biosensing Nanomed. IV 2011, 8099, 80990R. [Google Scholar] [CrossRef]
- Yariv, A.; Xu, Y.; Lee, R.K.; Scherer, A. Coupled-resonator optical waveguide: A proposal and analysis. Opt. Lett. 1999, 24, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, M.S.; Bailey, R.C. Silicon Photonic Microring Resonators for Quantitative Cytokine Detection and T-Cell Secretion Analysis. Anal. Chem. 2010, 82, 1975–1981. [Google Scholar] [CrossRef]
- Su, J. Label-Free Biological and Chemical Sensing Using Whispering Gallery Mode Optical Resonators: Past, Present, and Future. Sensors 2017, 17, 540. [Google Scholar] [CrossRef]
- Huertas, C.S.; Calvo-Lozano, O.; Mitchell, A.; Lechuga, L.M. Advanced Evanescent-Wave Optical Biosensors for the Detection of Nucleic Acids: An Analytic Perspective. Front. Chem. 2019, 7, 724. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Peters, E.A.; Schultz, Z.D. Bioanalytical applications of surface-enhanced Raman spectroscopy: De novo molecular identification. Rev. Anal. Chem. 2017, 36. [Google Scholar] [CrossRef]
- Kindt, J.T.; Bailey, R.C. Biomolecular analysis with microring resonators: Applications in multiplexed diagnostics and interaction screening. Curr. Opin. Chem. Biol. 2013, 17, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Ksendzov, A.; Lin, Y. Integrated optics ring-resonator sensors for protein detection. Opt. Lett. 2005, 30, 3344–3346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; White, I.M.; Suter, J.D.; Zourob, M.; Fan, X. Opto-fluidic micro-ring resonator for sensitive label-free viral detection. Analyst 2008, 133, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; White, I.M.; Suter, J.; Fan, X. Phage-based label-free biomolecule detection in an opto-fluidic ring resonator. Biosens. Bioelectron. 2008, 24, 461–466. [Google Scholar] [CrossRef]
- Bahadoran, M.; Noorden, A.F.A.; Mohajer, F.S.; Mubin, M.H.A.; Chaudhary, K.; Jalil, M.A.; Ali, J.; Yupapin, P. Detection ofSalmonella bacteriumin drinking water using microring resonator. Artif. Cells Nanomed. Biotechnol. 2014, 44, 315–321. [Google Scholar] [CrossRef]
- Baaske, M.D.; Foreman, M.R.; Vollmer, F. Single-molecule nucleic acid interactions monitored on a label-free microcavity biosensor platform. Nat. Nanotechnol. 2014, 9, 933–939. [Google Scholar] [CrossRef]
- Squires, T.M.; Messinger, R.J.; Manalis, S.R. Making it stick: Convection, reaction and diffusion in surface-based biosensors. Nat. Biotechnol. 2008, 26, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.T.; Long, M.; Piper, J.W.; Yago, T.; McEver, R.P.; Zhu, C. Direct observation of catch bonds involving cell-adhesion molecules. Nat. Cell Biol. 2003, 423, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Jia, Y.; Wang, P.; Sun, C. Progress of Inertial Microfluidics in Principle and Application. Sensors 2018, 18, 1762. [Google Scholar] [CrossRef]
- Van Oijen, A.M.; Blainey, P.C.; Crampton, D.J.; Richardson, C.C.; Ellenberger, T.; Xie, X.S. Single-Molecule Kinetics of Exonuclease Reveal Base Dependence and Dynamic Disorder. Science 2003, 301, 1235–1238. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Chemla, Y.R.; Smith, S.B.; Bustamante, C. Recent Advances in Optical Tweezers. Annu. Rev. Biochem. 2008, 77, 205–228. [Google Scholar] [CrossRef]
- Lionnet, T.; Allemand, J.-F.; Revyakin, A.; Strick, T.R.; Saleh, O.A.; Bensimon, D.; Croquette, V. Single-Molecule Studies Using Magnetic Traps. Cold Spring Harb. Protoc. 2011, 2012, 067488. [Google Scholar] [CrossRef][Green Version]
- Nakano, A.; Ros, A. Protein dielectrophoresis: Advances, challenges, and applications. Electrophoresis 2013, 34, 1085–1096. [Google Scholar] [CrossRef]
- Ding, J.; Lawrence, R.M.; Jones, P.V.; Hogue, B.G.; Hayes, M.A. Concentration of Sindbis virus with optimized gradient insulator-based dielectrophoresis. Analyst 2016, 141, 1997–2008. [Google Scholar] [CrossRef]
