A Low Spring Constant Piezoresistive Microcantilever for Biological Reagent Detection
Abstract
1. Introduction
2. Design and Fabrication
2.1. Design of Piezoresistive Microcantilever
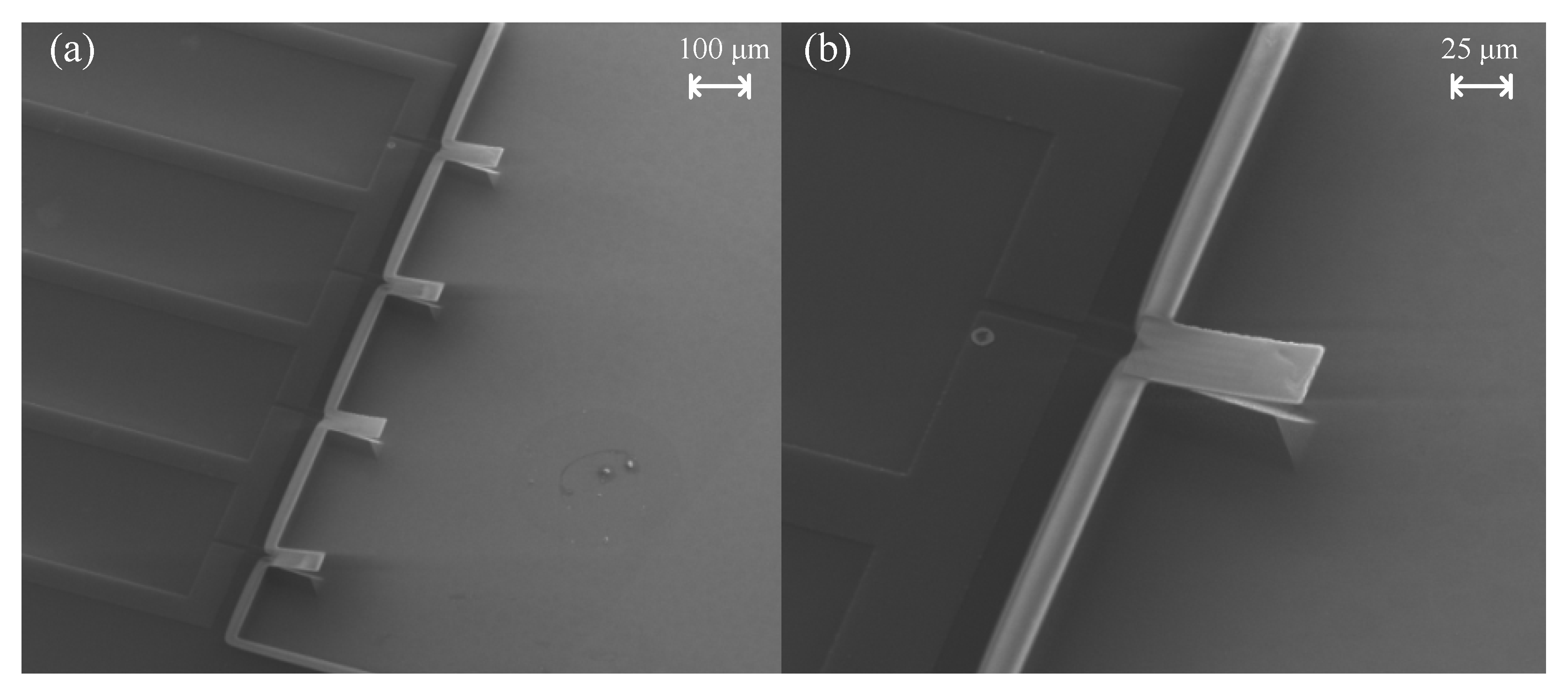
2.2. Fabrication
- (a)
- Firstly, LPCVD technology was used to deposit a 100 nm thick SiO2 on the surface of the silicon wafer as the bottom passive layer of the microcantilever. After the first photolithography, BHF was used to remove the SiO2 outside the pattern to define the SiO2 layer of microcantilever.
