Ultrasonic Retinal Neuromodulation and Acoustic Retinal Prosthesis
Abstract
1. Introduction
2. Ultrasonic Retinal Stimulation
2.1. Ultrasonic Neuromodulation
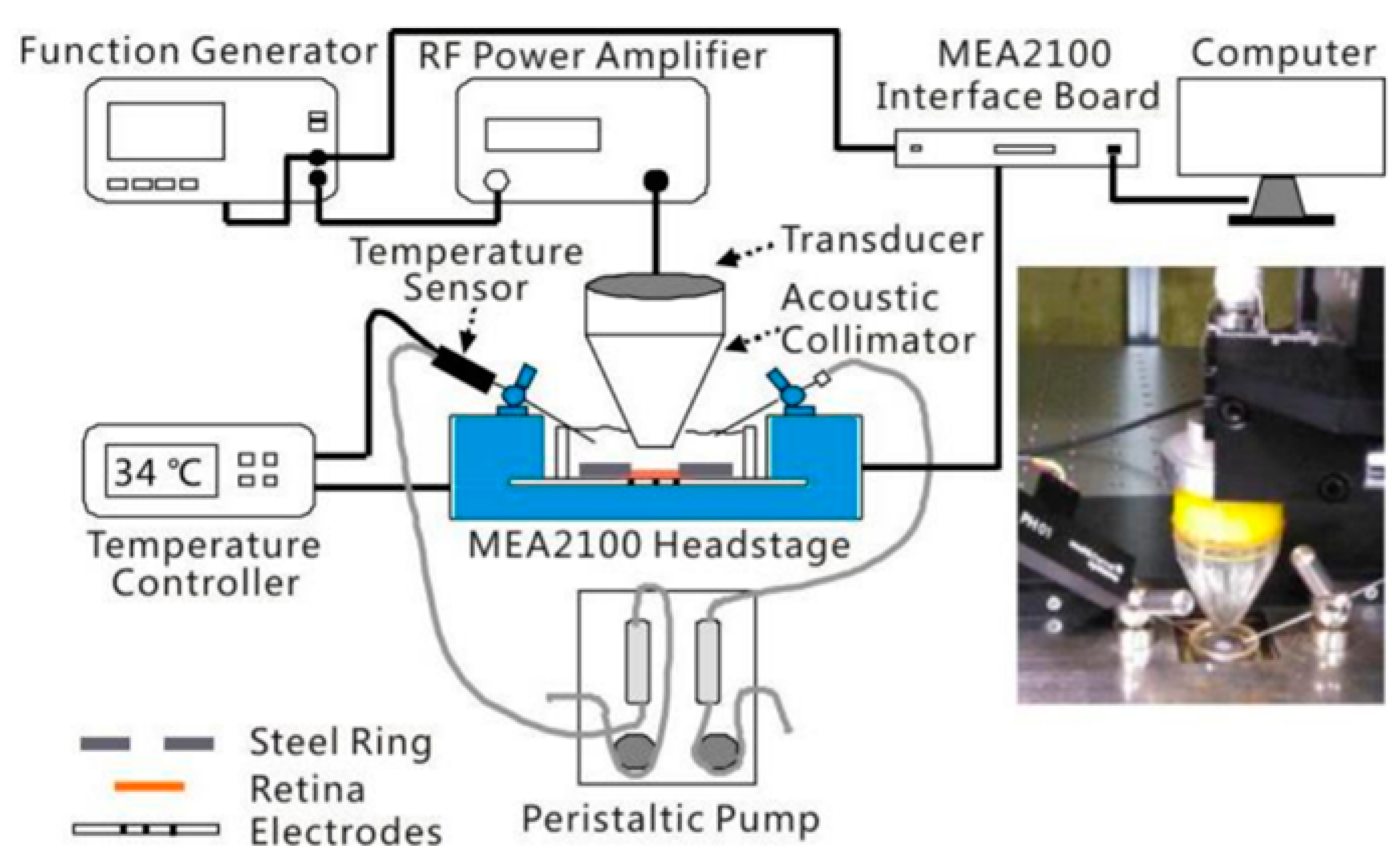
2.2. Ultrasonic Retinal Stimulation
2.3. Possible Mechanisms of Ultrasonic Neuromodulation
2.3.1. Thermal Effects
2.3.2. Nonthermal Effects
3. Spatiotemporal Characteristics of Retinal Response to Ultrasonic vs. Visual Stimulation
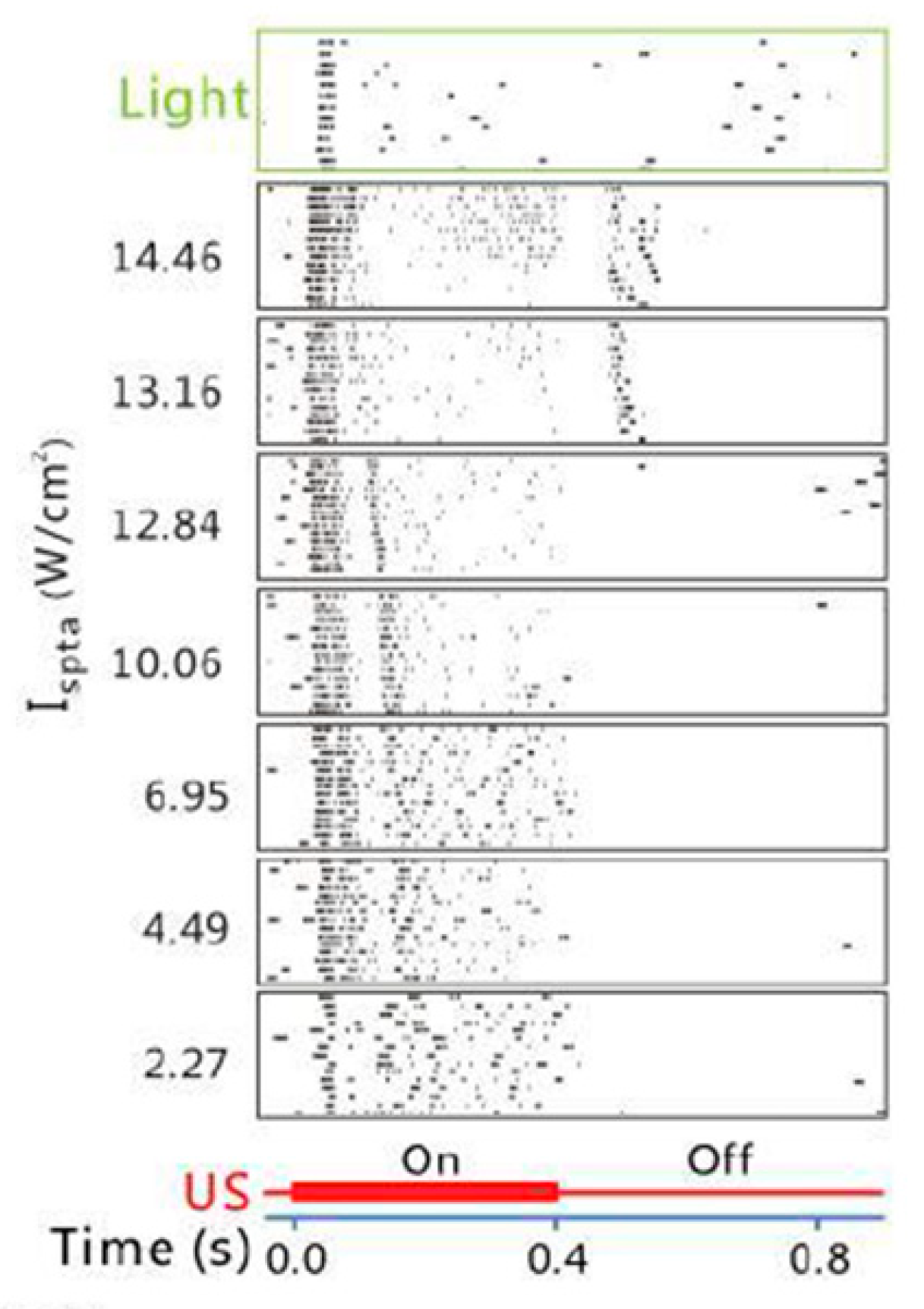
3.1. Temporal Characteristics
3.2. Spatial Characteristics
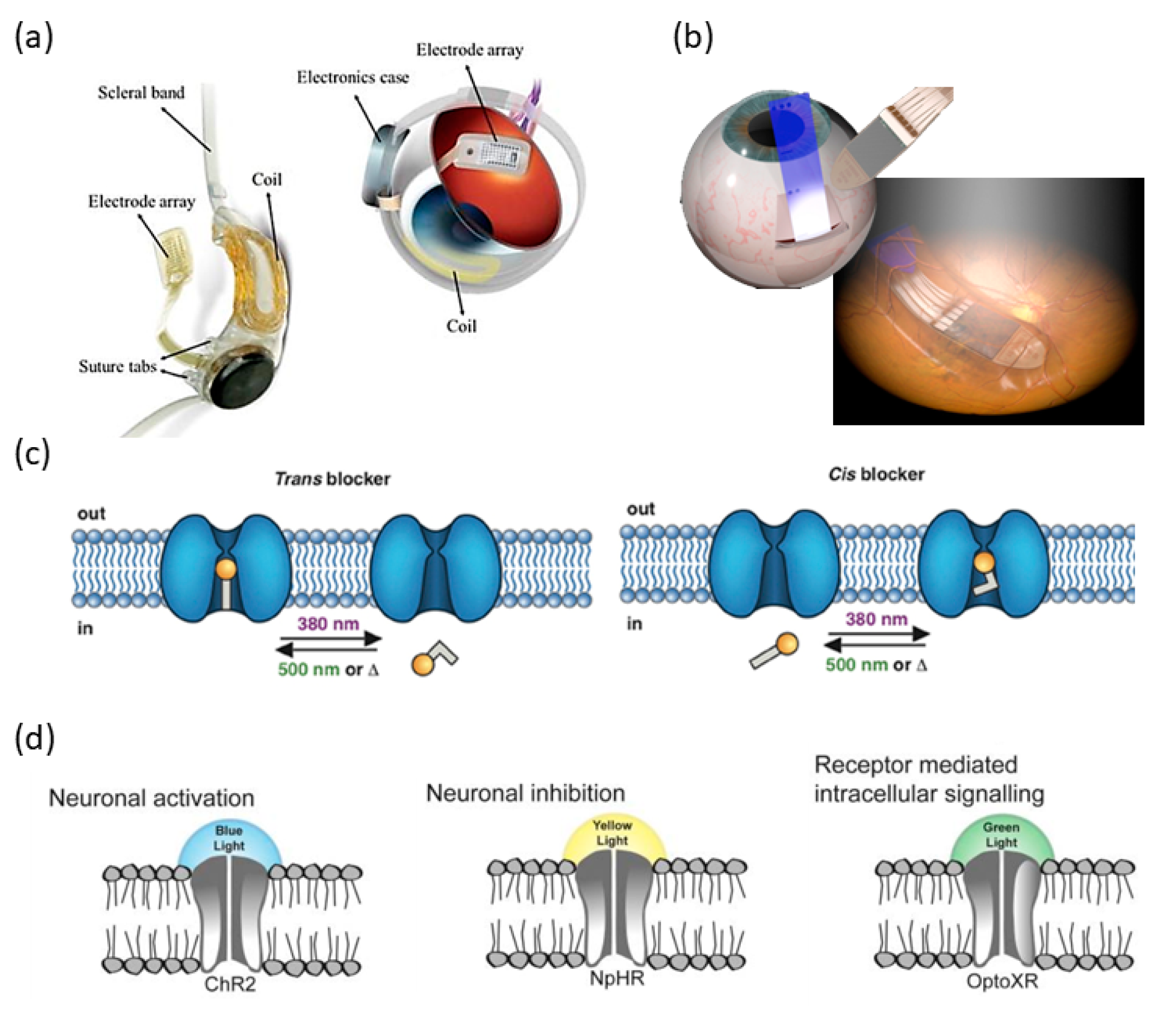
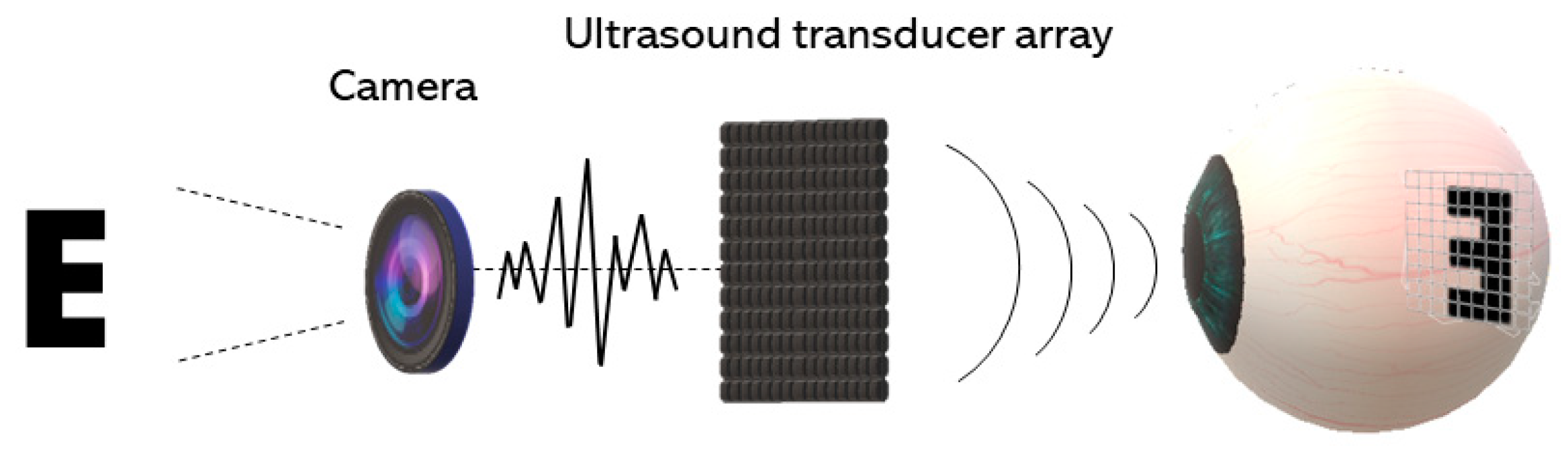
4. Acoustic Retinal Prosthesis (ARP)
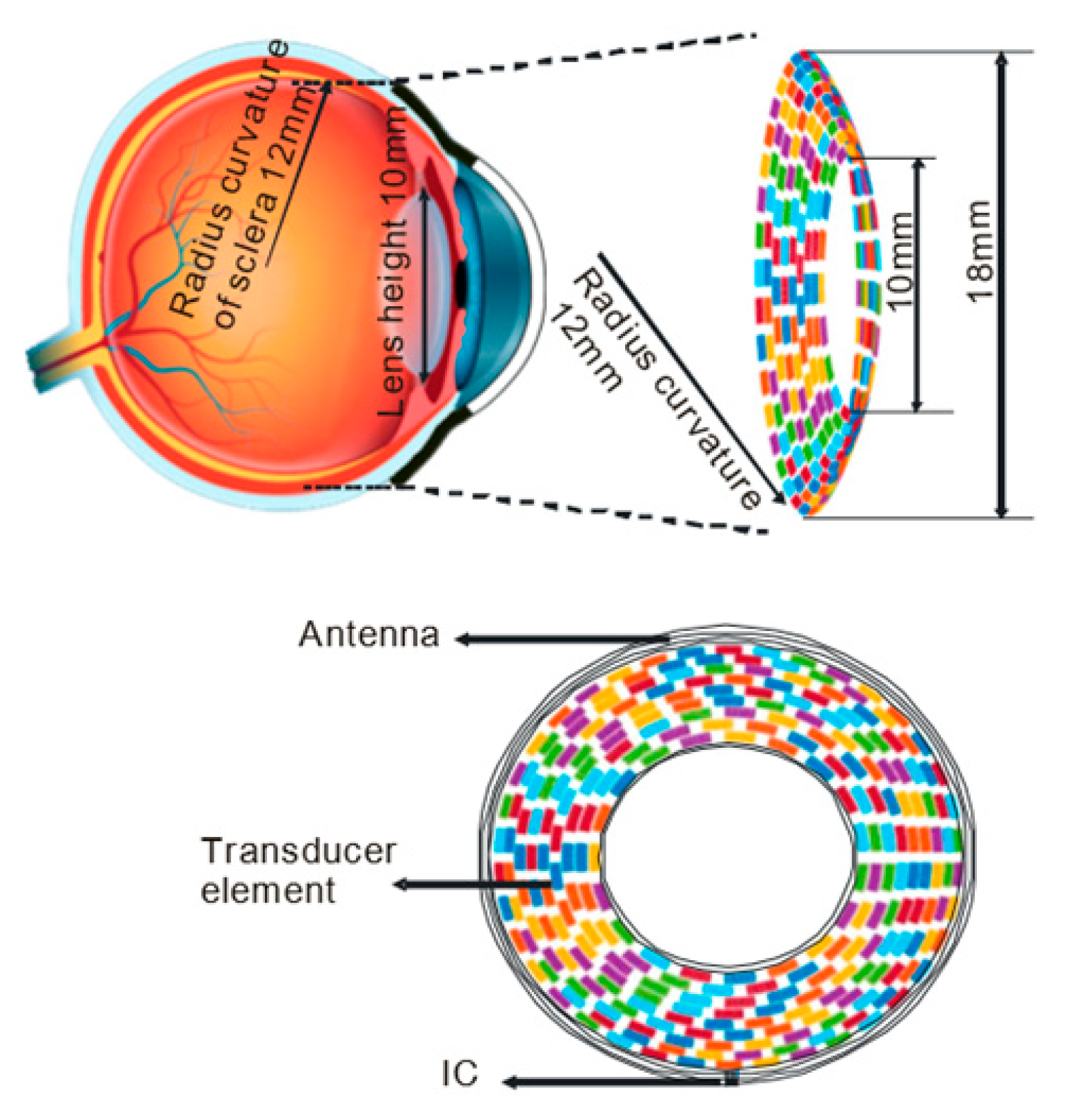
4.1. Basic Architecture and the Transducer Array
4.2. Algorithms for Generating Multifocal Ultrasonic Stimulation
4.3. Ultrasonic Stimulation Strategy
4.4. Safety Consideration
5. Ultrasonic Stimulation of the Visual Cortex
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Busskamp, V.; Duebel, J.; Balya, D.; Fradot, M.; Viney, T.J.; Siegert, S.; Groner, A.C.; Cabuy, E.; Forster, V.; Seeliger, M. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 2010, 329, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Owsley, C.; Jackson, G.R. Spare the rods, save the cones in aging and age-related maculopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2015–2018. [Google Scholar]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Jonas, J.B.; Bourne, R.R.; White, R.A.; Flaxman, S.R.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; Price, H.; Wong, T.Y. Visual impairment and blindness due to macular diseases globally: A systematic review and meta-analysis. Am. J. Ophthalmol. 2014, 158, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Yanai, A.; McNab, P.; Gregory-Evans, K. Retinal therapy with induced pluripotent stem cells; leading the way to human clinical trials. Expert Rev. Ophthalmol. 2019, 14, 53–59. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Ammann, F.; Klein, D.; Franceschetti, A. Genetic and epidemiological investigations on pigmentary degeneration of the retina and allied disorders in Switzerland. J. Neurol. Sci. 1965, 2, 183–196. [Google Scholar] [CrossRef]
- Haim, M. The epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol. Scand. 2002, 80, 1–34. [Google Scholar] [CrossRef]
- Boughman, J.A.; Conneally, P.M.; Nance, W.E. Population genetic studies of retinitis pigmentosa. Am. J. Hum. Genet. 1980, 32, 223. [Google Scholar]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Masland, R.H. The neuronal organization of the retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Strettoi, E. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res. 2003, 22, 607–655. [Google Scholar] [CrossRef]
- Bloch, E.; Luo, Y.; da Cruz, L. Advances in retinal prosthesis systems. Ther. Adv. Ophthalmol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Palanker, D.; Mer, Y.L.; Mohand-Said, S.; Muqit, M.; Sahel, J.A. Photovoltaic Restoration of Central Vision in Atrophic Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.L.; Cottriall, C.L.; Xue, K.; Simunovic, M.P.; Ramsden, J.D.; Zrenner, E.; MacLaren, R.E. Assessment of the electronic retinal implant alpha AMS in restoring vision to blind patients with end-stage retinitis pigmentosa. Ophthalmology 2018, 125, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wuyyuru, V.; Gonzalez-Calle, A.; Dorn, J.D.; Humayun, M.S. Retina–electrode interface properties and vision restoration by two generations of retinal prostheses in one patient—One in each eye. J. Neural Eng. 2020, 17, 026020. [Google Scholar] [CrossRef]
