Above and beyond C5a Receptor Targeting by Staphylococcal Leucotoxins: Retrograde Transport of Panton–Valentine Leucocidin and γ-Hemolysin
Abstract
:1. Introduction
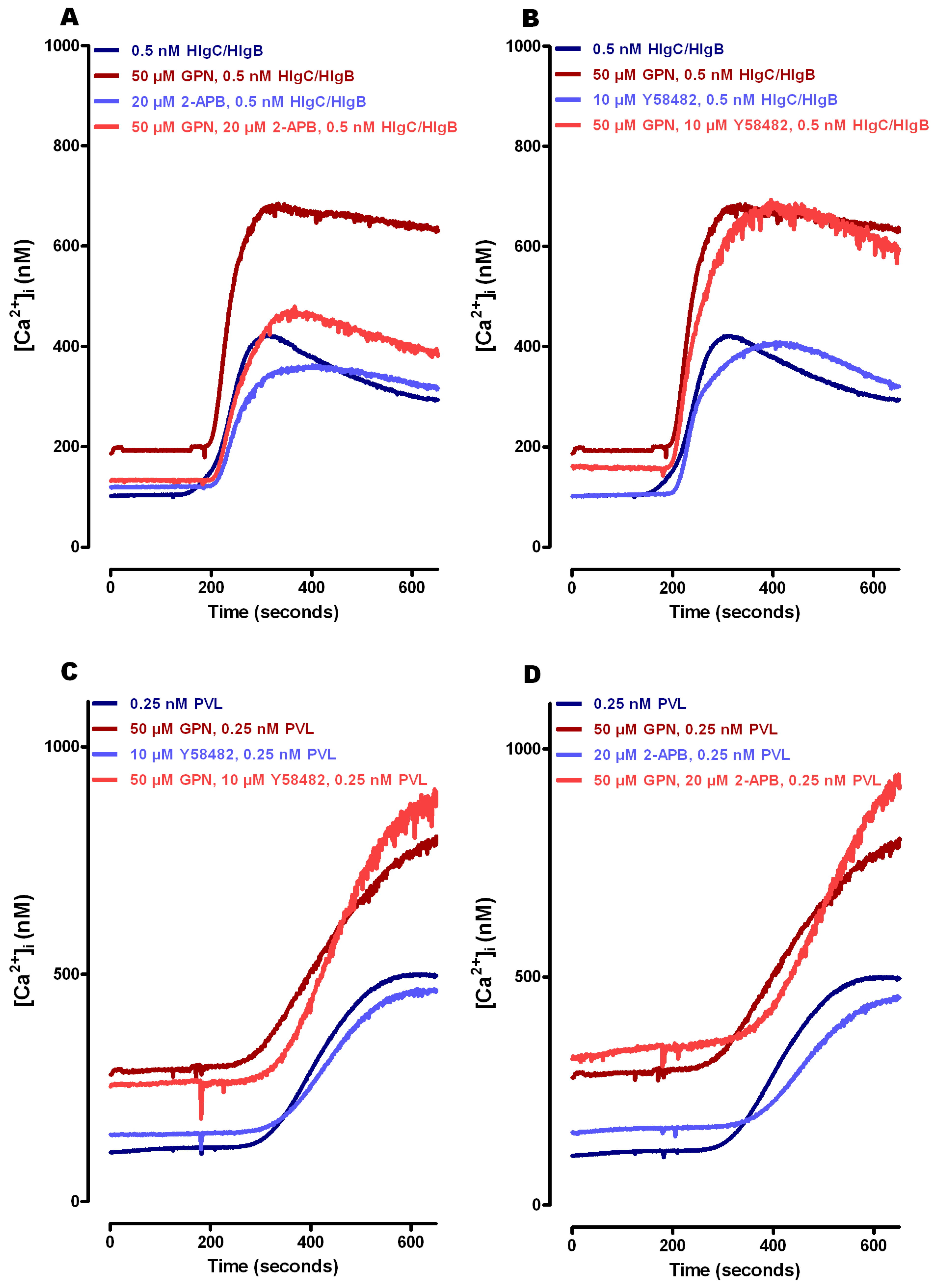
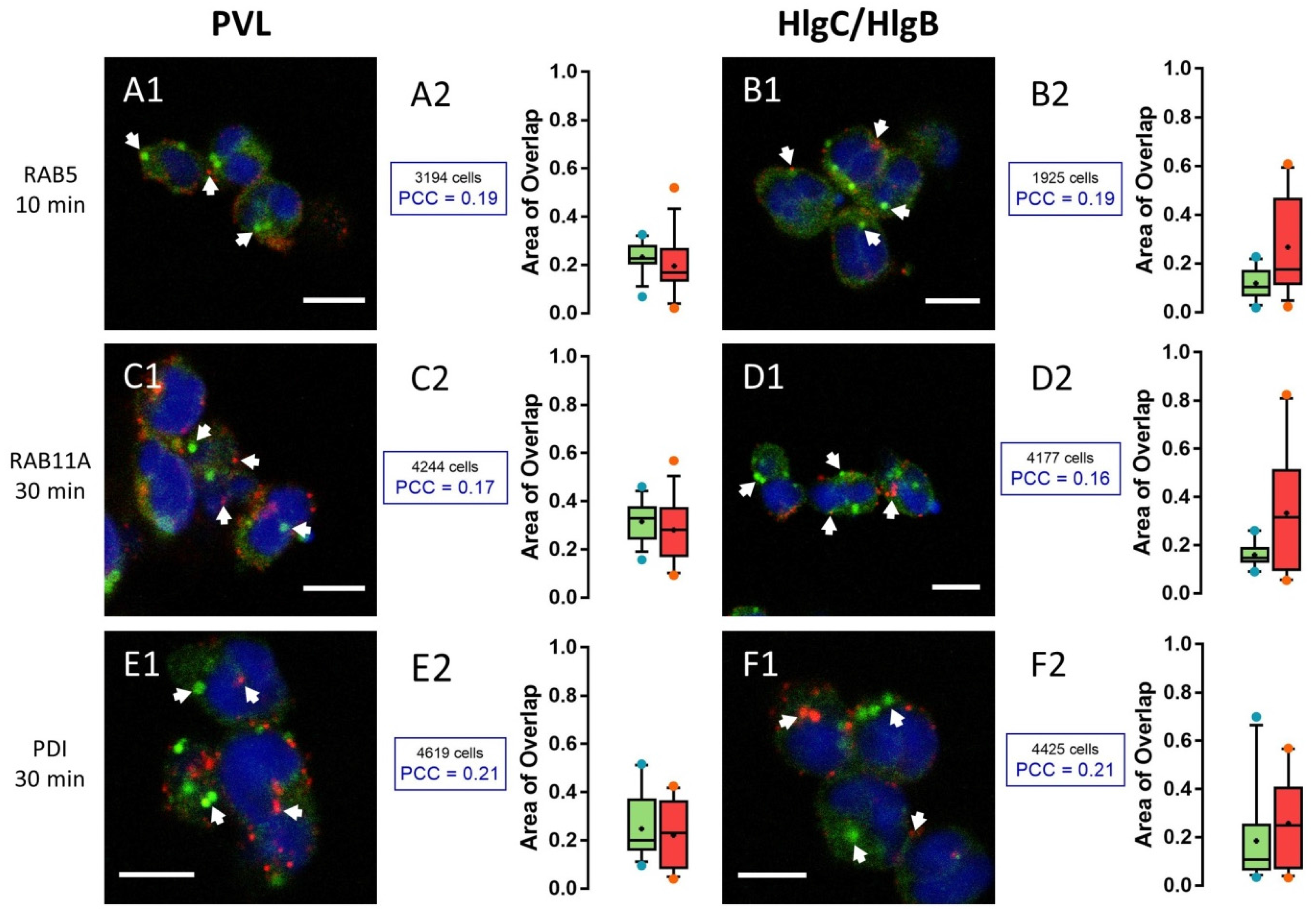
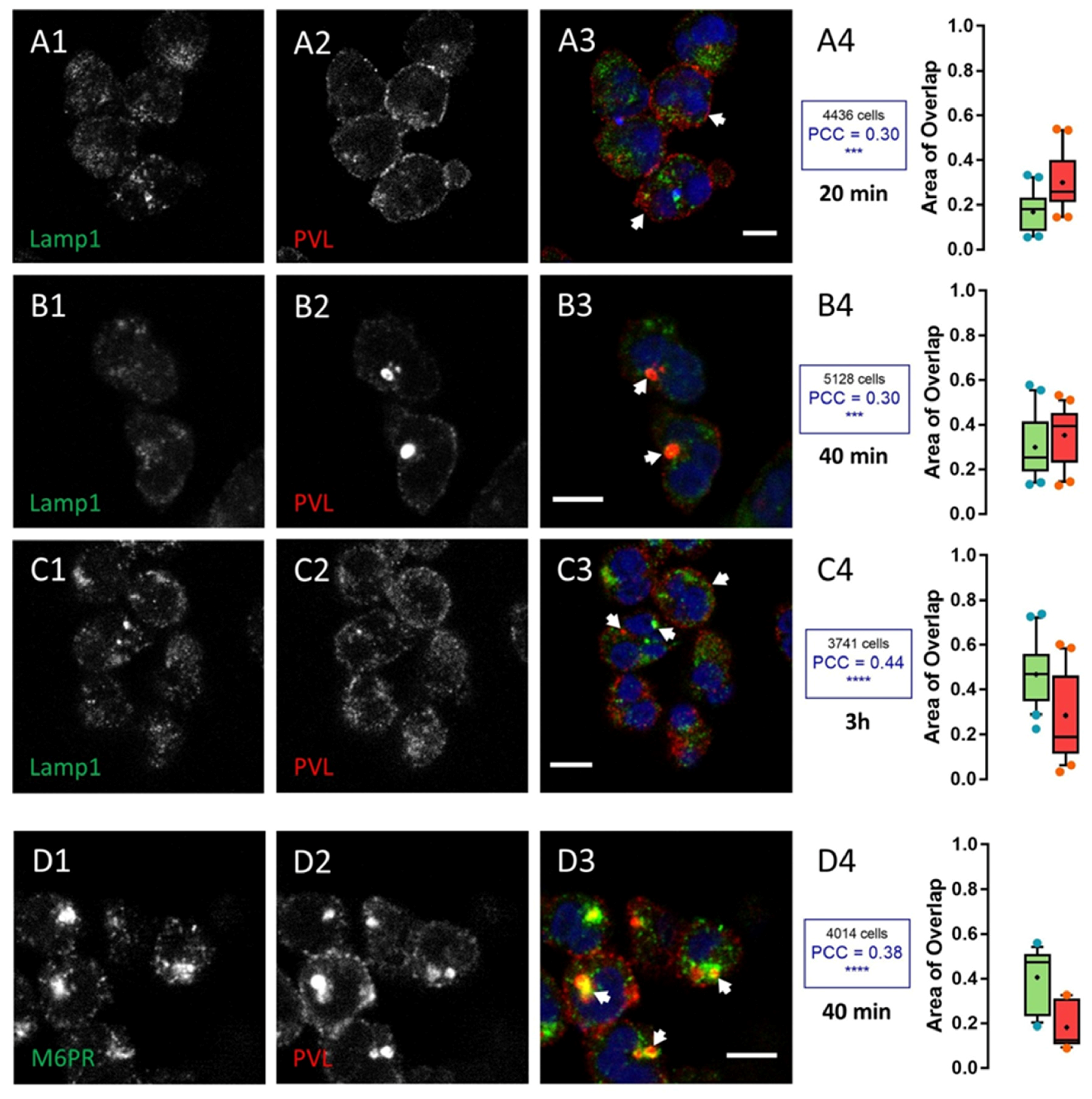
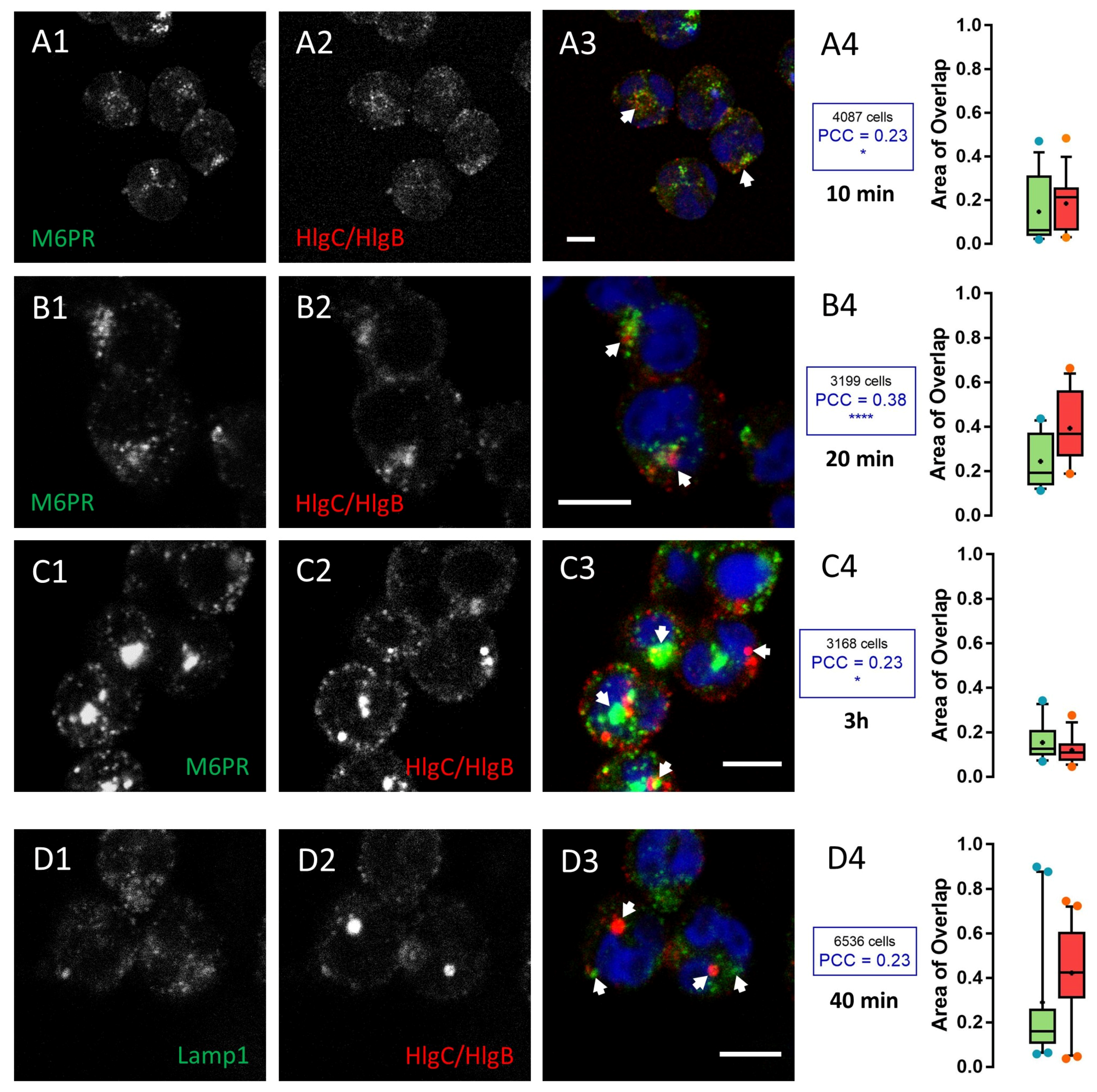
2. Results
2.1. Leucotoxins Progress into the Cell in Association with the C5a Receptor Following Endocytosis
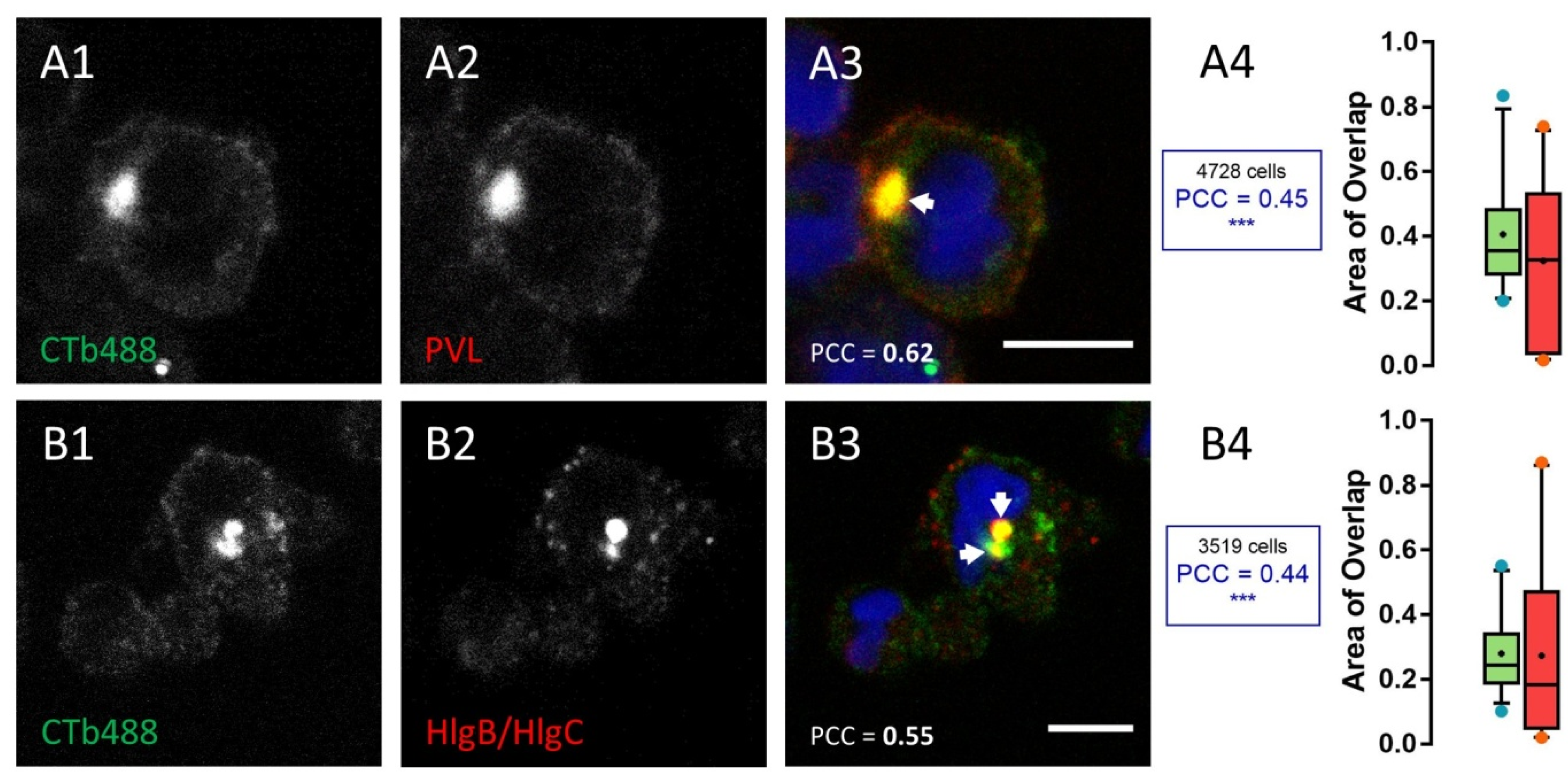
2.2. HlgC/HlgB Quickly Reaches the Golgi Apparatus, While the PVL Transits through the Lysosomal System
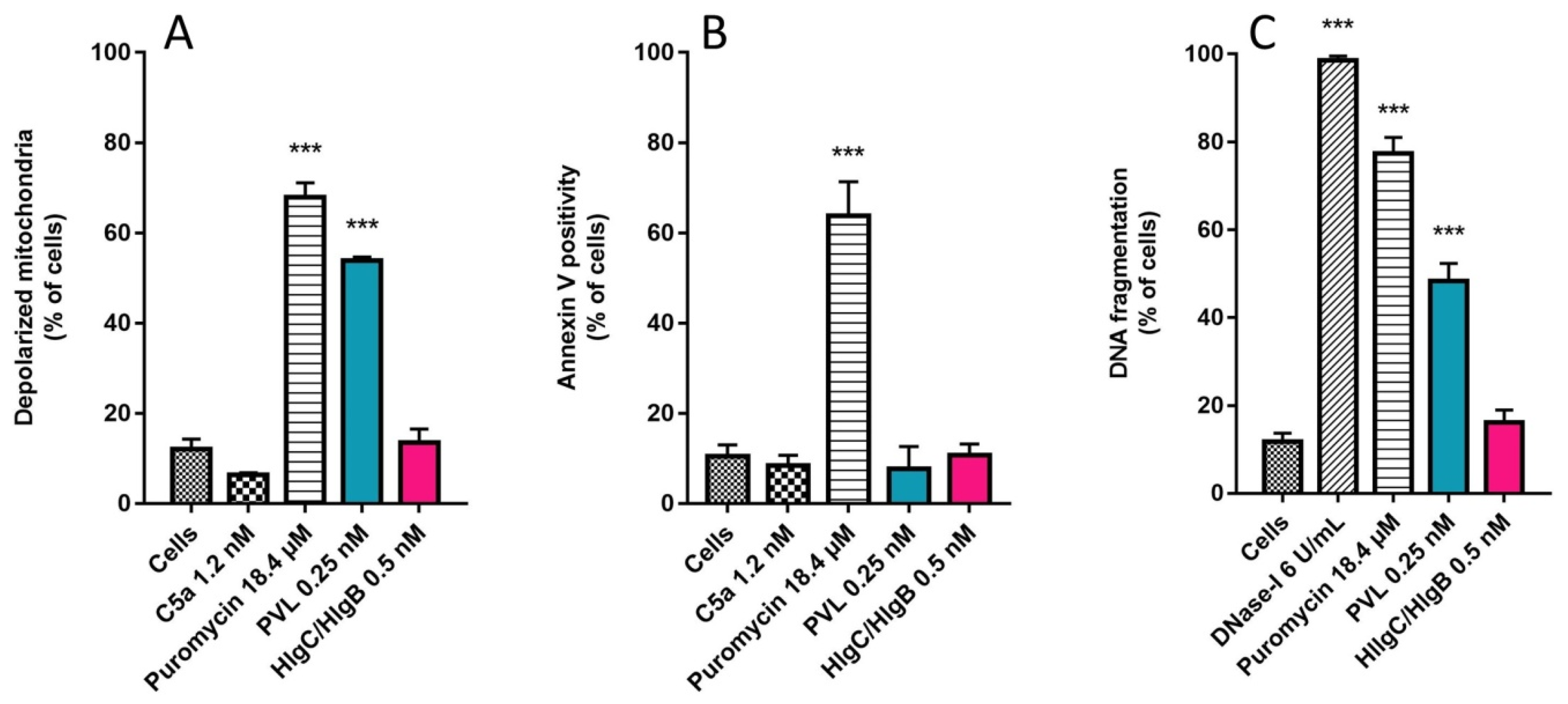
2.3. Do Leucotoxins Modify the Life Span of Human Neutrophils by Remaining in Intracellular Compartments?
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Drugs, Chemicals, and Antibodies
4.3. Preparation of Human Polymorphonuclear (hPMN) Cells
4.4. Leucotoxin Purification
4.5. Spectrofluorimetry
4.6. Mitochondrial Membrane Potential (Δψm) Estimates
4.7. Assessment of Neutrophil Apoptosis by Annexin-V Binding and TUNEL Assays
4.8. Immunocytochemistry
4.9. Image Analysis
4.10. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wertheim, H.F.L.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.; Rozwadowska-Dowzenko, M. Infection by penicillin-resistant staphylococci. Lancet 1948, 252, 641–644. [Google Scholar] [CrossRef]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- David, M.Z.; Boyle-Vavra, S.; Zychowski, D.L.; Daum, R.S. Methicillin-susceptible Staphylococcus aureus as a predominantly healthcare-associated pathogen: A possible reversal of roles? PLoS ONE 2011, 6, e18217. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.A.; Ohneck, E.A.; Ryan, C.; Alonzo, F.; Smith, H.; Narechania, A.; Kolokotronis, S.-O.; Satola, S.W.; Uhlemann, A.-C.; Sebra, R.; et al. Evolution of hypervirulence by a MRSA clone through acquisition of a transposable element. Mol. Microbiol. 2014, 93, 664–681. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Palazzolo-Ballance, A.M.; Tattevin, P.; Basuino, L.; Braughton, K.R.; Whitney, A.R.; Chen, L.; Kreiswirth, B.N.; Otto, M.; DeLeo, F.R.; et al. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS ONE 2008, 3, e3198. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.; Diep, B.A.; Mai, T.T.; Vo, N.H.; Warrener, P.; Suzich, J.; Stover, C.K.; Sellman, B.R. