Ochratoxin A: 50 Years of Research
Abstract
:1. Introduction
2. OTA Producers in Foodstuffs
3. OTA Chemistry
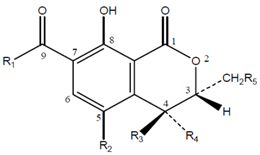
3.1. Chemical Characterization of OTA
4. OTA Analysis
5. Occurrence of OTA in Food and Feed
6. OTA Toxicity
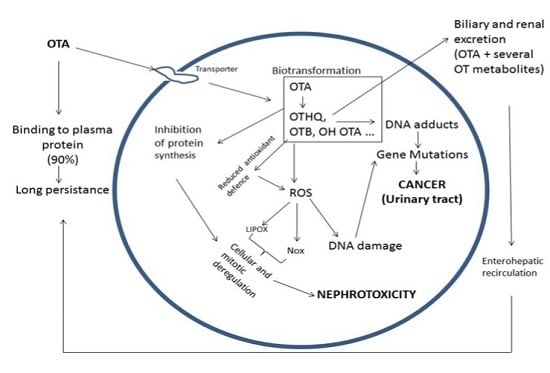
6.1. OTA Nephrotoxicity
6.2. OTA Carcinogenicity
7. OTA Biomarkers
7.1. OTA in Human Blood
7.2. OTA in Urine
7.3. OTA in Human Milk
7.4. OTA in Human Kidneys
8. Regulation of OTA in Food and Feed
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
Abbreviations
| 10-OHOA | 10-hydroxy ochratoxin A |
| 10-OHOA-Me | 10-hydroxy ochratoxin A methyl ester |
| 2′-DC-OTA | 2′-ochratoxin A decarboxylated |
| 2′R-OTA | 2′R-ochratoxin A |
| 4R-OHOA | 4R-hydroxy ochratoxin A |
| 4R-OHOA-Me | 4R-hydroxy ochratoxin A methyl ester |
| 4S-OHOA | 4S-hydroxy ochratoxin A |
| Acyl-hexose-OTA | conjugate ochratoxin A–acyl hexose |
| Acyl-pentose OTA | conjugate ochratoxin A–acyl pentose |
| BEN | Balkan endemic nephropathy |
| CAC | Codex Alimentarius Commission |
| CAS | Chemical Abstracts Services |
| CE-LIF | capillary electrophoresis with laser-induced fluorescence detection |
| CIN | chronic interstitial nephropathy |
| CIT | citrinin |
| DC-OA | ochratoxin A decarboxylated |
| DC-OTHQ | OTHQ decarboxylated |
| DNA aptamer | Artificial short single stranded oligonucleotides |
| DNA | Deoxyribonucleic acid |
| d-OA | d-ochratoxin A |
| EU | European Union |
| FB | fumonisin |
| FDA | Food and Drug Administration |
| FFDCA | Federal Food, Drug, and Cosmetic Act |
| GC-MS | gas chromatography–mass spectrometry |
| HPLC-FLD | high-performance liquid chromatography with fluorescence detection |
| HPLC-UVD | high-performance liquid chromatography with ultraviolet detection |
| IAC | immunoaffinity columns |
| TGFβ | profibrotic transforming growth factors β |
| ROS | reactive oxygen species |
| IARC | The International Agency for Research on Cancer |
| ICP-MS | inductively coupled plasma mass spectrometry |
| IgE | immunoglobulin E |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| IPCS | International Programme on Chemical Safety |
| IUPAC | International Union of Pure and Applied Chemistry |
| JECFA | The Joint FAO/WHO Expert Committee on Food Additives |
| LC-ESI-MS/MS | column liquid chromatography electrospray ionization tandem mass spectrometry |
| LC-MS | liquid chromatography–mass spectrometry |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MEKC | micellar electrokinetic capillary chromatography |
| MIPs | molecular imprinted polymers |
| M-Oα | Ochratoxin α ester methyl |
| OE-OA | ethylamide ochratoxin A |
| OM-OA | ochratoxin A O-methyl |
| OP-OTα | ochratoxin α open lactone |
| OP-OA | ochratoxin A open lactone |
| OP-OB | ochratoxin B open lactone |
| OP-OTα | ochratoxin α open lactone |
| OTα | ochratoxin α |
| OTβ | ochratoxin β |
| OTA | ochratoxin A |
| OTA-Me | ochratoxin A methyl ester |
| OTA-Tyrosine | tyrosine ochratoxin A |
| OTB | ochratoxin B |
| OTB-Et | ochratoxin B ethyl ester |
| OTB-Me | ochratoxin B methyl ester |
| OTC | ochratoxin C |
| OTHQ | ochratoxin A hydroquinone |
| OTQ | ochratoxin A quinone |
| OTQ-Glutathion | conjugate ochratoxin A quinone–glutathion |
| PCR | polymerase chain reaction |
| PTWI | provisional tolerable weekly intake |
| PFIA | fluorescence polarization immunoassay |
| RASFF | Rapid Alert System for Food and Feed |
| RIA | radioimmunoassay |
| RNA | ribonucleic acid |
| SPE | solid-phase extractions |
| TDI | tolerable daily intake |
| TLC | solid thin layer chromatography |
| TTIP | The Transatlantic Trade and Investment Partnership |
| TWI | tolerable weekly intake |
| UTT | urinary tract tumors |
| WHO | World Health Organization |
| WTO | World Trade Organization |
| EDI | exposure daily intake |
References
- Jørgensen, K. Survey of pork, poultry, coffee, beer and pulses for Ochratoxin A. Food Addit. Contam. 1998, 15, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Marin, S.; Sanchis, V.; Ramos, A.J. Screening of mycotoxin multicontamination in medicinal and aromatic herbs sampled in Spain. J. Sci. Food Agric. 2009, 89, 1802–1807. [Google Scholar] [CrossRef]
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L. Mycotoxins Part II. The constitution of Ochratoxins A, B and C, metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc. 1965, 204, 7083–7088. [Google Scholar] [CrossRef]
- Weidenbach, A.; Petzinger, E. Ochratoxin A: Toxicology of an abundant mycotoxin. Curr. Top. Pharmacol. 2004, 8, 235–250. [Google Scholar]
- Sava, V.; Reunova, O.; Velasquez, A.; Harbison, R.; Sanchez-Ramos, J. Acute neurotoxic effects of the fungal metabolite Ochratoxin A. Neurotoxicology 2006, 27, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Sava, V.; Velasquez, A.; Song, S.; Sanchez-Ramos, J. Adult hippocampal neural stem/progenitor cells in vitro are vulnerable to the mycotoxin Ochratoxin A. Toxicol. Sci. 2007, 98, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Manderville, R. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Novotna, E. Toxicity of the mycotoxin Ochratoxin A in the light of recent data. Toxin Rev. 2013, 32, 19–33. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Novotna, E. Ochratoxin A: Developmental and reproductive toxicity-An overview. Birth Defects Res. B 2013, 98, 493–502. [Google Scholar] [CrossRef] [PubMed]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Naturally Occuring Substances: Food Items and Costituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; Volume 56, pp. 489–524. [Google Scholar]
