Microfluidic In-Flow Decantation Technique Using Stepped Pillar Arrays and Hydraulic Resistance Tuners
Abstract
1. Introduction
2. Theory, Simulations and Experiments
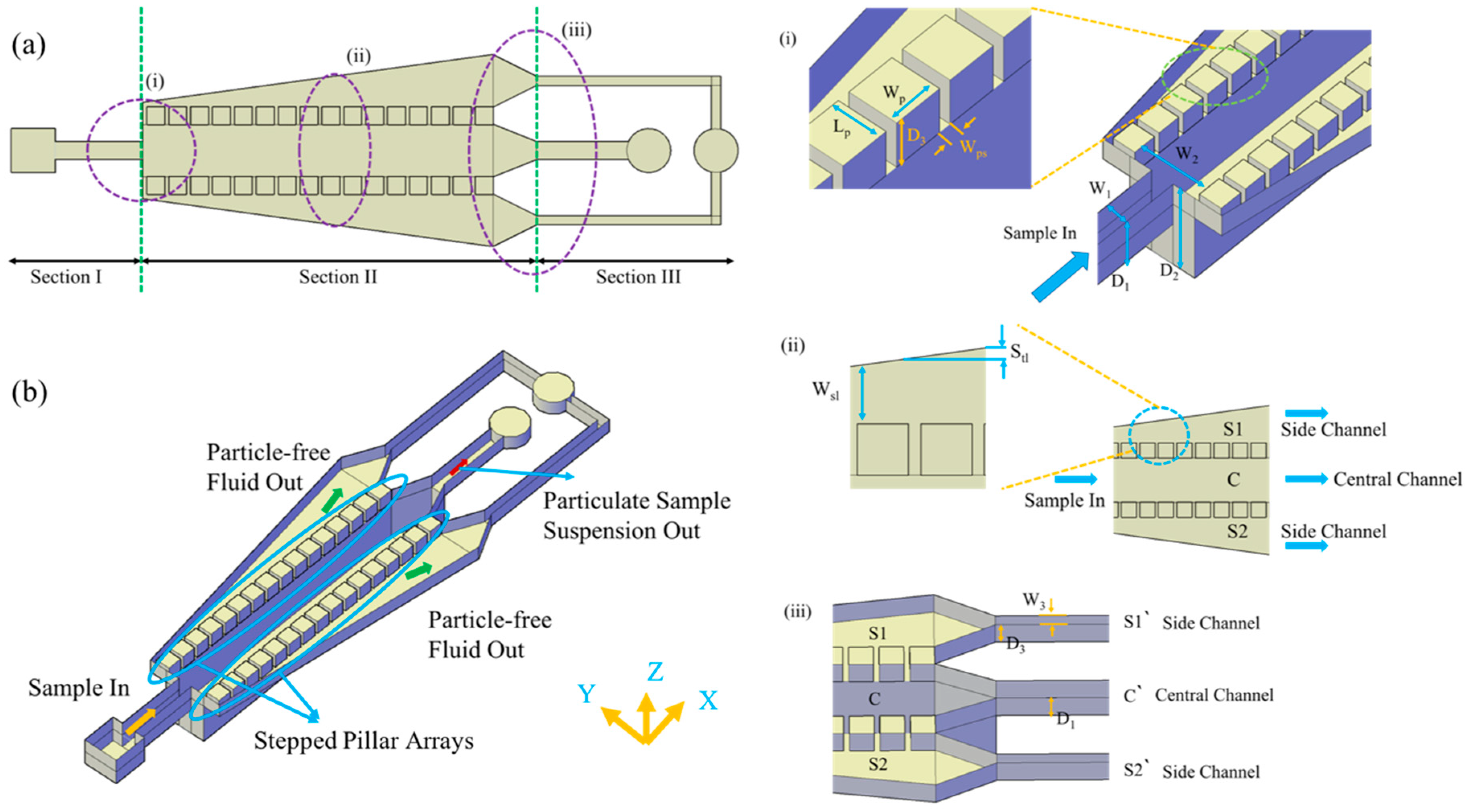
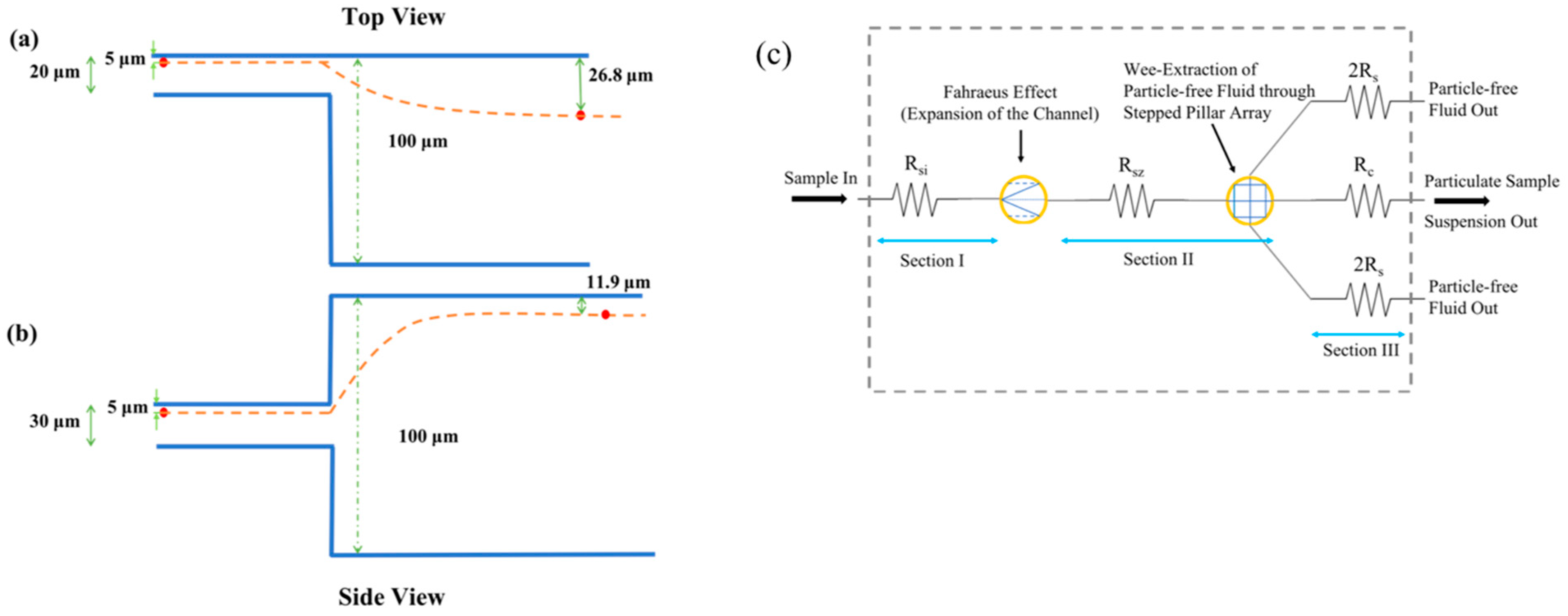
2.1. Theory and Principle of Operation
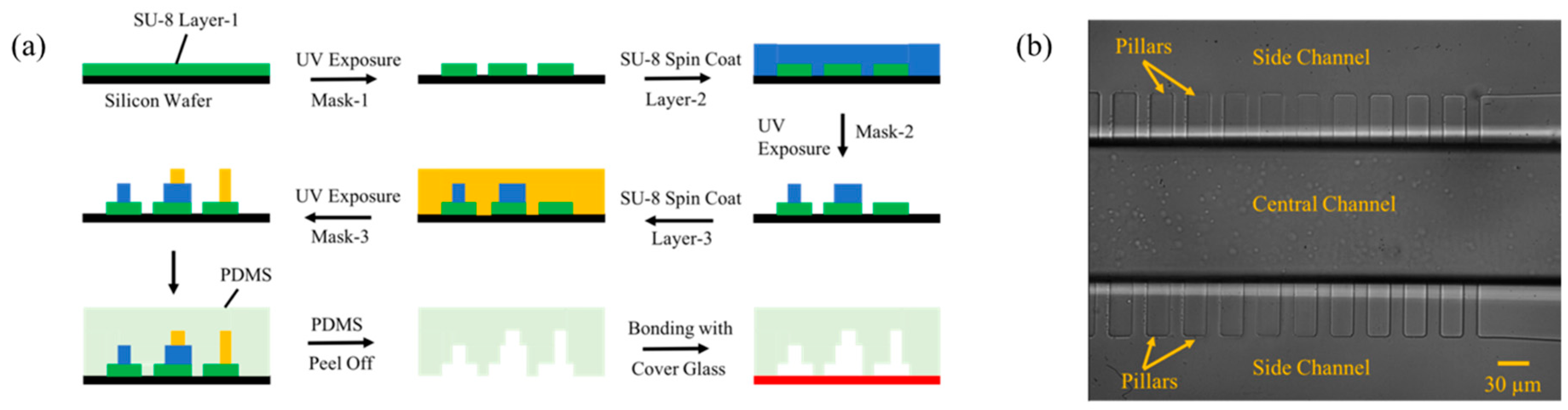
2.2. Device Fabrication
2.3. Sample Preparation
2.4. Simulations
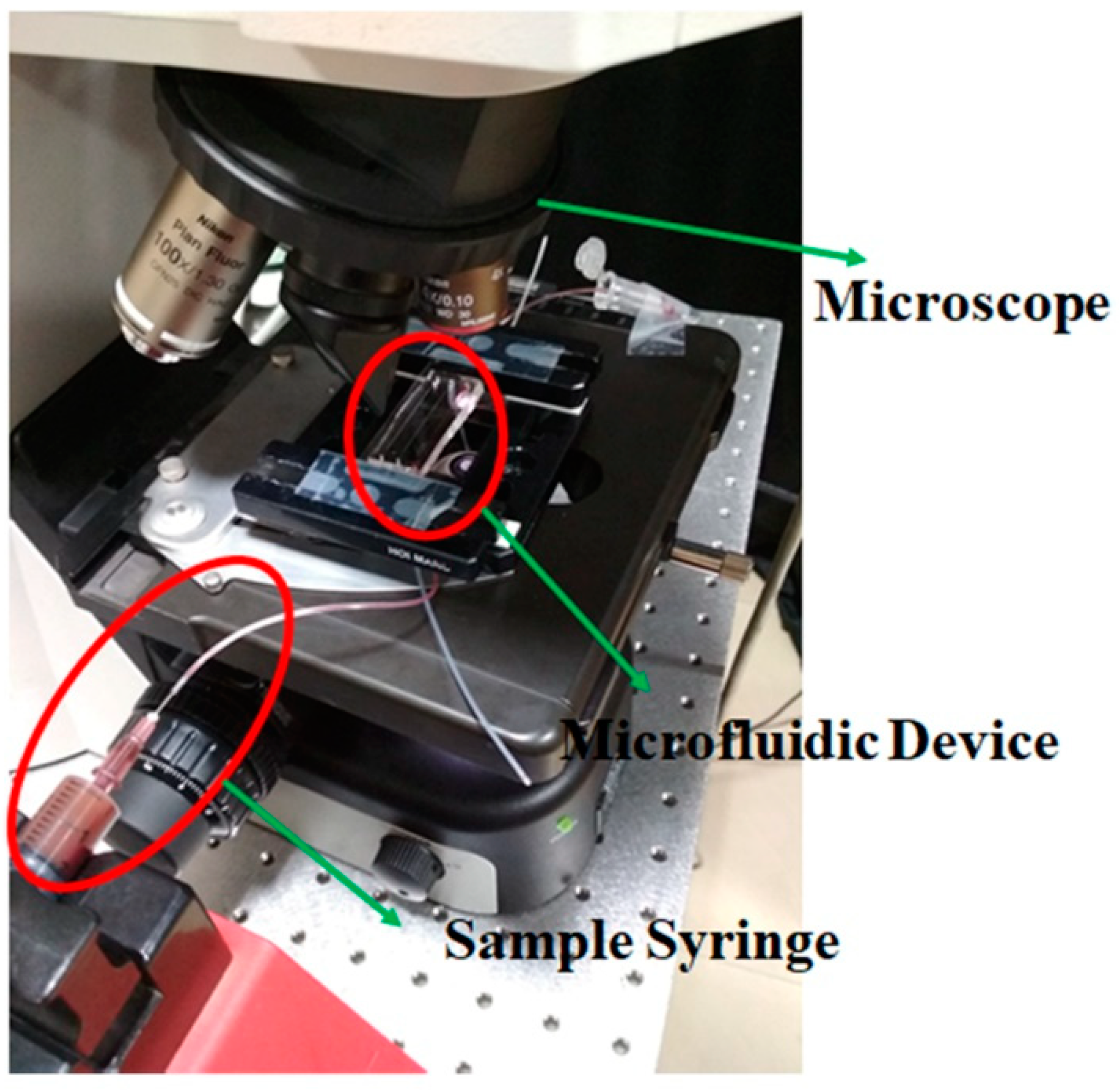
2.5. Experimental Procedure
3. Results and Discussion
3.1. Simulations
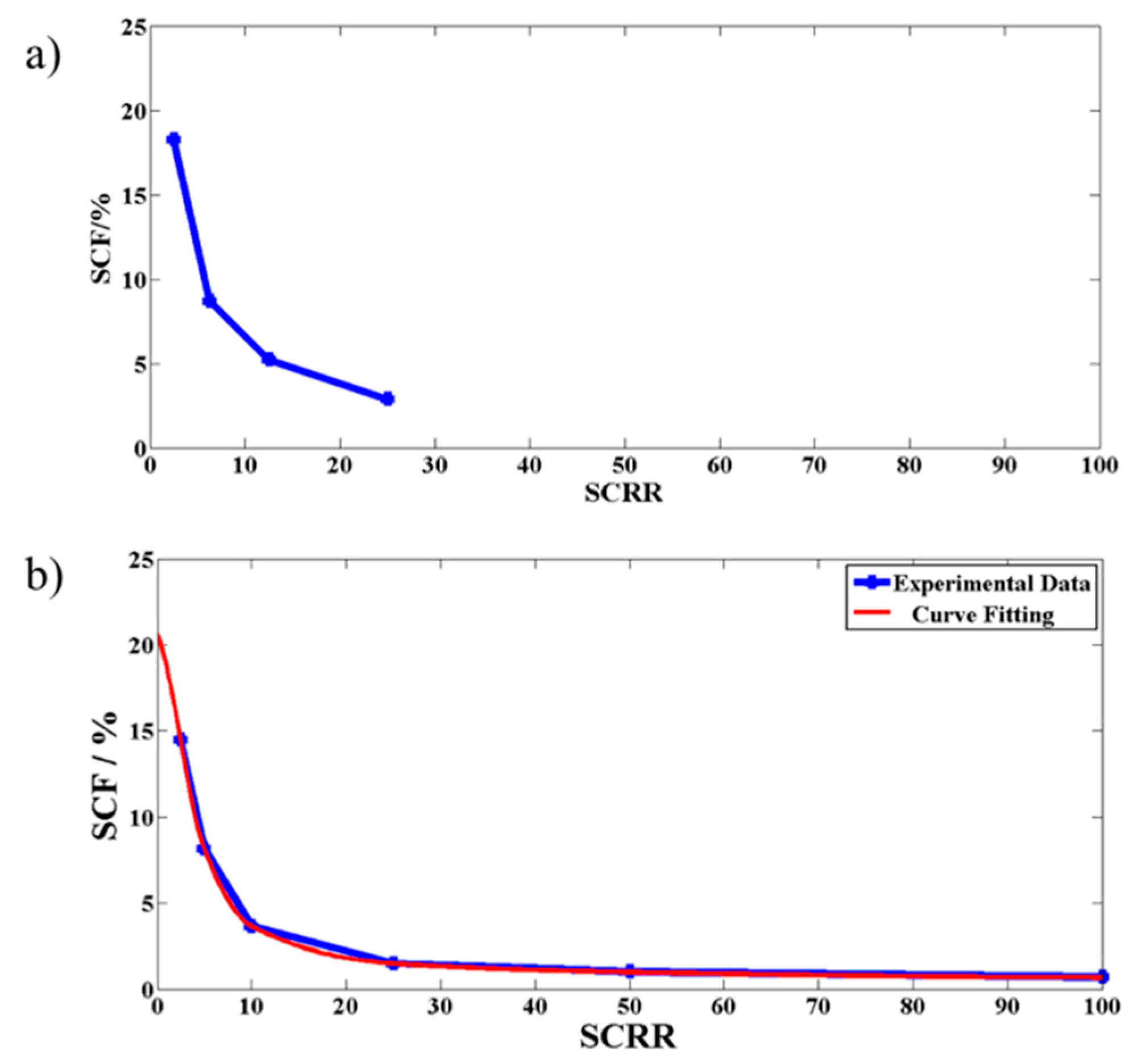
3.1.1. Effect of Section II Parameters [Height of Pillars (D3), Spacing between the Pillars (Wps), Number of Pillars (N), Slope of the Side Channel (Sslp), and Flow Rate (Q)] and Section III Parameters [Side Channel to Central Resistance Ratio (SCRR)] on Side Channel to Central Channel Flow Rate Ratio (SCF)