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Rose, H.R.; Holzman, R.S.; Altman, D.R.; Smyth, D.S.; Wasserman, G.A.; Kafer, J.M.; Wible, M.; Mendes, R.E.; Torres, V.J.; Shopsin, B. Cytotoxic virulence predicts mortality in nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 2015, 211, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Bae, T.; Schneewind, O.; Takeuchi, F.; Hiramatsu, K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: Polymorphism and evolution of two major pathogenicity islands. J. Bacteriol. 2008, 190, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.G.; Feil, E.J.; Lindsay, J.A.; Peacock, S.J.; Day, N.P.J.; Enright, M.C.; Foster, T.J.; Moore, C.E.; Hurst, L.; Atkin, R.; et al. Complete genomes of two clinical Staphylococcus aureus strains: Evidence for the rapid evolution of virulence and drug resistance. Proc. Natl. Acad. Sci. USA 2004, 101, 9786–9791. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, A.-C.; Otto, M.; Lowy, F.D.; DeLeo, F.R. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infect. Genet. Evol. 2014, 21, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Laabei, M.; Uhlemann, A.-C.; Lowy, F.D.; Austin, E.D.; Yokoyama, M.; Ouadi, K.; Feil, E.; Thorpe, H.A.; Williams, B.; Perkins, M.; et al. Evolutionary trade-offs underlie the multi-faceted virulence of Staphylococcus aureus. PLoS Biol. 2015, 13, e1002229. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.A.; Thaden, J.T.; Sharma-Kuinkel, B.K.; Fowler, V.G., Jr. Impact of bacterial and human genetic variation on Staphylococcus aureus infections. PLoS Pathog. 2016, 12, e1005330. [Google Scholar] [CrossRef] [PubMed]
- Peacock, S.J.; Moore, C.E.; Justice, A.; Kantzanou, M.; Story, L.; Mackie, K.; O’Neill, G.; Day, N.P.J. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 2002, 70, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.S.; Chen, L.; Joo, H.-S.; Cheung, G.Y.C.; Kreiswirth, B.N.; Otto, M. Distribution and regulation of the mobile genetic element-encoded phenol-soluble modulin PSM-mec in methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e28781. [Google Scholar] [CrossRef] [PubMed]
- DuMont, A.L.; Torres, V.J. Cell targeting by the Staphylococcus aureus pore-forming toxins: It’s not just about lipids. Trends Microbiol. 2014, 22, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.; Kamio, Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: Structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 2004, 68, 981–1003. [Google Scholar] [CrossRef] [PubMed]
