The Effects of Melittin and Apamin on Airborne Fungi-Induced Chemical Mediator and Extracellular Matrix Production from Nasal Polyp Fibroblasts
Abstract
:1. Introduction
2. Results
2.1. The Cytotoxicity of BV, Melittin, and Apamin
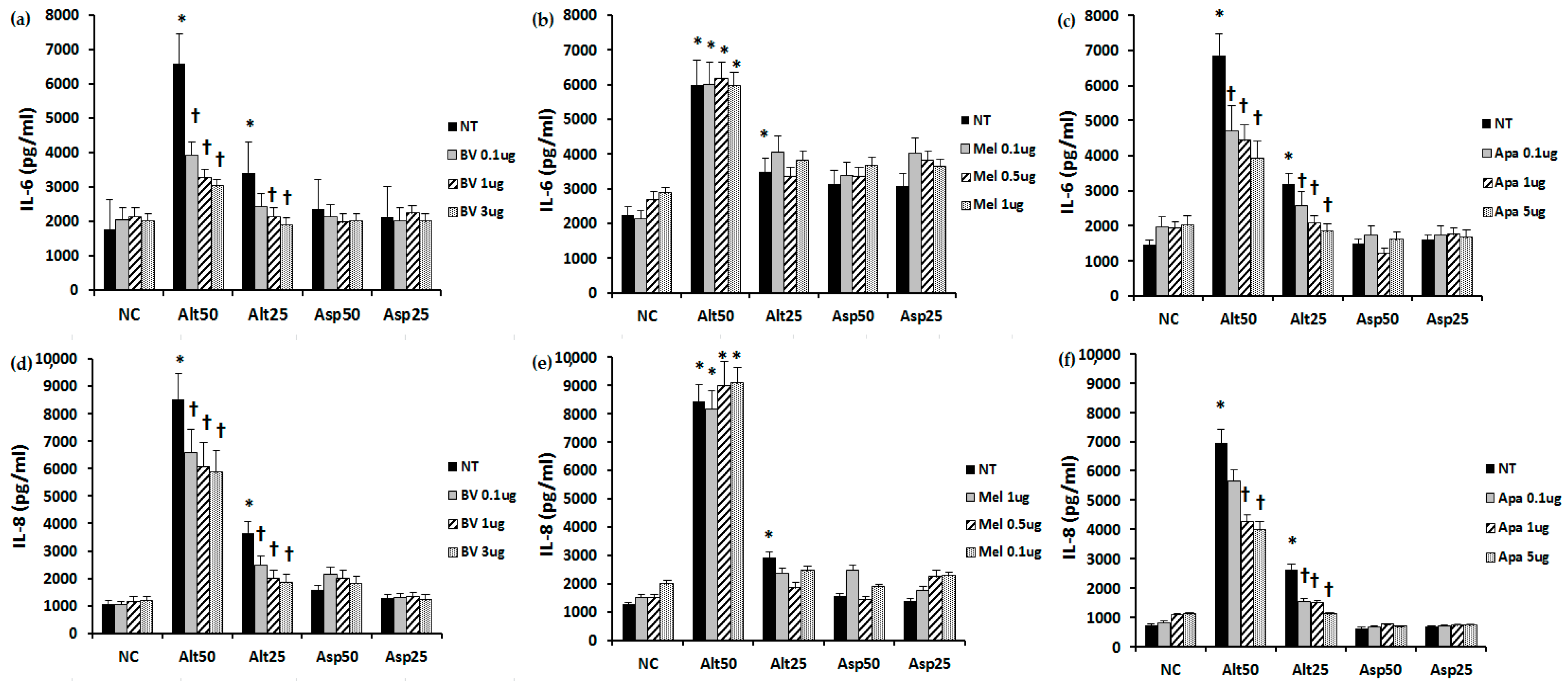
2.2. The Effect of BV, Melittin, and Apamin on the Production of Chemical Mediators
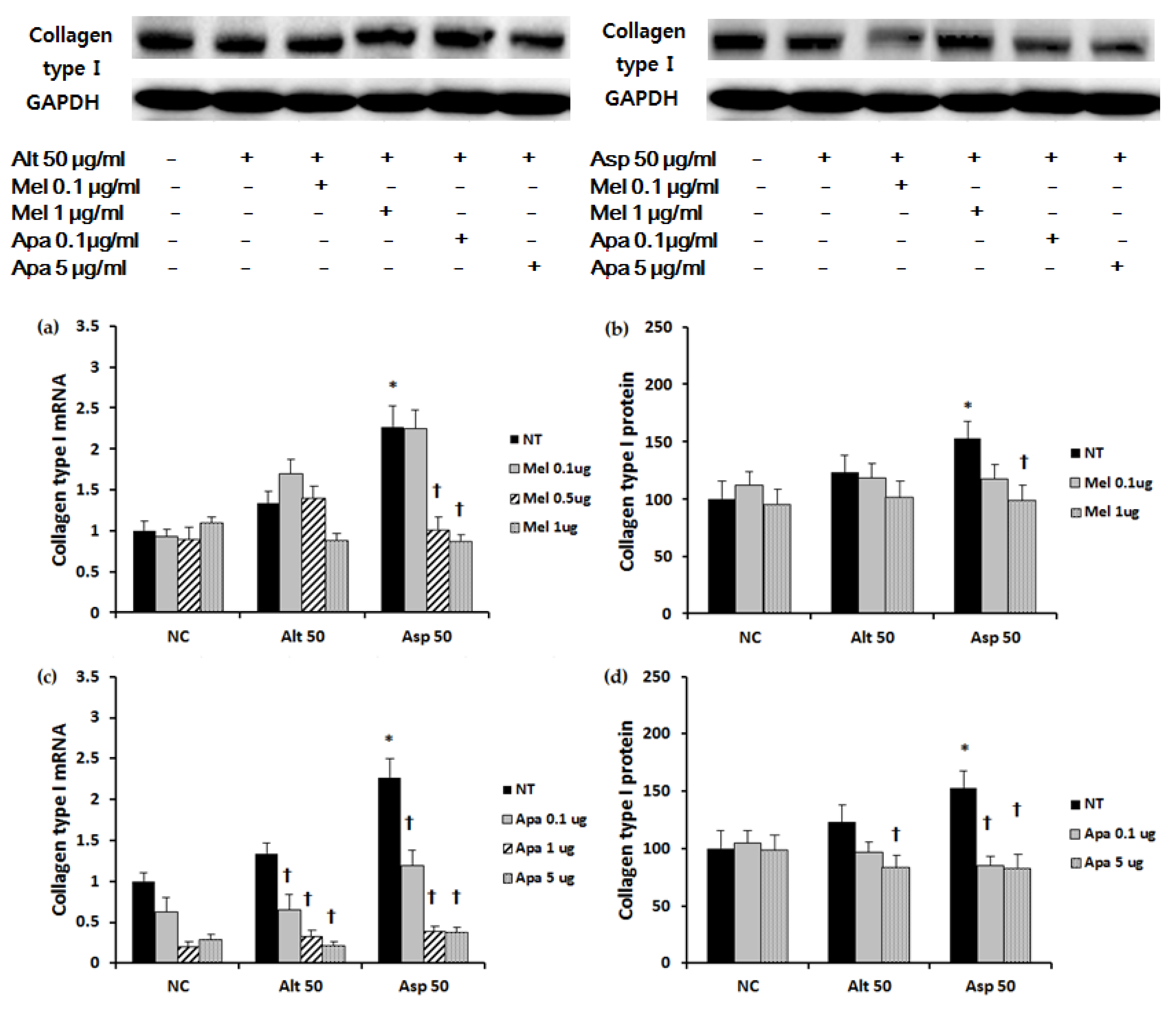
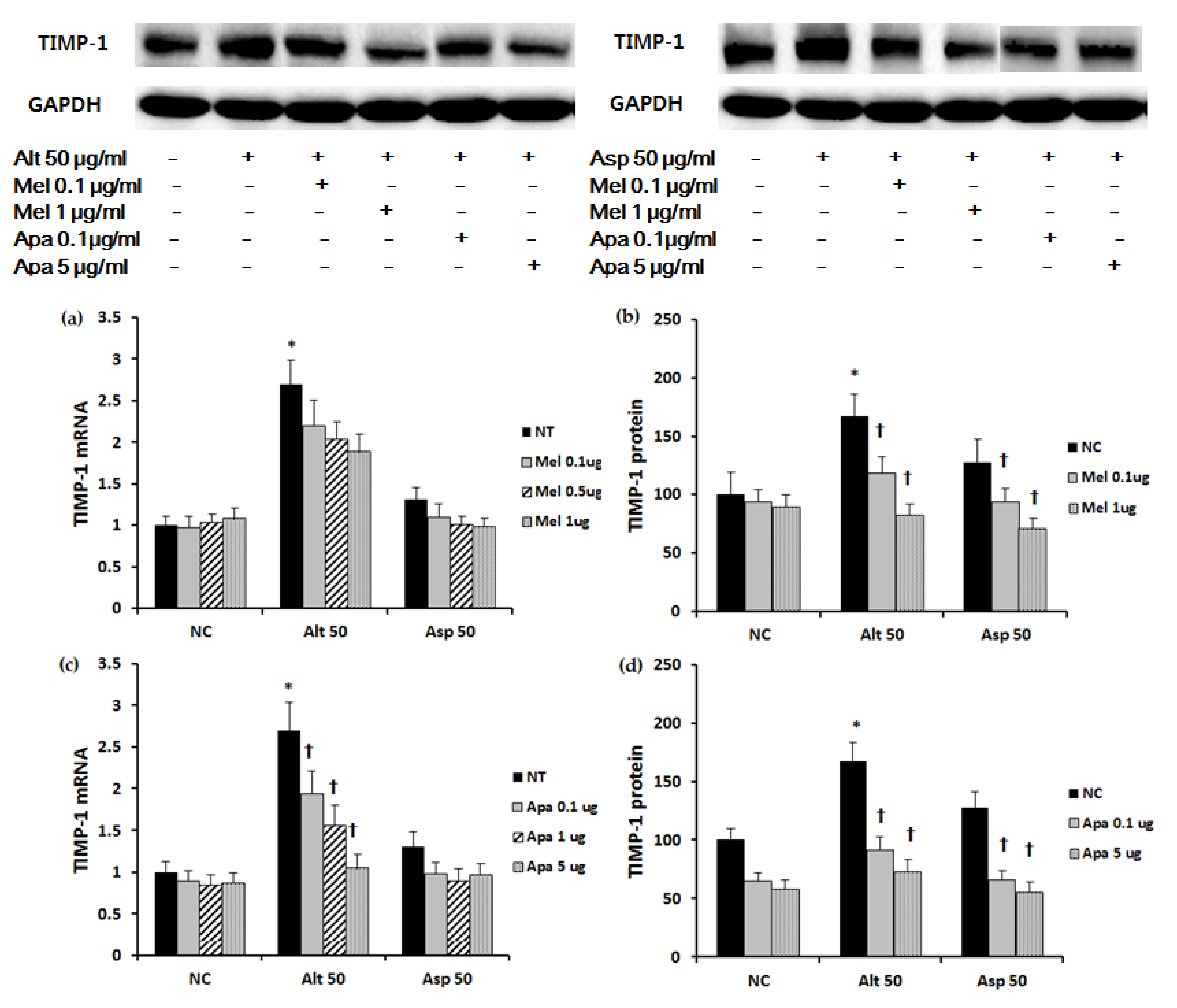
2.3. The Effect of Melittin and Apamin on the Expression of ECM
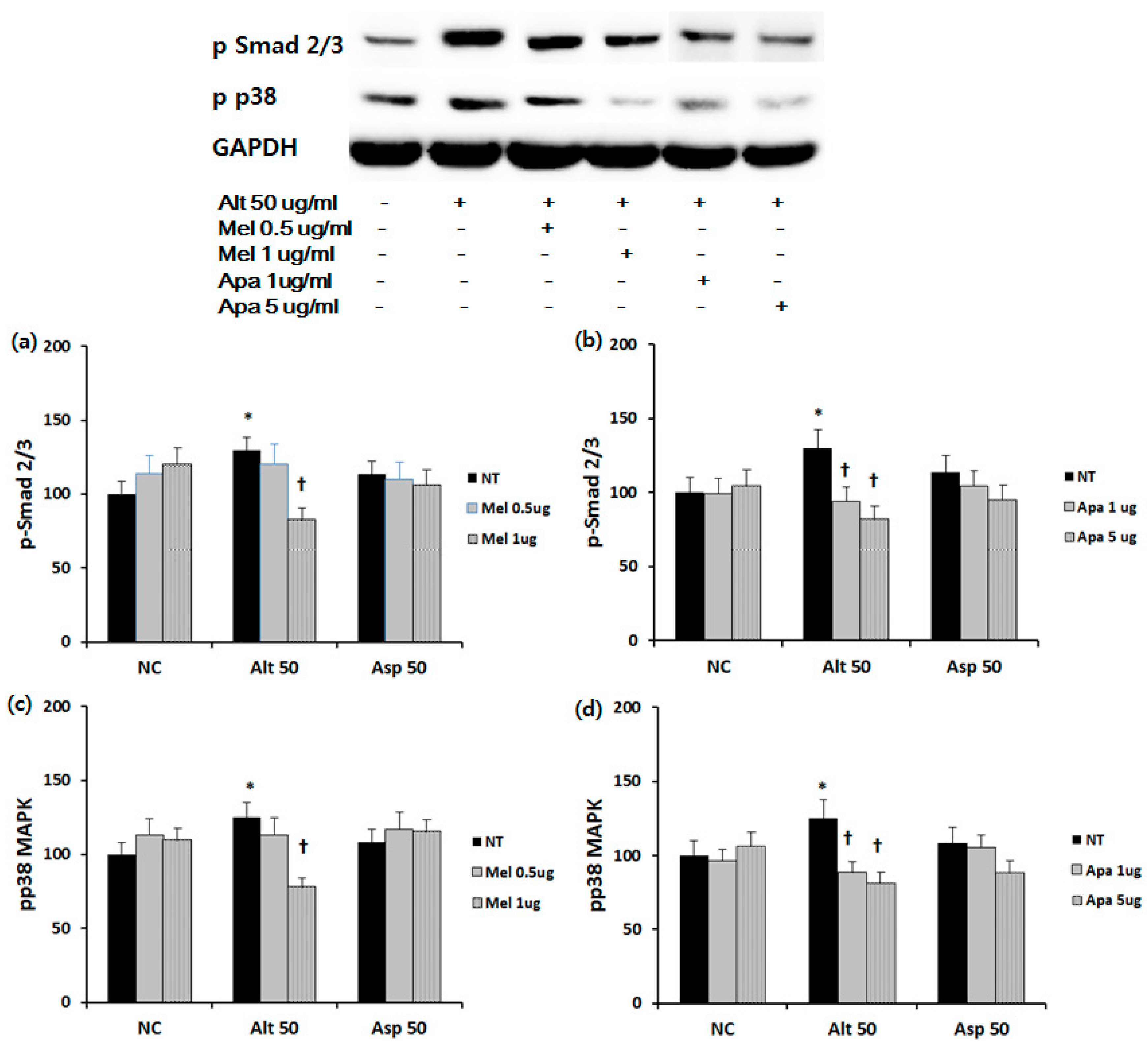
2.4. Effect of Melittin and Apamin on Phosphorylation of Smad 2/3 and p38 MAPK
3. Discussion
4. Materials and Methods
4.1. Isolation of Primary Nasal Polyp Fibroblasts
4.2. The Cytotoxic Effect of BV, Melittin, and Apamin on Nasal Polyp Fibroblasts
4.3. The Effect of Bee Venom, Melittin, and Apamin on IL-6 and IL-8 Production from Nasal Polyp Fibroblasts
4.4. Real Time Reverse Transcription–Polymerase Chain Reaction (RT-PCR) for ECM mRNA from Nasal Polyp Fibroblasts
4.5. Western Blot Analysis of Nasal Polyp Fibroblasts
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cho, J.S.; Moon, Y.M.; Park, I.H.; Um, J.Y.; Moon, J.H.; Park, S.J.; Lee, S.H.; Kang, H.J.; Lee, H.M. Epigenetic regulation of myofibroblast differentiation and extracellular matrix production in nasal polyp-derived fibroblasts. