Constructing a Dual-Function Surface by Microcasting and Nanospraying for Efficient Drag Reduction and Potential Antifouling Capabilities
Abstract
1. Introduction
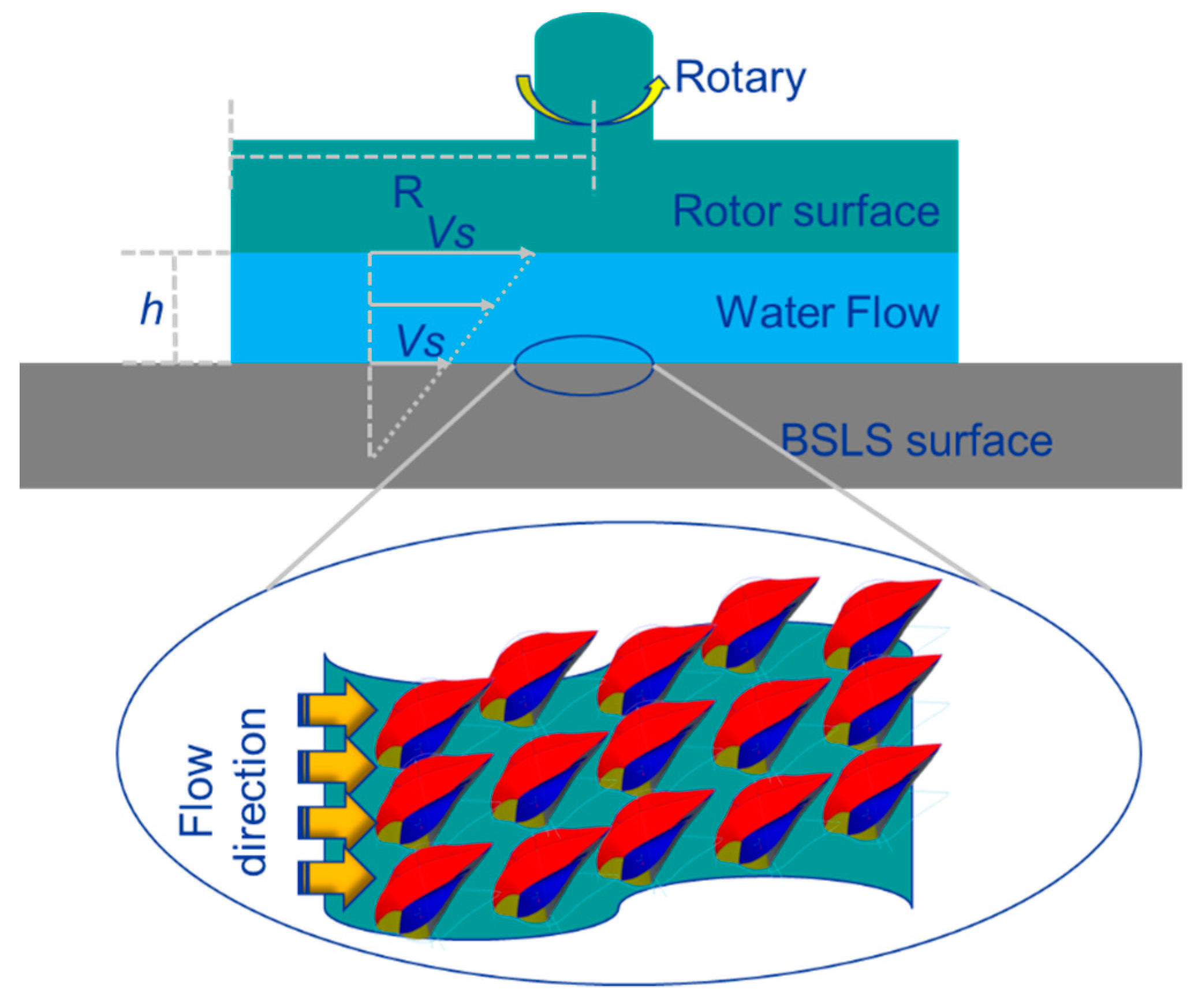
2. Materials and Methods
3. Results and Discussion
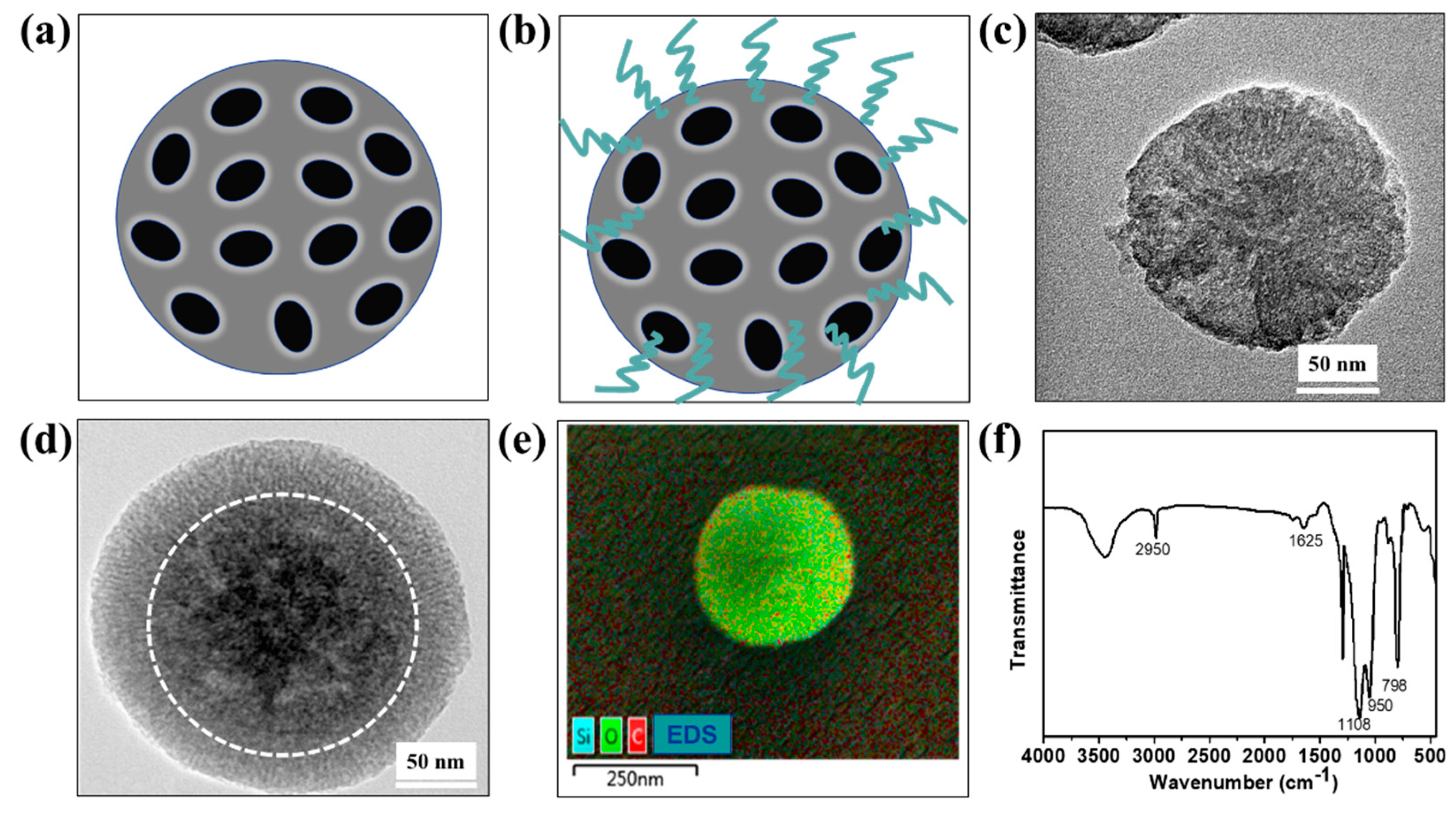
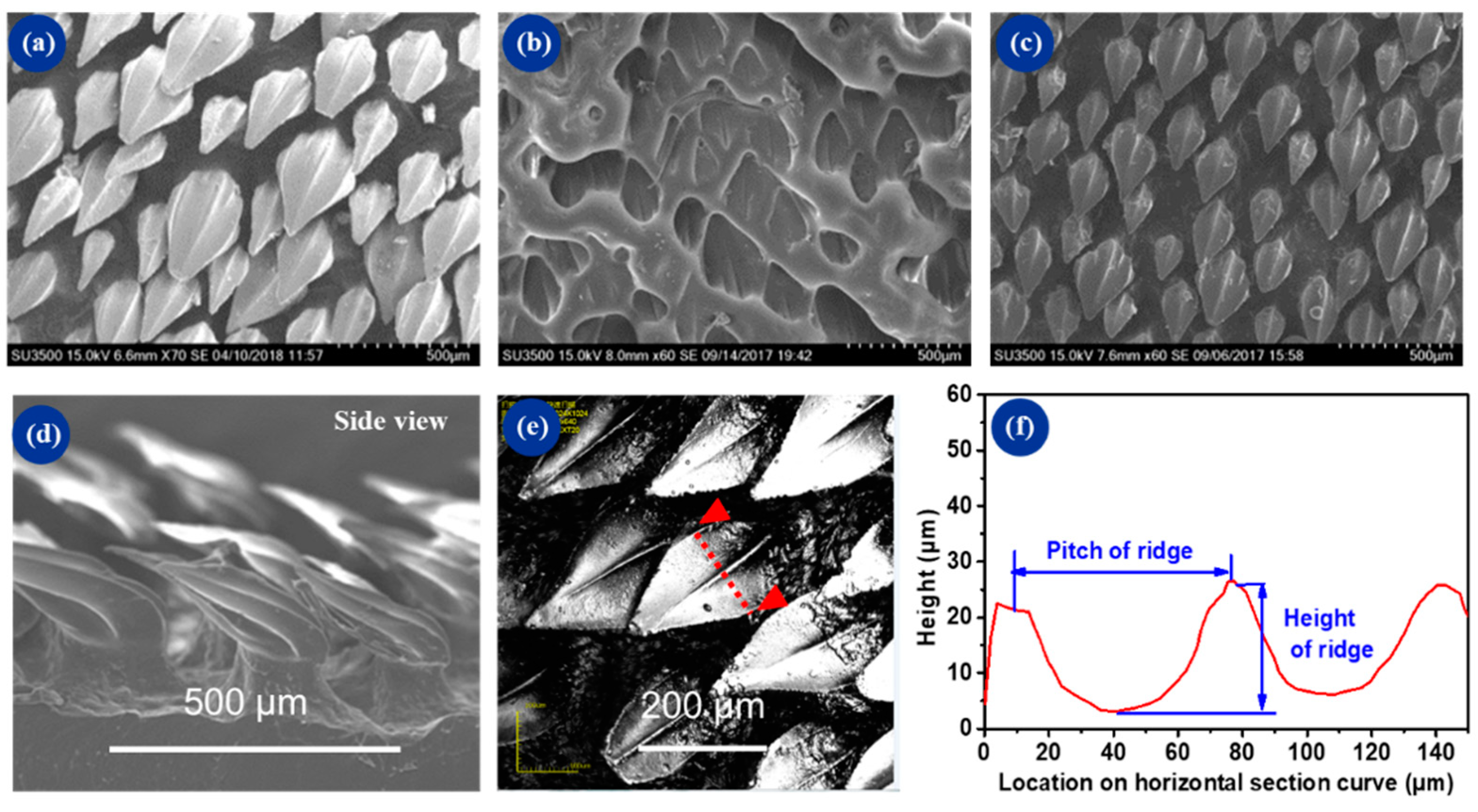
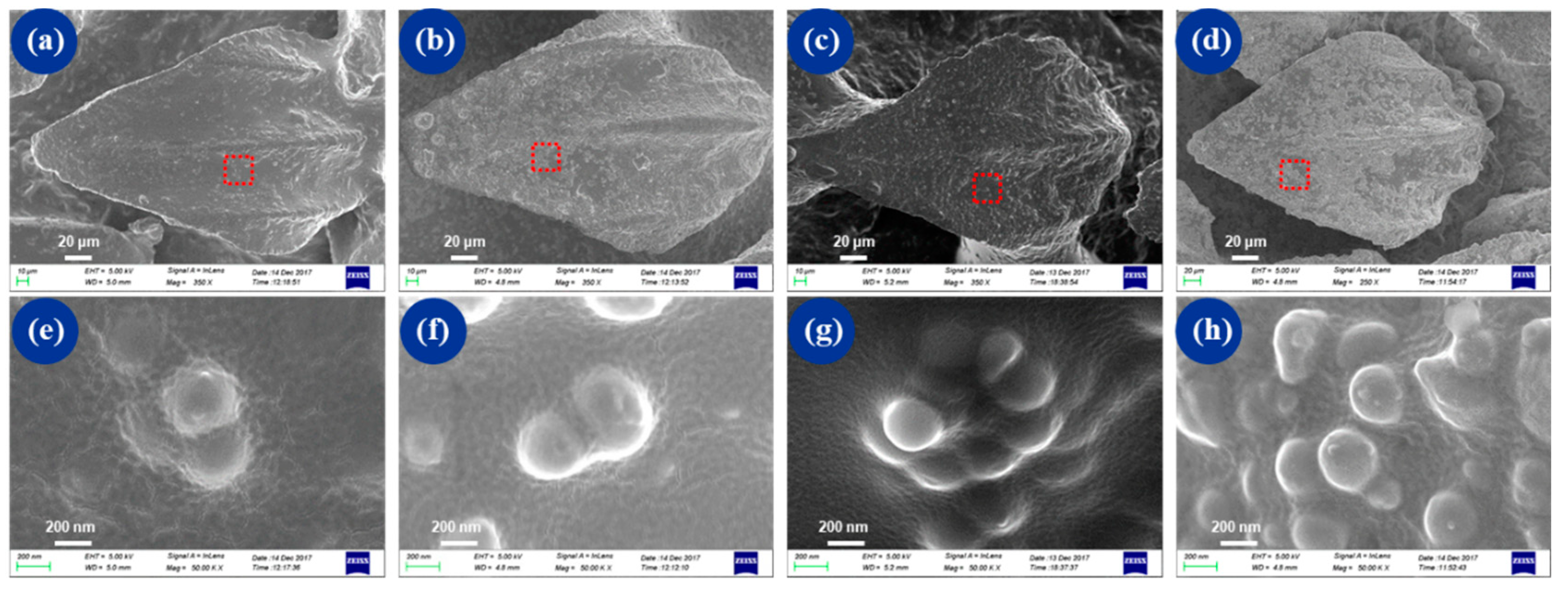
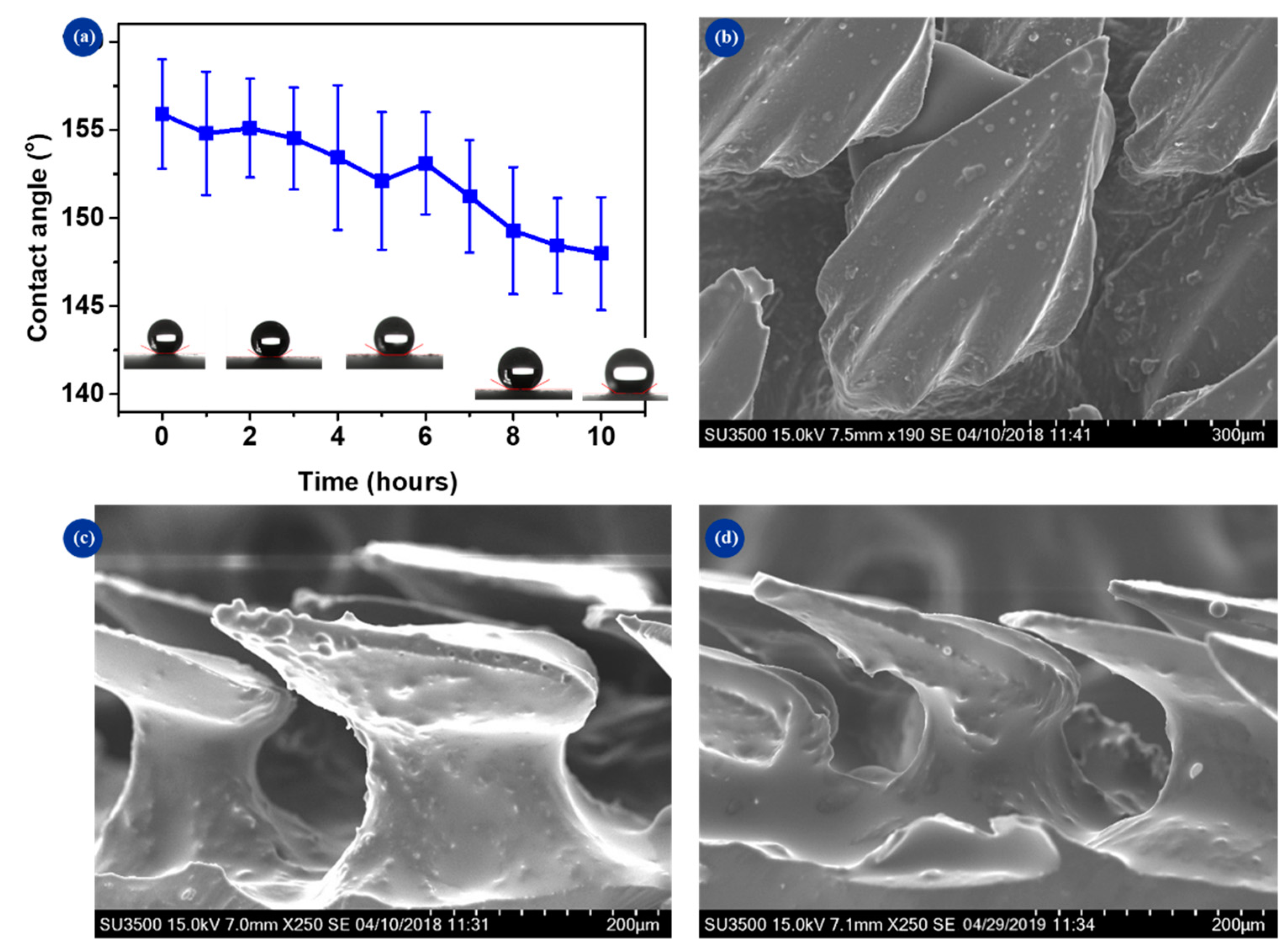
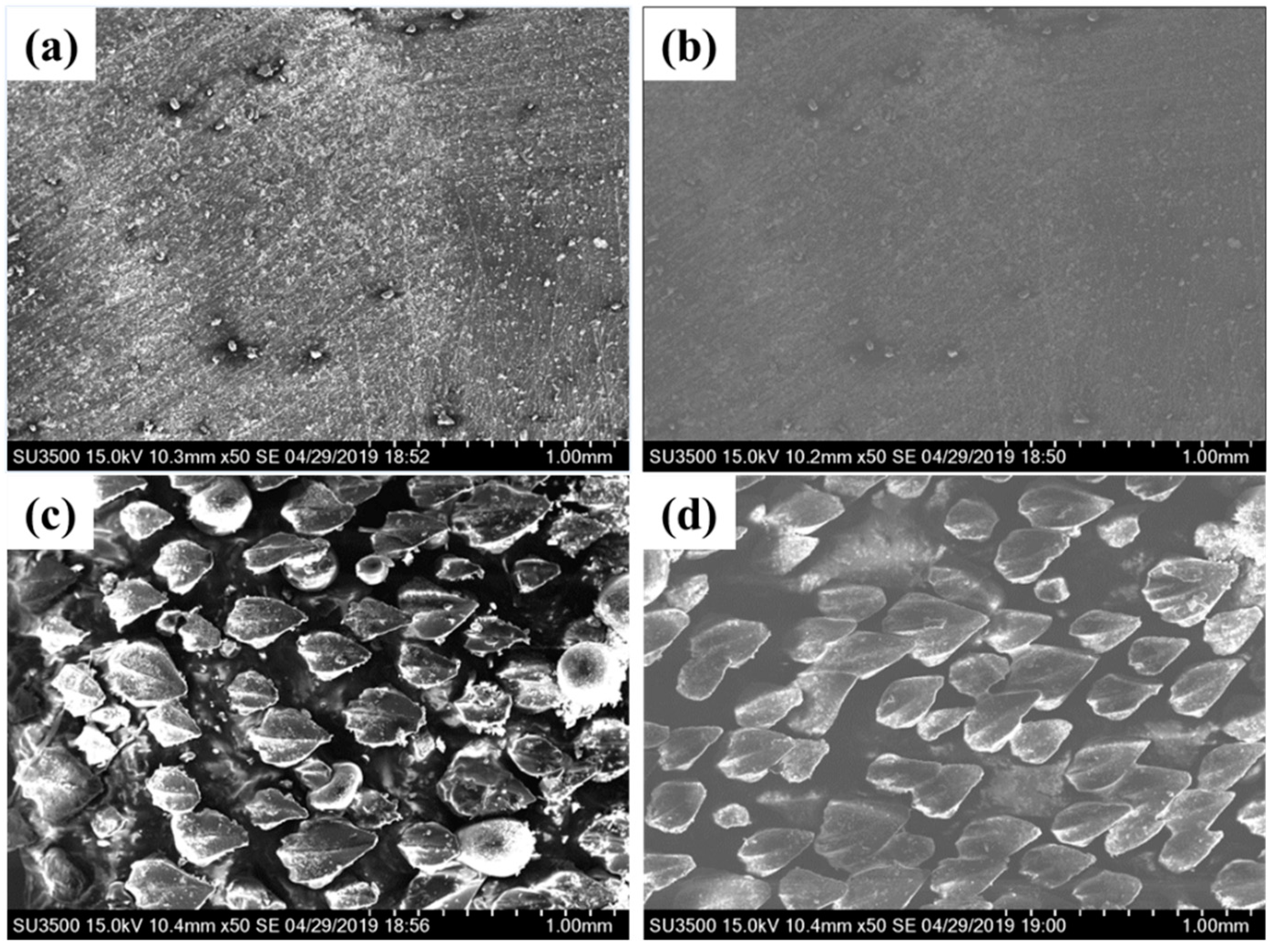
3.1. Surface Characterization
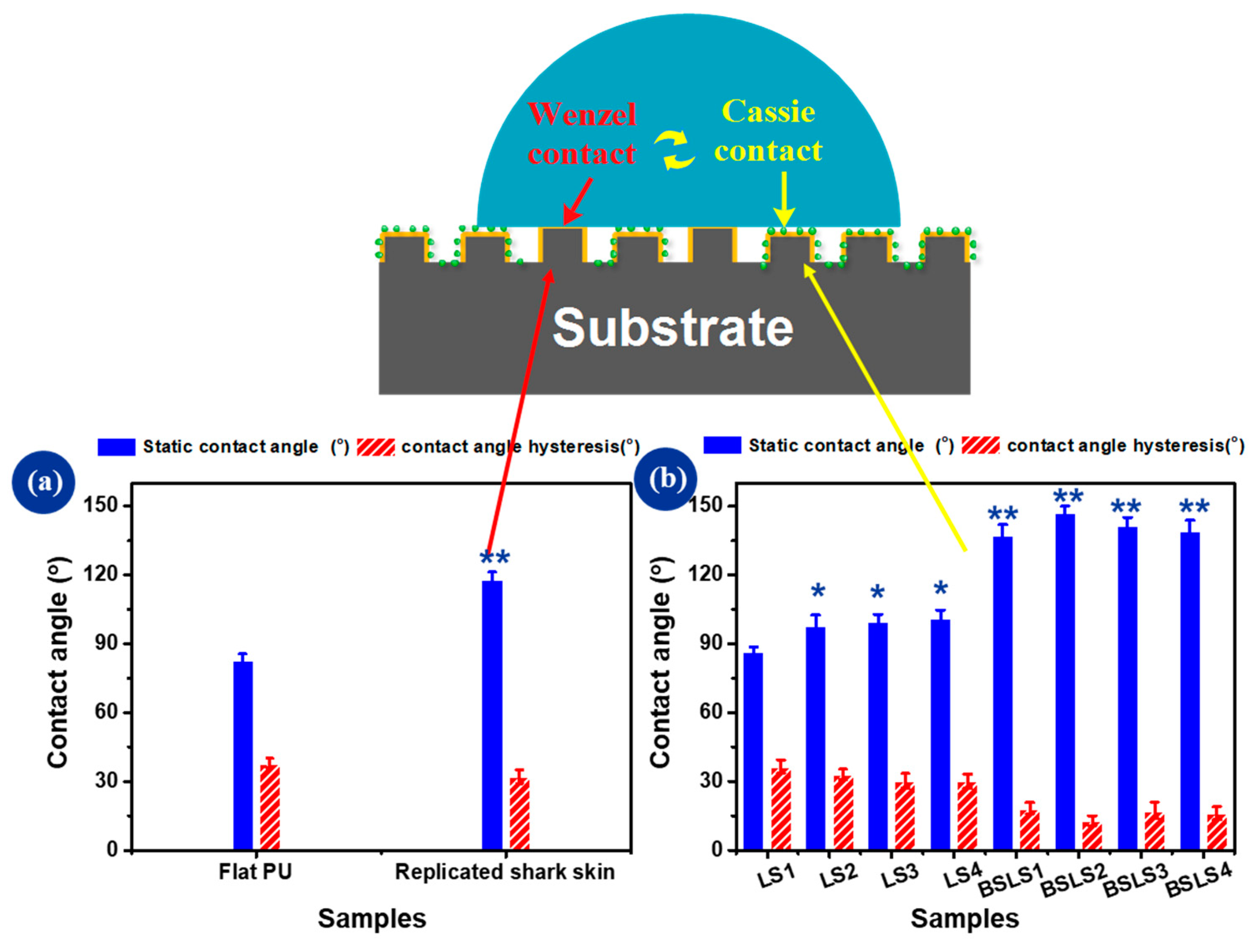
3.2. Surface Wettability
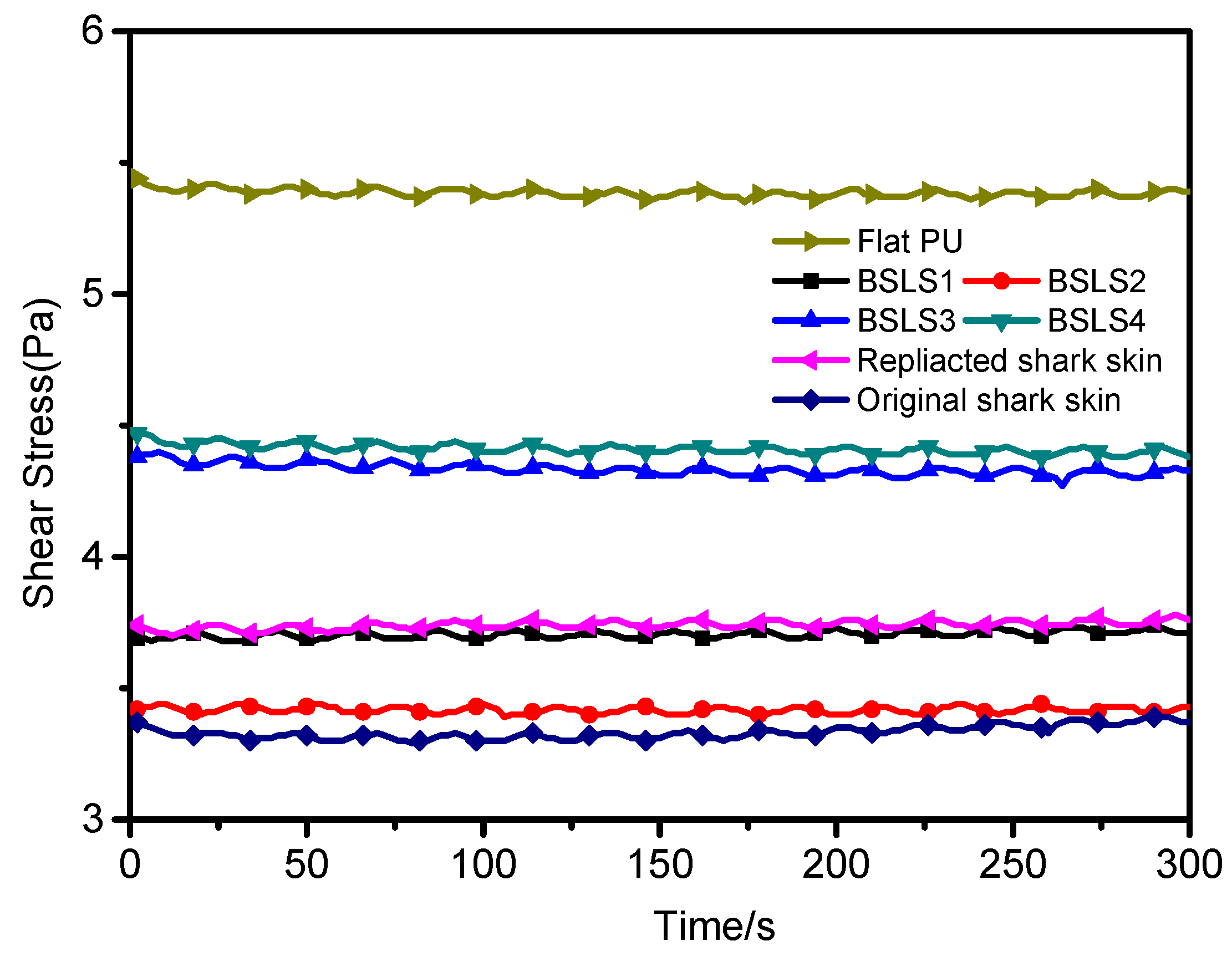
3.3. Drag Reduction Performance
3.4. Antifouling and Self-Cleaning Performance
3.5. Surface Duration
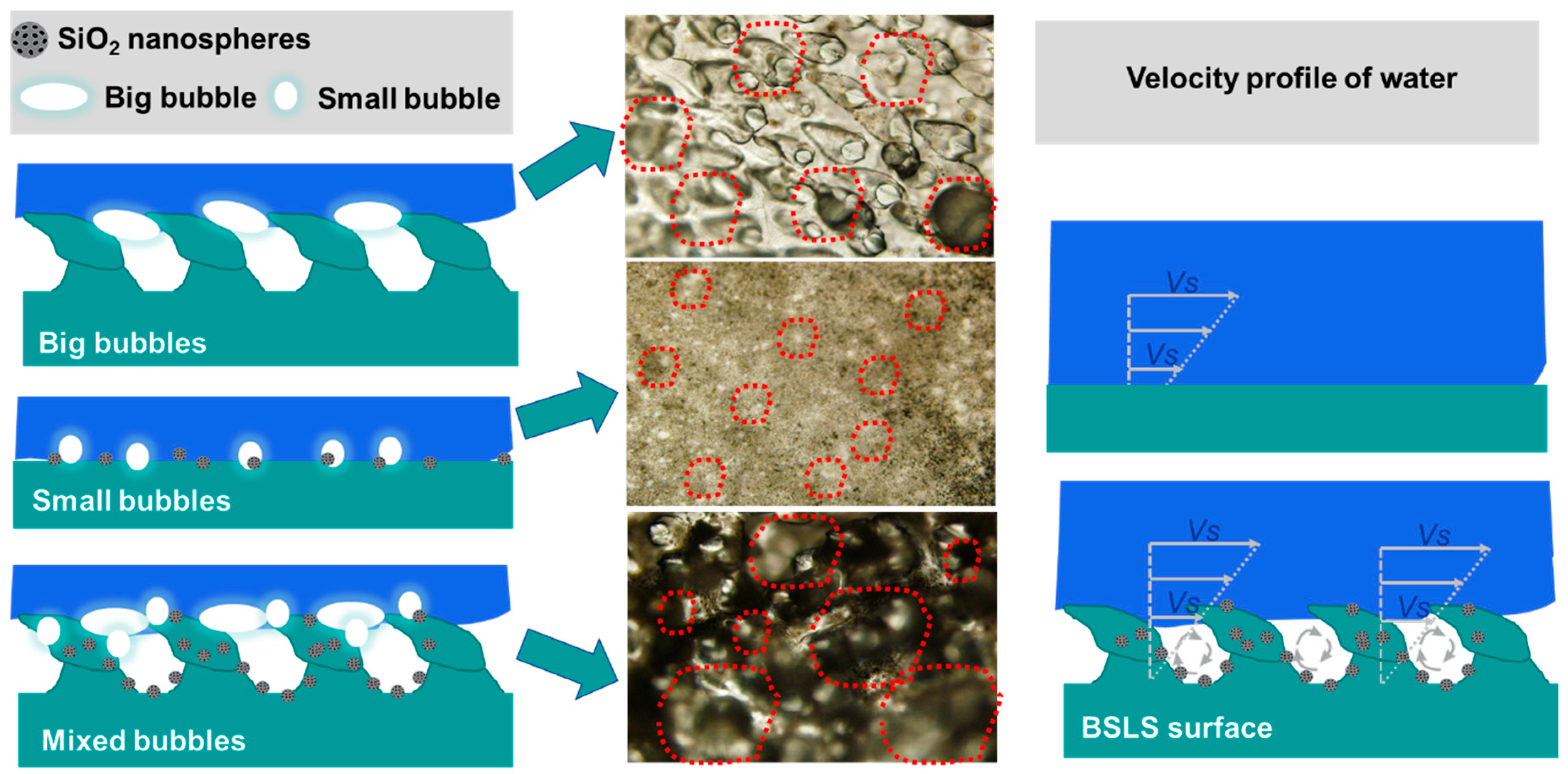
3.6. Antidrag and Antifouling Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Wu, W.; Wang, X.; Luo, Z.; Liang, Y.; Zhou, F. A replication strategy for complex micro/nanostructures with superhydrophobicity and superoleophobicity and high contrast adhesion. Soft Matter 2009, 5, 3097–3105. [Google Scholar] [CrossRef]