- Peraro, M.D.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Scali, F. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol. Lett. 2013, 150, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Badarau, A.; Rouha, H.; Malafa, S.; Logan, D.T.; Håkansson, M.; Stulik, L.; Dolezilkova, I.; Teubenbacher, A.; Gross, K.; Maierhofer, B.; et al. Structure-function analysis of heterodimer formation, oligomerization, and receptor binding of the Staphylococcus aureus bi-component toxin LukGH. J. Biol. Chem. 2015, 290, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Cooney, J.; Kienle, Z.; Foster, T.J.; O’Toole, P.W. The gamma-hemolysin locus of Staphylococcus aureus comprises three linked genes, two of which are identical to the genes for the f and s components of leukocidin. Infect. Immun. 1993, 61, 768–771. [Google Scholar] [PubMed]
- DuMont, A.L.; Yoong, P.; Day, C.J.; Alonzo, F.; McDonald, W.H.; Jennings, M.P.; Torres, V.J. Staphylococcus aureus lukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc. Natl. Acad. Sci. USA 2013, 110, 10794–10799. [Google Scholar] [CrossRef] [PubMed]
- Gravet, A.; Colin, D.A.; Keller, D.; Girardot, R.; Monteil, H.; Prévost, G. Characterization of a novel structural member, lukE-lukD, of the bi-component staphylococcal leucotoxins family. FEBS Lett. 1998, 436, 202–208. [Google Scholar] [CrossRef]
- Kaneko, J.; Muramoto, K.; Kamio, Y. Gene of LukF-PV-like component of Panton-Valentine leukocidin in Staphylococcus aureus P83 is linked with LukM. Biosci. Biotechnol. Biochem. 1997, 61, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Prévost, G.; Cribier, B.; Couppié, P.; Petiau, P.; Supersac, G.; Finck-Barbancon, V.; Monteil, H.; Piemont, Y. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 1995, 63, 4121–4129. [Google Scholar] [PubMed]
- Woodin, A.M. Fractionation of a leucocidin from Staphylococcus aureus. Biochem. J. 1959, 73, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Yanai, M.; Rocha, M.A.; Matolek, A.Z.; Chintalacharuvu, A.; Taira, Y.; Chintalacharuvu, K.; Beenhouwer, D.O. Separately or combined, lukG/lukH is functionally unique compared to other staphylococcal bicomponent leukotoxins. PLoS ONE 2014, 9, e89308. [Google Scholar] [CrossRef] [PubMed]
