Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA)
Abstract
:1. Introduction
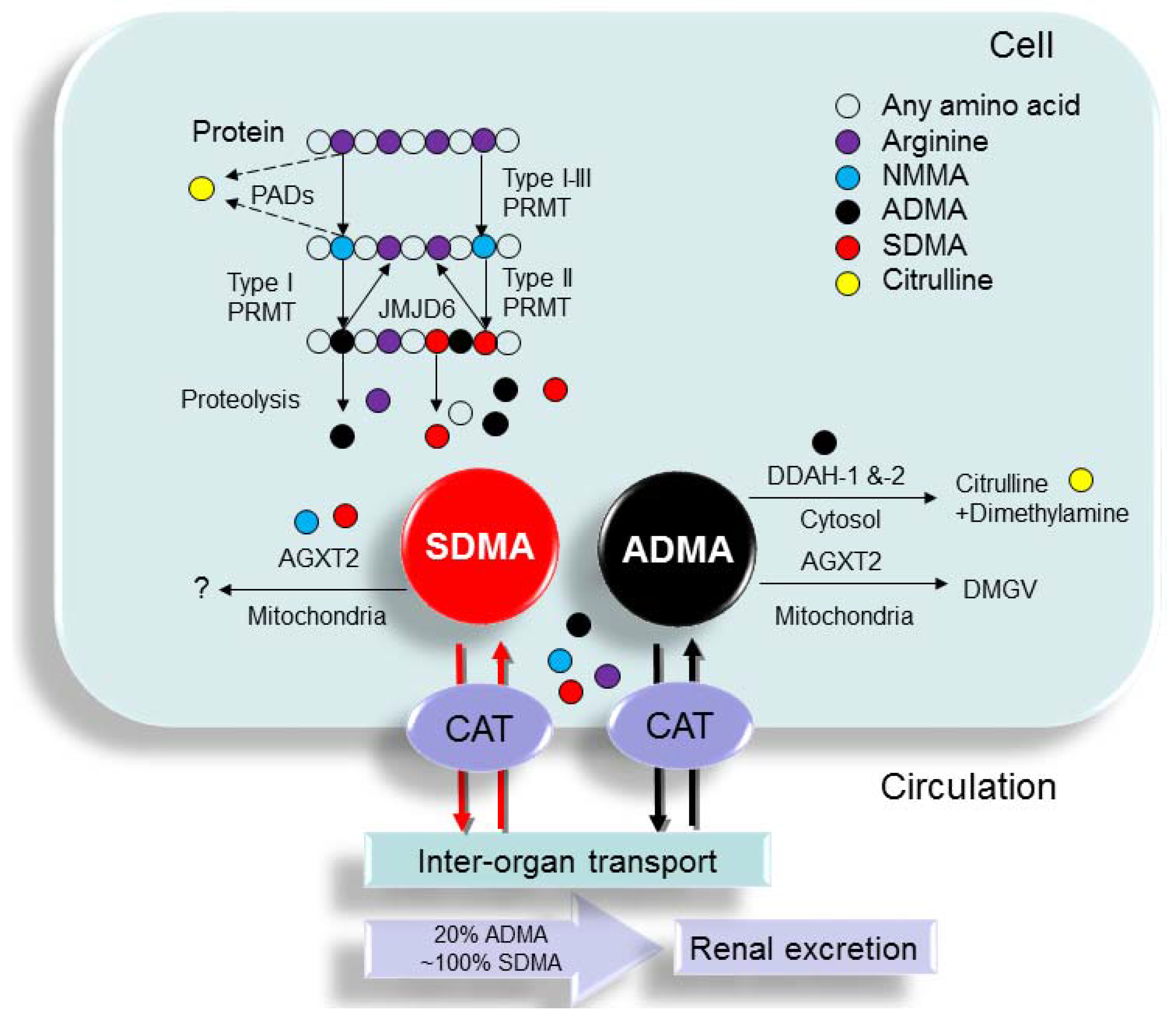
2. Synthesis and Metabolism of ADMA and SDMA
2.1. Synthesis of ADMA and SDMA
2.2. Metabolism of ADMA and SDMA
2.3. Quantification of ADMA and SDMA
3. Clinical Conditions Associated with Elevated ADMA and SDMA Levels
3.1. ADMA and SDMA: From Uremic Toxins to CVD Risk Factors
3.2. Clinical Conditions Associated with Elevated ADMA Levels
3.3. Clinical Conditions Associated with Elevated SDMA Levels
3.4. Causal Link between the Plasma Levels of ADMA or SDMA and Clinical Outcome
4. Pathophysiology of ADMA and SDMA
4.1. ADMA and SDMA: Inhibition of NO Synthesis
4.2. Tissue ADMA and SDMA Concentrations
4.3. ADMA: Multifunctional Effects
4.4. SDMA: Pro-Inflammatory and Pro-Oxidant Properties
5. Potential Therapies for Reducing ADMA and SDMA Levels
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kakimoto, Y.; Akazawa, S. Isolation and identification of NG,NG- and NG,N'G-dimethyl-arginine, Nε-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-δ-hydroxylysine from human urine. J. Biol. Chem. 1970, 245, 5751–5758. [Google Scholar] [PubMed]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [PubMed]
- Vallance, P.; Leiper, J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewicz, D.; Eickelberg, O. From arginine methylation to ADMA: A novel mechanism with therapeutic potential in chronic lung diseases. BMC Pulm. Med. 2009, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T. Asymmetric dimethylarginine: Clinical applications in pediatric medicine. J. Formos. Med. Assoc. 2011, 110, 70–77. [Google Scholar] [CrossRef]
- Leiper, J.; Nandi, M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat. Rev. Drug Discov. 2011, 10, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.T.; Hsieh, C.S.; Chang, K.A.; Tain, Y.L. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int. J. Mol. Sci. 2012, 13, 14606–14622. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Speer, T.; Bode-Böger, S.M.; Fliser, D.; Kielstein, J.T. Dimethylarginines ADMA and SDMA: The real water-soluble small toxins? Semin. Nephrol. 2014, 34, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, S.J.; de Boer, M.C.; Buijs, N.; van Leeuwen, P.A. Asymmetric dimethylarginine and critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Fliser, D. The past, presence and future of ADMA in nephrology. Nephrol. Ther. 2007, 3, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bode-Boger, S.M.; Scalera, F.; Kielstein, J.T.; Martens-Lobenhoffer, J.; Breithardt, G.; Fobker, M.; Reinecke, H. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J. Am. Soc. Nephrol. 2006, 17, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. Does ADMA cause endothelial dysfunction? Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; Münzel, T. ADMA and oxidative stress. Atheroscler. Suppl. 2003, 4, 41–51. [Google Scholar] [CrossRef]
- Schepers, E.; Glorieux, G.; Dhondt, A.; Leybaert, L.; Vanholder, R. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol. Dial. Transplant. 2009, 24, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wan, Y.N.; Zhao, J.H.; Wang, Y.J.; Wang, Y.X.; Yan, J.W.; Huang, X.L.; Wang, J. The association between systemic sclerosis, arginine and asymmetric dimethylarginine. Inflammation 2015, 38, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Barreto, D.V.; Liabeuf, S.; Glorieux, G.; Eloot, S.; Barreto, F.C.; Massy, Z.; Vanholder, R.; European Uremic Toxin Work Group (EUTox). Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2374–8233. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Oh, K.S.; Nho, J.H.; Kim, G.Y.; Kim, D.I. Asymmetric dimethylarginine (ADMA) treatment induces apoptosis in cultured rat mesangial cells via endoplasmic reticulum stress activation. Cell Biol. Int. 2016, 40, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, T.; Kako, K.; Shimada, T.; Nagashima, Y.; Nakamura, A.; Ishida, J.; Fukamizu, A. Production of free methylarginines via the proteasome and autophagy pathways in cultured cells. Mol. Med. Rep. 2011, 4, 615–620. [Google Scholar] [PubMed]
- Pekarova, M.; Kubala, L.; Martiskova, H.; Bino, L.; Twarogova, M.; Klinke, A.; Rudolph, T.K.; Kuchtova, Z.; Kolarova, H.; Ambrozova, G.; et al. Asymmetric dimethylarginine regulates the lipopolysaccharide-induced nitric oxide production in macrophages by suppressing the activation of NF-kappaB and iNOS expression. Eur. J. Pharmacol. 2013, 713, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Morales, Y.; Cáceres, T.; May, K.; Hevel, J.M. Biochemistry and regulation of the protein arginine methyltransferases (PRMTs). Arch. Biochem. Biophys. 2016, 590, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Raijmakers, R.; Zendman, A.J.; Egberts, W.V.; Vossenaar, E.R.; Raats, J.; Soede-Huijbregts, C.; Rutjes, F.P.; van Veelen, P.A.; Drijfhout, J.W.; Pruijn, G.J. Methylation of arginine residues interferes with citrullination by peptidylarginine deiminases in vitro. J. Mol. Biol. 2007, 367, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Chen, Y.; Zhao, Y.; Bruick, R.K. JMJD6 is a histone arginine demethylase. Science 2007, 318, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Böttger, A.; Islam, M.S.; Chowdhury, R.; Schofield, C.J.; Wolf, A. The oxygenase Jmjd6—A case study in conflicting assignments. Biochem. J. 2015, 468, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L.J. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, T.; Luo, Z.; Palm, F.; Wilcox, C.S. Cellular ADMA: Regulation and action. Pharmacol. Res. 2009, 60, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, R.N.; Martens-Lobenhoffer, J.; Brilloff, S.; Hohenstein, B.; Jarzebska, N.; Jabs, N.; Kittel, A.; Maas, R.; Weiss, N.; Bode-Böger, S.M. Role of alanine:glyoxylate aminotransferase 2 in metabolism of asymmetric dimethylarginine in the settings of asymmetric dimethylarginine overload and bilateral nephrectomy. Nephrol. Dial. Transplant. 2014, 29, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Palm, F.; Onozato, M.L.; Luo, Z.; Wilcox, C.S. Dimethylarginine dimethylaminohydrolase (DDAH): Expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3227–H3245. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Mazza, F.; Campisi, A.; Vanella, L.; Li, V.G.; Di, G.C. High glucose-mediated imbalance of nitric oxide synthase and dimethylarginine dimethylaminohydrolase expression in endothelial cells. Curr. Neurovasc. Res. 2006, 3, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Kao, Y.H.; Hsieh, C.S.; Chen, C.C.; Sheen, J.M.; Lin, I.C.; Huang, L.T. Melatonin blocks oxidative stress-induced increased asymmetric dimethylarginine. Free Radic. Biol. Med. 2010, 49, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Brands, M.W.; Bell, T.D.; Gibson, B. Nitric oxide may prevent hypertension early in diabetes by counteracting renal actions of superoxide. Hypertension 2004, 43, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kittel, A.; Maas, R.; König, J.; Mieth, M.; Weiss, N.; Jarzebska, N.; Hohenstein, B.; Martens-Lobenhoffer, J.; Bode-Böger, S.M.; Rodionov, R.N. In vivo evidence that Agxt2 can regulate plasma levels of dimethylarginines in mice. Biochem. Biophys. Res. Commun. 2013, 430, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. A critical review and discussion of analytical methods in the l-arginine/nitric oxide area of basic and clinical research. Anal. Biochem. 2008, 379, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, T.; Nijveldt, R.J.; de Jong, S.; van Leeuwen, P.A.M. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal. Biochem. 2002, 303, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Beckmann, B.; Gutzki, F.M.; Jordan, J. Simultaneous gas chromatography-tandem mass spectrometry quantification of symmetric and asymmetric dimethylarginine in human urine. Anal. Biochem. 2011, 413, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Wong, M.; Kim, J.-O.; Love, J.; Ansley, D.M.; Chen, D.D.Y. A new derivatization method coupled with LC-MS/MS to enable baseline separation and quantification of dimethylarginines in human plasma from patients to receive on-pump CABG surgery. Electrophoresis 2012, 33, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Krug, O.; Bode-Boger, S.M. Determination of arginine and asymmetric dimethylarginine (ADMA) in human plasma by liquid chromatography/mass spectrometry with the isotope dilution technique. J. Mass Spectrom. 2004, 39, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Boelaert, J.; Schepers, E.; Glorieux, G.; Eloot, S.; Vanholder, R.; Lynen, F. Determination of Asymmetric and Symmetric Dimethylarginine in Serum from Patients with Chronic Kidney Disease: UPLC-MS/MS versus ELISA. Toxins (Basel) 2016, 8, E149. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.; Wesemann, R.; Schwedhelm, E.; Sydow, K.; Albsmeier, J.; Cooke, J.P.; Böger, R.H. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin. Chem. Lab. Med. 2004, 42, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.D.; Heresztyn, T. An overview of plasma concentrations of asymmetric dimethylarginine (ADMA) in health and disease and in clinical studies: Methodological considerations. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 851, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Westphal, S.; Awiszus, F.; Bode-Boger, S.M.; Luley, C. Determination of asymmetric dimethylarginine: Liquid chromatography-mass spectrometry or ELISA? Clin. Chem. 2005, 51, 2188–2189. [Google Scholar] [CrossRef] [PubMed]
- Leiper, J.; Vallance, P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 1999, 43, 542–548. [Google Scholar] [CrossRef]
- Jacobi, J.; Tsao, P.S. Asymmetrical dimethylarginine in renal disease: Limits of variation or variation limits? A systematic review. Am. J. Nephrol. 2008, 28, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Salpeter, S.R.; Bode-Boeger, S.M.; Cooke, J.P.; Fliser, D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—A meta-analysis. Nephrol. Dial. Transplant. 2006, 21, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Bode-Böger, S.; Mallamaci, F.; Benedetto, F.; Tripepi, G.; Malatino, L.; Cataliotti, A.; Bellanuova, I.; Fermo, I.; Frölich, J.; et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 2001, 358, 2113–2117. [Google Scholar] [CrossRef]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and symmetric dimethylarginine as risk markers for total mortality and cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Khan, N.S.; Puri, B.K.; Hirsch, S.R. Elevated endogenous nitric oxide synthase inhibitor in schizophrenic plasma may reflect abnormalities in brain nitric oxide production. Neurosci. Lett. 1996, 215, 209–211. [Google Scholar] [CrossRef]
- Goonasekera, C.D.; Rees, D.D.; Woolard, P.; Frend, A.; Shah, V.; Dillon, M.J. Nitric oxide synthase inhibitors and hypertension in children and adolescents. J. Hypertens. 1997, 15, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H.; Bode-Böger, S.M.; Thiele, W.; Junker, W.; Alexander, K.; Frölich, J.C. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation 1997, 95, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H.; Bode-Böger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Matsuoka, H.; Miyazaki, H.; Ueda, S.; Okuda, S.; Imaizumi, T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci. 1998, 62, 2425–2430. [Google Scholar] [CrossRef]
- Pettersson, A.; Hedner, T.; Milsom, I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet. Gynecol. Scand. 1998, 77, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Fard, A.; Tuck, C.H.; Donis, J.A.; Sciacca, R.; Di Tullio, M.R.; Wu, H.D.; Bryant, T.A.; Chen, N.T.; Torres-Tamayo, M.; Ramasamy, R.; et al. Acute elevations of plasma asymmetric dimethylarginine and impaired endothelial function in response to a high-fat meal in patients with type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Gorenflo, M.; Zheng, C.; Werle, E.; Fiehn, W.; Ulmer, H.E. Plasma levels of asymmetrical dimethyl-l-arginine in patients with congenital heart disease and pulmonary hypertension. J. Cardiovasc. Pharmacol. 2001, 37, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Lee, S.C. Elevated levels of plasma homocyst(e)ine and asymmetric dimethylarginine in elderly patients with stroke. Atherosclerosis 2001, 158, 425–430. [Google Scholar] [CrossRef]