- (b)
- Then, a 40 nm thick Titanium was sputtered and patterned to form the Ti piezoresistor.
- (c)
- An 800 nm thick Al was sputtered, and Al connection wires and pads were patterned in the third photolithography.
- (d)
- After that, a 1.3 μm thick PI was spin coated on the surface of the substrate at 3000 rpm for 30 s. The wafer was then annealed in a nitrogen atmosphere with a gas flow of 4.5 L/min and a temperature of 350 °C for 90 min to complete the PI layer curing. Apart from the piezoresistor, the PI layer also fully encapsulates the aluminum wires to avoid electrical conduction in an aqueous environment.
- (e)
- E-beam evaporation was used to deposit a 10/50 nm Cr/Au modified layer on the surface of the PI layer. Cr was used as the adhesion layer between PI and Au. After the fourth photolithography, the Au/Cr was etched by wet etching technology to form a biochemical molecule modified layer in sensing microcantilevers.
- (f)
- At last, the microcantilever was patterned and defined by using a 6 μm thick photoresist as a mask. After PI was etched by anisotropic RIE oxygen plasma etching until the silicon wafer, a dry silicon etching techniques was used to etch the silicon wafer from front side until the microcantilever structure was completely released, and a reactive well was also formed at the same time. The traditional release of the microcantilever structure by the