- Woodin, A.M. Purification of the two components of leucocidin from Staphylococcus aureus. Biochem. J. 1960, 75, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Joubert, O.; Viero, G.; Keller, D.; Martinez, E.; Colin, D.A.; Monteil, H.; Mourey, L.; Dalla Serra, M.; Prévost, G. Engineered covalent leucotoxin heterodimers form functional pores: Insights into S-F interactions. Biochem. J. 2006, 396, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Meunier, O.; Ferreras, M.; Supersac, G.; Hoeper, F.; Baba-Moussa, L.; Monteil, H.; Colin, D.A.; Menestrina, G.; Prévost, G. A predicted β-sheet from class S components of staphylococcal γ-hemolysin is essential for the secondary interaction of the class F component. Biochim. Biophys. Acta (BBA) 1997, 1326, 275–286. [Google Scholar] [CrossRef]
- Bae, I.-G.; Tonthat, G.T.; Stryjewski, M.E.; Rude, T.H.; Reilly, L.F.; Barriere, S.L.; Genter, F.C.; Corey, G.R.; Fowler, V.G. Presence of genes encoding the Panton-Valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: Results of a multinational trial. J. Clin. Microbiol. 2009, 47, 3952–3957. [Google Scholar] [PubMed]
- Hamilton, S.M.; Bryant, A.E.; Carroll, K.C.; Lockary, V.; Ma, Y.; McIndoo, E.; Miller, L.G.; Perdreau-Remington, F.; Pullman, J.; Risi, G.F.; et al. In vitro production of Panton-Valentine leukocidin among strains of methicillin-resistant Staphylococcus aureus causing diverse infections. Clin. Infect. Dis. 2007, 45, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Lalani, T.; Federspiel, J.J.; Boucher, H.W.; Rude, T.H.; Bae, I.-G.; Rybak, M.J.; Tonthat, G.T.; Corey, G.R.; Stryjewski, M.E.; Sakoulas, G.; et al. Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J. Clin. Microbiol. 2008, 46, 2890–2896. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Kuinkel, B.K.; Ahn, S.H.; Rude, T.H.; Zhang, Y.; Tong, S.Y.C.; Ruffin, F.; Genter, F.C.; Braughton, K.R.; DeLeo, F.R.; Barriere, S.L.; et al. Presence of genes encoding Panton-Valentine leukocidin is not the primary determinant of outcome in patients with hospital-acquired pneumonia due to Staphylococcus aureus. J. Clin. Microbiol. 2012, 50, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Jover, E.; Tawk, M.Y.; Laventie, B.J.; Poulain, B.; Prévost, G. Staphylococcal leukotoxins trigger free intracellular Ca2+ rise in neurons, signaling through acidic stores and activation of store-operated channels. Cell Microbiol. 2013, 15, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Staali, L.; Monteil, H.; Colin, D.A. The staphylococcal pore-forming leukotoxins open Ca2+ channels in the membrane of human polymorphonuclear neutrophils. J. Membr. Biol. 1998, 162, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tawk, M.Y.; Zimmermann-Meisse, G.; Bossu, J.