- Castegnaro, M.; Mohr, U.; Pfohl-Leszkowicz, A.; Estève, J.; Steinmann, J.; Tillmann, T.; Michelon, J.; Bartsch, H. Sex-and strain-specific induction of renal tumors by Ochratoxin A in rats correlates with DNA adduction. IARC 1998, 77, 70–75. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Pinelli, E.; Bartsch, H.; Mohr, U.; Castegnaro, M. Sex and strain differences in Ochratoxin A metabolism and DNA adduction in two strains of rats. Mol. Carcinog. 1998, 23, 76–83. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Petkova-Bocharova, T.; Chernozemsky, I.; Castegnaro, M. Balkan Endemic Nephropathy and associated Urinary tract tumours: A review on aetiological causes and the potential role of mycotoxins. Food Addit. Contam. 2002, 19, 282–302. [Google Scholar] [CrossRef] [PubMed]
- Hsuuw, Y.; Chan, W.; Yu, J. Ochratoxin A inhibits mouse embryonic development by activating A mitochondrion-dependent apoptotic signaling pathway. Int. J. Mol. Sci. 2013, 14, 935–953. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Tozlovanu, M.; Manderville, R.; Peraica, M.; Castegnaro, M.; Stefanovic, V. New molecular and field evidences for the implication of mycotoxins but not aristolochic acid in human nephropathy and Urinary tract tumor. Mol. Nutr. Food Res. 2007, 51, 1131–1146. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A. Ochratoxin A and aristolochic acid involvement in nephropathies and associated Urothelial tract tumours. Arch. Ind. Hyg. Toxicol. 2009, 60, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Degen, G.; Mayer, S.; Blaszkewicz, M. Biomonitoring of Ochratoxin A in grain workers. Mycotoxin Res. 2007, 23, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Brera, C.; Carelli, G.; Caputi, R.; Marinaccio, A.; Miraglia, M. External and internal dose in subjects occupationally exposed to Ochratoxin A. Int. Arch. Occup. Environ. Health 2002, 75, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Halstensen, A.S.; Nordby, K.C.; Elen, O.; Eduard, W. Ochratoxin in grain dust-estimated exposure and relations to agricultural practices in grain production. Ann. Agric. Environ. Med. 2004, 11, 245–254. [Google Scholar] [PubMed]
- Abarca, M.L.; Bragulat, M.R.; Castella, G.; Cabañes, F.J. Ochratoxin A production by strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 1994, 60, 2650–2652. [Google Scholar] [PubMed]
- Teren, J.; Varga, J.; Hamari, Z.; Rinyu, E.; Kevei, E. Immunochemical detection of Ochratoxin A in black Aspergillus strains. Mycopathologia 1996, 134, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I. Biology and ecology of toxigenic Penicillium species. In Mycotoxins and Food Safety; DeVries, J.W., Truckseess, M.W., Jackson, L.S., Eds.; Kluwer Academic, Plenum Publisher: New York, NY, USA, 2002; pp. 29–41. [Google Scholar]
- Medina, A.; Mateo, R.; López-Ocaña, L.; Valle-Algarra, F.M.; Jiménez, M. Study of Spanish grape mycobiota and Ochratoxin A production by isolates of Aspergillus tubingensis and other members of Aspergillus section Nigri. Appl. Environ. Microbiol. 2005, 71, 4696–4702. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Frank, J.M.; Houbraken, J.A.M.P.; Kuijpers, A.F.A.; Samson, R.A. New Ochratoxin A producing species of Aspergillus section Circumdati. Stud. Mycol. 2004, 50, 23–43. [Google Scholar]
- Samson, R.A.; Houbraken, J.A.M.P.; Kuijpers, A.F.A.; Frank, J.M.; Frisvad, J.C. New Ochratoxin A or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 2004, 50, 45–61. [Google Scholar]
- Van Walbeek, W.; Scott, P.M.; Harwig, J.; Lawrence, J.W. Penicillium viridicatum Westling: A new source of Ochratoxin A. Can. J. Microbiol. 1969, 15, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Frisvad, J.C. Penicillium subgenus Penicillium: New taxonomic schemes and mycotoxins and other extrolites. Stud. Mycol. 2004, 49, 260. [Google Scholar]
- Ciegler, A.; Fennell, D.I.; Sansing, G.A.; Detroy, R.W.; Bennett, G.A. Mycotoxin-producing strains of Penicillium viridicatum: Classification into subgroups. Appl. Microbiol. 1973, 26, 271–278. [Google Scholar] [PubMed]
- Pitt, J.I. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces; Academic Press, Inc.: London, UK, 1979. [Google Scholar]
- Larsen, T.O.; Svendsen, A.; Smedsgaard, J. Biochemical characterization of Ochratoxin A-Producing strains of the genus Penicillium. Appl. Env. Microb. 2001, 67, 3630–3635. [Google Scholar] [CrossRef] [PubMed]
- Bogs, C.; Battilani, P.; Geisen, R. Development of a molecular detection and differentiation system for Ochratoxin A producing Penicillium species and its application to analyse the occurrence of Penicillium nordicum in cured meats. Int. J. Food Microbiol. 2006, 107, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Ruprich, J. Producers and important dietary sources of Ochratoxin A and citrinin. Toxins 2013, 5, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Budavari, S. (Ed.) The Merck Index, 11th ed.; Merck & Co.: Rahway, NJ, USA, 1989; p. 1068.
- Kuiper-Goodman, T.; Scott, P.M. Risk assessment of the mycotoxin Ochratoxin A. Biomed. Environ. Sci. 1989, 2, 179–248. [Google Scholar] [PubMed]
- Pohland, A.E.; Schuller, P.L.; Steyn, P.S.; van Egmond, H.P. Physico-chemical data for some selected mycotoxins. Pure Appl. Chem. 1982, 54, 2219–2228. [Google Scholar] [CrossRef]
- WHO. Selected Mycotoxins: Ochratoxins, Trichothecenes, Ergot; Environmental Health Criteria, WHO: Geneva, Switzerland, 1990; Volume 105, pp. 1–260. [Google Scholar]
- Müller, H.M. Decontamination of mycotoxins. 1. Physical process (Ger.). Übersicht. Tieremahr. 1982, 10, 95–122. [Google Scholar]
- Castegnaro, M.; Barek, J.; Frémy, J.-M.; Lafontaine, M.; Miraglia, M.; Sansone, E.B.; Telling, G.M. (Eds.) Laboratory Decontamination and Destruction of Carcinogens in Laboratory Wastes: Some Mycotoxins; IARC Scientific Publications: Lyon, France, 1991; Volume 113, pp. 9–16.