isotropic dry etching technology can cause serious lateral undercutting situation problems at the sidewall near the fixed end of the microcantilever. A combination of anisotropic and isotropic dry etching techniques was used to avoid this lateral undercutting problem of SiO2.
3. Performances
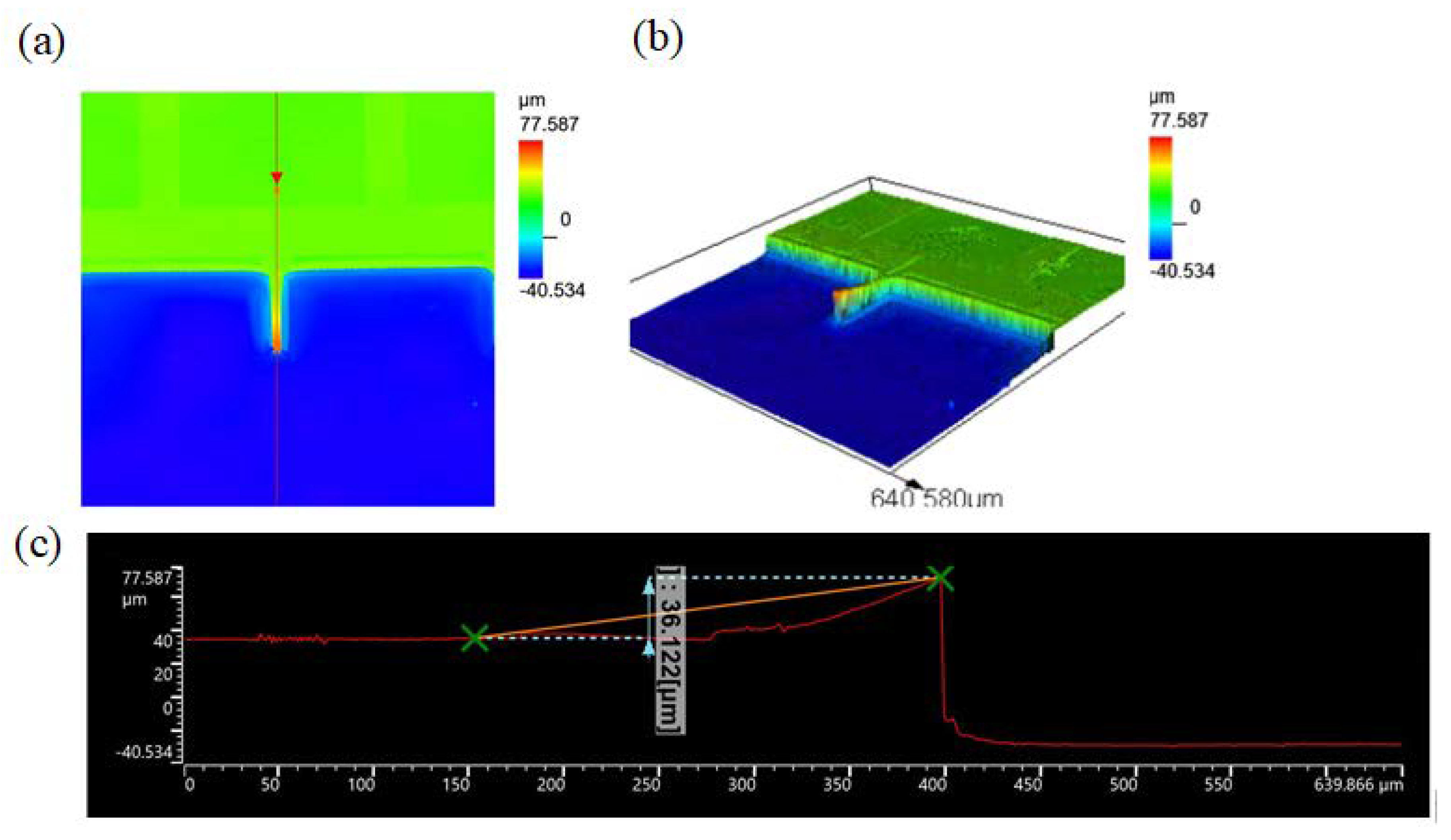
3.1. Spring Constant
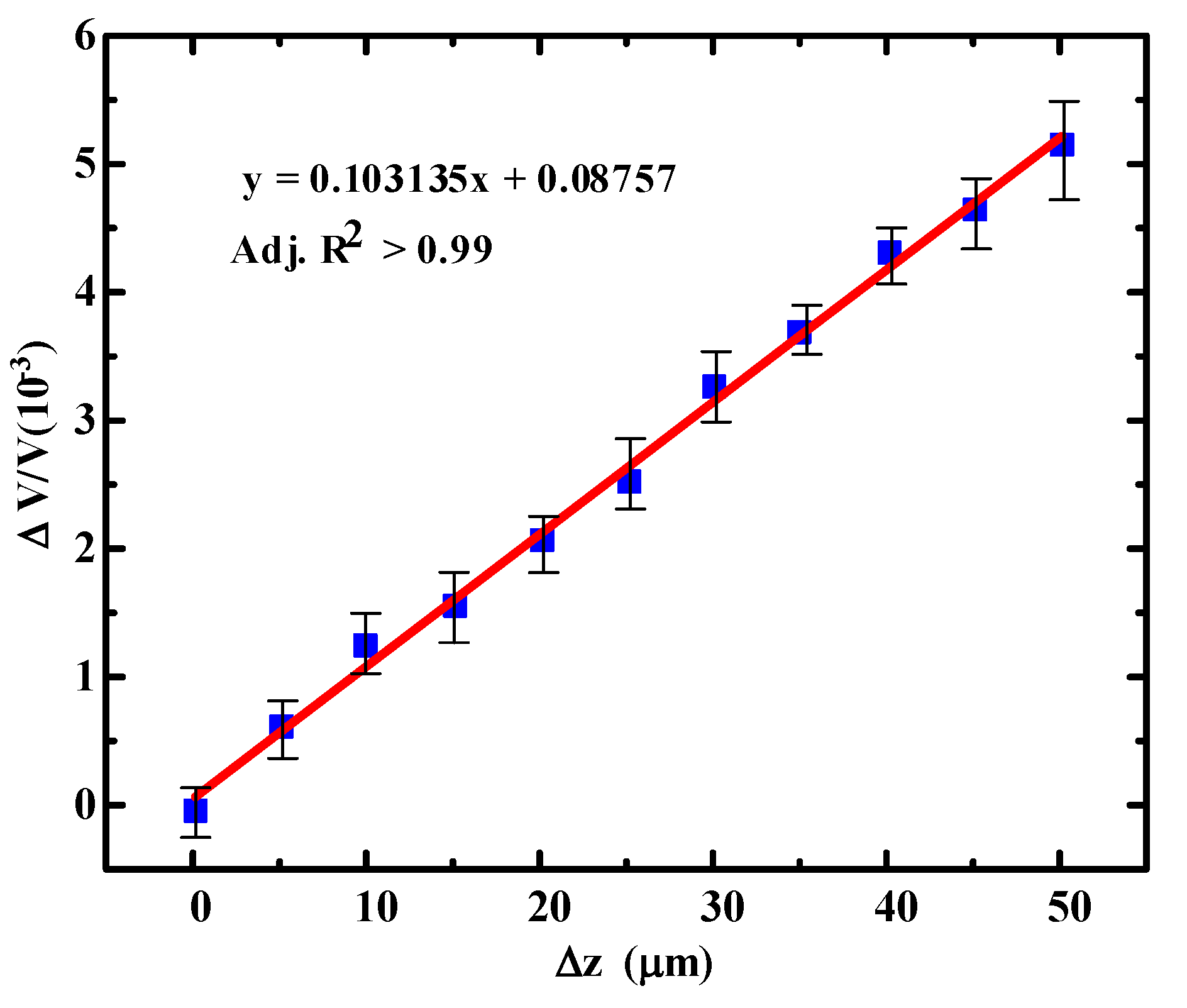
3.2. Sensitivity
3.3. Stability
4. Biological Detections
4.1. IgG Detections
- (a)
- The pre-processed microcantilever was immersed in 5 mg/mL DDPA for about 1 h to coat the carboxyl groups on the Au film surface of the sensing microcantilever
- (b)
- Then, it was soaked in the EDC/NHS mixture with a concentration of 5 mg/mLs, and a volume ratio of 3:1 for about 30 min to form a succinimide activator on the surface of the sensitive microcantilever.
- (c)
- After being repeatedly cleaned, the microcantilever was immersed in a 0.1 mg/mL streptavidin solution to react for about 1 h to cross-link the streptavidin molecule with the succinimide activator;
- (d)
- At last, a 1 M ethanolamine was injected to inactivate the residual carboxyl groups on the surface of the sensing microcantilever for 30 min after cleaning the microcantilever a few times.
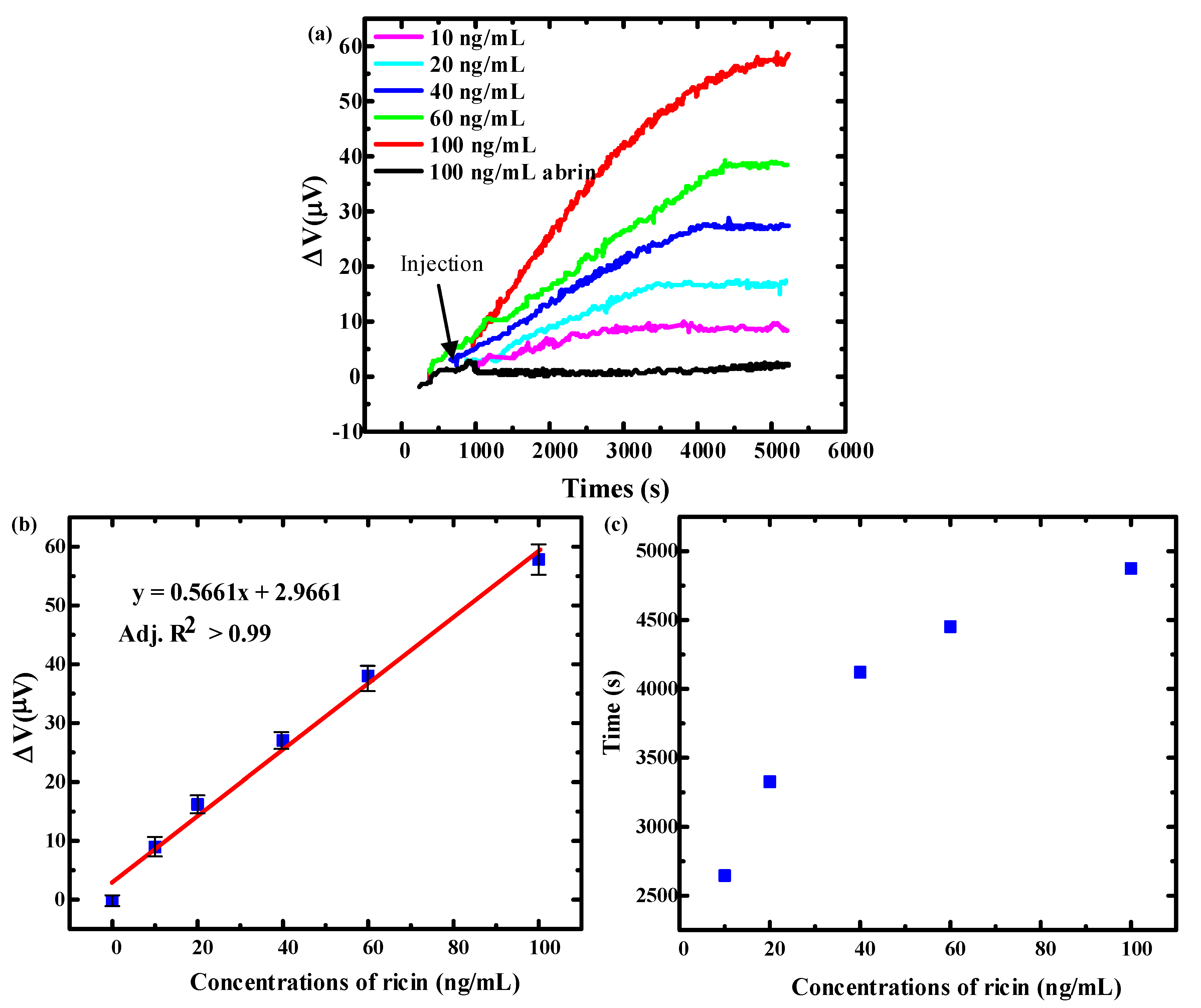
4.2. Ricin Detection