-L.; Potrich, C.; Bourcier, T.; Dalla Serra, M.; Poulain, B.; Prévost, G.; Jover, E. Internalization of staphylococcal leukotoxins that bind and divert the C5a receptor is required for intracellular Ca2+ mobilization by human neutrophils. Cell. Microbiol. 2015, 17, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Woodin, A.M.; Wieneke, A.A. The accumulation of calcium by the polymorphonuclear leucocyte treated with staphylococcal leucocidin and its significance in extrusion of protein. Biochem. J. 1963, 87, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.A.; Monteil, H. Control of the oxidative burst of human neutrophils by staphylococcal leukotoxins. Infect. Immun. 2003, 71, 3724–3729. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.F.; Kobayashi, S.D.; Braughton, K.R.; Whitney, A.R.; Sturdevant, D.E.; Rasmussen, D.L.; Kirpotina, L.N.; Quinn, M.T.; DeLeo, F.R. Sublytic concentrations of Staphylococcus aureus Panton-Valentine leukocidin alter human PMN gene expression and enhance bactericidal capacity. J. Leukoc. Biol. 2012, 92, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-Y.; Lin, C.-C.; Liao, I.-C.; Yao, Y.-C.; Shen, F.-C.; Liu, C.-C.; Lin, C.-F. Panton-valentine leukocidin facilitates the escape of Staphylococcus aureus from human keratinocyte endosomes and induces apoptosis. J. Infect. Dis. 2014, 209, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Genestier, A.-L.; Michallet, M.-C.; Prévost, G.; Bellot, G.; Chalabreysse, L.; Peyrol, S.; Thivolet, F.; Etienne, J.; Lina, G.; Vallette, F.M.; et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 2005, 115, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; Kobayashi, S.D.; Freedman, B.; Dorward, D.W.; DeLeo, F.R. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J. Immunol. 2013, 191, 6022–6029. [Google Scholar] [CrossRef] [PubMed]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [PubMed]
- Alonzo III, F.; Kozhaya, L.; Rawlings, S.A.; Reyes-Robles, T.; DuMont, A.L.; Myszka, D.G.; Landau, N.R.; Unutmaz, D.; Torres, V.J. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 2013, 493, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Robles, T.; Alonzo, F., III; Kozhaya, L.; Lacy, D.B.; Unutmaz, D.; Torres, V.J. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 2013, 14, 453–459. [Google Scholar] [PubMed]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.; van Kessel, K.P.; Vandenesch, F.; et al. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 2013, 13, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Schiepers, A.; de Haas, C.J.C.; van Hooijdonk, D.D.J.J.; Badiou, C.; Contamin, H.; Vandenesch, F.; Lina, G.; Gerard, N.P.; Gerard, C.; et al. Differential interaction of the staphylococcal toxins Panton-Valentine leukocidin and γ-Hemolysin CB with human C5a receptors. J. Immunol. 2015, 195, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Vrieling, M.; Wallet, P.; Badiou, C.; Reyes-Robles, T.; Ohneck, E.A.; Benito, Y.; de Haas, C.J.C.; Day, C.J.; Jennings, M.P.; et al. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Naik, N.; Giannini, E.; Brouchon, L.; Boulay, F. Internalization and recycling of the C5a anaphylatoxin receptor: Evidence that the agonist-mediated internalization is modulated by phosphorylation of the c-terminal domain. J. Cell Sci. 1997, 110, 2381–2390. [Google Scholar] [PubMed]
- Suvorova, E.S.; Gripentrog, J.M.; Miettinen, H.M. Different endocytosis pathways of the C5a receptor and the n-formyl peptide receptor. Traffic 2005, 6, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [PubMed]
- Togashi, K.; Inada, H.; Tominaga, M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB). Br. J. Pharmacol. 2008, 153, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Somasundaram, A.; Prakriya, M. Competitive modulation of Ca2+ release-activated Ca2+ channel gating by STIM1 and 2-aminoethyldiphenyl borate. J. Biol. Chem. 2011, 286, 9429–9442. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, M.; Höper, F.; Dalla Serra, M.; Colin, D.A.; Prévost, G.; Menestrina, G. The interaction of Staphylococcus aureus bi-component γ-hemolysins and leucocidins with cells and lipid membranes. Biochim. Biophys. Acta (BBA) 1998, 1414, 108–126. [Google Scholar] [CrossRef]
- Bastiaens, P.I.; Majoul, I.V.; Verveer, P.J.; Söling, H.D.; Jovin, T.M. Imaging the intracellular trafficking and state of the AB5 quaternary structure of cholera toxin. EMBO J. 1996, 15, 4246–4253. [Google Scholar] [PubMed]
- Thieblemont, N.; Wright, S.D. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J. Exp. Med. 1999, 190, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Nicolás-Ávila, J.A.; Hidalgo, A. Aging: A temporal dimension for neutrophils. Trends Immunol. 2016, 37, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Nagami, K.; Kawashima, Y.; Kuno, H.; Kemi, M.; Matsumoto, H. In vitro cytotoxicity assay to screen compounds for apoptosis-inducing potential on lymphocytes and neutrophils. J. Toxicol. Sci. 2002, 27, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Finck-Barbançon, V.; Duportail, G.; Meunier, O.; Colin, D.A. Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim. Biophys. Acta 1993, 1182, 275–282. [Google Scholar] [CrossRef]