- El Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Toman, J.; Bazin, I.; Roubal, T. Transfer of Ochratoxin A into tea and coffee beverages. Toxins 2014, 6, 3438–3453. [Google Scholar] [CrossRef] [PubMed]
- Bruinink, A.; Rasonyi, T.; Sidler, C. Differences in neurotoxic effects of Ochratoxin A, ochracin and ochratoxin-α in vitro. Nat. Toxins 1998, 6, 173–177. [Google Scholar] [CrossRef]
- Faucet-Marquis, V.; Pont, F.; Størmer, F.; Rizk, T.; Castegnaro, M.; Pfohl-Leszkowicz, A. Evidence of a new dechlorinated Ochratoxin A derivative formed in opossum kidney cell cultures after pretreatment by modulators of glutathione pathways: Correlation with DNA-adduct formation. Mol. Nutr. Food Res. 2006, 50, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Tozlovanu, M.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Manderville, R.A. Genotoxicity of the hydroquinone metabolite of Ochratoxin A: Structure-activity relationships for covalent DNA adduction. Chem. Res. Toxicol. 2006, 19, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Ringot, D.; Chango, A.; Schneider, Y.J.; Larondelle, Y. Toxicokinetics and toxicodynamics of Ochratoxin A, an update. Chem. Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef] [PubMed]
- Azpilicueta, C.A.; Arbeloa, M.I.; de Maquirriain, P.F.J. Natural and syntethic occurring forms of the ochratoxins. In Food Chemistry Research Developments; Papadopoulos, K.N., Ed.; Nova Science Publishers: New York, NY, USA, 2008. [Google Scholar]
- Wu, Q.; Dohnal, V.; Huang, L.; Kuca, K.; Wang, X.; Chen, G.; Yuan, Z. Metabolic pathways of Ochratoxin A. Curr. Drug Metab. 2011, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Tangni, E.K.; di Mavungu, J.D.; Vanhaecke, L.; de Saeger, S.; Wu, A.; Callebaut, A. In vitro glucuronidation of Ochratoxin A by rat liver microsomes. Toxins 2013, 5, 2671–2685. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, H.; de Saeger, S.; de Boevre, M.; Sun, F.; Zhang, S.; Cao, X.; Wang, Z. In vitro and in vivo metabolism of Ochratoxin A: A comparative study using ultra-performance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Bingle, L.E.H. Comparative Ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef] [PubMed]
- Grosse, Y.; Chekir-Ghedira, L.; Huc, A.; Obrecht-Pflumio, S.; Dirheimer, G.; Bacha, H.; Pfohl-Leszkowicz, A. Retinol, ascorbic acid and Α-tocopherol prevent DNA adduct formation in mice treated with the mycotoxins Ochratoxin A and zearalenone. Cancer Lett. 1997, 114, 225–229. [Google Scholar] [CrossRef]
- Galtier, P.; Alvinerie, M. In vitro Transformation of Ochratoxin A by animal microbioal floras. Ann. Rech. Vet. 1976, 7, 91–98. [Google Scholar] [PubMed]
- Pitout, M.J. The hydrolysis of Ochratoxin A by some proteolytic enzymes. Biochem. Pharmacol. 1969, 18, 485–491. [Google Scholar] [CrossRef]
- Hult, K.; Hokby, E.; Gatenbeck, S. Analysis of ochratoxin B alone and in the presence of Ochratoxin A, using carboxypeptidase A. Appl. Environ. Microbiol. 1977, 33, 1275–1277. [Google Scholar] [PubMed]
- Størmer, F.C.; Hansen, C.E.; Pedersen, J.I.; Hvistendahl, G.; Aasen, A.J. Formation of (4R)- and (4S)-4-hydroxyochratoxin A from Ochratoxin A by liver microsomes from various species. Appl. Environ. Microbiol. 1981, 42, 1051–1056. [Google Scholar] [PubMed]
- Størmer, F.C.; Storen, O.; Hansen, C.E.; Pedersen, J.I.; Aasen, A.J. Formation of (4R)- and (4S)-4-hydroxyochratoxin A and 10-hydroxyochratoxin A from Ochratoxin A by rabbit liver microsomes. Appl. Environ. Microbiol. 1983, 45, 1183–1187. [Google Scholar]
- Li, S.; Marquardt, R.; Frohlich, A. Identification of Ochratoxins and some of their metabolites in bile and urine of rats. Food. Chem. Toxicol. 2000, 38, 141–152. [Google Scholar] [CrossRef]
- Gillman, I.; Clark, T.; Manderville, R. Oxidation of Ochratoxin A by an Fe-porphyrin system: Model for enzymatic activation and DNA cleavage. Chem. Res. Toxicol. 1998, 12, 1066–1076. [Google Scholar] [CrossRef]
- Dai, J.; Park, G.; Wright, M.; Adams, M.; Akman, S.; Manderville, R. Detection and characterization of a glutathione conjugate of Ochratoxin A. Chem. Res. Toxicol. 2002, 15, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Gross-Steinmeyer, K.; Weymann, J.; Hege, H.; Metzler, M. Metabolism and lack of DNA reactivity of the mycotoxin Ochratoxin A in cultured rat and human primary hepatocytes. J. Agric. Food Chem. 2002, 50, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Marquardt, R.; Frohlich, A.; Ling, Y. Synthesis and structural elucidation of analogs of Ochratoxin A. J. Agric. Food Chem. 1995, 43, 524–530. [Google Scholar] [CrossRef]
- Creppy, E.; Chakor, K.; Fisher, M.; Dirheimer, G. The mycotoxin Ochratoxin A is a substrate for phenylalanine hydroxylase in isolated rat hepatocytes and in Vivo. Arch. Toxicol. 1990, 64, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Tozlovanu, M.; Canadas, D.; Pfohl-Leszkowicz, A.; Frenette, C.; Paugh, R.J.; Pfohl-Leszkowicz, A.; Frenette, C.; Paugh, R.J.; Manderville, R.A. Glutathione conjugates of ochratoxin A as biomarkers of exposure. Arh. Hig. Rada. Toksikol. 2012, 63, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Gabryelski, W.; Manderville, R. Formation of 2′-deoxyguanosine-carbon 8-bound Ochratoxin A adduct in rat kidney DNA. Mol. Nutr. Food Res. 2009, 53, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Hadjeba-Medjdoub, K.; Tozlovanu, M.; Pfohl-Leszkowicz, A.; Frenette, C.; Paugh, R.; Manderville, R. Structure–activity relationships imply different mechanisms of action for Ochratoxin A-mediated cytotoxicity and genotoxicity. Chem. Res. Toxicol. 2012, 25, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Cramer, B.; Königs, M.; Humpf, H. Identification and in vitro cytotoxicity of Ochratoxin A degradation products formed during coffee roasting. J. Agric. Food Chem. 2008, 56, 5673–5681. [Google Scholar] [CrossRef] [PubMed]