- Hermenegildo, C.; Medina, P.; Peiró, M.; Segarra, G.; Vila, J.M.; Ortega, J.; Lluch, S. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in hyperthyroid patients. J. Clin. Endocrinol. Metab. 2002, 87, 5636–5640. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Teerlink, T.; Van Der Hoven, B.; Siroen, M.P.; Kuik, D.J.; Rauwerda, J.A.; van Leeuwen, P.A. Asymmetrical dimethylarginine (ADMA) in critically ill patients: High plasma ADMA concentration is an independent risk factor of ICU mortality. Clin. Nutr. 2003, 22, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Lluch, P.; Torondel, B.; Medina, P.; Segarra, G.; Del Olmo, J.A.; Serra, M.A.; Rodrigo, J.M. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J. Hepatol. 2004, 41, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, L.; Hovind, P.; Teerlink, T.; Stehouwer, C.D.; Parving, H.H. Elevated plasma asymmetric dimethylarginine as a marker of cardiovascular morbidity in early diabetic nephropathy in type 1 diabetes. Diabetes Care 2004, 27, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Arnesen, H.; Hjerkinn, E.M.; Lyberg, T.; Seljeflot, I. Relationship between obesity, smoking, and the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine. Metabolism 2004, 53, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Bultink, I.E.; Teerlink, T.; Heijst, J.A.; Dijkmans, B.A.; Voskuyl, A.E. Raised plasma levels of asymmetric dimethylarginine are associated with cardiovascular events, disease activity, and organ damage in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2005, 64, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Bode-Böger, S.M.; Hesse, G.; Martens-Lobenhoffer, J.; Takacs, A.; Fliser, D.; Hoeper, M.M. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Lluch, P.; Mauricio, M.D.; Vila, J.M.; Segarra, G.; Medina, P.; Del Olmo, J.A.; Rodrigo, J.M.; Serra, M.A. Accumulation of symmetric dimethylarginine in hepatorenal syndrome. Exp. Biol. Med. (Maywood) 2006, 231, 70–75. [Google Scholar] [PubMed]
- Wang, J.; Sim, A.S.; Wang, X.L.; Salonikas, C.; Naidoo, D.; Wilcken, D.E. Relations between plasma asymmetric dimethylarginine (ADMA) and risk factors for coronary disease. Atherosclerosis 2006, 184, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Mittermayer, F.; Prusa, A.R.; Pollak, A.; Wolzt, M. Umbilical vein plasma concentrations of asymmetrical dimethylarginine are increased in male but not female neonates delivered preterm: A pilot study. Early Hum. Dev. 2006, 82, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Dooley, A.; Gao, B.; Bradley, N.; Abraham, D.J.; Black, C.M.; Jacobs, M.; Bruckdorfer, K.R. Abnormal nitric oxide metabolism in systemic sclerosis: Increased levels of nitrated proteins and asymmetric dimethylarginine. Rheumatology (Oxford) 2006, 45, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Charitidou, C.; Farmakiotis, D.; Zournatzi, V.; Pidonia, I.; Pegiou, T.; Karamanis, N.; Hatzistilianou, M.; Katsikis, I.; Panidis, D. The administration of estrogens, combined with anti-androgens, has beneficial effects on the hormonal features and asymmetric dimethyl-arginine levels, in women with the polycystic ovary syndrome. Atherosclerosis 2008, 196, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, Y.; Firat, H.; Simşek, B.; Torun, M.; Yardim-Akaydin, S. Circulating nitric oxide (NO), asymmetric dimethylarginine (ADMA), homocysteine, and oxidative status in obstructive sleep apnea-hypopnea syndrome (OSAHS). Sleep Breath 2008, 12, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, H.; Tsukahara, H.; Yorifuji, T.; Miida, T.; Murayama, K.; Tsuruoka, T.; Takatani, T.; Kanazawa, M.; Kobayashi, K.; Okano, Y.; et al. Evaluation of endogenous nitric oxide synthesis in congenital urea cycle enzyme defects. Metabolism 2009, 58, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Turiel, M.; Atzeni, F.; Tomasoni, L.; de Portu, S.; Delfino, L.; Bodini, B.D.; Longhi, M.; Sitia, S.; Bianchi, M.; Ferrario, P.; et al. Non-invasive assessment of coronary flow reserve and ADMA levels: A case-control study of early rheumatoid arthritis patients. Rheumatology (Oxford) 2009, 48, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Wang, Z.; Machado, R.F.; Blackwelder, W.C.; Taylor, J.G., 6th; Hazen, S.L. Endogenous nitric oxide synthase inhibitors in sickle cell disease: Abnormal levels and correlations with pulmonary hypertension, desaturation, haemolysis, organ dysfunction and death. Br. J. Haematol. 2009, 145, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, H.; Okano, Y.; Aizawa, M.; Miida, T.; Yorifuji, T.; Tajima, G.; Sakura, N.; Takatani, T.; Sanayama, Y.; Sugamoto, K.; et al. Altered metabolisms of mediators controlling vascular function and enhanced oxidative stress in asymptomatic children with congenital portosystemic venous shunt. Metabolism 2010, 59, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, N.; Cinemre, H.; Bilir, C.; Akin, O.; Akdemir, R. Increased serum asymmetric dimethylarginine levels in primary dysmenorrhea. Gynecol. Obstet. Investig. 2010, 69, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Cibor, D.; Mach, T. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), arginine, and 8-iso-prostaglandin F2α (8-iso-PGF2α) level in patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2010, 16, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; North, M.L.; Rafii, M.; Huang, H.; Pencharz, P.; Subbarao, P.; Belik, J.; Grasemann, H. Asymmetric dimethylarginine is increased in asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Kasumov, T.; Edmison, J.M.; Dasarathy, S.; Bennett, C.; Lopez, R.; Kalhan, S.C. Plasma levels of asymmetric dimethylarginine in patients with biopsy-proven nonalcoholic fatty liver disease. Metabolism 2011, 60, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Sarzi-Puttini, P.; Sitia, S.; Tomasoni, L.; Gianturco, L.; Battellino, M.; Boccassini, L.; De Gennaro Colonna, V.; Marchesoni, A.; Turiel, M. Coronary flow reserve and asymmetric dimethylarginine levels: New measurements for identifying subclinical atherosclerosis in patients with psoriatic arthritis. J. Rheumatol. 2011, 38, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Topal, G.; Donmez, A.; Doğan, B.S.; Kucur, M.; Cengiz, D.T.; Berkoz, F.B.; Erdogan, N. Asymmetric dimethylarginine (ADMA) levels are increased in patients with fibromyalgia: Correlation with tumor necrosis factor-α (TNF-α) and 8-iso-prostaglandin F2α (8-iso-PGF2α). Clin. Biochem. 