- Gauduchon, V.; Werner, S.; Prévost, G.; Monteil, H.; Colin, D.A. Flow cytometric determination of Panton-Valentine leucocidin s component binding. Infect. Immun. 2001, 69, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Gauduchon, V.R.; Cozon, G.; Vandenesch, F.O.; Genestier, A.-L.; Eyssade, N.; Peyrol, S.; Etienne, J.; Lina, G. Neutralization of Staphylococcus aureus Panton-Valentine leukocidin by intravenous immunoglobulin in vitro. J. Infect. Dis. 2004, 189, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, F.; Torres, V.J. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 2014, 78, 199–230. [Google Scholar] [CrossRef] [PubMed]
- Guillet, V.; Roblin, P.; Werner, S.; Coraiola, M.; Menestrina, G.; Monteil, H.; Prévost, G.; Mourey, L. Crystal structure of leucotoxin S component: New insight into the Staphilococcal β-Barrel Pore-Forming Toxins. J. Biol. Chem. 2004, 279, 41028–41037. [Google Scholar] [CrossRef] [PubMed]
- Menestrina, G.; Dalla Serra, M.; Comai, M.; Coraiola, M.; Viero, G.; Werner, S.; Colin, D.A.; Monteil, H.; Prévost, G. Ion channels and bacterial infection: The case of β-barrel pore-forming protein toxins of Staphylococcus aureus. FEBS Lett. 2003, 552, 54–60. [Google Scholar] [CrossRef]
- Yamashita, D.; Sugawara, T.; Takeshita, M.; Kaneko, J.; Kamio, Y.; Tanaka, I.; Tanaka, Y.; Yao, M. Molecular basis of transmembrane beta-barrel formation of staphylococcal pore-forming toxins. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Tisch-Idelson, D.; Sharabani, M.; Kloog, Y.; Aviram, I. Stimulation of neutrophils by prenylcysteine analogs: Ca2+ release and influx. Biochim. Biophys. Acta (BBA) 1999, 1451, 187–195. [Google Scholar] [CrossRef]
- Meyer, F.; Girardot, R.; Piémont, Y.; Prévost, G.; Colin, D.A. Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin f component binding. Infect. Immun. 2009, 77, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.; Gladstone, G.P.; Lenhart, N.A. The antigenicity in man of staphlylococcal leucocidin toxoid, with notes on therapeutic immunization in chronic osteomyelits. Br. J. Exp. Pathol. 1965, 46, 455–472. [Google Scholar] [PubMed]