- Bittner, A.; Cramer, B.; Harrer, H.; Humpf, H. Structure elucidation and in vitro cytotoxicity of ochratoxin Α amide, a new degradation product of Ochratoxin A. Mycotoxin Res. 2015, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nesheim, S.; Hardin, N.F.; Francis, O.J.; Langham, W.S. Analysis of Ochratoxins A and B and their esters in barley, using partition and thin-layer chromatography. I. Development of the method. J. Assoc. Off. Anal. Chem. 1973, 56, 817–821. [Google Scholar]
- Nesheim, S. Analysis of Ochratoxins A and B and their esters in barley, using partition and thin-layer chromatography. II. Collaborative study. J. Assoc. Off. Anal. Chem. 1973, 56, 822–826. [Google Scholar]
- Hult, K.; Gatenbeck, S. A spectrophotometric procedure, using carboxypeptidase A, for quantitative measurement of Ochratoxin A. J. Assoc. Off. Anal. Chem. 1976, 59, 128–129. [Google Scholar] [PubMed]
- Josefsson, E.; Möller, T. High pressure Iiquid chromatographic determination of Ochratoxin A and zearalenone in cereals. J. Assoc. Off. Anal. Chem. 1979, 62, 1165–1168. [Google Scholar] [PubMed]
- Schweighardt, H.; Schuh, M.; Abdelhamid, A.M.; Böhm, J.; Leibetseder, J. Method for quantitative determination of Ochratoxin A in foods and feeds by high-pressure Liquid chromatography (HPLC) (Ger.). Z. Lebensmittel. Untersuch. Forsch. 1980, 170, 355–359. [Google Scholar] [CrossRef]
- Hunt, D.C.; McConnie, B.R.; Crosby, N.T. Confirmation of Ochratoxin A by chemical derivatisation and high-performance liquid chromatography. Analyst 1980, 105, 89–90. [Google Scholar] [CrossRef]
- Howell, M.V.; Taylor, P.W. Determination of aflatoxins, Ochratoxin A, and zearalenone in mixed feeds, with detection by thin layer chromatography or high performance liquid chromatography. J. Assoc. Off. Anal. Chem. 1981, 64, 1356–1363. [Google Scholar] [PubMed]
- Aalund, O.; Brunfeldt, K.; Hald, B.; Krogh, P.; Poulsen, K. A radioimmunoassay for Ochratoxin A: A preliminary investigation. Acta Pathol. Microbiol. Scand. Sect. C 1975, 83, 390–392. [Google Scholar] [CrossRef]
- Pestka, J.J.; Steinert, B.W.; Chu, F.S. Enzyme-linked immunosorbent assay for detection of Ochratoxin A. Appl. Environ. Microbiol. 1981, 41, 1472–1474. [Google Scholar] [PubMed]
- Abramson, D. Measurement of Ochratoxin A in barley extracts by liquid—Chromatography-mass spectrometry. J. Chromatogr. 1987, 391, 315–320. [Google Scholar] [CrossRef]
- Breitholtz, A.; Olsen, M.; Dahlbäck, A.; Hult, K. Plasma Ochratoxin A levels in three Swedish population surveyed using an ion pair HPLC technique. Food Add. Contam. 1991, 8, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Blaas, W.; Rühl, C.; Weber, R. Identification of Ochratoxin A in food samples by chemical derivatization and gas chromatography-Mass spectrometry. J. Chromatogr. 1992, 595, 364–367. [Google Scholar] [CrossRef]
- Nesheim, S.; Stack, M.E.; Trucksess, M.W.; Eppley, R.M. Rapid solvent—efficient for liquid chromatographic determination of Ochratoxin A in corn, barley and kidney: Collaborative Study. J. AOAC Int. 1992, 75, 3, 481–487. [Google Scholar]
- Kawamura, O.; Maki, S.; Sato, S.; Ueno, Y. Ochratoxin A in livestock and human sera in Japan quantified by a sensitive ELISA. In Human ochratoxicosis and Its Pathologies; Creppy, E.E., Castegnaro, M., Dirheimer, G., Eds.; Colloque INSERM/John Libbey Eurotext: London, UK, 1993; Volume 231, pp. 159–165. [Google Scholar]
- Scott, P.M.; Trucksess, M.W. Application of immunoaffinity columns to mycotoxin analysis. J. AOAC Int. 1997, 80, 5, 941–949. [Google Scholar]
- Becker, M.; Degelman, P.; Herderich, M.; Schreier, P.; Humpf, H.-U. Column liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of Ochratoxin. J. Chromatogr. A 1998, 818, 260–264. [Google Scholar] [CrossRef]
- Jørgensen, K.; Vahl, M. Analysis of Ochratoxin A in pig kidney and rye flour using liquid chromatography tandem mass spectrometry (LC/MS/MS). Food Addit. Contam. 1999, 16, 451–456. [Google Scholar]
- Petkova-Bocharova, T.; Castegnaro, M.; Pfohl-Leszkowicz, A.; Garren, L.; Grosso, F.; Nikolov, I.; Vrabcheva, T.; Dragacci, S.; Chernozemsky, I.N. Analysis of Ochratoxin A in serum and urine of inhabitants from an area with Balkan endemic nephropathy: A one-month follow up study. Facta Univ. Ser. Med. Biol. 2003, 10, 62–68. [Google Scholar]
- Molinié, A.; Faucet, V.; Castegnaro, M.; Pfohl-Leszkowicz, A. Analysis of some breakfast cereals on the French market for their contents of Ochratoxin A, citrinin and fumonisin B1: Development of a method for simultaneous extraction of Ochratoxin A and citrinin. Food Chem. 2005, 92, 391–400. [Google Scholar] [CrossRef]
- Shim, W.-B.; Kolosova, A.Y.; Kim, Y.J.; Yang, Z.Y.; Park, S.J.; Eremin, S.A.; Lee, I.S.; Chung, D.-H. Fluorescence polarization immunoassay based on a monoclonal antibody for the detection of OTA. Int. J. Food Sci. Technol. 2004, 39, 827–837. [Google Scholar] [CrossRef]
- Cruz-Aguado, J.A.; Penner, G. Determination of Ochratoxin A with a DNA aptamer. J. Agric. Food Chem. 2008, 56, 10456–10461. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, D.; Kim, H.; Jahng, K.Y. Quantitative determination of mycotoxins in urine by LC-MS/MS. Food Addit. Contam. Part A 2010, 27, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Giesen, C.; Jakubowski, N.; Panne, U.; Weller, M.G. Comparison of ICP-MS and photometric detection of an immunoassay for the determination of Ochratoxin a in wine. J. Anal. At. Spectrom. 2010, 25, 1567–1572. [Google Scholar] [CrossRef]