2011, 44, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Sulicka, J.; Surdacki, A.; Strach, M.; Kwater, A.; Gryglewska, B.; Ćwiklińska, M.; Balwierz, W.; Grodzicki, T.K. Elevated asymmetric dimethylarginine in young adult survivors of childhood acute lymphoblastic leukemia: A preliminary report. Dis. Markers 2012, 33, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Javadiyan, S.; Burdon, K.P.; Whiting, M.J.; Abhary, S.; Straga, T.; Hewitt, A.W.; Mills, R.A.; Craig, J.E. Elevation of serum asymmetrical and symmetrical dimethylarginine in patients with advanced glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, V.; Matrozova, J.; Elenkova, A.; Vandeva, S.; Kirilov, G.; Zacharieva, S. Asymmetric dimethylarginine (ADMA) and soluble vascular cell adhesion molecule 1(sVCAM-1) as circulating markers for endothelial dysfunction in patients with pheochromocytoma. Exp. Clin. Endocrinol. Diabetes 2013, 121, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Mengeloglu, Z.; Sünnetcioglu, M.; Tosun, M.; Kücükbayrak, A.; Ceylan, M.R.; Baran, A.I.; Karahocagil, M.; Akdeniz, H. High asymmetric dimethylarginine (ADMA) levels in patients with brucellosis. Inflammation 2014, 37, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Senol, S.; Tekumit, H.; Akar, I.; Ince, I. The role of asymmetric and symmetric dimethylarginine in acute deep vein thrombosis. Ann. Vasc. Surg. 2015, 29, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Langen, J.; Kayacelebi, A.A.; Beckmann, B.; Weigt-Usinger, K.; Carmann, C.; Hörster, I.; Lilienthal, E.; Richter-Unruh, A.; Tsikas, D.; Lücke, T. Homoarginine (hArg) and asymmetric dimethylarginine (ADMA) in short stature children without and with growth hormone deficiency: hArg and ADMA are involved differently in growth in the childhood. Amino Acids 2015, 47, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Altintas, N.; Cem Mutlu, L.; Bilir, B.; Oran, M.; Tülübaş, F.; Topçu, B.; Tayfur, İ.; Küçükyalçin, V.; Kaplan, G.; et al. Asymmetric dimethylarginine contributes to airway nitric oxide deficiency in patients with COPD. Clin. Respir. J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Relationship between asymmetric dimethylarginine and nocturia in the general elderly population: The HEIJO-KYO cohort. Neurourol. Urodyn. 2015, 34, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, O.; Ozcan, B.; Yucel, H.; Bas, A.Y.; Demirel, N. Asymmetric dimethylarginine and l-arginine levels in neonatal sepsis and septic shock. J. Matern. Fetal Neonatal. Med. 2015, 28, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Isik, D.U.; Bas, A.Y.; Demirel, N.; Kavurt, S.; Aydemir, O.; Kavurt, A.V.; Cetin, I. Increased asymmetric dimethylarginine levels in severe transient tachypnea of the newborn. J. Perinatol. 2016, 36, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Carvalho, D.R.; Brum, J.M.; Ünal, Ö.; Coskun, T.; Weisfeld-Adams, J.D.; Schrager, N.L.; Scholl-Bürgi, S.; Schlune, A.; Donner, M.G.; et al. Clinical phenotype, biochemical profile, and treatment in 19 patients with arginase 1 deficiency. J. Inherit. Metab. Dis. 2016, 39, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Kirbas, S.; Kirbas, A.; Tufekci, A.; Cumhur Cure, M.; Cakmak, S.; Yazici, T.; Cure, E. Serum levels of homocysteine, asymmetric dimethylarginine and nitric oxide in patients with Parkinson’s disease. Acta Clin. Belg. 2016, 71, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; Fortmann, S.P.; Fair, J.M.; Varady, A.; Hlatky, M.A.; Go, A.S.; Iribarren, C.; Tsao, P.S.; ADVANCE Investigators. Distribution of asymmetric dimethylarginine among 980 healthy, older adults of different ethnicities. Clin. Chem. 2010, 56, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Vida, G.; Sulyok, E.; Ertl, T.; Martens-Lobenhoffer, J.; Bode-Boger, S.M. Plasma asymmetric dimethylarginine concentration during the perinatal period. Neonatology 2007, 92, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lücke, T.; Kanzelmeyer, N.; Kemper, M.J.; Tsikas, D.; Das, A.M. Developmental changes in the l-arginine/nitric oxide pathway from infancy to adulthood: Plasma asymmetric dimethylarginine levels decrease with age. Clin. Chem. Lab. Med. 2007, 45, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Brunetti-Pierri, N.; Erez, A.; Shchelochkov, O.; Craigen, W.; Lee, B. Systemic hypertension in two patients with ASL deficiency: A result of nitric oxide deficiency? Mol. Genet. Metab. 2009, 98, 195–197. [Google Scholar] [CrossRef] [PubMed]
- MacAllister, R.J.; Rambausek, M.H.; Vallance, P.; Williams, D.; Hoffmann, K.H.; Ritz, E. Concentration of dimethyl-l-arginine in the plasma of patients with end-stage renal failure. Nephrol. Dial. Transplant. 1996, 11, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, K.; Mittermayer, F.; Shnawa, N.; Hofer, M.; Schnabler, J.; Etmüller, Y.; Kapiotis, S.; Wolzt, M.; Schernthaner, G. Asymmetrical dimethylarginine is related to renal function, chronic inflammation and macroangiopathy in patients with Type 2 diabetes and albuminuria. Diabet. Med. 2007, 24, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, R.P.; Malaki, M.; Davies, N.A.; Hodges, S.J.; Dalton, R.N.; Turner, C.; Sen, S.; Williams, R.; Leiper, J.; Vallance, P.; et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology 2007, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Tong, W.; Shrestha, K.; Wang, Z.; Levison, B.S.; Delfraino, B.; Hu, B.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur. Heart J. 2008, 29, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.; Carter, A.M.; Schwedhelm, E.; Ajjan, R.; Maas, R.; von Holten, R.A.; Atzler, D.; Grant, P.J.; Böger, R.H. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis 2010, 208, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, K.; Kay, A.R.; Leiper, J.; Barry, J.A.; Hardiman, P.J. Symmetric dimethylarginine (SDMA) is raised in women with polycystic ovary syndrome: A pilot study. J. Obstet. Gynaecol. 2011, 31, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Braekke, K.; Ueland, P.M.; Harsem, N.K.; Staff, A.C. Asymmetric dimethylarginine in the maternal and fetal circulation in preeclampsia. Pediatr. Res. 2009, 66, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Tenderenda-Banasiuk, E.; Wasilewska, A.; Taranta-Janusz, K.; Korzeniecka-Kozerska, A. Asymmetric and symmetric dimethylarginine in adolescents with hyperuricemia. Dis. Mark. 2013, 35, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.B.; Yeo, T.W.; Mukemba, J.P.; Florence, S.M.; Volkheimer, A.D.; Wang, H.; Chen, Y.; Rubach, M.; Granger, D.L.; Mwaikambo, E.D.; et al. Dimethylarginines: Endogenous inhibitors of nitric oxide synthesis in children with falciparum malaria. J. Infect. Dis. 2014, 210, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Veldink, H.; Martens-Lobenhoffer, J.; Haller, H.; Burg, M.; Lorenzen, J.M.; Lichtinghagen, R.; Bode-Böger, S.M.; Kliem, V. SDMA is an early marker of change in GFR after living-related kidney donation. Nephrol. Dial. Transplant. 