- He, X.; Cao, P.; Tian, F.; Bai, X.; Yuan, C. Autoclaving-Induced in-Situ Grown Hierarchical Structures for Construction of Superhydrophobic Surfaces: A New Route to Fabricate Antifouling Coatings. Surf. Coat. Technol. 2019, 357, 180–188. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Nadhe, S.; Wadhwani, S.; Shedbalkar, U.; Chopade, B.A. Nanoparticles for Control of Biofilms of Acinetobacter Species. Materials 2016, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Sun, Y.; Lang, Y.; Wang, L.; Liu, B.; Zhang, Z. Effect of CNT/PDMS Nanocomposites on the Dynamics of Pioneer Bacterial Communities in the Natural Biofilms of Seawater. Materials 2018, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Kumar, A.; Mills, S.; Bazaka, K.; Bajema, N.; Atkinson, I.; Jacob, M.V. Biodegradable optically transparent terpinen-4-ol thin films for marine antifouling applications. Surf. Coat. Technol. 2018, 349, 426–433. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic hierarchically structured surfaces in biology: Evolution, structural principles and biomimetic applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20160191. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, G.J. A New Method for Producing Lotus Effect on a Biomimetic Shark Skin. J. Colloid Interface Sci. 2012, 388, 235–242. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-inspired design of multiscale structures for function integration. Nano Today 2011, 6, 155–175. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter 2008, 4, 1943. [Google Scholar] [CrossRef]
- Bixler, G.D.; Theiss, A.; Bhushan, B.; Lee, S.C. Anti-fouling properties of microstructured surfaces bio-inspired by rice leaves and butterfly wings. J. Colloid Interface Sci. 2014, 419, 114–133. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Bio-Inspired Self-Cleaning Surfaces. Annu. Rev. Mater. Res. 2012, 42, 231–263. [Google Scholar] [CrossRef]
- Wang, N.; Tang, L.; Cai, Y.; Tong, W.; Xiong, D. Scalable superhydrophobic coating with controllable wettability and investigations of its drag reduction. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 290–295. [Google Scholar] [CrossRef]
- Tuo, Y.; Chen, W.; Zhang, H.; Li, P.; Liu, X. One-Step Hydrothermal Method to Fabricate Drag Reduction Superhydrophobic Surface on Aluminum Foil. Appl. Surf. Sci. 2018, 446, 230–235. [Google Scholar] [CrossRef]
- Li, Y.; Mao, H.; Hu, P.; Hermes, M.; Lim, H.; Yoon, J.; Luhar, M.; Chen, Y.; Wu, W. Bioinspired Functional Surfaces Enabled by Multiscale Stereolithography. Adv. Mater. Technol. 2019, 4, 1800638. [Google Scholar] [CrossRef]