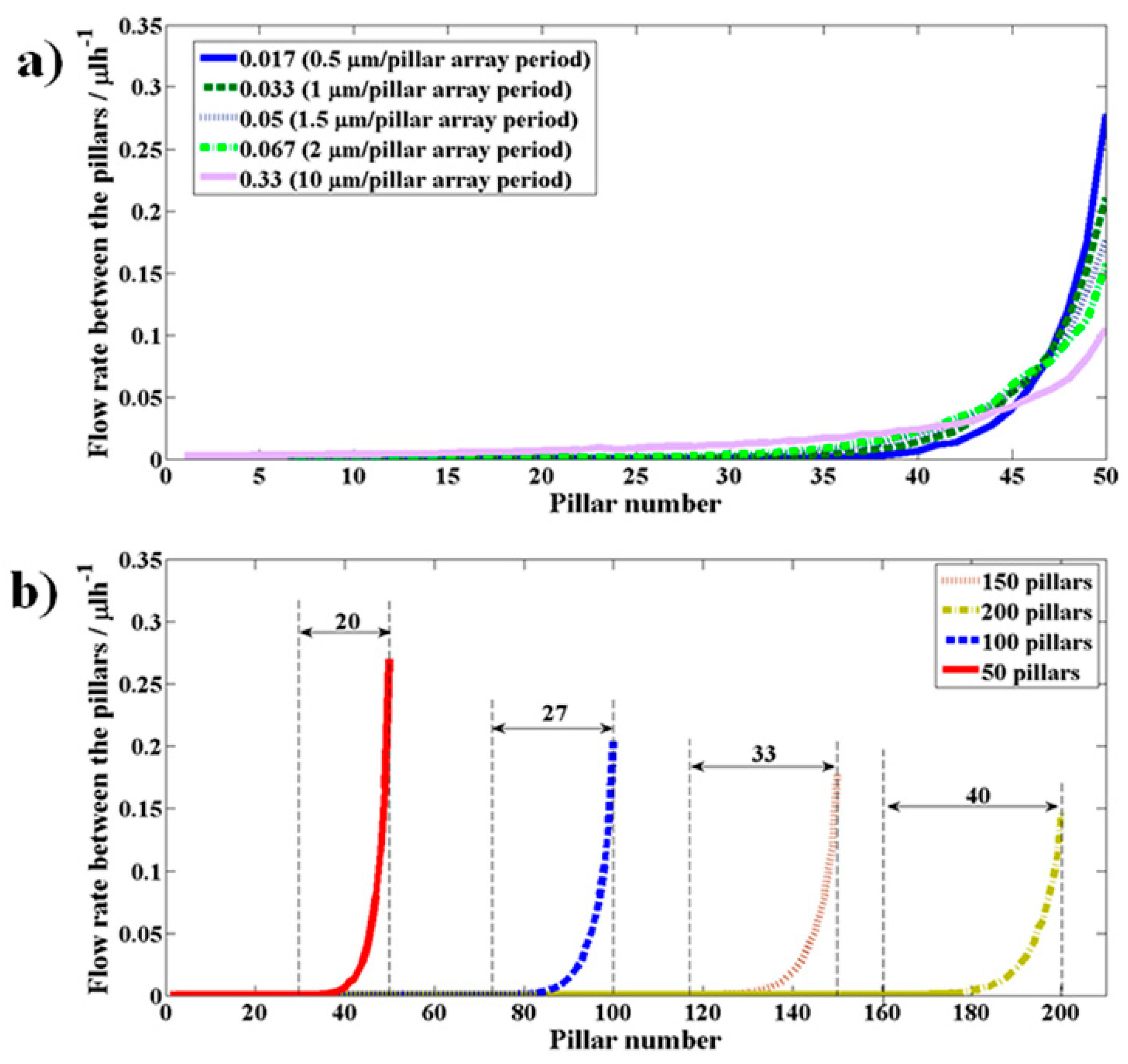
3.1.2. Effect of Section II Parameters [Number of Pillars (N) and Side Channel Slope (Sslp)] on the Uniformity of the Flow Across the Pillar Gaps
3.2. Experimental
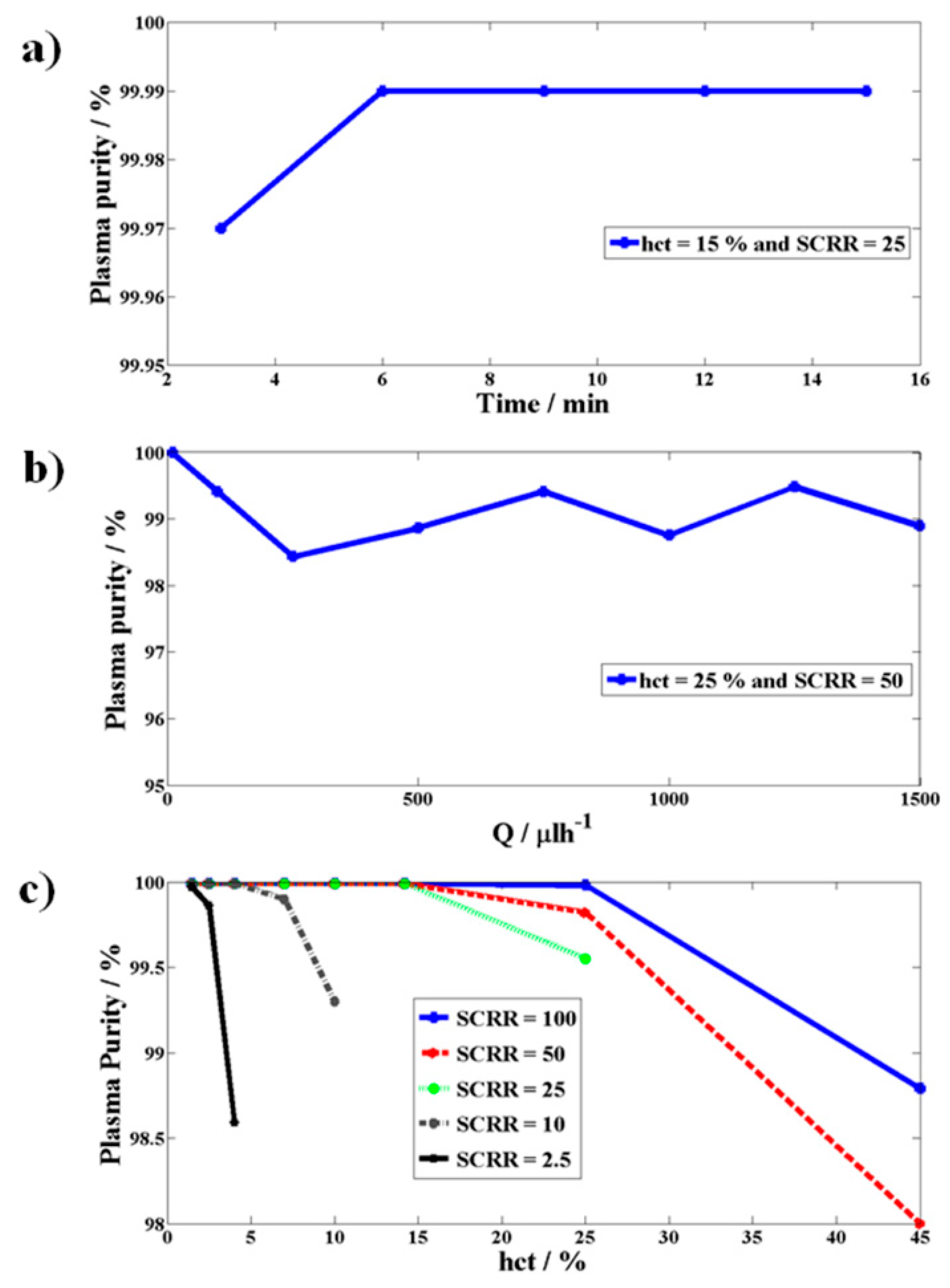
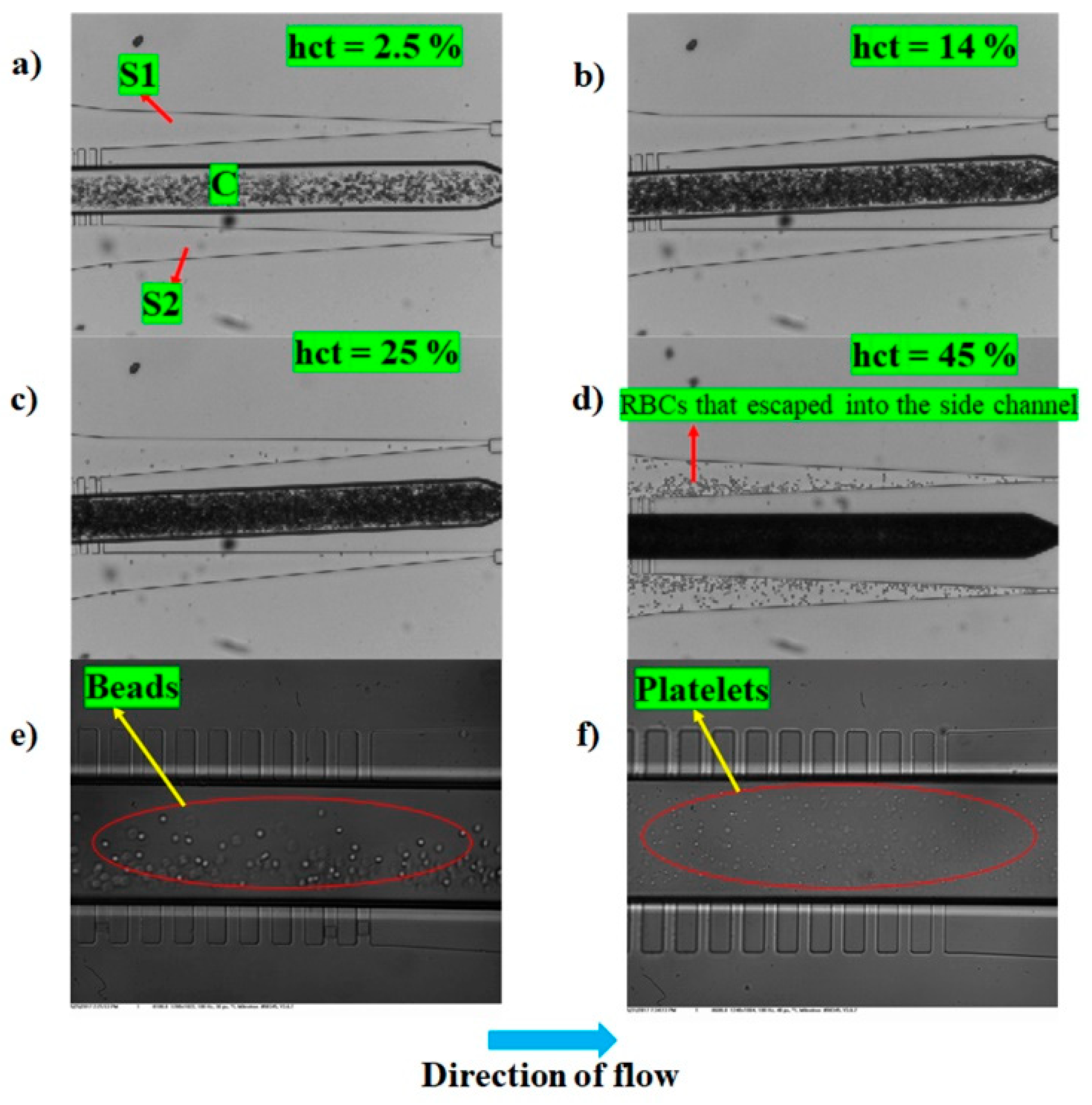
Variation of Purity of Plasma Generated as a Function of Time, Flow Rate (Q), Hematocrit, and SCRR
3.3. Discussion on the Choice of Parameters for Wee-Extraction
3.4. Enhancing the Yield through Multiplexing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arifin, D.R.; Yeo, L.Y.; Friend, J.R. Microfluidic blood plasma separation via bulk electrohydrodynamic flows. Biomicrofluidics 2006, 1, 014103. [Google Scholar] [CrossRef]
