Protection of the Furin Cleavage Site in Low-Toxicity Immunotoxins Based on Pseudomonas Exotoxin A
Abstract
:1. Introduction
2. Results
2.1. Design and Production of the DS-PE24 Immunotoxins
2.2. The Disulfide Bond around the Furin Cleavage Site Is Formed
2.3. Cytotoxicity Assays
2.4. Stability Studies
2.5. Half-Life Studies in Mice
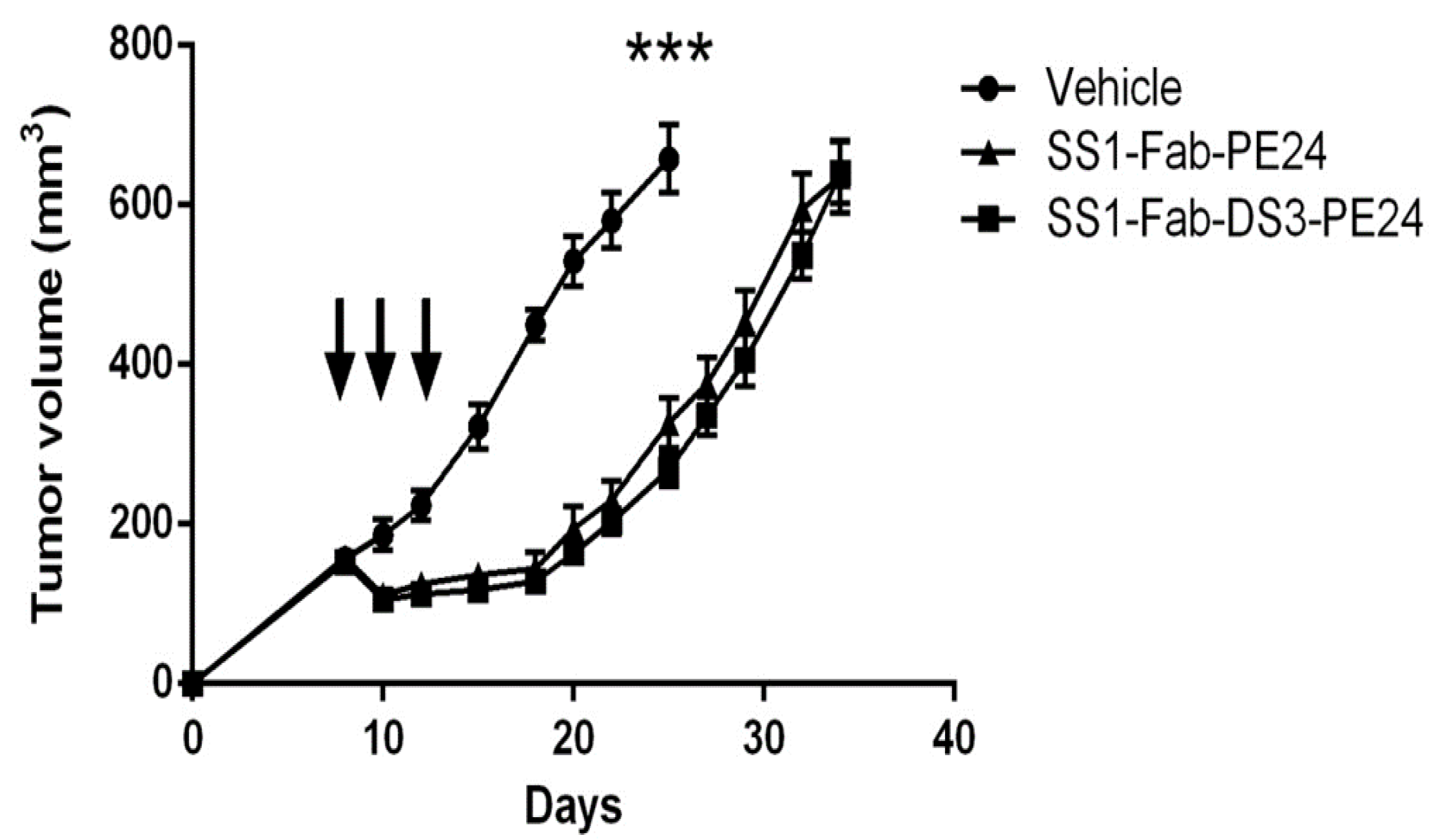
2.6. Anti-Tumor Experiments
3. Discussion
3.1. DS-PE24 Immunotoxins Are Producible, Stable and Cytotoxic
3.2. Furin Cleavage Correlates with Immunotoxin Activity
3.3. Importance of Serum Half-Life of Protein Therapeutics
3.4. Importance of Proteolytic Stability in the Tumor Microenvironment
3.5. Summary
4. Materials and Methods
4.1. Construction, Expression and Protein Purification
4.2. Computational Design of DS-PE24 Linkers
4.3. Cell Growth Inhibition Assay
4.4. In Vitro Furin Cleavage
4.5. Immunotoxin Stability in Human Serum
4.6. In Vivo Half-Life
4.7. Anti-Tumor Activity
4.8. Graphs and Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RIT | Recombinant immunotoxins |
| PE | Pseudomonas exotoxin |
| PC | Pro-protein convertase |
| TGN | Trans-golgi network |
| eEF2 | Elongation factor 2 |
| ADA | Anti-drug antibodies |
| FCS | Furin Cleavage Site |
| E. coli | Escherichia coli |
| PBS | Phosphate buffered saline |
References
- Weidle, U.H.; Tiefenthaler, G.; Schiller, C.; Weiss, E.H.; Georges, G.; Brinkmann, U. Prospects of bacterial and plant protein-based immunotoxins for treatment of cancer. Cancer Genom. Proteom. 2014, 11, 25–38. [Google Scholar]
- Wolf, P.; Elsasser-Beile, U. Pseudomonas exotoxin a: From virulence factor to anti-cancer agent. Int. J. Med. Microb. 2009, 299, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Alewine, C.; Hassan, R.; Pastan, I. Advances in anticancer immunotoxin therapy. Oncologist 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Weldon, J.E.; Pastan, I. A guide to taming a toxin—Recombinant immunotoxins constructed from pseudomonas exotoxin a for the treatment of cancer. FEBS J. 2011, 278, 4683–4700. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Onda, M.; Pastan, I. Immunogenicity of therapeutic recombinant immunotoxins. Immunol. Rev. 2016, 270, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Miller, A.C.; Sharon, E.; Thomas, A.; Reynolds, J.C.; Ling, A.; Kreitman, R.J.; Miettinen, M.M.; Steinberg, S.M.; Fowler, D.H.; et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Weldon, J.E.; Xiang, L.; Chertov, O.; Margulies, I.; Kreitman, R.J.; FitzGerald, D.J.; Pastan, I. A protease-resistant immunotoxin against cd22 with greatly increased activity against cll and diminished animal toxicity. Blood 2009, 113, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Crown, D.; Addissie, S.; Jang, Y.; Kaplan, G.; Pastan, I. Elimination of murine and human T cell epitopes in recombinant immunotoxin eliminates neutralizing and anti-drug antibodies in vivo. Cell. Mol. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Eberle, J.A.; Hu, X.; Vassall, A.N.; Onda, M.; Beers, R.; Lee, E.C.; Kreitman, R.J.; Lee, B.; Baker, D.; et al. Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human t-cell epitopes. Proc. Natl. Acad. Sci. USA 2014, 111, 8571–8576. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.K.; Weldon, J.E.; Xiang, L.; Beers, R.; Onda, M.; Pastan, I. A recombinant immunotoxin targeting cd22 with low immunogenicity, low nonspecific toxicity, and high antitumor activity in mice. J. Immunother. 2010, 33, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Weldon, J.E.; Xiang, L.; Zhang, J.; Beers, R.; Walker, D.A.; Onda, M.; Hassan, R.; Pastan, I. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol. Cancer Ther. 2013, 12, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002, 3, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.S.; Thomas, L.; VanSlyke, J.K.; Stenberg, P.E.; Thomas, G. Intracellular trafficking and activation of the furin proprotein convertase: Localization to the tgn and recycling from the cell surface. EMBO J. 1994, 13, 18–33. [Google Scholar] [PubMed]
- Klimpel, K.R.; Molloy, S.S.; Thomas, G.; Leppla, S.H. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc. Natl. Acad. Sci. USA 1992, 89, 10277–10281. [Google Scholar] [CrossRef] [PubMed]
- Hollevoet, K.; Mason-Osann, E.; Liu, X.F.; Imhof-Jung, S.; Niederfellner, G.; Pastan, I. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin rg7787 in pancreatic cancer. Mol. Cancer Ther. 2014, 13, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verschraegen, C.F.; Mendoza, J.; Hassan, R. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, ss1(dsfv)pe38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer Res. 2004, 24, 1327–1335. [Google Scholar] [PubMed]
- Alewine, C.; Xiang, L.; Yamori, T.; Niederfellner, G.; Bosslet, K.; Pastan, I. Efficacy of rg7787, a next-generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol. Cancer Ther. 2014, 13, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Feng, M.; Fisher, R.J.; Rader, C.; Pastan, I. A novel high-affinity human monoclonal antibody to mesothelin. Int. J. Cancer 2011, 128, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Feng, M.; Gao, W.; Phung, Y.; Chen, W.; Chaudhary, A.; St Croix, B.; Qian, M.; Dimitrov, D.S.; Ho, M. A human single-domain antibody elicits potent antitumor activity by targeting an epitope in mesothelin close to the cancer cell surface. Mol. Cancer Ther. 2013, 12, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011, 22, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Vugmeyster, Y.; Xu, X.; Theil, F.P.; Khawli, L.A.; Leach, M.W. Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World J. Biol. Chem. 2012, 3, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Sporn, M.B. The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer 2007, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Watson, P.H.; Paterson, J.A.; Seidah, N.; Chretien, M.; Shiu, R.P. Pro-protein convertase gene expression in human breast cancer. Int. J. Cancer 1997, 71, 966–971. [Google Scholar] [CrossRef]