- Herrmann, N.; Neubauer, P.; Birkholz, M. Spiral microfluidic devices for cell separation and sorting in bioprocesses. Biomicrofluidics 2019, 13, 061501. [Google Scholar] [CrossRef]
- Abt, V.; Gringel, F.; Han, A.; Neubauer, P.; Birkholz, M. Separation, Characterization, and Handling of Microalgae by Dielectrophoresis. Microorganisms 2020, 8, 540. [Google Scholar] [CrossRef]
- Viefhues, M.; Eichhorn, R. DNA dielectrophoresis: Theory and applications a review. Electrophoresis 2017, 38, 1483–1506. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Alanis, F.; Ros, A. Protein dielectrophoresis and the link to dielectric properties. Bioanalysis 2015, 7, 353–371. [Google Scholar] [CrossRef]
- Li, M.; Li, W.H.; Zhang, J.; Alici, G.; Wen, W. A review of microfabrication techniques and dielectrophoretic microdevices for particle manipulation and separation. J. Phys. D Appl. Phys. 2014, 47, 63001. [Google Scholar] [CrossRef]
- Pethig, R. Review—Where Is Dielectrophoresis (DEP) Going? J. Electrochem. Soc. 2016, 164, B3049–B3055. [Google Scholar] [CrossRef]
- Washizu, M.; Suzuki, S.; Kurosawa, O.; Nishizaka, T.; Shinohara, T. Molecular dielectrophoresis of biopolymers. IEEE Trans. Ind. Appl. 1994, 30, 835–843. [Google Scholar] [CrossRef]
- Hölzel, R.; Calander, N.; Chiragwandi, Z.; Willander, M.; Bier, F.F. Trapping Single Molecules by Dielectrophoresis. Phys. Rev. Lett. 2005, 95, 128102. [Google Scholar] [CrossRef]
- Otto, S.; Kaletta, U.; Bier, F.F.; Wenger, C.; Hölzel, R. Dielectrophoretic immobilisation of antibodies on microelectrode arrays. Lab Chip 2014, 14, 998. [Google Scholar] [CrossRef]
- Laux, E.-M.; Bier, F.F.; Holzel, R. Electrode-based AC electrokinetics of proteins: A mini-review. Bioelectrochemistry 2018, 120, 76–82. [Google Scholar] [CrossRef]
- Li, S.; Cui, H.; Yuan, Q.; Wu, J.; Wadhwa, A.; Eda, S.; Jiang, H. AC electrokinetics-enhanced capacitive immunosensor for point-of-care serodiagnosis of infectious diseases. Biosens. Bioelectron. 2014, 51, 437–443. [Google Scholar] [CrossRef]
- Freedman, K.J.; Otto, L.M.; Ivanov, A.P.; Barik, A.; Oh, S.-H.; Edel, J.B. Nanopore sensing at ultra-low concentrations using single-molecule dielectrophoretic trapping. Nat. Commun. 2016, 7, 10217. [Google Scholar] [CrossRef]
- Jäger, M.; Becherer, T.; Bruns, J.; Haag, R.; Petermann, K. Antifouling coatings on SOI microring resonators for bio sensing applications. Sensors Actuators B Chem. 2016, 223, 400–405. [Google Scholar] [CrossRef]
- Duarte, V.C.; Prata, J.G.; Ribeiro, C.F.; Nogueira, R.N.; Winzer, G.; Zimmermann, L.; Walker, R.; Clements, S.; Filipowicz, M.; Napierała, M.; et al. Modular coherent photonic-aided payload receiver for communications satellites. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Duarte, V.C.; Ribeiro, C.F.; Prata, J.G.; Winzer, G.; Petousi, D.; Zimmermann, L.; Nogueira, R.; Drummond, M.V. Reconfigurable monitoring and control system for tunable optical delay lines. Opt. Lett. 2018, 43, 2543–2546. [Google Scholar] [CrossRef]
- Sharma, A.; Han, C.-H.; Jang, J. Rapid electrical immunoassay of the cardiac biomarker troponin I through dielectrophoretic concentration using imbedded electrodes. Biosens. Bioelectron. 2016, 82, 78–84. [Google Scholar] [CrossRef]
- Gong, J.-R. Label-Free Attomolar Detection of Proteins Using Integrated Nanoelectronic and Electrokinetic Devices. Small 2010, 6, 967–973. [Google Scholar] [CrossRef]
- Jäger, M.; Bruns, J.; Ehrentreich-Förster, E.; Petermann, K. Arrays of Individually Addressable SOI Micro Ring Resonators for Bio Sensing. Integr. Photonics Res. Silicon Nanophotonics Photonics Switch. 2013, 4, 3. [Google Scholar] [CrossRef]