- Tang, J.; Qin, N.; Chong, Y.; Diao, Y.; Wang, Z.; Xue, T.; Jiang, M.; Zhang, J.; Zheng, G. Nanowire arrays restore vision in blind mice. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Ghezzi, D.; Antognazza, M.R.; Colombo, E.; Mete, M.; Feyen, P.; Desii, A.; Buschiazzo, A.; Di Paolo, M.; Di Marco, S. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 2017, 16, 681–689. [Google Scholar] [CrossRef]
- Degenaar, P.; McGovern, B.; Berlinguer-Palmini, R.; Vysokov, N.; Grossman, N.; Pohrer, V.; Drakakis, E.; Neil, M. Individually addressable optoelectronic arrays for optogenetic neural stimulation. In Proceedings of the 2010 Biomedical Circuits and Systems Conference (BioCAS), Paphos, Cyprus, 3–5 November 2010; pp. 170–173. [Google Scholar]
- Farah, N.; Reutsky, I.; Shoham, S. Patterned optical activation of retinal ganglion cells. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 6368–6370. [Google Scholar]
- Mourot, A.; Tochitsky, I.; Kramer, R.H. Light at the end of the channel: Optical manipulation of intrinsic neuronal excitability with chemical photoswitches. Front. Mol. Neurosci. 2013, 6, 5. [Google Scholar] [CrossRef]
- Reutsky-Gefen, I.; Golan, L.; Farah, N.; Schejter, A.; Tsur, L.; Brosh, I.; Shoham, S. Holographic optogenetic stimulation of patterned neuronal activity for vision restoration. Nat. Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Pama, E.; Colzato, L.S.; Hommel, B. Optogenetics as a neuromodulation tool in cognitive neuroscience. Front. Psychol. 2013, 4, 610. [Google Scholar] [CrossRef]
- Bystritsky, A.; Korb, A.S.; Douglas, P.K.; Cohen, M.S.; Melega, W.P.; Mulgaonkar, A.P.; DeSalles, A.; Min, B.K.; Yoo, S.S. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011, 4, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Harvey, E.N. The effect of high frequency sound waves on heart muscle and other irritable tissues. Am. J. Physiol. Leg. Content 1929, 91, 284–290. [Google Scholar] [CrossRef]
- Fry, F.; Ades, H.; Fry, W. Production of reversible changes in the central nervous system by ultrasound. Science 1958, 127, 83–84. [Google Scholar] [CrossRef]
- Wulff, V.; Fry, W.; Tucker, D.; Fry, F.J.; Melton, C. Effects of Ultrasonic Vibrations on Nerve Tissues. Proc. Soc. Exp. Biol. Med. 1951, 76, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Fry, W.J. Use of intense ultrasound in neurological research. Am. J. Phys. Med. 1958, 37, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, L.; Tsirulnikov, E.; Davies, I.a.I. Application of focused ultrasound for the stimulation of neural structures. Ultrasound Med. Biol. 1996, 22, 179–192. [Google Scholar] [CrossRef]
- Gavrilov, L.; Gersuni, G.; Ilyinsky, O.; Sirotyuk, M.; Tsirulnikov, E.; Shchekanov, E. The effect of focused ultrasound on the skin and deep nerve structures of man and animal. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1976; Volume 43, pp. 279–292. [Google Scholar]