- Schalken, J.A.; Roebroek, A.J.; Oomen, P.P.; Wagenaar, S.S.; Debruyne, F.M.; Bloemers, H.P.; Van de Ven, W.J. Fur gene expression as a discriminating marker for small cell and nonsmall cell lung carcinomas. J. Clin. Investig. 1987, 80, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Bassi, D.E.; Mahloogi, H.; Al-Saleem, L.; Lopez De Cicco, R.; Ridge, J.A.; Klein-Szanto, A.J. Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol. Carcinog. 2001, 31, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Lopez de Cicco, R.; Bassi, D.E.; Page, R.; Klein-Szanto, A.J. Furin expression in squamous cell carcinomas of the oral cavity and other sites evaluated by tissue microarray technology. Acta Odontol. Latinoam. 2002, 15, 29–37. [Google Scholar] [PubMed]
- Arsenault, D.; Lucien, F.; Dubois, C.M. Hypoxia enhances cancer cell invasion through relocalization of the proprotein convertase furin from the trans-golgi network to the cell surface. J. Cell. Physiol. 2012, 227, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Mason-Osann, E.; Hollevoet, K.; Niederfellner, G.; Pastan, I. Quantification of recombinant immunotoxin delivery to solid tumors allows for direct comparison of in vivo and in vitro results. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Onda, M.; Kreitman, R.J.; Pastan, I. An improved recombinant fab-immunotoxin targeting cd22 expressing malignancies. Leuk. Res. 2014, 38, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; Beers, R.; Bera, T.K. Recombinant immunotoxins in the treatment of cancer. Methods Mol. Biol. 2004, 248, 503–518. [Google Scholar] [PubMed]
- Leaver-Fay, A.; Tyka, M.; Lewis, S.M.; Lange, O.F.; Thompson, J.; Jacak, R.; Kaufman, K.; Renfrew, P.D.; Smith, C.A.; Sheffler, W.; et al. Rosetta3: An object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 2011, 487, 545–574. [Google Scholar] [PubMed]
- Huang, P.S.; Ban, Y.E.; Richter, F.; Andre, I.; Vernon, R.; Schief, W.R.; Baker, D. Rosettaremodel: A generalized framework for flexible backbone protein design. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Onda, M.; Willingham, M.; Nagata, S.; Bera, T.K.; Beers, R.; Ho, M.; Hassan, R.; Kreitman, R.J.; Pastan, I. New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence-activated cell sorting, western blotting, and elisa. Clin. Cancer Res. 2005, 11, 5840–5846. [Google Scholar] [CrossRef] [PubMed]
- Onda, M.; Nagata, S.; FitzGerald, D.J.; Beers, R.; Fisher, R.J.; Vincent, J.J.; Lee, B.; Nakamura, M.; Hwang, J.; Kreitman, R.J.; et al. Characterization of the b cell epitopes associated with a truncated form of pseudomonas exotoxin (pe38) used to make immunotoxins for the treatment of cancer patients. J. Immunol. 2006, 177, 8822–8834. [Google Scholar] [CrossRef] [PubMed]
- Note: Author’s contribution to the Work was done as part of the Author’s official duties as a NIH employee and is a Work of the United States Government. Therefore, copyright may not be established in the United States.