- Moock, P.; Kasper, L.; Jäger, M.; Stolarek, D.; Richter, H.; Bruns, J.; Petermann, K. TDM-controlled ring resonator arrays for fast, fixed-wavelength optical biosensing. Opt. Express 2018, 26, 22356–22365. [Google Scholar] [CrossRef]
- Schwelb, O. Transmission, Group Delay, and Dispersion in Single-Ring Optical Resonators and Add/Drop Filters—A Tutorial Overview. J. Light. Technol. 2004, 22, 1380–1394. [Google Scholar] [CrossRef]
- Lima, L.F.; Vieira, A.L.; Mukai, H.; Andrade, C.M.; Fernandes, P.R. Electric impedance of aqueous KCl and NaCl solutions: Salt concentration dependence on components of the equivalent electric circuit. J. Mol. Liq. 2017, 241, 530–539. [Google Scholar] [CrossRef]
- Myers, T.L.; Tonkyn, R.G.; Danby, T.O.; Taubman, M.S.; Bernacki, B.E.; Birnbaum, J.C.; Sharpe, S.W.; Johnson, T.J. Accurate Measurement of the Optical Constants n and k for a Series of 57 Inorganic and Organic Liquids for Optical Modeling and Detection. Appl. Spectrosc. 2017, 72, 535–550. [Google Scholar] [CrossRef]
- Saunders, J.E.; Sanders, C.; Chen, H.; Loock, H.-P. Refractive indices of common solvents and solutions at 1550 nm. Appl. Opt. 2016, 55, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Kittler, M.; Yu, X.; Vyvenko, O.; Birkholz, M.; Seifert, W.; Reiche, M.; Wilhelm, T.; Arguirov, T.; Wolff, A.; Fritzsche, W.; et al. Self-organized pattern formation of biomolecules at silicon surfaces: Intended application of a dislocation network. Mater. Sci. Eng. C 2006, 26, 902–910. [Google Scholar] [CrossRef]
- Kim, D.; Sonker, M.; Ros, A. Dielectrophoresis: From Molecular to Micrometer-Scale Analytes. Anal. Chem. 2018, 91, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, R.; Pethig, R. Protein Dielectrophoresis: I. Status of Experiments and an Empirical Theory. Micromachines 2020, 11, 533. [Google Scholar] [CrossRef]
- Wirth, J.C. Engineering Sensitivity: An Optical Optimization of Ring Resonator Arrays for Label-Free Whole Bacterial Sensing. Ph.D. Thesis, Purdue University Graduate School, West Lafayette, IN, USA, 2019. [Google Scholar] [CrossRef]
- Ramachandran, A.; Wang, S.; Clarke, J.; Ja, S.; Goad, D.; Wald, L.; Flood, E.; Knobbe, E.; Hryniewicz, J.; Chu, S.; et al. A universal biosensing platform based on optical micro-ring resonators. Biosens. Bioelectron. 2008, 23, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, A.K.T. Determination of viable legionellae in engineered water systems: Do we find what we are looking for? Water Res. 2016, 93, 276–288. [Google Scholar] [CrossRef] [PubMed]



| Material | Relative Permittivity | Electric Conductivity (S/m) |
|---|---|---|
| SiO2 | 3.74 | 10−14 |
| Medium (PBS Puffer) | 80 | 1 |
| Si | 11.7 | 10 |
| n+-Si | 11.7 | 2 × 104 |
| Metal 1 | 1 | 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriksson, A.; Kasper, L.; Jäger, M.; Neubauer, P.; Birkholz, M. An Approach to Ring Resonator Biosensing Assisted by Dielectrophoresis: Design, Simulation and Fabrication. Micromachines 2020, 11, 954. https://doi.org/10.3390/mi11110954
Henriksson A, Kasper L, Jäger M, Neubauer P, Birkholz M. An Approach to Ring Resonator Biosensing Assisted by Dielectrophoresis: Design, Simulation and Fabrication. Micromachines. 2020; 11(11):954. https://doi.org/10.3390/mi11110954
Chicago/Turabian StyleHenriksson, Anders, Laura Kasper, Matthias Jäger, Peter Neubauer, and Mario Birkholz. 2020. "An Approach to Ring Resonator Biosensing Assisted by Dielectrophoresis: Design, Simulation and Fabrication" Micromachines 11, no. 11: 954. https://doi.org/10.3390/mi11110954
APA StyleHenriksson, A., Kasper, L., Jäger, M., Neubauer, P., & Birkholz, M. (2020). An Approach to Ring Resonator Biosensing Assisted by Dielectrophoresis: Design, Simulation and Fabrication. Micromachines, 11(11), 954. https://doi.org/10.3390/mi11110954