- Ediage, E.N.; di Mavungu, J.D.; Song, S.; Wu, A.; van Peteghem, C.; de Saeger, S. A direct assessment of mycotoxin biomarkers in human urine samples by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2012, 741, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Hayat, A.; Hernandez, D.B.; Meraihi, Z.; Munoz, R.; Marty, J.-L. Development of an automated flow-based electrochemical aptasensor for on-line detection of Ochratoxin A. Sens. Actuators B Chem. 2013, 176, 1160–1166. [Google Scholar] [CrossRef]
- Wen, J.; Kong, W.; Hu, Y.; Wang, J.; Yang, M. Multi-mycotoxins analysis in ginger and related products by UHPLC-FLR detection and LC-MS/MS confirmation. Food Control 2014, 43, 82–87. [Google Scholar] [CrossRef]
- Cramer, B.; Osteresch, B.; Muñoz, K.; Hillmann, H.; Sibrowski, W.; Humpf, H. Biomonitoring using dried blood spots: Detection of Ochratoxin A and its degradation product 2’R-ochratoxin A in blood from coffee drinkers. Mol. Nutr. Food Res. 2015, 59, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.V.; Zherdev, A.V.; Petrakova, A.V.; Sadykhov, E.G.; Koroleva, O.V.; Dzantiev, B.B. Rapid multiple immunoenzyme assay of mycotoxins. Toxins 2015, 7, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Todescato, F.; Antognoli, A.; Meneghello, A.; Cretaio, E.; Signorini, R.; Bozio, R. Sensitive detection of Ochratoxin A in food and drinks using metal-enhanced fluorescence. Biosens. Bioelectron. 2014, 57, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Li, R.; Lin, C.; Guo, L.; Qiu, B.; Lin, Z.; Chen, G. Electrochemiluminescence biosensor for ultrasensitive determination of Ochratoxin A in corn samples based on aptamer and hyperbranched rolling circle amplification. Biosens. Bioelectron. 2015, 70, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.; Castro, M.; Machado, S.; Barroso, M.; Nouws, H.; Delerue-Matos, C. Molecularly imprinted electrochemical sensor for Ochratoxin A detection in food samples. Sens. Actuators B Chem. 2015, 215, 107–112. [Google Scholar] [CrossRef]
- Sanzani, S.; Reverberi, M.; Fanelli, C.; Ippolito, A. Detection of Ochratoxin A using molecular beacons and Real-time PCR thermal cycler. Toxins 2015, 7, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Van Egmond, H.P. Methods for determining Ochratoxin A and other nephrotoxic mycotoxins. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumors; Castegnaro, M., Plestina, R., Dirheimer, G., Eds.; IARC Sci. Publ.: Lyon, France, 1991; Volume 115, pp. 57–70. [Google Scholar]
- Paulsch, W.E.; van Egmond, H.P.; Schuller, P.-L. Thin layer chromatographic method for analysis and chemical confirmation of Ochratoxin A in kidneys of pigs. In Proceedings of the International IUPAC Symposium on Mycotoxins and Phycotoxins, Vienna, Austria, 1–3 September 1982; Austrian Chemical Society: Vienna, Austria, 1982; pp. 40–43. [Google Scholar]
- Cohen, H.; Lapointe, M. Determination of Ochratoxin A in animal feed and cereal grains by liquid chromatography with fluorescence detection. J. Assoc. Off. Anal. Chem. 1986, 69, 957–959. [Google Scholar] [PubMed]
- International Program on Chemical Safety (IPCS). Environmental Health Criteria; World Health Organization: Geneva, Switzerland, 1990; No. 105, 27. [Google Scholar]
- Ueno, Y.; Kawamura, O.; Sugiura, Y.; Horiguchi, K.; Nakajima, M.; Yamamoto, K.; Sato, S. Use of monoclonal antibodies, enzyme–linked immunosorbent assay and immunoaffinity column chromatography to determine Ochratoxin A in porcine sera, coffee products and toxin-producing fungi. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumors; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I., Bartsch, H., Eds.; IARC Sci. Publ.: Lyon, France, 1991; Volume 115, pp. 71–75. [Google Scholar]
- Zimmerli, B.; Dick, R. Determination of Ochratoxin A at the ppt level in human blood, serum, milk and some foodstuffs by high performance liquid chromatography with enhanced fluorescence detection and immunoaffinity column cleanup: Methodology and Swiss data. J. Chromatogr. B Biomed. Appl. 1995, 666, 85–99. [Google Scholar] [CrossRef]
- Prelle, A.; Spadaro, D.; Denca, A.; Garibaldi, A.; Gullino, M.L. Comparison of clean-up methods for Ochratoxin A on wine, beer, roasted coffee and chilli commercialized in Italy. Toxins 2013, 5, 1827–1844. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Kong, W.; Zhou, S.; Yin, L.; Wan, L.; Yang, M. Molecularly imprinted polymer-based solid phase clean-up for analysis of Ochratoxin A in beer, red wine, and grape juice. J. Sep. Sci. 2013, 36, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Castellari, M.; Fabbri, S.; Fabiani, A.; Amati, A.; Galassi, S. Comparison of different immunoaffinity clean-up procedures for high-performance liquid chromatographic analysis of Ochratoxin A in wines. J. Chromatogr. A 2000, 888, 129–136. [Google Scholar] [CrossRef]
- Castegnaro, M.; Tozlovanu, M.; Wild, C.; Molinié, A.; Sylla, A.; Pfohl-Leszkowicz, A. Advantages and drawbacks of immunoaffinity columns in analysis of mycotoxins in food. Mol. Nutr. Food Res. 2006, 50, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Polisenska, I.; Pfohl-Leszkowicz, A.; Hadjeba, K.; Dohnal, V.; Jirsa, O.; Denesova, O.; Jezkova, A.; Macharackova, P. Occurrence of Ochratoxin A and citrinin in Czech cereals and comparison of two HPLC methods for Ochratoxin A detection. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk. Assess. 2010, 27, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Tozlovanu, M.; Pfohl-Leszkowicz, A. Ochratoxin A in roasted coffee purchased in French super market. Transfer in coffee beverage: Comparison of several methods. Toxins 2010, 2, 1928–1949. [Google Scholar] [CrossRef] [PubMed]
- Bazin, I.; Faucet-Marquis, V.; Monje, M.; El Khoury, M.; Marty, J.; Pfohl-Leszkowicz, A. Impact of pH on the stability and the cross-reactivity of Ochratoxin A and citrinin. Toxins 2013, 5, 2324–2340. [Google Scholar] [CrossRef] [PubMed]