- Tufail, Y.; Matyushov, A.; Baldwin, N.; Tauchmann, M.L.; Georges, J.; Yoshihiro, A.; Tillery, S.I.H.; Tyler, W.J. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 2010, 66, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J.; Tufail, Y.; Finsterwald, M.; Tauchmann, M.L.; Olson, E.J.; Majestic, C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 2008, 3, e3511. [Google Scholar] [CrossRef] [PubMed]
- Tufail, Y.; Yoshihiro, A.; Pati, S.; Li, M.M.; Tyler, W.J. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 2011, 6, 1453. [Google Scholar] [CrossRef]
- Li, G.; Qiu, W.; Zhang, Z.; Jiang, Q.; Su, M.; Cai, R.; Li, Y.; Cai, F.; Deng, Z.; Xu, D. Noninvasive ultrasonic neuromodulation in freely moving mice. IEEE Trans. Biomed. Eng. 2018, 66, 217–224. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.; Jung, Y.; Song, I.U.; Chung, Y.A.; Yoo, S.S. Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci. Rep. 2015, 5, 8743. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, H.C.; Jung, Y.; Chung, Y.A.; Song, I.U.; Lee, J.H.; Yoo, S.S. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci. Rep. 2016, 6, 34026. [Google Scholar] [CrossRef] [PubMed]
- Naor, O.; Hertzberg, Y.; Zemel, E.; Kimmel, E.; Shoham, S. Towards multifocal ultrasonic neural stimulation II: Design considerations for an acoustic retinal prosthesis. J. Neural Eng. 2012, 9. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, G.; Zhao, H.; Sheng, W.; Yue, L.; Su, M.; Weng, S.; Chan, L.L.H.; Zhou, Q.; Humayun, M.S. Temporal neuromodulation of retinal ganglion cells by low-frequency focused ultrasound stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Fukada, Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vis. Res. 1971, 11, 209–226. [Google Scholar] [CrossRef]
- Fukada, Y.; Saito, H.A. The relationship between response characteristics to flicker stimulation and receptive field organization in the cat’s optic nerve fibers. Vis. Res. 1971, 11, 227–240. [Google Scholar] [CrossRef]
- Cleland, B.; Dubin, M.; Levick, W. Sustained and transient neurones in the cat’s retina and lateral geniculate nucleus. J. Physiol. 1971, 217, 473–496. [Google Scholar] [CrossRef]
- Hamasaki, D.; Winters, R. A review of the properties of sustained and transient retinal ganglion cells. Experientia 1974, 30, 713–719. [Google Scholar] [CrossRef]
- Speed, C. Therapeutic ultrasound in soft tissue lesions. Rheumatology 2001, 40, 1331–1336. [Google Scholar] [CrossRef]
- Abramowicz, J.S.; Barnett, S.B.; Duck, F.A.; Edmonds, P.D.; Hynynen, K.H.; Ziskin, M.C. Fetal thermal effects of diagnostic ultrasound. J. Ultrasound Med. 2008, 27, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.G.; Homma, K.; Villarreal, S.; Richter, C.P.; Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 2012, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Farah, N.; Zoubi, A.; Matar, S.; Golan, L.; Marom, A.; Butson, C.R.; Brosh, I.; Shoham, S. Holographically patterned activation using photo-absorber induced neural–thermal stimulation. J. Neural Eng. 2013, 10, 056004. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.; Kao, C.; Konrad, P.; Milner, T.; Kim, J.; Mahadevan-Jansen, A.; Jansen, E.D. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys. J. 2007, 93, 2567–2580. [Google Scholar] [CrossRef]
- Liu, Q.; Frerck, M.J.; Holman, H.A.; Jorgensen, E.M.; Rabbitt, R.D. Exciting cell membranes with a blustering heat shock. Biophys. J. 2014, 106, 1570–1577. [Google Scholar] [CrossRef]