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2012, 42, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Yamane, H.; Nakai, Y.; Shigeta, T.; Takashima, T.; Takeda, Z. Comparative assessment of cell proliferation and accumulation of extracellular matrix in nasal polyps. Acta Oto-Laryngol. Suppl. 1998, 538, 205–208. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, Y.H.; Jin, H.S.; Kang, S.H. Alternaria induces production of thymic stromal lymphopoietin in nasal fibroblasts through toll-like receptor 2. Allergy Asthma Immunol. Res. 2016, 8, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.S.; Cao, T.M.; Gao, Y.Y.; Liu, M.J.; Liu, Y.Q.; Wang, Z. Effects of chronic exposure to Aspergillus fumigatus on epidermal growth factor receptor expression in the airway epithelial cells of asthmatic rats. Exp. Lung Res. 2014, 40, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Roeder, A.; Kirschning, C.J.; Rupec, R.A.; Schaller, M.; Korting, H.C. Toll-like receptors and innate antifungal responses. Trends Microbiol. 2004, 12, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Yang, E.J.; Lee, M.S.; Kim, Y.S.; Huh, Y.; Cho, I.H.; Kang, S.; Koh, H.K. Bee venom reduces neuroinflammation in the mptp-induced model of parkinson’s disease. Int. J. Neurosci. 