- Lenshof, A.; Ahmad-Tajudin, A.; Järås, K.; Swärd-Nilsson, A.-M.; Åberg, L.; Marko-Varga, G.; Malm, J.; Lilja, H.; Laurell, T. Acoustic Whole Blood Plasmapheresis Chip for Prostate Specific Antigen Microarray Diagnostics. Anal. Chem. 2009, 81, 6030–6037. [Google Scholar] [CrossRef]
- Jiang, H.; Weng, X.; Chon, C.H.; Wu, X.; Li, D. A microfluidic chip for blood plasma separation using electro-osmotic flow control. J. Micromech. Microeng. 2011, 21, 085019. [Google Scholar] [CrossRef]
- Shih, C.-H.; Lu, C.-H.; Yuan, W.-L.; Chiang, W.-L.; Lin, C.-H. Supernatant decanting on a centrifugal platform. Biomicrofluidics 2011, 5, 013414. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, P.-H.; Chung, C.-K. Microfluidic chip for plasma separation from undiluted human whole blood samples using low voltage contactless dielectrophoresis and capillary force. Lab Chip 2014, 14, 1996–2001. [Google Scholar] [CrossRef]
- Chen, X.; Kuo, J. Blood separation and plasma preparation on a compact disk microfluidic chip. In Proceedings of the 9th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Waikiki Beach, HI, USA, 13–16 April 2014; pp. 47–50. [Google Scholar]
- Szydzik, C.; Khoshmanesh, K.; Mitchell, A.; Karnutsch, C. Microfluidic platform for separation and extraction of plasma from whole blood using dielectrophoresis. Biomicrofluidics 2015, 9, 064120. [Google Scholar] [CrossRef]
- Shimizu, H.; Kumagai, M.; Mori, E.; Mawatari, K.; Kitamori, T. Whole blood analysis using microfluidic plasma separation and enzyme-linked immunosorbent assay devices. Anal. Methods 2016, 8, 7597–7602. [Google Scholar] [CrossRef]