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyer, G.; Amer, N.M. Erratum: Novel optical approach to atomic force microscopy [Appl. Phys. Lett. 5 3, 1045 (1988)]. Appl. Phys. Lett. 1988, 53, 2400–2402. [Google Scholar] [CrossRef]
- Lavrik, N.V.; Sepaniak, M.J.; Datskos, P.G. Cantilever transducers as a platform for chemical and biological sensors. Rev. Sci. Instrum. 2004, 75, 2229–2253. [Google Scholar] [CrossRef]
- Gunter, R.; Delinger, W.G.; Manygoats, K.; Kooser, A.; Porter, T. Viral detection using an embedded piezoresistive microcantilever sensor. Sens. Actuators A Phys. 2003, 107, 219–224. [Google Scholar] [CrossRef]
- Kooser, A.; Gunter, R.L.; Delinger, W.; Porter, T.L.; Eastman, M.P. Gas sensing using embedded piezoresistive microcantilever sensors. Sens. Actuators B Chem. 2004, 99, 474–479. [Google Scholar] [CrossRef]
- Porter, T.; Eastman, M.; Pace, D.; Bradley, M. Sensor based on piezoresistive microcantilever technology. Sens. Actuators A Phys. 2001, 88, 47–51. [Google Scholar] [CrossRef]
- Amírola, J.; Rodríguez, A.; Castañer, L.; Santos, J.; Gutiérrez, J.; Horrillo, M. Micromachined silicon microcantilevers for gas sensing applications with capacitive read-out. Sens. Actuators B Chem. 2005, 111, 247–253. [Google Scholar] [CrossRef]
- Napoli, M.; Bamieh, B.; Turner, K. A capacitive microcantilever: Modelling, validation, and estimation using current measurements. J. Dyn. Sys. Meas. Control 2004, 126, 319–326. [Google Scholar] [CrossRef]