- Studer-Rohr, I.; Dietrich, D.R.; Schlatter, J.; Schlatter, C. The occurrence of Ochratoxin A in coffee. Food Chem. Toxicol. 1995, 33, 341–355. [Google Scholar] [CrossRef]
- ISO. Enviromental Management Systems—Requirements with Guidance for Use; EN ISO 45001:1989; International Organisation for Standardisation: Geneva, Switzerland, 1989. [Google Scholar]
- ISO. General Requirements for the Competence of Testing and Calibration Laboratories; ISO/IEC 17025:2005; International Organisation for Standardisation: Geneva, Switzerland, 2005. [Google Scholar]
- Chen, J.; Fang, Z.; Liu, J.; Zeng, L. A simple and rapid biosensor for Ochratoxin A based on a structure-switching signalling aptamer. Food Control 2012, 25, 555–560. [Google Scholar] [CrossRef]
- Ruprich, J.; Ostry, V. Enzymo-immunological assay of the mycotoxin Ochratoxin A (in Czech). Vet. Med. 1991, 36, 245–249. [Google Scholar]
- Ricciardi, C.; Castagna, R.; Ferrante, I.; Frascella, F.; Luigi Marasso, S.; Ricci, A.; Canavese, G.; Lorè, A.; Prelle, A.; Lodovica Gullino, M.; et al. Development of a microcantilever-based immunosensing method for mycotoxin detection. Biosens. Bioelectron. 2013, 40, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Frennette, C.; Paugh, R.; Tozlovanu, M.; Juzio, M.; Pfohl-Leszkowicz, A.; Manderville, R. Structure-activity relationships for the fluorescence of ochratoxin A: Insight for detection of ochratoxin A metabolites. Anal. Chim. Acta 2008, 617, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Al-Taher, F.; Banaszewski, K.; Jackson, L.; Zweingenbaum, J.; Ryu, D.; Cappozzo, J. Rapid method for the determination of multiple mycotoxins in wines and beers using a stable isotope dilution assay. J. Agric. Food Chem. 2013, 61, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.S.; Chang, F.C.C.; Hinsdill, R.D. Production of antibody against Ochratoxin A. Appl. Environ. Microbiol. 1976, 31, 831–835. [Google Scholar] [PubMed]
- Rousseau, D.M.; Slegers, G.A.; van Peteghem, C.H. Radioimmunoassay of Ochratoxin A in barley. Appl. Environ. Microbiol. 1985, 50, 529–531. [Google Scholar] [PubMed]
- Rousseau, D.M.; Slegers, G.A.; van Peteghem, C.H. Solid phase radioimmunoassay of Ochratoxin A in serum. J. Agric. Food Chem. 1986, 34, 862–865. [Google Scholar] [CrossRef]
- Fukal, I. A survey of cereals, cereals products, feedstuffs and porcine kidney by radioimmunoassay. Food Addit. Contam. 1990, 7, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Fukal, I. Spontaneous occurrence of Ochratoxin A residues in Czechoslovak slaughter pigs determined by radioimmunoassay. Dtsch. Lebensm. Rundsch. 1991, 87, 316–319. [Google Scholar]
- Meulenberg, E.P. Immunochemical methods for Ochratoxin A detection: A review. Toxins 2012, 4, 244–266. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.M. Analysis of mycotoxins with capillary electrophoresis. Semin. Food Anal. 1998, 3, 353–373. [Google Scholar]
- Böhns, B.; Seidel, V.; Lindner, W. Analysis of selected mycotoxins by capillary electrophoresis. Chromatographia 1995, 41, 631–637. [Google Scholar]
- Corneli, S.; Maragos, C.M. Capillary electrophoresis with laser-induced fluorescence: Method for the mycotoxin Ochratoxin A. J. Agric. Food Chem. 1998, 46, 3162–3165. [Google Scholar] [CrossRef]
- Holland, R.D.; Sepaniak, M.J. Qualitative analysis of mycotoxins using micellar electrokinetic capillary chromatography. Anal. Chem. 1993, 65, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. Molecular imprinting in cross-linked materials with the aid of molecular templates—A way towards artificial antibodies. Angew. Chem. Int. Ed. 1995, 34, 1812–1832. [Google Scholar] [CrossRef]
- Shephard, G.S. Analytical methodology for mycotoxins: Recent advances and future challenges. In Mycotoxins and Phycotoxins Perspective at the Turn of the Millennium; De Koe, W.J., Samson, R.A., van Egmond, H.P., Gilbert, J., Sabino, M., Eds.; Publisher: W.J. de Koe, Wageningen, Netherlands, 2001. [Google Scholar]
- Yu, J.C.C.; La, E.P.C. Molecularly imprinted polymers for Ochratoxin A extraction and analysis. Toxins 2010, 2, 1536–1553. [Google Scholar] [CrossRef] [PubMed]
- Ngundi, M.M.; Shriver-Lake, L.C.; Moore, M.H.; Lassman, M.E.; Ligler, F.S.; Taitt, C.R. Array biosensor for detection of Ochratoxin A in cereals and beverages. Anal. Chem. 2005, 77, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Duan, N.; Hun, X.; Wu, S. Electrochemiluminescent aptamer biosensor for the determination of Ochratoxin A at a gold-nanoparticles-modified gold electrode using N-(aminobutyl)-N-ethylisoluminol as a luminescent label. Anal. Bioanal. Chem. 2010, 398, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Bonel, L.; Vidal, J.C.; Duato, P.; Castillo, J.R. An electrochemical competitive biosensor for Ochratoxin A based on a DNA biotinylated aptamer. Biosens. Bioelectron. 2011, 26, 3254–3259. [Google Scholar] [CrossRef] [PubMed]
- Shotwell, O.L.; Hesseltine, C.W.; Goulden, M.L. Ochratoxin A: Occurrence as natural contaminant of a corn sample. Appl. Microbiol. 1969, 17, 765–766. [Google Scholar] [PubMed]
- Scott, P.M.; van Walbeek, W.; Harwig, J.; Fennell, D.I. Occurrence of a mycotoxin, Ochratoxin A, in wheat and isolation of Ochratoxin A and citrinin producing strains of Penicillium viridicatum. Canad. J. Plant Sci. 1970, 50, 583–585. [Google Scholar] [CrossRef]
- Scott, P.M.; van Walbeek, W.; Kennedy, B.; Anyeti, D. Mycotoxins (Ochratoxin A, citrinin, and sterigmatocystin) and toxigenic fungi in grains and agricultural products. J. Agric. Food Chem. 1972, 20, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Hald, B.; Krogh, P. Ochratoxin residues in bacon pigs. In Proceedings of the IUPAC: Symposium on the Control of Mycotoxins, Göteborg, Sweden, 21–22 August 1972.