- Eom, K.; Byun, K.M.; Jun, S.B.; Kim, S.J.; Lee, J. Theoretical study on gold-nanorod-enhanced near-infrared neural stimulation. Biophys. J. 2018, 115, 1481–1497. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Numata, T.; Morone, N.; Kaneko, S.; Mori, Y.; Imahori, H.; Murakami, T. Thermosensitive Ion Channel Activation in Single Neuronal Cells by Using Surface-Engineered Plasmonic Nanoparticles. Angew. Chem. Int. Ed. 2015, 54, 11725–11729. [Google Scholar] [CrossRef]
- Albert, E.; Bec, J.M.; Desmadryl, G.; Chekroud, K.; Travo, C.; Gaboyard, S.; Bardin, F.; Marc, I.; Dumas, M.; Lenaers, G. TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. J. Neurophysiol. 2012, 107, 3227–3234. [Google Scholar] [CrossRef]
- Yoo, S.S.; Bystritsky, A.; Lee, J.H.; Zhang, Y.; Fischer, K.; Min, B.K.; McDannold, N.J.; Pascual-Leone, A.; Jolesz, F.A. Focused ultrasound modulates region-specific brain activity. Neuroimage 2011, 56, 1267–1275. [Google Scholar] [CrossRef]
- Wright, C.; Rothwell, J.; Saffari, N. Ultrasonic stimulation of peripheral nervous tissue: An investigation into mechanisms. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2015; Volume 581, p. 012003. [Google Scholar]
- Menz, M.D.; Oralkan, Ö.; Khuri-Yakub, P.T.; Baccus, S.A. Precise neural stimulation in the retina using focused ultrasound. J. Neurosci. 2013, 33, 4550–4560. [Google Scholar] [CrossRef]
- Yao, J.; Liu, B.; Qin, F. Rapid temperature jump by infrared diode laser irradiation for patch-clamp studies. Biophys. J. 2009, 96, 3611–3619. [Google Scholar] [CrossRef]
- Baniasad, F.; Makkiabadi, B.; Solgi, R.; Ghadiri, H. Transcranial Focused Ultrasound Modulates Electrical Behavior of the Neurons: Design and Implementation of a Model. J. Biomed. Phys. Eng. 2020, 10, 65. [Google Scholar] [CrossRef]
- Ebbini, E.S.; Cain, C.A. Multiple-focus ultrasound phased-array pattern synthesis: Optimal driving-signal distributions for hyperthermia. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1989, 36, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Krasovitski, B.; Frenkel, V.; Shoham, S.; Kimmel, E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. USA 2011, 108, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Torr, G. The acoustic radiation force. Am. J. Phys. 1984, 52, 402–408. [Google Scholar] [CrossRef]
- Westervelt, P.J. The theory of steady forces caused by sound waves. J. Acoust. Soc. Am. 1951, 23, 312–315. [Google Scholar] [CrossRef]
- Beyer, R.T. Radiation pressure—The history of a mislabeled tensor. J. Acoust. Soc. Am. 1978, 63, 1025–1030. [Google Scholar] [CrossRef]
- Nyborg, W. Physical Acoustics; Academic Press: New York, NY, USA, 1965; Volume 2. [Google Scholar]
- Miller, M.W.; Miller, D.L.; Brayman, A.A. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med. Biol. 1996, 22, 1131–1154. [Google Scholar] [CrossRef]
- Dalecki, D. Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 2004, 6, 229–248. [Google Scholar] [CrossRef]
- Wu, J.; Nyborg, W.L. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug Deliv. Rev. 2008, 60, 1103–1116. [Google Scholar] [CrossRef]
- Plaksin, M.; Shoham, S.; Kimmel, E. Intramembrane cavitation as a predictive bio-piezoelectric mechanism for ultrasonic brain stimulation. Phys. Rev. X 2014, 4, 011004. [Google Scholar] [CrossRef]