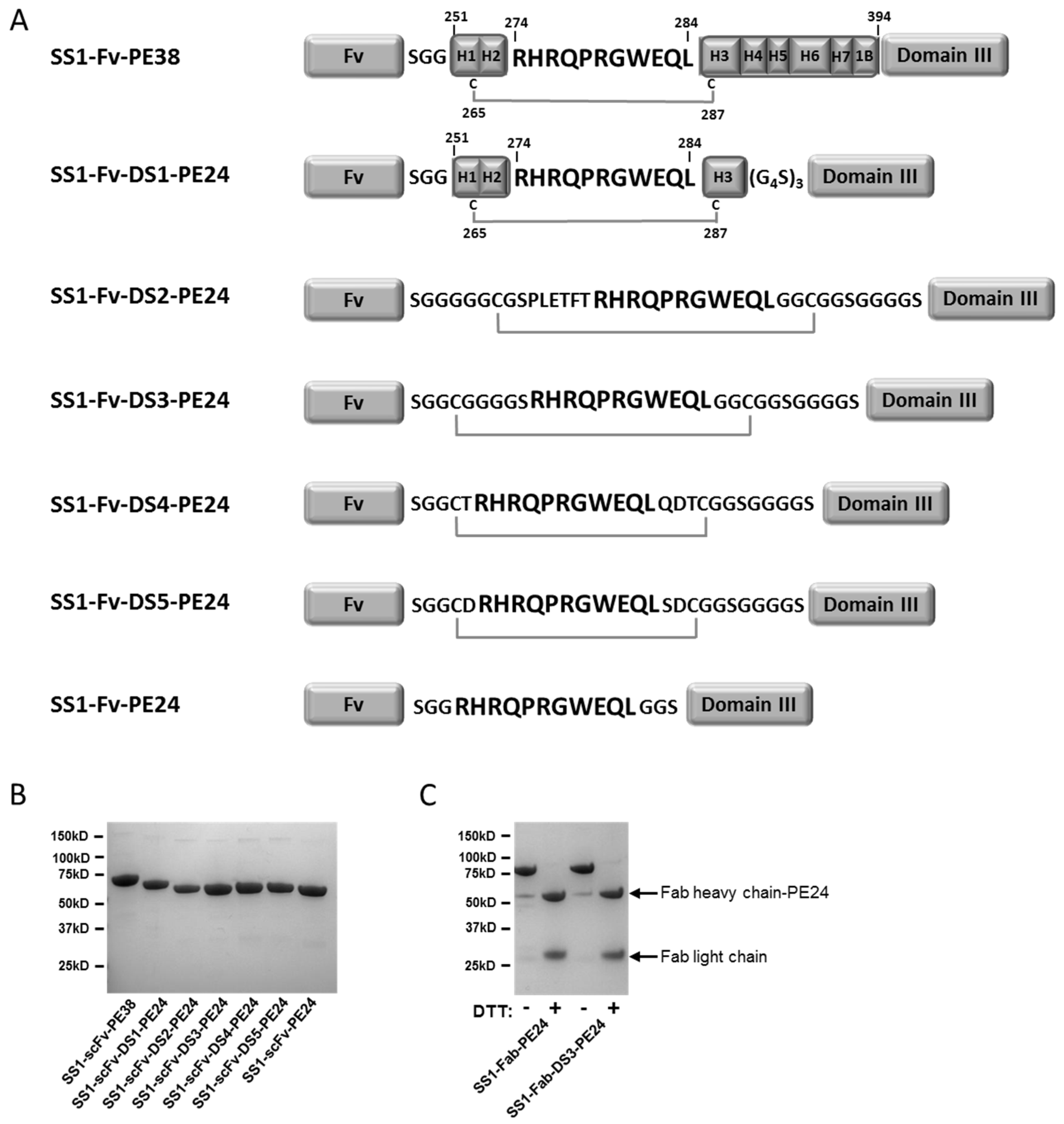
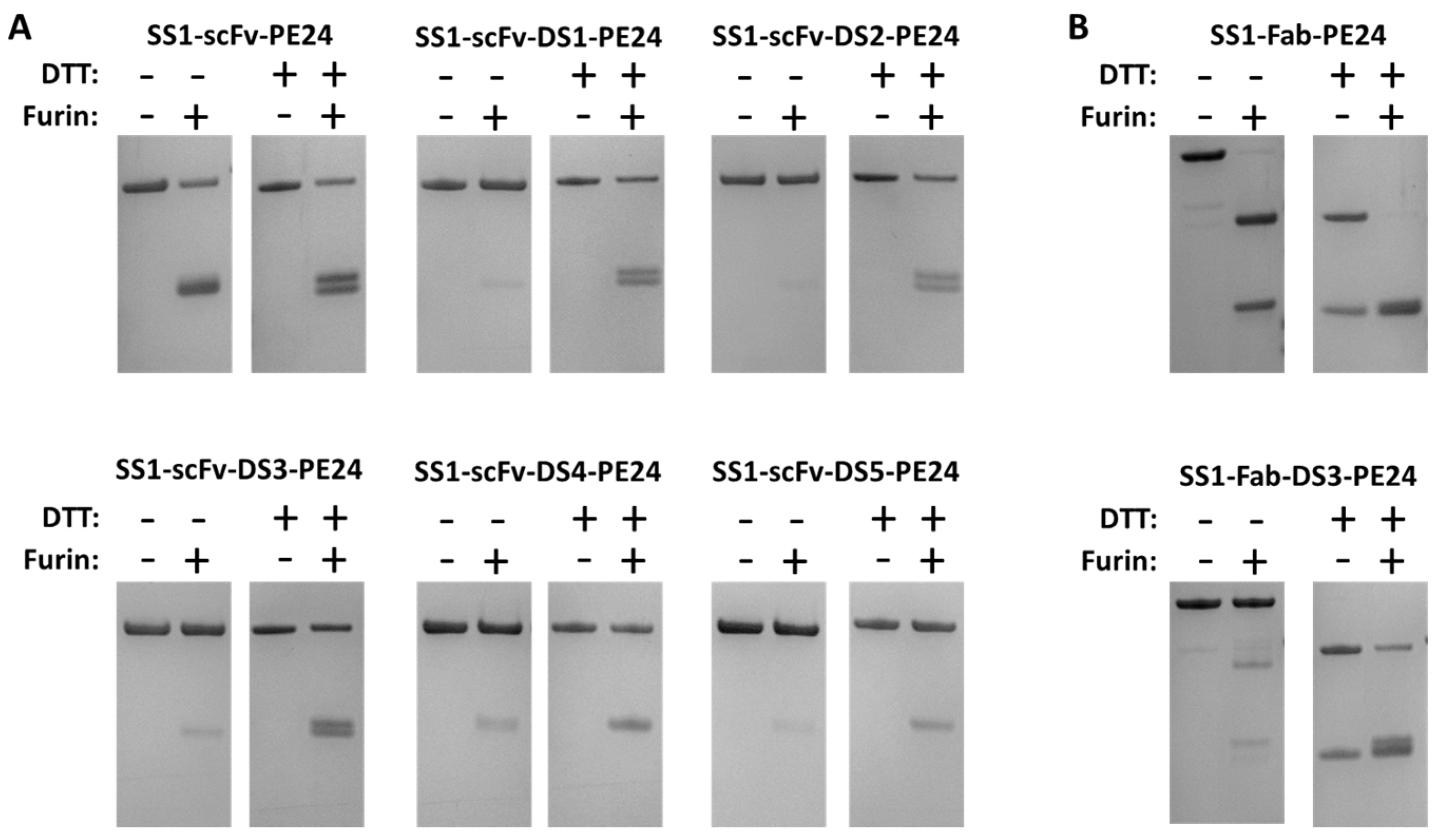
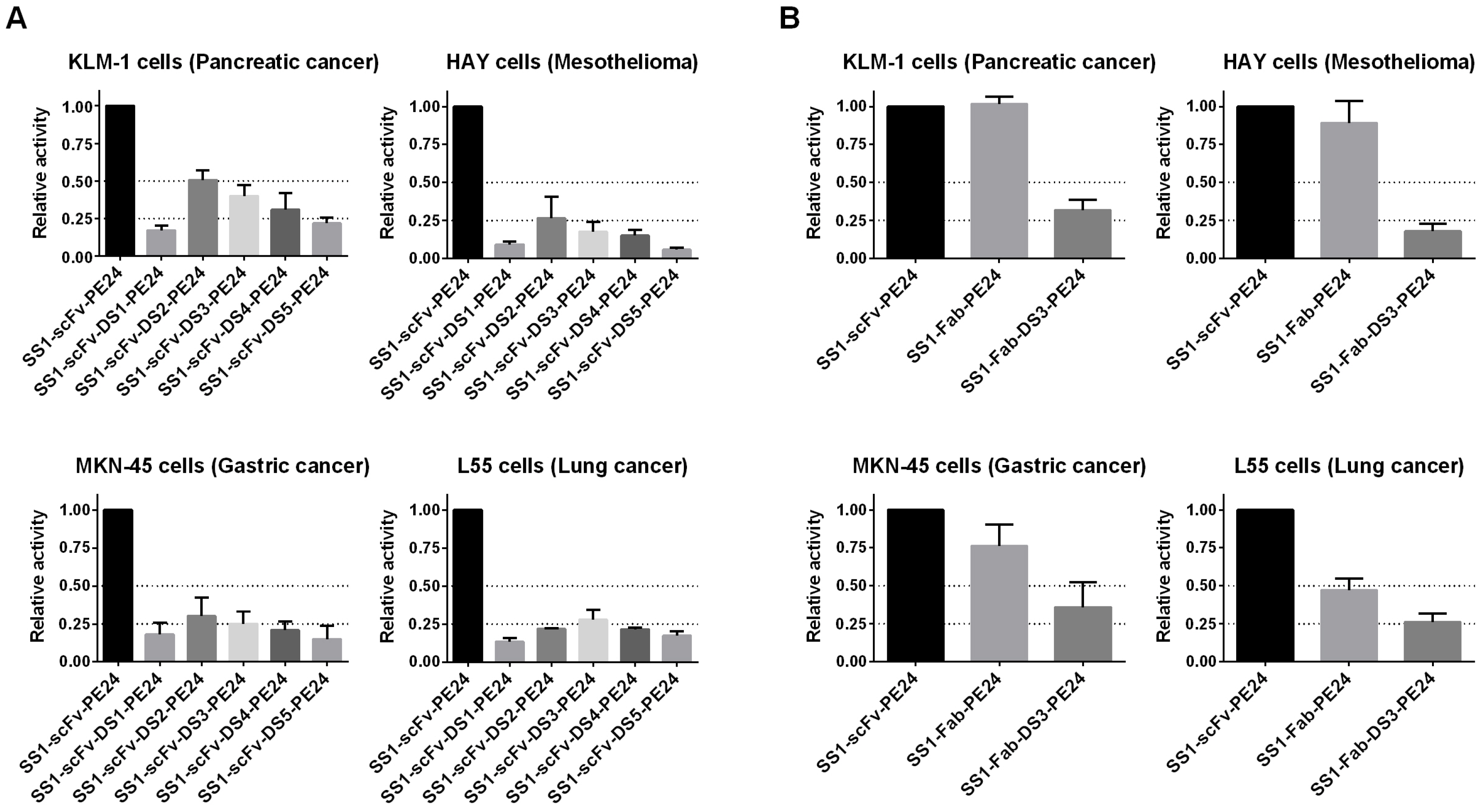
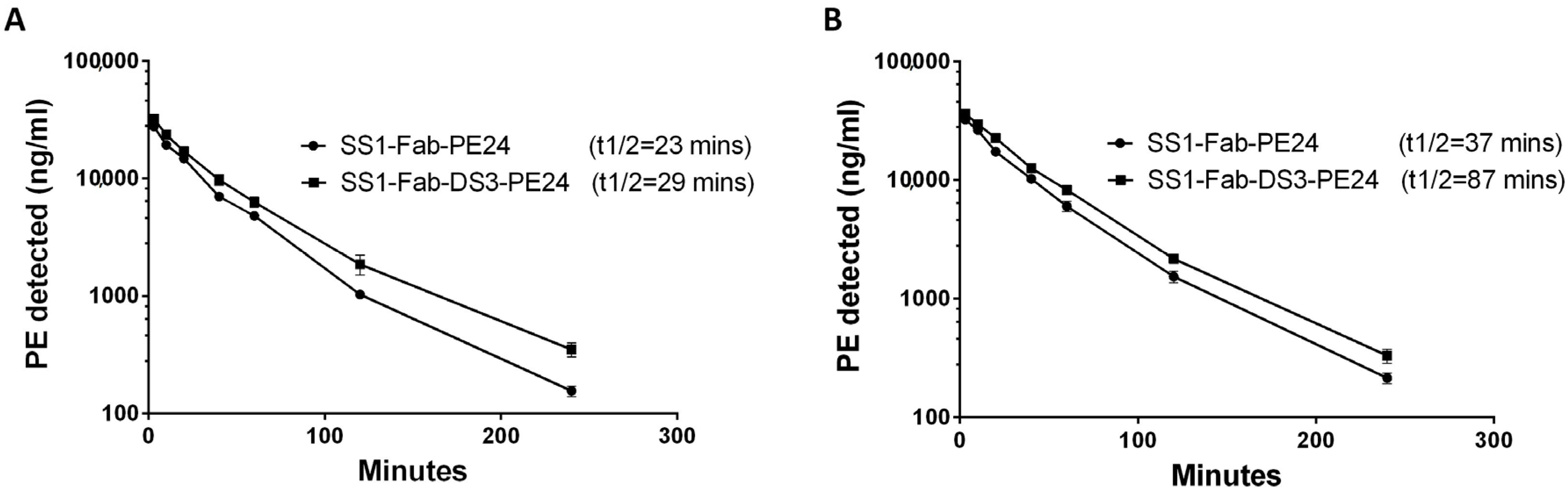
| Construct | Yield (mg) |
|---|---|
| SS1-scFv-PE24 | 26 |
| SS1-scFv-DS1-PE24 | 12 |
| SS1-scFv-DS2-PE24 | 6.5 |
| SS1-scFv-DS3-PE24 | 13 |
| SS1-scFv-DS4-PE24 | 7.7 |
| SS1-scFv-DS5-PE24 | 11.8 |
| SS1-Fab-PE24 | 8.3 |
| SS1-Fab-DS3-PE24 | 8.9 |
| Construct | KLM1 | MKN45 | HAY | L55 |
|---|---|---|---|---|
| SS1-scFv-PE24 | 6 | 2 | 6 | 22 |
| SS1-scFv-DS1-PE24 | 36 | 15 | 70 | 191 |
| SS1-scFv-DS2-PE24 | 12 | 7 | 36 | 102 |
| SS1-scFv-DS3-PE24 | 17 | 8 | 37 | 98 |
