The Heat-Stable Enterotoxin Receptor, Guanylyl Cyclase C, as a Pharmacological Target in Colorectal Cancer Immunotherapy: A Bench-to-Bedside Current Report
Abstract
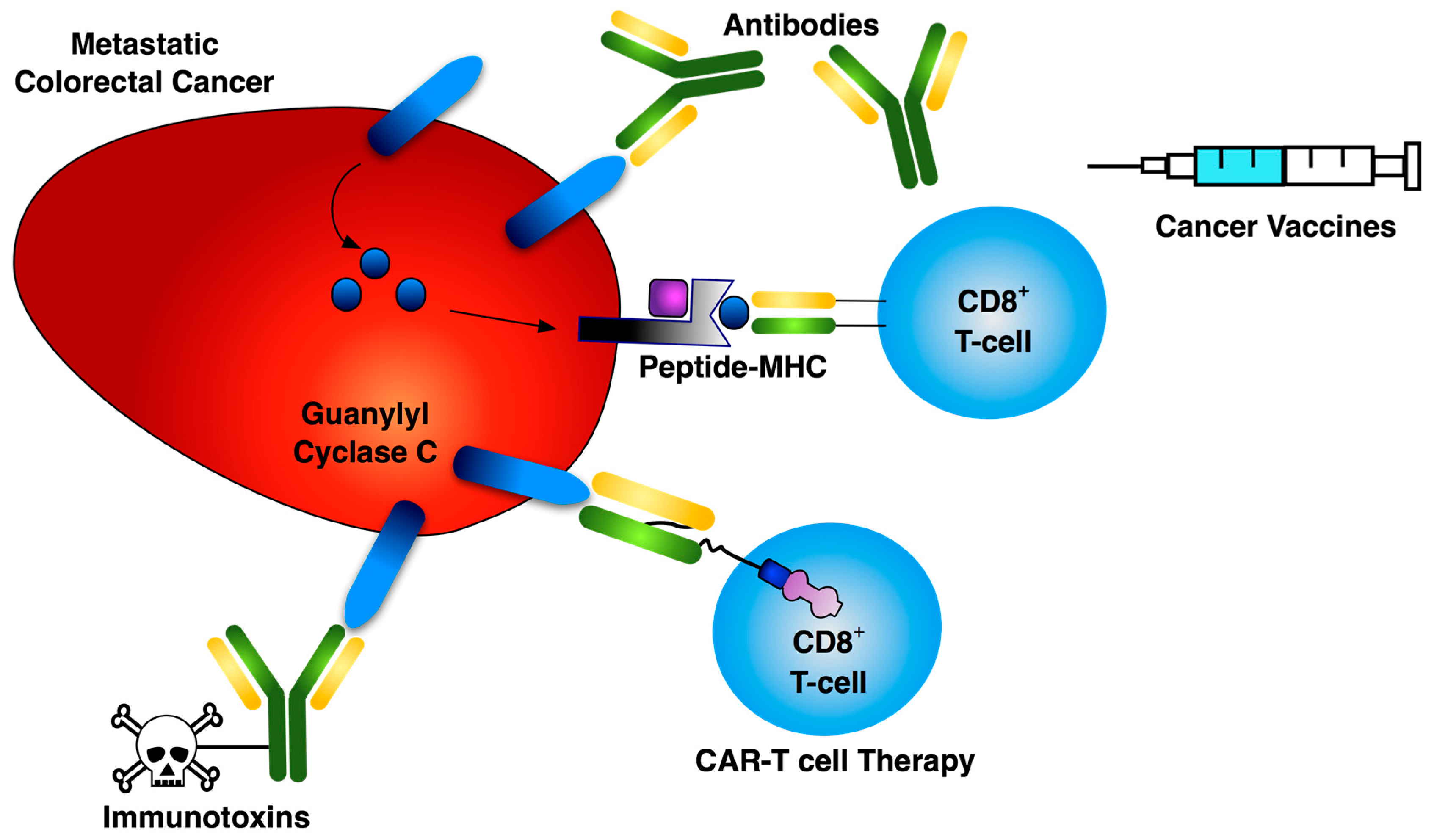
:1. Introduction
2. Guanylyl Cyclase C
3. Vaccines
4. Immunotoxins and Antibody-Drug Conjugates
5. Chimeric Antigen Receptor T Cells
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (checkmate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Goldinger, S.M. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr. Opin. Oncol. 2017, 29, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Gjerstorff, M.F.; Andersen, M.H.; Ditzel, H.J. Oncogenic cancer/testis antigens: Prime candidates for immunotherapy. Oncotarget 2015, 6, 15772–15787. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, M.R.; Yang, J.C.; Langan, R.C.; Dudley, M.E.; Nathan, D.A.; Feldman, S.A.; Davis, J.L.; Morgan, R.A.; Merino, M.J.; Sherry, R.M.; et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2011, 19, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Snook, A.E.; Eisenlohr, L.C.; Rothstein, J.L.; Waldman, S.A. Cancer mucosa antigens as a novel immunotherapeutic class of tumor-associated antigen. Clin. Pharmacol. Ther. 2007, 82, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Snook, A.E.; Stafford, B.J.; Eisenlohr, L.C.; Rothstein, J.L.; Waldman, S.A. Mucosally restricted antigens as novel immunological targets for antitumor therapy. Biomark. Med. 2007, 1, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lin, J.E.; Marszlowicz, G.P.; Valentino, M.A.; Chang, C.; Schulz, S.; Pitari, G.M.; Waldman, S.A. Gcc signaling in colorectal cancer: Is colorectal cancer a paracrine deficiency syndrome? Drug News Perspect. 2009, 22, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Frick, G.S.; Pitari, G.M.; Weinberg, D.S.; Hyslop, T.; Schulz, S.; Waldman, S.A. Guanylyl cyclase c: A molecular marker for staging and postoperative surveillance of patients with colorectal cancer. Expert Rev. Mol. Diagn. 2005, 5, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Carrithers, S.L.; Barber, M.T.; Biswas, S.; Parkinson, S.J.; Park, P.K.; Goldstein, S.D.; Waldman, S.A. Guanylyl cyclase c is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc. Natl. Acad. Sci. USA 1996, 93, 14827–14832. [Google Scholar] [CrossRef] [PubMed]
- Waldman, S.A.; Hyslop, T.; Schulz, S.; Barkun, A.; Nielsen, K.; Haaf, J.; Bonaccorso, C.; Li, Y.; Weinberg, D.S. Association of gucy2c expression in lymph nodes with time to recurrence and disease-free survival in pn0 colorectal cancer. JAMA 2009, 301, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Carrithers, S.L.; Parkinson, S.J.; Goldstein, S.; Park, P.; Robertson, D.C.; Waldman, S.A. Escherichia coli heat-stable toxin receptors in human colonic tumors. Gastroenterology 1994, 107, 1653–1661. [Google Scholar] [CrossRef]