2011, 26, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Calver, A.; Collier, J.; Leone, A.; Moncada, S.; Vallance, P. Effect of local intra-arterial asymmetric dimethylarginine (ADMA) on the forearm arteriolar bed of healthy volunteers. J. Hum. Hypertens. 1993, 7, 193–194. [Google Scholar] [PubMed]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Brouns, R.; Marescau, B.; Possemiers, I.; Sheorajpanday, R.; De Deyn, P.P. Dimethylarginine levels in cerebrospinal fluid of hyperacute ischemic stroke patients are associated with stroke severity. Neurochem. Res. 2009, 34, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Hsu, C.N.; Huang, C.F.; Lo, M.H.; Chien, S.J.; Tain, Y.L. Urinary arginine methylation index associated with ambulatory blood pressure abnormalities in children with chronic kidney disease. J. Am. Soc. Hypertens. 2012, 6, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Huang, L.T.; Lau, Y.T.; Lin, C.Y.; Tain, Y.L. The combined ratios of l-arginine and asymmetric and symmetric dimethylarginine as biomarkers in spontaneously hypertensive rats. Transl. Res. 2012, 159, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Kuo, H.C.; Hsu, C.N.; Huang, L.T.; Tain, Y.L. Metformin reduces asymmetric dimethylarginine and prevents hypertension in spontaneously hypertensive rats. Transl. Res. 2014, 164, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Li Volti, G.; Salomone, S.; Sorrenti, V.; Mangiameli, A.; Urso, V.; Siarkos, I.; Galvano, F.; Salamone, F. Effect of silibinin on endothelial dysfunction and ADMA levels in obese diabetic mice. Cardiovasc. Diabetol. 2011, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, D.; Takahashi, M.; Kanemitsu, Y.; Ishida, A.; Abe, T.; Yamakuni, T.; Suzuki, N.; Tomioka, Y. Determination of Asymmetric Dimethylarginine and Symmetric Dimethylarginine in Biological Samples of Mice Using LC/MS/MS. Am. J. Anal. Chem. 2011, 2, 303–313. [Google Scholar] [CrossRef]
- Wang, D.; Gill, P.S.; Chabrashvili, T.; Onozato, M.L.; Raggio, J.; Mendonca, M.; Dennehy, K.; Li, M.; Modlinger, P.; Leiper, J.; et al. Isoform-specific regulation by NG,NG-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ. Res. 2007, 101, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.T.; Fox, M.F.; Vallance, P.; Leiper, J.M. Chromosomal localization, gene structure, and expression pattern of DDAH1: Comparison with DDAH2 and implications for evolutionary origins. Genomics 2000, 68, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, D.E.; Sim, A.S.; Wang, J.; Wang, X.L. Asymmetric dimethylarginine (ADMA) in vascular, renal and hepatic disease and the regulatory role of l-arginine on its metabolism. Mol. Genet. Metab. 2007, 91, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsieh, C.S.; Chen, C.C.; Sheen, J.M.; Lee, C.T.; Huang, L.T. Melatonin prevents increased asymmetric dimethylarginine in young rats with bile duct ligation. J. Pineal Res. 2010, 48, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.M.; Huang, L.T.; Hsieh, C.S.; Chen, C.C.; Wang, J.Y.; Tain, Y.L. Bile duct ligation in developing rats: Temporal progression of liver, kidney, and brain damage. J. Pediatr. Surg. 2010, 45, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Cardounel, A.J.; Cui, H.; Samouilov, A.; Johnson, W.; Kearns, P.; Tsai, A.L.; Berka, V.; Zweier, J.L. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J. Biol. Chem. 2007, 282, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Anthony, S.; Hubank, M.; Leiper, J.M.; Vallance, P. Effects of ADMA upon gene expression: An insight into the pathophysiological significance of raised plasma ADMA. PLoS Med. 2005, 2, e264. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Wang, K.; He, J.; Qiu, Y.; Xie, G.; Su, M.; Jia, W.; Li, H. Effects of ADMA on gene expression and metabolism in serum-starved LoVo cells. Sci. Rep. 2016, 6, 25892. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Targeting on Asymmetric dimethylarginine-related nitric oxide-reactive oxygen species imbalance to reprogram the development of hypertension. Int. J. Mol. Sci. 2016, 17, E2020. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Glorieux, G.; Dou, L.; Cerini, C.; Gayrard, N.; Louvet, L.; Preus, P.; Rodriguez-Ortiz, M.; Argiles, A.; Brunet, P.; et al. Guanidino compounds as cause of cardiovascular damage in chronic kidney disease: An in vitro evaluation. Blood Purif. 2010, 30, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Speer, T.; Rohrer, L.; Blyszczuk, P.; Shroff, R.; Kuschnerus, K.; Kränkel, N.; Kania, G.; Zewinger, S.; Akhmedov, A.; Shi, Y.; et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 2013, 38, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Anderstam, B.; Katzarski, K.; Bergstrom, J. Serum levels of NG, NG-dimethyl-l-arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J. Am. Soc. Nephrol. 1997, 8, 1437–1442. [Google Scholar] [PubMed]
- Bełtowski, J.; Kedra, A. Asymmetric dimethylarginine (ADMA) as a target for pharmacotherapy. Pharmacol. Rep. 2006, 58, 159–178. [Google Scholar] [PubMed]
- Hu, H.; Qian, K.; Ho, M.C.; Zheng, Y.G. Small molecule inhibitors of protein arginine methyltransferases. Expert Opin. Investig. Drugs 2016, 25, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, R.N.; Jarzebska, N.; Weiss, N.; Lentz, S.R. AGXT2: A promiscuous aminotransferase. Trends Pharmacol. Sci. 2014, 35, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.L.; Li, N.S.; Li, Y.J.; Deng, H.W. Probucol preserves endothelial function by reduction of the endogenous nitric oxide synthase inhibitor level. Br. J. Pharmacol. 2002, 135, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhu, H.Q.; Jiang, D.J.; Jiang, J.L.; Deng, H.W.; Li, Y.J. 17β-estradiol preserves endothelial function by reduction of the endogenous nitric oxide synthase inhibitor level. Int. J. Cardiol. 