- Bausells, J. Piezoresistive cantilevers for nanomechanical sensing. Microelectron. Eng. 2015, 145, 9–20. [Google Scholar] [CrossRef]
- Zhao, R.; Jia, D.; Wen, Y.; Yu, X. Cantilever-based aptasensor for trace level detection of nerve agent simulant in aqueous matrices. Sens. Actuators B Chem. 2017, 238, 1231–1239. [Google Scholar] [CrossRef]
- Patkar, R.S.; Vinchurkar, M.; Ashwin, M.; Rao, V.R. A Novel PET-Based Piezoresistive MEMS Sensor Platform for Agricultural Applications. JMemS 2017, 26, 746–748. [Google Scholar] [CrossRef]
- Ku, Y.-F.; Huang, L.-S.; Yen, Y.-K. A real-time thermal self-elimination method for static mode operated freestanding piezoresistive microcantilever-based biosensors. Biosensors 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bertke, M.; Li, X.; Mu, H.; Zhou, H.; Yu, F.; Hamdana, G.; Schmidt, A.; Bremers, H.; Peiner, E. Fabrication of ZnO nanorods and Chitosan@ZnO nanorods on MEMS piezoresistive self-actuating silicon microcantilever for humidity sensing. Sens. Actuators B Chem. 2018, 273, 276–287. [Google Scholar] [CrossRef]
- Xu, J.; Setiono, A.; Peiner, E. Piezoresistive Microcantilever with SAM-Modified ZnO-Nanorods@ Silicon-Nanopillars for Room-Temperature Parts-per-Billion NO2 Detection. ACS Appl. Nano Mater. 2020, 3, 6609–6620. [Google Scholar] [CrossRef]