- Faivre, M.; Abkarian, M.; Bickraj, K.; Stone, H.A. Geometrical focusing of cells in a microfluidic device: An approach to separate blood plasma. Biorheology 2006, 43, 147–159. [Google Scholar]
- VanDelinder, V.; Groisman, A. Separation of Plasma from Whole Human Blood in a Continuous Cross-Flow in a Molded Microfluidic Device. Anal. Chem. 2006, 78, 3765–3771. [Google Scholar] [CrossRef]
- Rodríguez-Villarreal, A.I.; Arundell, M.; Carmona, M.; Samitier, J. High flow rate microfluidic device for blood plasma separation using a range of temperatures. Lab Chip 2010, 10, 211–219. [Google Scholar] [CrossRef]
- Kersaudy-Kerhoas, M.; Dhariwal, R.; Desmulliez, M.P.Y.; Jouvet, L. Hydrodynamic blood plasma separation in microfluidic channels. Microfluid. Nanofluid. 2010, 8, 105. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, Y.V.B.; Agrawal, A.; Prabhakar, A.; Joshi, S.S. Microdevice for plasma separation from whole human blood using bio-physical and geometrical effects. Sci. Rep. 2016, 6, 26749. [Google Scholar] [CrossRef]
- Yang, S.; Undar, A.; Zahn, J.D. A microfluidic device for continuous, real time blood plasma separation. Lab Chip 2006, 6, 871–880. [Google Scholar] [CrossRef]
- Lee, M.G.; Shin, J.H.; Choi, S.; Park, J.-K. Enhanced blood plasma separation by modulation of inertial lift force. Sens. Actuators B: Chem. 2014, 190, 311–317. [Google Scholar] [CrossRef]
- Hou, H.W.; Bhagat, A.A.S.; Lee, W.C.; Huang, S.; Han, J.; Lim, C.T. Microfluidic Devices for Blood Fractionation. Micromachines 2011, 2, 319–343. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, Y.V.B.V.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromech. Microeng. 2015, 25, 083001. [Google Scholar] [CrossRef]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef]