| SS1-scFv-DS4-PE24 | 28 | 9 | 46 | 100 |
| SS1-scFv-DS5-PE24 | 30 | 19 | 112 | 132 |
| SS1-Fab-PE24 | 6 | 2 | 7 | 47 |
| SS1-Fab-DS3-PE24 | 20 | 6 | 38 | 86 |
| Incubation (h) | SS1-scFv-PE24 | SS1-scFv-DS3-PE24 | SS1-Fab-PE24 | SS1-Fab-DS3-PE24 |
|---|---|---|---|---|
| 0 | 5 (0.5) | 14 (2.7) | 6 (0.7) | 15 (3.0) |
| 1 | 5 (0.8) | 13 (2.6) | 6 (2.8) | 14 (0.8) |
| 6 | 6 (0.4) | 12 (2.5) | 9 (2.8) | 15 (1.0) |
| 24 | 9 * (2.1) | 26 * (6.6) | 10 * (1.1) | 22 (2.2) |
| Minutes | Experiment 1 | Experiment 2 | ||
|---|---|---|---|---|
| SS1-Fab-PE24 | SS1-Fab-DS3-PE24 | SS1-Fab-PE24 | SS1-Fab-DS3-PE24 | |
| 3 | 27,682 | 32,139 | 32,202 | 36,114 |
| 10 | 19,297 | 23,574 | 26,330 | 29,584 |
| 20 | 14,171 | 17,061 | 17,338 | 22,608 |
| 40 | 7009 | 9742 | 10,260 | 12,627 |
| 60 | 4813 | 6296 | 6010 | 8266 |
| 120 | 1029 | 1860 | 1541 | 2175 |
| 240 | 156 | 350 | 215 | 331 |
| Parameter | Experiment 1 | Experiment 2 | ||
|---|---|---|---|---|
| SS1-Fab-PE24 | SS1-Fab-DS3-PE24 | SS1-Fab-PE24 | SS1-Fab-DS3-PE24 | |
| Dose (µg) | 30 | 30 | 30 | 30 |
| Bleed volume (µL) | 40 | 40 | 10 | 10 |
| KFast (1/min) | 0.24 | 0.11 | 0.05 | 0.03 |
| KSlow (1/min) | 0.03 | 0.02 | 0.02 | 0.01 |
| Half-life (Fast) | 2.88 | 6.39 | 14.2 | 22.14 |