- Maingret, F.; Fosset, M.; Lesage, F.; Lazdunski, M.; Honoré, E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J. Biol. Chem. 1999, 274, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Kubanek, J.; Shi, J.; Marsh, J.; Chen, D.; Deng, C.; Cui, J. Ultrasound modulates ion channel currents. Sci. Rep. 2016, 6, 24170. [Google Scholar] [CrossRef]
- Tyler, W.J. The mechanobiology of brain function. Nat. Rev. Neurosci. 2012, 13, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Menz, M.D.; Ye, P.; Firouzi, K.; Nikoozadeh, A.; Pauly, K.B.; Khuri-Yakub, P.; Baccus, S.A. Radiation force as a physical mechanism for ultrasonic neurostimulation of the ex vivo retina. J. Neurosci. 2019, 39, 6251–6264. [Google Scholar] [CrossRef]
- Cai, C.F.; Zhou, Y.; Liu, X.; Liang, P.J.; Zhang, P.M. Possible mechanism of dual-peak response in retinal ganglion cells: A computational study. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–4. [Google Scholar]
- Zhou, Y.; Liu, X.; Liang, P.J. The dual-peak light response of ganglion cells in chicken retina. Brain Res. 2007, 1138, 104–110. [Google Scholar] [CrossRef]
- Yan, R.J.; Gong, H.Q.; Zhang, P.M.; He, S.G.; Liang, P.J. Temporal properties of dual-peak responses of mouse retinal ganglion cells and effects of inhibitory pathways. Cogn. Neurodyn. 2016, 10, 211–223. [Google Scholar] [CrossRef]
- Eiber, C.D.; Lovell, N.H.; Suaning, G.J. Attaining higher resolution visual prosthetics: A review of the factors and limitations. J. Neural Eng. 2013, 10, 011002. [Google Scholar] [CrossRef]
- Azhari, H. Basics of Biomedical Ultrasound for Engineers; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Chivers, R.; Round, W.; Zieniuk, J. Investigation of ultrasound axially traversing the human eye. Ultrasound Med. Biol. 1984, 10, 173–188. [Google Scholar] [CrossRef]
- Ye, S.; Harasiewicz, K.; Pavlin, C.; Foster, F. Ultrasound characterization of normal ocular tissue in the frequency range from 50 MHz to 100 MHz. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995, 42, 8–14. [Google Scholar] [CrossRef]
- De Korte, C.; Van Der Steen, A.; Thijssen, J. Acoustic velocity and attenuation of eye tissues at 20 MHz. Ultrasound Med. Biol. 1994, 20, 471–480. [Google Scholar] [CrossRef]
- Albano, D.; Aringhieri, G.; Messina, C.; De Flaviis, L.; Sconfienza, L.M. High-frequency and ultra-high frequency ultrasound: Musculoskeletal imaging up to 70 MHz. In Seminars in Musculoskeletal Radiology; Thieme Medical Publishers: New York, NY, USA, 2020; Volume 24, pp. 125–134. [Google Scholar]
- Hayashi, A.; Visconti, G.; Yamamoto, T.; Giacalone, G.; Hayashi, N.; Handa, M.; Yoshimatsu, H.; Salgarello, M. Intraoperative imaging of lymphatic vessel using ultra high-frequency ultrasound. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 778–780. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Chiu, C.T.; Chen, X.; Chen, Z.; Ma, J.; Zhu, B.; Shung, K.K.; Zhou, Q. Ultrahigh frequency (100 MHz–300 MHz) ultrasonic transducers for optical resolution medical imagining. Sci. Rep. 2016, 6, 28360. [Google Scholar] [CrossRef] [PubMed]
- King, R.L.; Brown, J.R.; Newsome, W.T.; Pauly, K.B. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med. Biol. 2013, 39, 312–331. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, S.W. Neurons in the retina: Organization, inhibition and excitation problems. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 1952; Volume 17, pp. 281–292. [Google Scholar]