- Camci, C.; Şahin, A.; Sevinc, A.; Kalender, M.E.; Öztuzcu, S.; Sever, Ö.N.; Özkara, E.; Demiryürek, A.T. Peripheral blood guanylyl cyclase c (gcc) expressions are associated with prognostic parameters and response to therapy in colorectal cancer patients. Tumor Biol. 2011, 32, 1265. [Google Scholar] [CrossRef] [PubMed]
- Almenoff, J.S.; Williams, S.I.; Scheving, L.A.; Judd, A.K.; Schoolnik, G.K. Ligand-based histochemical localization and capture of cells expressing heat-stable enterotoxin receptors. Mol. Microbiol. 1993, 8, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Cohen, M.B.; Overmann, G.; Thompson, M.R.; Giannella, R.A. Binding of e. Coli heat-stable enterotoxin to rat intestinal brush borders and to basolateral membranes. Dig. Dis. Sci. 1987, 32, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Wiedenmann, B.; Kruhoffer, M.; Adermann, K.; Ankorina-Stark, I.; Schlatter, E.; Ahnert-Hilger, G.; Forssmann, W.G.; Kuhn, M. Guanylin stimulates regulated secretion from human neuroendocrine pancreatic cells. Gastroenterology 1998, 114, 791–797. [Google Scholar] [CrossRef]
- Kloeters, O.; Friess, H.; Giese, N.; Buechler, M.W.; Cetin, Y.; Kulaksiz, H. Uroguanylin inhibits proliferation of pancreatic cancer cells. Scand. J. Gastroenterol. 2008, 43, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Schulz, S.; Haaf, J.; Kairys, J.C.; Waldman, S.A. Ectopic expression of guanylyl cyclase c in adenocarcinomas of the esophagus and stomach. Cancer Epidemiol. Biomark. Prev. 2002, 11, 739–744. [Google Scholar]
- Snook, A.E.; Stafford, B.J.; Li, P.; Tan, G.; Huang, L.; Birbe, R.; Schulz, S.; Schnell, M.J.; Thakur, M.; Rothstein, J.L.; et al. Guanylyl cyclase c-induced immunotherapeutic responses opposing tumor metastases without autoimmunity. J. Natl. Cancer Inst. 2008, 100, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Snook, A.E.; Li, P.; Stafford, B.J.; Faul, E.J.; Huang, L.; Birbe, R.C.; Bombonati, A.; Schulz, S.; Schnell, M.J.; Eisenlohr, L.C.; et al. Lineage-specific t-cell responses to cancer mucosa antigen oppose systemic metastases without mucosal inflammatory disease. Cancer Res. 2009, 69, 3537–3544. [Google Scholar] [CrossRef] [PubMed]