2004, 96, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Wakino, S.; Hayashi, K.; Tatematsu, S.; Hasegawa, K.; Takamatsu, I.; Kanda, T.; Homma, K.; Yoshioka, K.; Sugano, N.; Saruta, T. Pioglitazone lowers systemic asymmetric dimethylarginine by inducing dimethylarginine dimethylaminohydrolase in rats. Hypertens. Res. 2005, 28, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Chouinard, M.; Cox, A.L.; Sipes, P.; Marcelo, M.; Ficorilli, J.; Li, S.; Gao, H.; Ryan, T.P.; Michael, M.D.; et al. Farnesoid X receptor agonist reduces serum asymmetric dimethylarginine levels through hepatic dimethylarginine dimethylaminohydrolase-1 gene regulation. J. Biol. Chem. 2006, 281, 39831–39838. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Jiang, D.J.; Huang, H.; Jia, S.J.; Jiang, J.L.; Hu, C.P.; Li, Y.J. Taurine protects against low-density lipoprotein-induced endothelial dysfunction by the DDAH/ADMA pathway. Vascul. Pharmacol. 2007, 46, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Altun, Z.S.; Uysal, S.; Guner, G.; Yilmaz, O.; Posaci, C. Effects of oral l-arginine supplementation on blood pressure and asymmetric dimethylarginine in stress-induced preeclamptic rats. Cell Biochem. Funct. 2008, 26, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Delemasure, S.; Korandji, C.; Segueira-Le Grand, A.; Lauzier, B.; Guilland, J.C.; Duvillard, L.; Zeller, M.; Cottin, Y.; Vergely, C.; et al. Anti-hypertensive effects of Rosuvastatin are associated with decreased inflammation and oxidative stress markers in hypertensive rats. Free Radic. Res. 2008, 42, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Onozato, M.L.; Tojo, A.; Leiper, J.; Fujita, T.; Palm, F.; Wilcox, C.S. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: Effects of angiotensin II receptor blockers. Diabetes 2008, 57, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, X.J.; Liu, Y.Z.; Zhang, Y.S.; Yuan, Q.; Tan, N.; Xiang, D.X.; Peng, J. The role of the DDAH-ADMA pathway in the protective effect of resveratrol analog BTM-0512 on gastric mucosal injury. Can. J. Physiol. Pharmacol. 2010, 88, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Lin, I.C.; Lau, Y.T.; Lin, C.Y. Melatonin prevents hypertension and increased asymmetric dimethylarginine in young spontaneous hypertensive rats. J. Pineal Res. 2010, 49, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Lin, C.Y.; Huang, L.T.; Lau, Y.T. Aliskiren prevents hypertension and reduces asymmetric dimethylarginine in young spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 670, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Liu, Y.; Liu, Y.; Chen, M. The effect of nebivolol on asymmetric dimethylarginine system in spontaneously hypertension rats. Vascul. Pharmacol. 2011, 54, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Ma, P.; Wang, X.; Zhang, W.; Zhang, X.; Zheng, P.; Yan, L.; Xu, Q.; Dai, G. Rosuvastatin attenuates monocrotaline-induced pulmonary hypertension via regulation of Akt/eNOS signaling and asymmetric dimethylarginine metabolism. Eur. J. Pharmacol. 2011, 666, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniyan, V.; Wright, G.; Sharma, V.; Davies, N.A.; Sharifi, Y.; Habtesion, A.; Mookerjee, R.P.; Jalan, R. Ammonia reduction with ornithine phenylacetate restores brain eNOS activity via the DDAH-ADMA pathway in bile duct-ligated cirrhotic rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G145–G152. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xia, K.; Zhao, Z.; Deng, X.; Yang, T. Atorvastatin modulates the DDAH1/ADMA system in high-fat diet-induced insulin-resistant rats with endothelial dysfunction. Vasc. Med. 2012, 17, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Makino, T.; Ono, T.; Mizukami, H. Anti-hypertensive effects of shichimotsukokato in 5/6 nephrectomized Wistar rats mediated by the DDAH-ADMA-NO pathway. J. Nat. Med. 2012, 66, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Lee, T.Y.; Huang, Y.T.; Chan, C.C.; Yeh, Y.C.; Lee, F.Y.; Lee, S.D.; Lin, H.C. Asymmetric dimethylarginine (ADMA) determines the improvement of hepatic endothelial dysfunction by vitamin E in cirrhotic rats. Liver Int. 2012, 32, 48–57. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, X.; Wang, H.; Zhang, W.; Jiang, H.; Wang, S.; Yuan, L.; Liu, Y.; Liu, X. Effects of salvianolic acid A on plasma and tissue dimethylarginine levels in a rat model of myocardial infarction. J. Cardiovasc. Pharmacol. 2013, 61, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Ojima, A.; Ishibashi, Y.; Matsui, T.; Maeda, S.; Nishino, Y.; Takeuchi, M.; Fukami, K.; Yamagishi, S. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am. J. Pathol. 2013, 182, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.C.; Tsai, C.M.; Hsu, C.N.; Huang, L.T.; Tain, Y.L. N-acetylcysteine prevents hypertension via regulation of the ADMA-DDAH pathway in young spontaneously hypertensive rats. Biomed. Res. Int. 2013, 2013, 696317. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.J.; Lin, K.M.; Kuo, H.C.; Huang, C.F.; Lin, Y.J.; Huang, L.T.; Tain, Y.L. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: l-citrulline and nitrate. Transl. Res. 2014, 163, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Yan, W.J.; Sun, B.; Zou, Z.P. Synergistic effects of atorvastatin and rosiglitazone on endothelium protection in rats with dyslipidemia. Lipids Health Dis. 2014, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Liu, Y.K.; Liu, J.; Wang, X.; Xu, Q.; Wang, Y.; Xu, X.; Dai, G. Cardioprotective effects of oxymatrine on isoproterenol-induced heart failure via regulation of DDAH/ADMA metabolism pathway in rats. Eur. J. Pharmacol. 2014, 745, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sasser, J.M.; Cunningham, M.W., Jr.; Baylis, C. Serelaxin reduces oxidative stress and asymmetric dimethylarginine in angiotensin II-induced hypertension. Am. J. Physiol. Renal Physiol. 2014, 307, F1355–F1362. [Google Scholar] [CrossRef] [PubMed]