- Lu, Y. Fabrication of a lotus leaf-like hierarchical structure to induce an air lubricant for drag reduction. Surf. Coat. Technol. 2017, 331, 48–56. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Bioinspired micro/nanostructured surfaces for oil drag reduction in closed channel flow. Soft Matter 2013, 9, 1620–1635. [Google Scholar] [CrossRef]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef]
- West, J.; Critchlow, G.; Lake, D.; Banks, R. Development of a superhydrophobic polyurethane-based coating from a two-step plasma-fluoroalkyl silane treatment. Int. J. Adhes. Adhes. 2016, 68, 195–204. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, M.; Li, Z.; Song, X.; Wang, H.; Pei, X.; Zhou, F. Slip flow of diverse liquids on robust superomniphobic surfaces. J. Colloid Interface Sci. 2014, 414, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xue, Y.; Pei, X.; Cai, M.; Duan, H.; Huck, W.T.S.; Zhou, F.; Xue, Q. Adhesion-Regulated Switchable Fluid Slippage on Superhydrophobic Surfaces. J. Phys. Chem. C 2014, 118, 2564–2569. [Google Scholar] [CrossRef]
- Cheng, Y.; Wei, H.; Sun, R.; Tian, Z.; Zheng, X. Rapid Method for Protein Quantitation by Bradford Assay after Elimination of the Interference of Polysorbate 80. Anal. Biochem. 2016, 494, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, T.A.; Dibrell, M.M.; Li, Z.; Thomas, C.R.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar]
- Angelos, S.; Liong, M.; Choi, E.; Zink, J.I. Mesoporous silicate materials as substrates for molecular machines and drug delivery. Chem. Eng. J. 2008, 137, 4–13. [Google Scholar] [CrossRef]
- Michailidis, M.; Sorzabal-Bellido, I.; Adamidou, E.A.; Diaz-Fernandez, Y.A.; Aveyard, J.; Wengier, R.; Grigoriev, D.; Raval, R.; Benayahu, Y.; Shchukin, D.; et al. Modified Mesoporous Silica Nanoparticles with a Dual Synergetic Antibacterial Effect. ACS Appl. Mater. Interfaces 2017, 9, 38364–38372. [Google Scholar] [CrossRef]
- Qin, L.; Feng, X.; Hafezi, M.; Zhang, Y.; Guo, J.; Dong, G.; Qin, Y. Investigating the Tribological and Biological Performance of Covalently Grafted Chitosan Coatings on Co-Cr-Mo Alloy. Tribol. Int. 2018, 127, 302–312. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–550. [Google Scholar] [CrossRef]