- Snook, A.E.; Huang, L.; Schulz, S.; Eisenlohr, L.C.; Waldman, S.A. Cytokine adjuvanation of therapeutic anti-tumor immunity targeted to cancer mucosa antigens. Clin. Transl. Sci. 2008, 1, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, C.; Xie, X.; Zhao, P.; Wei, X.; Sun, W.; Liu, H.C.; Alexandrou, A.T.; Jones, J.; Zhao, R.; et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PLoS ONE 2014, 9, e93886. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Xing, W.; Yang, J.; Zheng, Y.; Jia, X.; Zhang, B.; Ren, H. An effective cytokine adjuvant vaccine induces autologous t-cell response against colon cancer in an animal model. BMC Immunol. 2016, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Niedzwiecki, D.; Marshall, J.L.; Garrett, C.; Chang, D.Z.; Aklilu, M.; Crocenzi, T.S.; Cole, D.J.; Dessureault, S.; Hobeika, A.C.; et al. A randomized phase ii study of immunization with dendritic cells modified with poxvectors encoding cea and muc1 compared with the same poxvectors plus gm-csf for resected metastatic colorectal cancer. Ann. Surg. 2013, 258, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Snook, A.E.; Magee, M.S.; Schulz, S.; Waldman, S.A. Selective antigen-specific cd4+ t-cell, but not cd8+ t- or b-cell, tolerance corrupts cancer immunotherapy. Eur. J. Immunol. 2014, 44, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Baybutt, T.R.; Berman-Booty, L.; Magee, M.S.; Waldman, S.A.; Alexeev, V.Y.; Snook, A.E. Prime-boost immunization eliminates metastatic colorectal cancer by producing high-avidity effector cd8⁺ t cells. J. Immunol. 2017, 198, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Snook, A.E.; Baybutt, T.R.; Hyslop, T.; Waldman, S.A. Preclinical evaluation of a replication-deficient recombinant adenovirus serotype 5 vaccine expressing guanylate cyclase c and the padre t-helper epitope. Hum. Gene Ther. Methods 2016, 27, 238–250. [Google Scholar] [CrossRef] [PubMed]
- del Guercio, M.F.; Alexander, J.; Kubo, R.T.; Arrhenius, T.; Maewal, A.; Appella, E.; Hoffman, S.L.; Jones, T.; Valmori, D.; Sakaguchi, K.; et al. Potent immunogenic short linear peptide constructs composed of b cell epitopes and pan dr t helper epitopes (padre) for antibody responses in vivo. Vaccine 1997, 15, 441–448. [Google Scholar] [CrossRef]
- Snook, A.; Baybutt, T.; Mastrangelo, M.; Lewis, N.; Goldstein, S.; Kraft, W.; Oppong, Y.; Hyslop, T.; Myers, R.; Alexeev, V.; et al. A phase i study of ad5-gucy2c-padre in stage i and ii colon cancer patients. J. Immunother. Cancer 2015, 3, P450. [Google Scholar] [CrossRef]
- Marszalowicz, G.P.; Snook, A.E.; Magee, M.S.; Merlino, D.; Berman-Booty, L.D.; Waldman, S.A. Gucy2c lysosomotropic endocytosis delivers immunotoxin therapy to metastatic colorectal cancer. Oncotarget 2014, 5, 9460–9471. [Google Scholar] [CrossRef] [PubMed]