- Verkaik, N.J.; Dauwalder, O.; Antri, K.; Boubekri, I.; de Vogel, C.P.; Badiou, C.d.; Bes, M.l.; Vandenesch, F.o.; Tazir, M.; Hooijkaas, H.; et al. Immunogenicity of toxins during Staphylococcus aureus infection. Clin. Infect. Dis. 2010, 50, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Badiou, C.; Dumitrescu, O.; George, N.; Forbes, A.R.N.; Drougka, E.; Chan, K.S.; Ramdani-Bouguessa, N.; Meugnier, H.; Bes, M.; Vandenesch, F.; et al. Rapid detection of Staphylococcus aureus Panton-Valentine leukocidin in clinical specimens by enzyme-linked immunosorbent assay and immunochromatographic tests. J. Clin. Microbiol. 2010, 48, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Popovich, K.J.; Hayden, M.K. Delineating the epidemiology-host-microbe relationship for methicillin-resistant Staphylococcus aureus infection. J. Infect. Dis. 2015, 211, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Colin, D.A.; Coraiola, M.; Menestrina, G.; Monteil, H.; Prévost, G. Retrieving biological activity from LukF-PV mutants combined with different S components implies compatibility between the stem domains of these staphylococcal bicomponent leucotoxins. Infect. Immun. 2002, 70, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Finck-Barbançon, V.; Prévost, G.; Piémont, Y. Improved purification of leukocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res. Microbiol. 1991, 142, 75–85. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. Cellprofiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann-Meisse, G.; Prévost, G.; Jover, E. Above and beyond C5a Receptor Targeting by Staphylococcal Leucotoxins: Retrograde Transport of Panton–Valentine Leucocidin and γ-Hemolysin. Toxins 2017, 9, 41. https://doi.org/10.3390/toxins9010041
Zimmermann-Meisse G, Prévost G, Jover E. Above and beyond C5a Receptor Targeting by Staphylococcal Leucotoxins: Retrograde Transport of Panton–Valentine Leucocidin and γ-Hemolysin. Toxins. 2017; 9(1):41. https://doi.org/10.3390/toxins9010041
Chicago/Turabian StyleZimmermann-Meisse, Gaëlle, Gilles Prévost, and Emmanuel Jover. 2017. "Above and beyond C5a Receptor Targeting by Staphylococcal Leucotoxins: Retrograde Transport of Panton–Valentine Leucocidin and γ-Hemolysin" Toxins 9, no. 1: 41. https://doi.org/10.3390/toxins9010041
APA StyleZimmermann-Meisse, G., Prévost, G., & Jover, E. (2017). Above and beyond C5a Receptor Targeting by Staphylococcal Leucotoxins: Retrograde Transport of Panton–Valentine Leucocidin and γ-Hemolysin. Toxins, 9(1), 41. https://doi.org/10.3390/toxins9010041