- Bal, F.; Bekpinar, S.; Unlucerci, Y.; Kusku-Kiraz, Z.; Önder, S.; Uysal, M.; Gurdol, F. Antidiabetic drug metformin is effective on the metabolism of asymmetric dimethylarginine in experimental liver injury. Diabetes Res. Clin. Pract. 2014, 106, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N.; Lee, C.T. Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell Longev. 2014, 2014, 283180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, Q.; Ji, Y.; Wang, G.; He, X.; Tian, W.; Xu, H.; Lei, T.; Wang, Y. Effect of 18β-glycyrrhetinic acid on cerebral vasospasm caused by asymmetric dimethylarginine after experimental subarachnoid hemorrhage in rats. Neurol. Res. 2015, 37, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Sahna, E.; Ozguler, I.M.; Burma, O.; Ilhan, N. Effects of apocynin, an NADPH oxidase inhibitor, on levels of ADMA, MPO, iNOS and TLR4 induced by myocardial ischemia reperfusion. Perfusion 2015, 30, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lee, C.T.; Huang, L.T.; Tain, Y.L. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liang, Q.; Zeng, F.; Mai, Z.; Cai, A.; Qiu, R.; Xu, R.; Li, D.; Mai, W. Atorvastatin protects endothelium by decreasing asymmetric dimethylarginine in dyslipidemia rats. Lipids Health Dis. 2015, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xie, Y.; Jiang, J.; Wang, Y.; Xu, X.; Zhao, C.; Huang, F. Pleiotropic effects of fenofibrate therapy on rats with hypertriglycemia. Lipids Health Dis. 2015, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Hewedy, W.A.; Mostafa, D.K. Nebivolol suppresses asymmetric dimethylarginine and attenuates cyclosporine-induced nephrotoxicity and endothelial dysfunction in rats. Pharmacol. Rep. 2016, 68, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; İlhan, S.; Onat, E.; Kara, M.; Şahna, E. The effects of novokinin, an AT2 agonist, on blood pressure, vascular responses, and levels of ADMA, NADPH oxidase, and Rho kinase in hypertension induced by NOS inhibition and salt. Turk. J. Med. Sci. 2016, 46, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.M.; Chen, Y.C.; Hsu, M.H.; Tain, Y.L.; Yu, H.R.; Huang, L.T. Combined intraperitoneal and intrathecal etanercept reduce increased brain tumor necrosis factor-alpha and asymmetric dimethylarginine levels and rescues spatial deficits in young rats after bile duct ligation. Front. Cell Neurosci. 2016, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Villamandos, B.; Arnalich-Montiel, A.; Arribas, S.; Lüneburg, N.; Böger, R.H.; Delgado-Martos, M.J.; Fernández-Criado, C.; Delgado-Baeza, E.; González, M.C. Early regression of coronary artery remodeling with esmolol and DDAH/ADMA pathway in hypertensive rats. Hypertens. Res. 2016, 39, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.K.; El Azhary, N.M.; Nasra, R.A. The hydrogen sulfide releasing compounds ATB-346 and diallyl trisulfide attenuate streptozotocin-induced cognitive impairment, neuroinflammation, and oxidative stress in rats: Involvement of asymmetric dimethylarginine. Can. J. Physiol. Pharmacol. 2016, 94, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, K.Q.; Li, B.; Sun, D.Q.; Zhang, H.; Fu, Q. Epigallocatechin-3-gallate ameliorates erectile function in aged rats via regulation of PRMT1/DDAH/ADMA/NOS metabolism pathway. Asian J. Androl. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tai, I.H.; Sheen, J.M.; Lin, Y.J.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Tain, Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide 2016, 53, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Ma, P.; Xiong, A.; Xu, Y.; Wang, Y.; Xu, Q. Protective effects of low dose rosuvastatin on isoproterenol-induced chronic heart failure in rats by regulation of DDAH-ADMA-NO pathway. Cardiovasc. Ther. 2016. [Google Scholar] [CrossRef] [PubMed]
| Patient Population | N | Correlation with Clinical Outcome | Year of First Report | Ref. |
|---|---|---|---|---|
| CKD/ESRD | >1500 | ND | 1992 | [3,46] |
| Schizophrenia | 16 | ND | 1996 | [47] |
| Childhood hypertension | 38 | ND | 1997 | [48] |
| Peripheral arterial occlusive disease (PAOD) | 77 | ND | 1997 | [49] |
| Hypercholesteremia | 49 | ND | 1998 | [50] |
| Congestive heart failure | 84 | ADMA positively correlates with severity of heart failure | 1998 | [51] |
| Preeclampsia | 12 | ND | 1998 | [52] |
| Type 2 diabetes | 50 | ADMA correlates with brachial arterial dilation | 2000 | [53] |
| Congenital heart disease (CHD) | 20 | Elevated ADMA in CHD with pulmonary hypertension | 2001 | [54] |
| Stroke | 52 | ADMA correlates with homocysteine level | 2001 | [55] |
| Hyperthyroidism | 19 | ADMA correlates with free T4 level | 2002 | [56] |
| Critical illness in intensive care unit | 52 | ADMA increases risk for ICU death | 2003 | [57] |
| Liver cirrhosis | 11 | ND | 2004 | [58] |
| Type 1 diabetes | 408 | ADMA correlates with CVD events | 2004 | [59] |
| Obesity | 563 | ND | 2004 | [60] |
| Systemic lupus erythematous | 107 | ADMA correlates with CVD events | 2005 | [61] |
| Idiopathic pulmonary arterial hypertension | 57 | ND | 2005 | [62] |
| Hepatorenal syndrome | 11 | ND | 2006 | [63] |
| Coronary artery disease | 145 | ADMA correlates with homocysteine level; ADMA negatively correlates with GFR | 2006 | [64] |
| Prematurity | 19 | Elevated ADMA in male premature | 2006 | [65] |
| Systemic sclerosis | 21 | Elevated ADMA in diffuse systemic sclerosis | 2006 | [66] |
| Polycystic ovary syndrome (PCOS) | 106 | ND | 2008 | [67] |
| Obstructive sleep apnea-hypopnea syndrome (OSAHS) | 34 | ND | 2008 | [68] |
| Congenital urea cycle enzyme defects | 15 | Elevated ADMA in argininosuccinate synthase (ASS) deficiency and argininosuccinate lyase (ASL) deficiency | 2009 | [69] |
| Rheumatiod arthritis (RA) | 25 | ND | 2009 | [70] |
| Sickle cell disease (SCD) | 177 | ADMA correlates with mortality | 2009 | [71] |
| Congenital portosystemic venous shunt (PSVS) | 14 | ND | 2010 | [72] |
| Primary dysmenorrhea | 33 | ND | 2010 | [73] |
| Inflammatory bowel diseases (IBD) | 63 | ADMA correlates with Crohn’s disease activity | 2010 | [74] |
| Asthma | 17 | ND | 2011 | [75] |
| Nonalcoholic fatty liver disease (NAFLD) | 35 | ND | 2011 | [76] |
| Psoriatic arthritis | 22 | ADMA correlates with coronary flow reserve | 2011 | [77] |
| Fibromyalgia | 27 | ND | 2011 | [78] |
| Childhood acute lymphoblastic leukemia (ALL) | 25 | ND | 2012 | [79] |
| Glaucoma | 210 | Elevated ADMA in advanced glaucoma | 2012 | [80] |
| Pheochromocytoma | 18 | ND | 2013 | [81] |
| Brucellosis | 39 | ND | 2014 | [82] |
| Deep vein thrombosis (DVT) | 34 | ND | 2015 | [83] |
| Short stature | 66 | ND | 2015 | [84] |
| COPD | 58 | ND | 2015 | [85] |
| Nocturia | 262 | ND | 2015 | [86] |
| Neonatal sepsis | 31 | ADMA correlates with disease severity | 2015 | [87] |
| Transient tachypnea of the newborn (TTN) | 36 | ND | 2016 | [88] |
| Arginase 1 deficiency | 19 | ND | 2016 | [89] |
| Idiopathic Parkinson’s disease (PD) | 82 | ND | 2016 | [90] |
| Patient Population | N | Correlation with Clinical Outcome | Year of First Report | Ref. |
|---|---|---|---|---|
| CKD/ESRD | 10 | SDMA correlates with renal function | 1996 | [44,95] |