- Famiglietti, E.; Kolb, H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science 1976, 194, 193–195. [Google Scholar] [CrossRef]
- Enroth-Cugell, C.; Robson, J.G. The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol. 1966, 187, 517–552. [Google Scholar] [CrossRef] [PubMed]
- Deffieux, T.; Younan, Y.; Wattiez, N.; Tanter, M.; Pouget, P.; Aubry, J.F. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr. Biol. 2013, 23, 2430–2433. [Google Scholar] [CrossRef]
- Li, G.F.; Zhao, H.X.; Zhou, H.; Yan, F.; Wang, J.Y.; Xu, C.X.; Wang, C.Z.; Niu, L.L.; Meng, L.; Wu, S. Improved anatomical specificity of non-invasive neuro-stimulation by high frequency (5 MHz) ultrasound. Sci. Rep. 2016, 6, 24738. [Google Scholar] [CrossRef]
- Casper, A.; Liu, D.; Ebbini, E.S. Realtime control of multiple-focus phased array heating patterns based on noninvasive ultrasound thermography. IEEE Trans. Biomed. Eng. 2011, 59, 95–105. [Google Scholar] [CrossRef]
- Li, G.; Qiu, W.; Hong, J.; Jiang, Q.; Su, M.; Mu, P.; Yang, G.; Li, Y.; Wang, C.; Zhang, H. Imaging-guided dual-target neuromodulation of the mouse brain using array ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1583–1589. [Google Scholar] [CrossRef]
- Kolb, H. Gross anatomy of the eye. In Webvision: The Organization of the Retina and Visual System; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 2007. [Google Scholar]
- Gao, M.; Yu, Y.; Zhao, H.; Li, G.; Jiang, H.; Wang, C.; Cai, F.; Chan, L.L.H.; Chiu, B.; Qian, W. Simulation study of an ultrasound retinal prosthesis with a novel contact-lens array for noninvasive retinal stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Bekerman, I.; Gottlieb, P.; Vaiman, M. Variations in eyeball diameters of the healthy adults. J. Ophthalmol. 2014, 2014, 503645. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Cai, F.; Su, M.; Jiang, Q.; Zhou, Q.; Humayun, M.S.; Qiu, W.; Zheng, H. A Novel Racing Array Transducer for Noninvasive Ultrasonic Retinal Stimulation: A Simulation Study. Sensors 2019, 19, 1825. [Google Scholar] [CrossRef]
- Hand, J.; Shaw, A.; Sadhoo, N.; Rajagopal, S.; Dickinson, R.; Gavrilov, L. A random phased array device for delivery of high intensity focused ultrasound. Phys. Med. Biol. 2009, 54, 5675. [Google Scholar] [CrossRef] [PubMed]
- Ibbini, M.S.; Cain, C.A. A field conjugation method for direct synthesis of hyperthermia phases-array heating patterns. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1989, 36, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gerchberg, R.W. A practical algorithm for the determination of phase from image and diffraction plane pictures. Optik 1972, 35, 237–246. [Google Scholar]
- Hertzberg, Y.; Naor, O.; Volovick, A.; Shoham, S. Towards multifocal ultrasonic neural stimulation: Pattern generation algorithms. J. Neural Eng. 2010, 7, 056002. [Google Scholar] [CrossRef]
- Goodman, J.W. Introduction to Fourier Optics; Roberts and Company Publishers: Greenwood Village, CO, USA, 2005. [Google Scholar]
- Prince, J.L.; Links, J.M. Medical Imaging Signals and Systems; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Wu, X.; Kumar, M. An ultrasound-based noninvasive neural interface to the retina. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 2623–2626. [Google Scholar]