2011, 121, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin, apamin and mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.M.; Yeo, J.H.; Seo, H.S. Melittin, a honeybee venomderived antimicrobial peptide, may target methicillinresistant staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Anti-inflammatory applications of melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Cho, H.J.; Jeong, Y.J.; Shin, J.M.; Kang, J.H.; Park, K.K.; Choe, J.Y.; Park, Y.Y.; Bae, Y.S.; Han, S.M.; et al. Melittin inhibits tgf-beta-induced pro-fibrotic gene expression through the suppression of the tgfbetarii-smad, erk1/2 and jnk-mediated signaling pathway. Am. J. Chin. Med. 2014, 42, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; An, H.J.; Kim, W.H.; Park, Y.Y.; Park, K.D.; Park, K.K. Apamin suppresses biliary fibrosis and activation of hepatic stellate cells. Int. J. Mol. Med. 2017, 39, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Baek, Y.H.; Lee, M.H.; Choi, D.Y.; Park, D.S.; Lee, J.D. Bee venom inhibits tumor angiogenesis and metastasis by inhibiting tyrosine phosphorylation of vegfr-2 in llc-tumor-bearing mice. Cancer Lett. 2010, 292, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Bilo, B.M.; Rueff, F.; Mosbech, H.; Bonifazi, F.; Oude-Elberink, J.N. Diagnosis of hymenoptera venom allergy. Allergy 2005, 60, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Lazdunski, M.; Fosset, M.; Hughes, M.; Mourre, C.; Romey, G.; Schmid-Antomarchi, H. The apamin-sensitive Ca2+-dependent K+ channel molecular properties, differentiation and endogenous ligands in mammalian brain. Biochem. Soc. Symp. 1985, 50, 31–42. [Google Scholar] [PubMed]