- Qin, L.; Lin, P.; Zhang, Y.; Dong, G.; Zeng, Q. Influence of Surface Wettability on the Tribological Properties of Laser Textured Co-Cr-Mo Alloy in Aqueous Bovine Serum Albumin Solution. Appl. Surf. Sci. 2013, 268, 79–86. [Google Scholar] [CrossRef]
- Baron, A.; Quadrio, M.; Vigevano, L. On the boundary layer/riblets interaction mechanisms and the prediction of turbulent drag reduction. Int. J. Heat Fluid Flow 1993, 14, 324–332. [Google Scholar] [CrossRef]
- Dean, B.; Bhushan, B. Shark-Skin Surfaces for Fluid-Drag Reduction in Turbulent Flow: A Review. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 5737. [Google Scholar] [CrossRef]
- Jafargholinejad, S.; Pishevar, A.; Sadeghy, K. On the Use of Rotating-Disk Geometry for Evaluating the Drag-Reducing Efficiency of Polymeric and Surfactant Additives. J. Appl. Fluid Mech. 2011, 4, 1–5. [Google Scholar]
- Pertsin, A.J.; Grunze, M. Computer Simulation of Water near the Surface of Oligo(ethylene glycol)-Terminated Alkanethiol Self-Assembled Monolayers. Langmuir 2000, 16, 8829–8841. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic degradation of bisphenol-A with immobilized laccase on TiO2 solgel coated PVDF membrane. J. Membr. Sci. 2014, 469, 19–30. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R. Novel membrane surface modification to enhance anti-oil fouling property for membrane distillation application. J. Membr. Sci. 2013, 447, 26–35. [Google Scholar] [CrossRef]