- Rotake, D.R.; Darji, A.; Kale, N.S. Highly selective piezoresistive sensor for mercury (Hg2+) ions detection using mercaptosuccinic acid-functionalized microcantilevers with cross-linked pyridinedicarboxylic acid. SeRv 2020, 40, 543–558. [Google Scholar] [CrossRef]
- Kandpal, M.; Behera, S.N.; Singh, J.; Palaparthy, V.; Singh, S. Residual stress compensated silicon nitride microcantilever array with integrated poly-Si piezoresistor for gas sensing applications. Microsystem Technol. 2020, 26, 1379–1385. [Google Scholar] [CrossRef]
- Zhu, W.; Park, J.S.; Sessler, J.L.; Gaitas, A. A colorimetric receptor combined with a microcantilever sensor for explosive vapor detection. Appl. Phys. Lett. 2011, 98, 123501. [Google Scholar] [CrossRef]
- Liu, Y.; Schweizer, L.M.; Wang, W.; Reuben, R.L.; Schweizer, M.; Shu, W. Label-free and real-time monitoring of yeast cell growth by the bending of polymer microcantilever biosensors. Sens. Actuators B Chem. 2013, 178, 621–626. [Google Scholar] [CrossRef]
- Yu, X.; Thaysen, J.; Hansen, O.; Boisen, A. Optimization of sensitivity and noise in piezoresistive cantilevers. J. Appl. Phys. 2002, 92, 6296–6301. [Google Scholar] [CrossRef]
- Beckwith, T.G.; Marangoni, R.D.; Lienhard, J.H. Mechanical Measurements; Pearson: London, UK, 2009; p. 360. [Google Scholar]
- Temperature Coefficient of Resistance. Available online: http://www.radio-electronics.com/info/formulae/resistance/resistance-temperature-coefficient.php (accessed on 11 November 2020).