| Half-life (Slow) | 23.01 | 29.77 | 37.64 | 87.91 |
| Vd (mL) | 0.85 | 0.80 | 0.83 | 0.76 |
| Cl (mL/min) | 0.033 | 0.025 | 0.025 | 0.020 |
| AUC (ng·min/mL) | 0.91 × 106 | 1.2 × 106 | 1.19 × 106 | 1.52 × 106 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, G.; Lee, F.; Onda, M.; Kolyvas, E.; Bhardwaj, G.; Baker, D.; Pastan, I. Protection of the Furin Cleavage Site in Low-Toxicity Immunotoxins Based on Pseudomonas Exotoxin A. Toxins 2016, 8, 217. https://doi.org/10.3390/toxins8080217
Kaplan G, Lee F, Onda M, Kolyvas E, Bhardwaj G, Baker D, Pastan I. Protection of the Furin Cleavage Site in Low-Toxicity Immunotoxins Based on Pseudomonas Exotoxin A. Toxins. 2016; 8(8):217. https://doi.org/10.3390/toxins8080217
Chicago/Turabian StyleKaplan, Gilad, Fred Lee, Masanori Onda, Emily Kolyvas, Gaurav Bhardwaj, David Baker, and Ira Pastan. 2016. "Protection of the Furin Cleavage Site in Low-Toxicity Immunotoxins Based on Pseudomonas Exotoxin A" Toxins 8, no. 8: 217. https://doi.org/10.3390/toxins8080217
APA StyleKaplan, G., Lee, F., Onda, M., Kolyvas, E., Bhardwaj, G., Baker, D., & Pastan, I. (2016). Protection of the Furin Cleavage Site in Low-Toxicity Immunotoxins Based on Pseudomonas Exotoxin A. Toxins, 8(8), 217. https://doi.org/10.3390/toxins8080217