- Yoo, S.S.; Kim, H.; Min, B.K.; Franck, S.P.E. Transcranial focused ultrasound to the thalamus alters anesthesia time in rats. Neuroreport 2011, 22, 783. [Google Scholar] [CrossRef]
- Kim, H.; Chiu, A.; Park, S.; Yoo, S.S. Image-guided navigation of single-element focused ultrasound transducer. Int. J. Imaging Syst. Technol. 2012, 22, 177–184. [Google Scholar] [CrossRef]
- Yang, P.S.; Kim, H.; Lee, W.; Bohlke, M.; Park, S.; Maher, T.J.; Yoo, S.S. Transcranial focused ultrasound to the thalamus is associated with reduced extracellular GABA levels in rats. Neuropsychobiology 2012, 65, 153–160. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.D.; Chiu, A.; Yoo, S.S.; Park, S. Estimation of the spatial profile of neuromodulation and the temporal latency in motor responses induced by focused ultrasound brain stimulation. Neuroreport 2014, 25, 475. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chiu, A.; Lee, S.D.; Fischer, K.; Yoo, S.S. Focused ultrasound-mediated non-invasive brain stimulation: Examination of sonication parameters. Brain Stimul. 2014, 7, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, J.; Long, X.; Qiao, Y.; Ma, T.; He, Q.; Cao, P.; Zhang, X.; Zheng, H. A Magnetic Resonance-guided Focused Ultrasound Neuromodulation System with a Whole Brain Coil Array for Nonhuman Primates at 3 T. IEEE Trans. Med. Imaging 2020. [Google Scholar] [CrossRef]
- Lu, G.; Qian, X.; Castillo, J.; Li, R.; Jiang, L.; Lu, H.; Shung, K.K.; Humayun, M.S.; Thomas, B.B.; Zhou, Q. Transcranial Focused Ultrasound for Non-invasive Neuromodulation of the Visual Cortex. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020. [Google Scholar] [CrossRef]
- Chaplin, V.; Phipps, M.; Jonathan, S.V.; Grissom, W.A.; Yang, P.; Chen, L.M.; Caskey, C.F. On the accuracy of optically tracked transducers for image-guided transcranial ultrasound. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1317–1327. [Google Scholar] [CrossRef]
- Ter Haar, G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Duck, F.A. Medical and non-medical protection standards for ultrasound and infrasound. Prog. Biophys. Mol. Biol. 2007, 93, 176–191. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.D.; Park, M.Y.; Foley, L.; Purcell-Estabrook, E.; Kim, H.; Fischer, K.; Maeng, L.-S.; Yoo, S.S. Image-guided focused ultrasound-mediated regional brain stimulation in sheep. Ultrasound Med. Biol. 2016, 42, 459–470. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.Y.; Lee, S.D.; Lee, W.; Chiu, A.; Yoo, S.-S. Suppression of EEG visual-evoked potentials in rats via neuromodulatory focused ultrasound. Neuroreport 2015, 26, 211. [Google Scholar] [CrossRef]

| Index | Definition | Safety Limit |
|---|---|---|
| Spatial peak time average intensity (ISPTA) | The maximum intensity measured within the sound field averaged over the sonication time. | ISPTA ≤ 50 mW/cm2 |
| Spatial peak pulse average intensity (ISPPA) | The maximum intensity measured within the sound field averaged over the duration of a single pulse | ISPPA ≤ 50 mW/cm2 |
| Mechanical index (MI) | MI ≤ 0.23 | |
| Thermal index (TI) | TI ≤ 1 |