- Hunt, D.C.; Philip, L.A.; Crosby, N.T. Determination of ochratoxin A in pig’s kidney using enzymatic digestion, dialysis and high-performance liquid chromatography with post-column derivatization. Analyst 1979, 104, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Dofkova, M.; Skarkova, J.; Pfohl-Leszkowicz, A.; Ruprich, J. Ochratoxin A dietary exposure of ten population groups in the Czech Republic: Comparison with data over the world. Toxins 2015, 7, 3608–3635. [Google Scholar] [CrossRef] [PubMed]
- European Union. Assessment of Dietary Intake of Ochratoxin A by the Population of EU Member States. Report of Experts Participating in Task 3.2.7 Reports on Tasks for Scientific Cooperation. 2002. Available online: http://ec.europa.eu/food/fs/scoop/index_en.html (accessed on 7 April 2016).
- RASFF Portal. Available online: https://webgate.ec.europa.eu/rasffwindow/portal/?event=SearchForm&cleanSearch=1 (accessed on 11 April 2016).
- EFSA. Opinion of the Scientific Panel on Contaminants in Food Chain on a request from the Commission related to Ochratoxin A (OTA) as undesirable substance in animal feed. EFSA J. 2004, 101, 1–36. [Google Scholar]
- Fuchs, R.; Peraica, M. Ochratoxin A in human kidney diseases. Food Addit. Contam. 2005, 22, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Maaroufi, K.; Achour, A.; Hammami, M.; El May, M.; Betbeder, A.; Ellouz, F.; Creppy, E.; Bacha, H. Ochratoxin A in human blood in relation to nephropathy in Tunisia. Hum. Exp. Toxicol. 1995a, 14, 609–614. [Google Scholar] [CrossRef]
- Grosso, F.; Saı̈d, S.; Mabrouk, I.; Fremy, J.; Castegnaro, M.; Jemmali, M.; Dragacci, S. New data on the occurrence of Ochratoxin A in human sera from patients affected or not by renal diseases in Tunisia. Food. Chem. Toxicol. 2003, 41, 1133–1140. [Google Scholar] [CrossRef]
- Wafa, E.; Yahya, R.; Sobh, M.; Eraky, I.; El-Baz, M.; El-Gayar, H.; Betbeder, A.; Creppy, E. Human ochratoxicosis and nephropathy in Egypt: A preliminary study. Hum. Exp. Toxicol. 1998, 17, 124–129. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Memorandum. The Endemic Nephropathy of South-eastern Europe. Bull. World Health Organ. 1965, 32, 441–448. [Google Scholar]
- Plestina, R.; Stavljenic, A.; Ceovic, R.; Fuchs, R. Haematological features of the population of the area of Croatia, Yugoslavia, endemic for Balkan nephropathy. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Bartsch, H., Eds.; IARC: Lyon, France, 1991; pp. 43–46. [Google Scholar]
- Stefanović, V.; Polenaković, M. Balkan nephropathy. Kidney disease beyond the Balkans? Am. J. Nephrol. 1991, 11, 1–11. [Google Scholar] [PubMed]
- Plestina, R. Some features of Balkan endemic nephropathy. Food Chem. Toxicol. 1992, 30, 177–181. [Google Scholar] [CrossRef]
- Akhmeteli, M.A. Epidemiology of endemic nephropathy. In Endemic Nephropathy, Proceedings of the Second International Symposium on Endemic Nephropathy, Sofia, Sofia, Bulgaria, 9–12 November 1972; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1972; pp. 9–23. [Google Scholar]
- Krogh, P. Mycotoxic porcine nephropathy—A possible model for Balkan (endemic) nephropathy. In Endemic Nephropathy, Proceedings of the Second International Symposium on Endemic Nephropathy, Sofia, Bulgaria, 9–12 November 1972; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1972; pp. 266–277. [Google Scholar]
- Ribelin, W.E.; Fukushima, K.; Still, P. The toxicity of Ochratoxin A to ruminants. Can. J. Comp. Med. 1978, 42, 172–176. [Google Scholar] [PubMed]
- Castegnaro, M.; Bartsch, H.; Chernozemsky, I.N. Endemic nephropathy and urinary tract tumours in the Balkans. Cancer Res. 1987, 47, 3608–3609. [Google Scholar]
- Rahimtula, A.D.; Chong, X. Alteration in calcium homeostasis as a possible cause of Ochratoxin A nephrotoxicity. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumors; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I., Bartsch, H., Eds.; IARC Sci. Publ.: Lyon, France, 1991; Volume 115, pp. 245–253. [Google Scholar]
- Vukelic, M.; Sostaric, B.; Belicza, M. Pathomorphology of Balkan endemic nephropathy. Food Chem. Toxicol. 1992, 30, 193–200. [Google Scholar] [CrossRef]
- Gekle, M.; Silbernagl, S. Mechanism of Ochratoxin A-induced reduction of glomerular filtration rate in rats. J. Pharmacol. Exp. Ther. 1993, 267, 316–321. [Google Scholar] [PubMed]
- Gekle, M.; Silbernagl, S. The role of proximal tubule in Ochratoxin A nephrotoxicity in vivo: Toxodynamic and toxokinetic aspects. Renal Physiol. Biochem. 1994, 17, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kozaczynski, W. Experimental ochratoxicosis A in chickens. Histopatological and histochemical study. Arch. Vet. Pol. 1994, 34, 205–219. [Google Scholar] [PubMed]
- Gekle, M.; Pollock, C.A.; Silbernagl, S. Time and concentration-dependent biphasic efect of Ochratoxin A on growth of proximal tubular cells in primary culture. J. Pharmacol. Exper. Ther. 1995, 275, 397–404. [Google Scholar]
- Gekle, M.; Silbernagl, S. Renal toxicodynamics of Ochratoxin A: A pathophysiological approach. Kidney Blood. Press Res. 1996, 19, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, G.; Gekle, M.; Silbernagl, S. Ochratoxin A disturbs pH homeostasis in the kidney: Increases in pH and HCO 3—In the tubules and vasa recta. Pflugers Arch. 1997, 434, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Atroshi, F.; Biese, I.; Saloniemi, H.; Ali-Vehmas, T.; Saari, S.; Rizzo, A.; Veijalainen, P. Significance of apoptosis and its relationship to antioxidants after Ochratoxin A administration in mice. J. Pharm. Pharm. Sci. 2000, 3, 281–291. [Google Scholar] [PubMed]