- Almhanna, K.; Kalebic, T.; Cruz, C.; Faris, J.E.; Ryan, D.P.; Jung, J.; Wyant, T.; Fasanmade, A.A.; Messersmith, W.; Rodon, J. Phase i study of the investigational anti-guanylyl cyclase antibody-drug conjugate tak-264 (mln0264) in adult patients with advanced gastrointestinal malignancies. Clin. Cancer Res. 2016, 22, 5049–5057. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, E.S.; Smallshaw, J.E.; Coleman, E.; Jafri, H.; Foster, C.; Munford, R.; Schindler, J. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc. Natl. Acad. Sci. USA 2006, 103, 2268–2273. [Google Scholar] [CrossRef] [PubMed]
- Magee, M.S.; Snook, A.E. Challenges to chimeric antigen receptor (car)-t cell therapy for cancer. Discov. Med. 2014, 18, 265–271. [Google Scholar] [PubMed]
- Magee, M.S.; Snook, A.E.; Waldman, S.A. Adoptive cell therapy: At the forefront of cancer immunotherapy. In Horizons in Cancer Research; Watanabe, H.S., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; Volume 51. [Google Scholar]
- Magee, M.S.; Kraft, C.L.; Abraham, T.S.; Baybutt, T.R.; Marszalowicz, G.P.; Li, P.; Waldman, S.A.; Snook, A.E. Gucy2c-directed car-t cells oppose colorectal cancer metastases without autoimmunity. Oncoimmunology 2016, 5, e1227897. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and toxicity management of 19–28z car t cell therapy in b cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014, 6, 224ra225. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baybutt, T.R.; Aka, A.A.; Snook, A.E. The Heat-Stable Enterotoxin Receptor, Guanylyl Cyclase C, as a Pharmacological Target in Colorectal Cancer Immunotherapy: A Bench-to-Bedside Current Report. Toxins 2017, 9, 282. https://doi.org/10.3390/toxins9090282
Baybutt TR, Aka AA, Snook AE. The Heat-Stable Enterotoxin Receptor, Guanylyl Cyclase C, as a Pharmacological Target in Colorectal Cancer Immunotherapy: A Bench-to-Bedside Current Report. Toxins. 2017; 9(9):282. https://doi.org/10.3390/toxins9090282
Chicago/Turabian StyleBaybutt, Trevor R., Allison A. Aka, and Adam E. Snook. 2017. "The Heat-Stable Enterotoxin Receptor, Guanylyl Cyclase C, as a Pharmacological Target in Colorectal Cancer Immunotherapy: A Bench-to-Bedside Current Report" Toxins 9, no. 9: 282. https://doi.org/10.3390/toxins9090282
APA StyleBaybutt, T. R., Aka, A. A., & Snook, A. E. (2017). The Heat-Stable Enterotoxin Receptor, Guanylyl Cyclase C, as a Pharmacological Target in Colorectal Cancer Immunotherapy: A Bench-to-Bedside Current Report. Toxins, 9(9), 282. https://doi.org/10.3390/toxins9090282