- Allen, M. The effect of tension on the electrical resistance of single bismuth crystals. Phys. Rev. 1932, 42, 848. [Google Scholar] [CrossRef]
- Hoffmann, K. Applying the Wheatstone Bridge Circuit; HBM Germany: Darmstadt, Germany, 1974; p. 25. [Google Scholar]
- Eastman, T.; Zhu, D.-M. Adhesion forces between surface-modified AFM tips and a mica surface. Langmuir 1996, 12, 2859–2862. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, W.; Wen, Y.; Yang, J.; Yu, X. Trace level detections of abrin with high SNR piezoresistive cantilever biosensor. Sens. Actuators B Chem. 2015, 212, 112–119. [Google Scholar] [CrossRef]
- Moulin, A.; O’shea, S.; Welland, M.E. Microcantilever-based biosensors. Ultmi 2000, 82, 23–31. [Google Scholar] [CrossRef]
- Shekhawat, G.; Tark, S.-H.; Dravid, V.P. MOSFET-embedded microcantilevers for measuring deflection in biomolecular sensors. Science 2006, 311, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Sun, Y. Polymeric flexible immunosensor based on piezoresistive micro-cantilever with PEDOT/PSS conductive layer. Sensors 2018, 18, 451. [Google Scholar] [CrossRef]
- Nan, T.; Wu, S.; Zhao, H.; Tan, W.; Li, Z.; Zhang, Q.; Wang, B. Development of a secondary antibody thio-functionalized microcantilever immunosensor and an ELISA for measuring ginsenoside Re content in the herb ginseng. Anal. Chem. 2012, 84, 4327–4333. [Google Scholar] [CrossRef]
- Kanamori-Kataoka, M.; Kato, H.; Uzawa, H.; Ohta, S.; Takei, Y.; Furuno, M.; Seto, Y. Determination of ricin by nano liquid chromatography/mass spectrometry after extraction using lactose-immobilized monolithic silica spin column. J. Mass Spectrom. 2011, 46, 821–829. [Google Scholar] [CrossRef]
- Campos, A.R.; Gao, Z.; Blaber, M.G.; Huang, R.; Schatz, G.C.; Van Duyne, R.P.; Haynes, C.L. Surface-Enhanced Raman spectroscopy detection of ricin B chain in human blood. J. Phys. Chem. C 2016, 120, 20961–20969. [Google Scholar] [CrossRef]
- Shyu, R.-H.; Shyu, H.-F.; Liu, H.-W.; Tang, S.-S. Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon 2002, 40, 255–258. [Google Scholar] [CrossRef]
- Anderson, G.P.; Glaven, R.H.; Algar, W.R.; Susumu, K.; Stewart, M.H.; Medintz, I.L.; Goldman, E.R. Single domain antibody-quantum dot conjugates for ricin detection by both fluoroimmunoassay and surface plasmon resonance. Anal. Chim. Acta 2013, 786, 132–138. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Tong, Z.-Y.; Liu, B.; Hao, L.-Q.; Mu, X.-H.; Zhang, J.-P.; Gao, C. Piezoresistive microcantilever aptasensor for ricin detection and kinetic analysis. AIP Adv. 2015, 5, 041324. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Zhao, R.; Liu, Y.; Yu, X. A Low Spring Constant Piezoresistive Microcantilever for Biological Reagent Detection. Micromachines 2020, 11, 1001. https://doi.org/10.3390/mi11111001
Tian Y, Zhao R, Liu Y, Yu X. A Low Spring Constant Piezoresistive Microcantilever for Biological Reagent Detection. Micromachines. 2020; 11(11):1001. https://doi.org/10.3390/mi11111001
Chicago/Turabian StyleTian, Yuan, Rui Zhao, Yi Liu, and Xiaomei Yu. 2020. "A Low Spring Constant Piezoresistive Microcantilever for Biological Reagent Detection" Micromachines 11, no. 11: 1001. https://doi.org/10.3390/mi11111001
APA StyleTian, Y., Zhao, R., Liu, Y., & Yu, X. (2020). A Low Spring Constant Piezoresistive Microcantilever for Biological Reagent Detection. Micromachines, 11(11), 1001. https://doi.org/10.3390/mi11111001