- Xuan, X.; Zhu, J.; Church, C. Particle focusing in microfluidic devices. Microfluid. Nanofluid. 2010, 9, 1–16. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.-T.; Warkiani, M.E.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2015, 16, 10–34. [Google Scholar] [CrossRef]
- Torino, S.; Iodice, M.; Rendina, I.; Coppola, G.; Schonbrun, E. Hydrodynamic self-focusing in a parallel microfluidic device through cross-filtration. Biomicrofluidics 2015, 9, 064107. [Google Scholar] [CrossRef]
- Eluru, G.; Julius, L.A.N.; Gorthi, S.S. Single-layer microfluidic device to realize hydrodynamic 3D flow focusing. Lab Chip 2016, 16, 4133–4141. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eluru, G.; Nagendra, P.; Gorthi, S.S. Microfluidic In-Flow Decantation Technique Using Stepped Pillar Arrays and Hydraulic Resistance Tuners. Micromachines 2019, 10, 471. https://doi.org/10.3390/mi10070471
Eluru G, Nagendra P, Gorthi SS. Microfluidic In-Flow Decantation Technique Using Stepped Pillar Arrays and Hydraulic Resistance Tuners. Micromachines. 2019; 10(7):471. https://doi.org/10.3390/mi10070471
Chicago/Turabian StyleEluru, Gangadhar, Pavan Nagendra, and Sai Siva Gorthi. 2019. "Microfluidic In-Flow Decantation Technique Using Stepped Pillar Arrays and Hydraulic Resistance Tuners" Micromachines 10, no. 7: 471. https://doi.org/10.3390/mi10070471
APA StyleEluru, G., Nagendra, P., & Gorthi, S. S. (2019). Microfluidic In-Flow Decantation Technique Using Stepped Pillar Arrays and Hydraulic Resistance Tuners. Micromachines, 10(7), 471. https://doi.org/10.3390/mi10070471