- Shin, S.H.; Ye, M.K.; Lee, Y.H. Fungus culture of the nasal secretion of chronic rhinosinusitis patients: Seasonal variations in Daegu, Korea. Am. J. Rhinol. 2007, 21, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Ye, M.K.; Kim, Y.H.; Kim, J.K. Role of TLRs in the production of chemical mediators in nasal polyp fibroblasts by fungi. Auris Nasus Larynx 2016, 43, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Watelet, J.B.; Bachert, C.; Claeys, C.; Van Cauwenberge, P. Matrix metalloproteinases mmp-7, mmp-9 and their tissue inhibitor timp-1: Expression in chronic sinusitis vs. nasal polyposis. Allergy 2004, 59, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Ye, M.K.; Choi, S.Y.; Kim, Y.H. Effect of eosinophils activated with Alternaria on the production of extracellular matrix from nasal fibroblasts. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2016, 116, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Matsuwaki, Y.; Wada, K.; White, T.A.; Benson, L.M.; Charlesworth, M.C.; Checkel, J.L.; Inoue, Y.; Hotta, K.; Ponikau, J.U.; Lawrence, C.B.; et al. Recognition of fungal protease activities induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils. J. Immunol. (1950) 2009, 183, 6708–6716. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, J.; Gong, W.; Iribarren, P.; Dunlop, N.M.; Wang, J.M. Toll-like receptors in inflammation, infection and cancer. Int. Immunopharmacol. 2007, 7, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, Y.H. Airborne fungi induce nasal polyp epithelial cell activation and toll-like receptor expression. Int. Arch. Allergy Immunol. 2010, 153, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Chen, P.H.; Wu, T.Y.; Lin, Y.C.; Tsai, M.S.; Lee, P.H.; Tai, T.S.; Chang, H.R.; Sun, C.K. Lipopolysaccharides induce smad2 phosphorylation through pi3k/akt and mapk cascades in hsc-t6 hepatic stellate cells. Life Sci. 2017, 184, 37–46. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kim, K.H.; Lee, W.R.; Kim, J.Y.; Lee, S.J.; Pak, S.C.; Han, S.M.; Park, K.K. Anti-fibrotic effect of natural toxin bee venom on animal model of unilateral ureteral obstruction. Toxins 2015, 7, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jeong, Y.J.; Park, K.K.; Cho, H.J.; Chung, I.K.; Min, K.S.; Kim, M.; Lee, K.G.; Yeo, J.H.; Park, K.K.; et al. Melittin suppresses pma-induced tumor cell invasion by inhibiting nf-kappab and ap-1-dependent mmp-9 expression. Mol. Cells 2010, 29, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Lee, S.J.; Han, S.M.; Pak, S.C.; Park, K.K. Apamin inhibits hepatic fibrosis through suppression of transforming growth factor