- Hashino, M.; Hirami, K.; Ishigami, T.; Ohmukai, Y.; Maruyama, T.; Kubota, N.; Matsuyama, H. Effect of kinds of membrane materials on membrane fouling with BSA. J. Membr. Sci. 2011, 384, 157–165. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Cottin-Bizonne, C.; Barentin, C.; Bocquet, L. Scaling laws for slippage on superhydrophobic fractal surfaces. Phys. Fluids 2012, 24, 12001. [Google Scholar] [CrossRef]
- Cao, M.; Li, Z.; Ma, H.; Geng, H.; Yu, C.; Jiang, L. Is Superhydrophobicity Equal to Underwater Superaerophilicity: Regulating the Gas Behavior on Superaerophilic Surface via Hydrophilic Defects. ACS Appl. Mater. Interfaces 2018, 10, 20995–21000. [Google Scholar] [CrossRef]
- Fan, L.; Li, B.; Zhang, J. Antibioadhesive Superhydrophobic Syringe Needles Inspired by Mussels and Lotus Leafs. Adv. Mater. Interfaces 2015, 2, 1500019. [Google Scholar] [CrossRef]


| Samples | Flat PU | LS1 | LS2 | LS3 | LS4 | Replicated Shark Skin | BSLS1 | BSLS2 | BSLS3 | BSLS4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Δm (mg) | 13.4 ± 2.3 | 12.1 ± 2.4 | 12.5 ± 2.6 | 13.2 ± 3.1 | 13.1 ± 2.9 | 9.2 ± 1.9 | 3.5 ± 0.9 | 3.1 ± 0.8 | 3.4 ± 1.1 | 4.1 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Hafezi, M.; Yang, H.; Dong, G.; Zhang, Y. Constructing a Dual-Function Surface by Microcasting and Nanospraying for Efficient Drag Reduction and Potential Antifouling Capabilities. Micromachines 2019, 10, 490. https://doi.org/10.3390/mi10070490
Qin L, Hafezi M, Yang H, Dong G, Zhang Y. Constructing a Dual-Function Surface by Microcasting and Nanospraying for Efficient Drag Reduction and Potential Antifouling Capabilities. Micromachines. 2019; 10(7):490. https://doi.org/10.3390/mi10070490
Chicago/Turabian StyleQin, Liguo, Mahshid Hafezi, Hao Yang, Guangneng Dong, and Yali Zhang. 2019. "Constructing a Dual-Function Surface by Microcasting and Nanospraying for Efficient Drag Reduction and Potential Antifouling Capabilities" Micromachines 10, no. 7: 490. https://doi.org/10.3390/mi10070490
APA StyleQin, L., Hafezi, M., Yang, H., Dong, G., & Zhang, Y. (2019). Constructing a Dual-Function Surface by Microcasting and Nanospraying for Efficient Drag Reduction and Potential Antifouling Capabilities. Micromachines, 10(7), 490. https://doi.org/10.3390/mi10070490