| Authors | Transducer | Acoustic Frequency (MHz) | Resolution * (mm) | ISPPA (W/cm2) | PRF (Hz) | Duty Cycle (%) | Stimulation Time (s) | Region | Species | Major Findings and Experimental Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Yoo et al. [52] | single element | 0.69 | 2.3 | 3.3–12.6 | 10–1000 | 5 | 0.5–2, 9 | V1, M1 | Rabbit (in vivo) | Ultrasound-induced excitation and inhibition of the neural activity. |
| Lee et al. [112] | single element | 0.25 | 47 | 1.7–14.3 | 500 | 50 | 0.3 | V1 | Sheep (in vivo) | Highly variable threshold acoustic intensity for focused ultrasonic stimulation. Possibility of hemorrhage. |
| Lee et al. [36] | single element | 0.27 | 3 | 16.6 | 500 | 50 | 0.3 | V1 | Human (in vivo) | Demonstrated ultrasound modulated activities in the primary somatosensory cortex and ultrasound induced phosphene perception. |
| Kim et al. [113] | single element | 0.35 | 3.7 (The full-width at 90% maximum) | 1, 3, 5 | 100 | 1, 5, 8.3 | 150 | Visual cortex | Rat (in vivo) | VEP was evoked or suppressed depending on the intensity and duty cycle of the acoustic wave |
| Naor et al. [37] | phased array | 0.5, 1 | 0.4–0.53 | 0.1–0.4, 5.2–8.5 | 1900–2000, 1667 | 10~20 | 5~20 | RGCs | Rat (in vivo) | Conceptualized an acoustic retinal prosthesis and adapted the algorithms to generate spatially patterned multifocal stimulation. |
| Menz et al. [54] | single element | 43 | ~0.1 | 20~60 | 0.5–1 M | 100 | 1 | RGCs | Tiger salamander (in vitro) | Conducted high frequency retinal stimulation and demonstrated a spatial precision of ~100 um. |
| Jiang et al. [38] | single element | 2 | 1.6 | 12.84 (ISPTA) | 1000 | 50 | 0.4 | RGCs | Rat (in vitro) | Found the difference in the response pattern of the RGCs to light vs. ultrasound stimuli, and the dual-peak responses to ultrasound that are intensity dependent. |
| Gao et al. [91] | contact lens | 6~0.3 | 12.5–5 | 8.1, 9.3, 10 | 1000 | - | 0.3 | RGCs | Simulation | Proposed a contact lens form transducer array that utilizes the tear film for acoustic coupling. |
| Yu et al. [93] | racing array | 2.5, 5, 10 | 1.3, 0.6, 0.26 | 0.2–0.6 | - | - | - | RGCs | Simulation | Proposed a racing ring lens design to avoid the acoustic exposure of the lens suitable for high frequency stimulation. |
| Lu et al. [107] | single element | 0.5 | 2.4 (The full-width at 25% maximum) | 115.8 | 100–500 | 33.3–50 | 0.002–0.03 | Visual cortex | Rat (in vivo) | Demonstrated VEP elicited by focused transcranial ultrasonic stimulation in both normal and retinal degenerative rats. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, P.-A.; Huang, K.; Zhou, Q.; Humayun, M.S.; Yue, L. Ultrasonic Retinal Neuromodulation and Acoustic Retinal Prosthesis. Micromachines 2020, 11, 929. https://doi.org/10.3390/mi11100929
Lo P-A, Huang K, Zhou Q, Humayun MS, Yue L. Ultrasonic Retinal Neuromodulation and Acoustic Retinal Prosthesis. Micromachines. 2020; 11(10):929. https://doi.org/10.3390/mi11100929
Chicago/Turabian StyleLo, Pei-An, Kyana Huang, Qifa Zhou, Mark S. Humayun, and Lan Yue. 2020. "Ultrasonic Retinal Neuromodulation and Acoustic Retinal Prosthesis" Micromachines 11, no. 10: 929. https://doi.org/10.3390/mi11100929
APA StyleLo, P.-A., Huang, K., Zhou, Q., Humayun, M. S., & Yue, L. (2020). Ultrasonic Retinal Neuromodulation and Acoustic Retinal Prosthesis. Micromachines, 11(10), 929. https://doi.org/10.3390/mi11100929