beta1-induced hepatocyte epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2014, 450, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Pak, S.C.; Park, K.K. The protective effect of Bee venom on Fibrosis causing inflammatory diseases. Toxins 2015, 16, 4758–4772. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.-H.; Ye, M.-K.; Choi, S.-Y.; Park, K.-K. The Effects of Melittin and Apamin on Airborne Fungi-Induced Chemical Mediator and Extracellular Matrix Production from Nasal Polyp Fibroblasts. Toxins 2017, 9, 348. https://doi.org/10.3390/toxins9110348
Shin S-H, Ye M-K, Choi S-Y, Park K-K. The Effects of Melittin and Apamin on Airborne Fungi-Induced Chemical Mediator and Extracellular Matrix Production from Nasal Polyp Fibroblasts. Toxins. 2017; 9(11):348. https://doi.org/10.3390/toxins9110348
Chicago/Turabian StyleShin, Seung-Heon, Mi-Kyung Ye, Sung-Yong Choi, and Kwan-Kyu Park. 2017. "The Effects of Melittin and Apamin on Airborne Fungi-Induced Chemical Mediator and Extracellular Matrix Production from Nasal Polyp Fibroblasts" Toxins 9, no. 11: 348. https://doi.org/10.3390/toxins9110348
APA StyleShin, S.-H., Ye, M.-K., Choi, S.-Y., & Park, K.-K. (2017). The Effects of Melittin and Apamin on Airborne Fungi-Induced Chemical Mediator and Extracellular Matrix Production from Nasal Polyp Fibroblasts. Toxins, 9(11), 348. https://doi.org/10.3390/toxins9110348