| Childhood hypertension | 38 | SDMA correlates with GFR | 1997 | [48] |
| Hyperthyroidism | 19 | ND | 2002 | [56] |
| Critical illness in intensive care unit | 52 | SDMA correlates with creatinine level | 2003 | [57] |
| Hepatorenal syndrome | 11 | ND | 2006 | [63] |
| Coronary artery disease | 145 | SDMA negatively correlates with GFR | 2006 | [64] |
| Type 2 diabetes mellitus (DM) | 103 | Elevated SDMA in type 2 DM with albuminuria | 2007 | [96] |
| Alcoholic hepatitis | 52 | ND | 2007 | [97] |
| Heart failure | 132 | ND | 2008 | [98] |
| Preeclampsia | 47 | ND | 2009 | [99] |
| Stroke | 394 | SDMA predicts all-cause mortality | 2010 | [100] |
| Polycystic ovary syndrome (PCOS) | 16 | ND | 2011 | [101] |
| Glaucoma | 210 | Elevated SDMA in advanced glaucoma | 2012 | [80] |
| Hyperuricemia | 58 | SDMA correlates with uric acid level | 2013 | [102] |
| Malaria | 123 | ND | 2014 | [103] |
| Animal Models | Intervention | Protective Effects | Year of First Report | Ref. |
|---|---|---|---|---|
| LDL injection-induced endothelial dysfunction in rat | Probucol | Preserve endothelial function | 2002 | [129] |
| LDL injection-induced endothelial dysfunction in rat | 17β-estradiol | Preserve endothelial function | 2004 | [130] |
| Spontaneously hypertensive rat (SHR) | Pioglitazone | Increase renal DDAH-2 expression; Prevent hypertension | 2005 | [131] |
| Zucker diabetic fatty rat | Farnesoid X receptor agonist | Increase hepatic DDAH-1 expression; Prevent atherosclerosis | 2006 | [132] |
| LDL injection-induced endothelial dysfunction in rat | Taurine | Preserve endothelial function | 2007 | [133] |
| Stress-induced preeclampsia in pregnant rat | l-arginine | Prevent hypertension and proteinuria | 2008 | [134] |
| SHR | Rosuvastatin | Attenuate hypertension | 2008 | [135] |
| STZ-induced diabetic rat | Telmisartan | Reduce renal PRMT-1 expression; Increase renal DDAH-1 expression | 2008 | [136] |
| Ethanol-induced gastric mucosal injury in rat | Resveratrol analog BTM-0512 | Prevent gastric mucosa injury; Increase DDAH activity | 2010 | [137] |
| Bile duct-ligated cirrhotic rat | Melatonin | Prevent liver damage; Increase DDAH activity | 2010 | [117] |
| SHR | Melatonin | Prevent hypertension; Increase DDAH activity | 2010 | [138] |
| SHR | Aliskiren | Prevent hypertension | 2011 | [139] |
| SHR | Nebivolol | Prevent hypertension | 2011 | [140] |
| Monocrotaline-induced pulmonary hypertension in rat | Rosuvastatin | Prevent pulmonary hypertension | 2011 | [141] |
| Bile duct-ligated cirrhotic rat | Ornithine phenylacetate | Prevent liver damage | 2012 | [142] |
| High-fat diet in rat | Atorvastatin | Improve endothelial function; Increase DDAH activity | 2012 | [143] |
| 5/6 nephrectomized rats | Shichimotsukokato | Prevent hypertension; Increase DDAH-2 level | 2012 | [144] |
| Bile duct-ligated cirrhotic rat | Vitamin E | Improve endothelial function; Increase hepatic DDAH-2 level | 2012 | [145] |
| Coronary artery-ligated rat | Salvianolic acid A | Improve cardiac damage; Increase DDAH activity | 2013 | [146] |
| STZ-induced diabetic pregnant rat | l-citrulline | Prevent offspring hypertension; Increase renal DDAH-2 level | 2013 | [112] |
| STZ-induced diabetic rat | Glucagon-like peptide-1 receptor agonist | Protect diabetic nephropathy; Reduce PRMT-1 expression | 2013 | [147] |
| SHR | N-acetylcysteine | Prevent gastric mucosa injury; Increase DDAH activity | 2013 | [148] |
| Prenatal dexamethasone exposure in rat | l-citrulline | Prevent offspring hypertension | 2014 | [149] |
| SHR | l-citrulline | Prevent hypertension | 2014 | [150] |
| SHR | Sodium nitrate | Prevent hypertension | 2014 | [150] |
| High-fat and high-cholesterol diet in rat | Atorvastatin plus rosiglitazone | Protect endothelial function | 2014 | [151] |
| Isoproterenol-induced heart failure in rat | Oxymatrine | Ameliorate ventricular function and hypertrophy; Increase DDAH-2 expression | 2014 | [152] |
| Angiotensin II-induced hypertension in rat | Serelaxin | Attenuate hypertension and proteinuria | 2014 | [153] |
| Lipopolysaccharide/D-galactosamine-induced liver injury | Metformin | Protect liver injury/Increase DDAH activity | 2014 | [154] |
| SHR | Metformin | Prevent hypertension | 2014 | [110] |
| Maternal caloric restriction.rat | Melatonin | Prevent offspring hypertension | 2014 | [155] |
| Constriction of artery-induced subarachnoid hemorrhage in rat | 18β-glycyrrhetinic acid | Improve neurological outcome | 2015 | [156] |
| Myocardial ischemia/reperfusion injury in rat | Apocynin | Protect myocardial injury | 2015 | [157] |
| Maternal caloric restriction.rat | Aliskiren | Prevent offspring hypertension | 2015 | [158] |
| High-fat and high-cholesterol diet in rat | Atorvastatin | Protective endothelial function | 2015 | [159] |
| 10% furctose administration rat | Fenofibrate | Reduce triglyceride level | 2015 | [160] |
| Cyclosporine-induced nephrotoxicity | Nebivolol | Ameliorate endothelial function | 2016 | [161] |
| l-NAME induced hypertension in rat | Novokinin | Prevent hypertension | 2016 | [162] |
| Bile duct-ligated cirrhotic rat | Etanercept | Prevent brain damage | 2016 | [163] |
| 2016 | [164] | |||
| STZ-induced cognitive impairment in rat | H2S releasing compounds ATB-346 and diallyl trisulfide | Ameliorate behavior performance | 2016 | [165] |
| Aged rat | Epigallocatechin-3-gallate | Ameliorate erectile function; reduce PRMT-1 expression; Increase DDAH activity | 2016 | [166] |
| Prenatal dexamethasone plus postnatal high-fat diet in rat | N-acetylcysteine | Prevent hypertension | 2016 | [167] |
| Isoproterenol-induced heart failure in rat | Rosuvastatin | Ameliorate ventricular function and hypertrophy; Reduce PRMT-1 expression; Increase DDAH-2 expression | 2016 | [168] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.; Hsu, C. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. https://doi.org/10.3390/toxins9030092
Tain Y, Hsu C. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins. 2017; 9(3):92. https://doi.org/10.3390/toxins9030092
Chicago/Turabian StyleTain, You‐Lin, and Chien‐Ning Hsu. 2017. "Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA)" Toxins 9, no. 3: 92. https://doi.org/10.3390/toxins9030092
APA StyleTain, Y., & Hsu, C. (2017). Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins, 9(3), 92. https://doi.org/10.3390/toxins9030092