- Di Paolo, N.; Guarnieri, A.; Loi, F.; Sacchi, G.; Mangiarotti, A.; di Paolo, M. Acute renal failure from inhalation of mycotoxins. Nephron 1993, 64, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Godin, M.; Fillastre, J.-P.; Simon, P.; Francois, A.; le Roy, F.; Morin, J.-P. Is Ochratoxin A nephrotoxic in human beings? Adv. Nephrol. Necker Hosp. 1997, 26, 181–206. [Google Scholar] [PubMed]
- Schwerdt, G.; Freundiger, R.; Mildenberger, S.; Silbernagl, S.; Gekle, M. The nephrotoxin Ochratoxin A induces apoptosis in cultured human proximal tubule cells. Cell Biol. Toxicol. 1999, 15, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Mally, A.; Völkel, W.; Amberg, A.; Kurz, M.; Wanek, P.; Eder, E.; Hard, G.; Dekant, W. Functional, biochemical, and pathological effects of repeated oral administration of Ochratoxin A to Rats. Chem. Res. Toxicol. 2005, 18, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Gekle, M.; Sauvant, C.; Schwerdt, G. Ochratoxin A at nanomolar concentrations: A signal modulator in renal cells. Mol. Nutr. Food Res. 2005, 49, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Schwerdt, G.; Holzinger, H.; Sauvant, C.; Königs, M.; Humpf, H.; Gekle, M. Long-term effects of Ochratoxin A on fibrosis and cell death in human proximal tubule or fibroblast cells in primary culture. Toxicology 2007, 232, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Jennings-Gee, J.; Tozlovanu, M.; Manderville, R.; Miller, M.; Pfohl-Leszkowicz, A.; Schwartz, G. Ochratoxin A: In utero exposure in mice induces adducts in testicular DNA. Toxins 2010, 2, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Limonciel, A.; Jennings, P. A review of the evidence that Ochratoxin A is an Nrf2 inhibitor: Implications for nephrotoxicity and renal carcinogenicity. Toxins 2014, 6, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Kanisawa, M.; Suzuki, S. Induction of renal and hepatic tumors in mice by Ochratoxin A, a mycotoxin. Gann 1978, 69, 599–600. [Google Scholar] [PubMed]
- Bendele, A.M.; Carlton, W.W.; Krogh, P.; Lillehoj, E.B. Ochratoxin A carcinogenesis in the (C57B1/6J × C3H) F1 mouse. J. Nat. Cancer Int. 1985, 75, 733–742. [Google Scholar]
- Kanisawa, M. Synergistic effect of citrinin on hepatorenal carcinogenesis of Ochratoxin A in mice. Dev. Food Sci. 1984, 7, 245–254. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs; IARC: Lyon, France, 1987; Volume 1–42, (Suppl. 7), pp. 1–403. [Google Scholar]
- Boorman, G. (Ed.) NTP Technical Report on the Toxicology and Carcinogenesis Studies of Ochratoxin A (CAS No. 30-47-9) in F344/N Rats (Gavage Studies), NIH Publication No. 89-2813; U.S. Department of Health and Human Services, National Institutes of Health: Research Triangle Park, NC, USA, 1989.
- Dirheimer, G.; Creppy, E.E. Mechanism of Action of Ochratoxin A; IARC Sci. Publ.: Lyon, France, 1991; Volume 115, pp. 171–186. [Google Scholar]
- Pfohl-Leszkowicz, A.; Chakor, K.; Creppy, E.E.; Dirheimer, G. DNA adducts formation in mice treated with Ochratoxin A. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumors; Castegnaro, M., Plestina, R., Dirheimer, G., Eds.; IARC Sci. Publ.: Lyon, France, 1991; Volume 115, pp. 245–253. [Google Scholar]
- Pfohl-Leszkowicz, A.; Grosse, Y.; Kane, A.; Gharbi, Y.; Baudrimont, I.; Obrecht, S.; Creppy, E.E.; Dirheimer, G. Is the oxydative pathway implicated in the genotoxicity of ochratoxin A? In Human ochratoxicosis and related pathologies; John Libbey Eurotext: London Colloque INSERM, 1993; Volume 231, pp. 177–187. [Google Scholar]
- Pfohl-Leszkowicz, A.; Grosse, Y.; Kane, A.; Creppy, E.; Dirheimer, G. Differential DNA adduct formation and disappearance in three mouse tissues after treatment with the mycotoxin Ochratoxin A. Mutat. Res. 1993, 289, 265–273. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Grosse, Y.; Castegnaro, M.; Petkova-Bocharova, T. Ochratoxin A Related DNA Adducts in Urinary Tract Tumours of Bulgarian Subjects; Phillips, D.H., Castegnaro, M., Bartsch, H., Eds.; IARC Sci. Publ.: Lyon, France, 1993; Volume 124, pp. 115–122. [Google Scholar]
- Maaroufi, K.; Pfohl-Leszkowicz, A.; Achour, A.; El May, M.; Grosse, Y.; Hammami, M.; Ellouz, F.; Creppy, E.E.; Bacha, H. Genotoxicity of Ochratoxin A, relation to renal tumors. Arch. Inst. Pasteur Tunis 1994, 71, 21–31. [Google Scholar] [PubMed]
- Azémar, B.; Pinelli, E.; Escourrou, G.; Plante, P.; Pfohl-Leszkowicz, A.P. Evidence that DNA adducts in some human kidney tumours in France are related to Ochratoxin A. Mutat. Res. 1997, 379, S157. [Google Scholar] [CrossRef]
- Arlt, V.M.; Pfohl-Leszkowicz, A.; Cosyns, J.P.; Schmeiser, H.H. Analyses of DNA adducts formed by Ochratoxin A and aristolochic acid in patients with Chinese herbs nephropathy. Mutat. Res. 2001, 494, 143–150. [Google Scholar] [CrossRef]
- Faucet, V.; Pfohl-Leszkowicz, A.; Dai, J.; Castegnaro, M.; Manderville, R. Evidence for covalent DNA adduction by Ochratoxin A following chronic exposure to rat and subacute exposure to pig. Chem. Res. Toxicol. 2004, 17, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Bartsch, H.; Azemar, B.; Mohr, U.; Esteve, J.; Castegnaro, M. MESNA protects rats against nephro-toxicity but not carcinogenicity induced by Ochratoxin A, implicating two separate pathways. Facta Univ. Ser. Med. Biol. 2002, 9, 57–63. [Google Scholar]
- Petkova-Bocharova, T.; Stoichev, I.; Chernozemsky, I.; Castegnaro, M.; Pfohl-Leszkowicz, A. Formation of DNA adducts in tissues of mouse progeny through transplacental contamination and/or lactation after administration of a single dose of Ochratoxin A to the pregnant mother. Environ. Mol. Mutagen. 1998, 32, 155–162. [Google Scholar] [CrossRef]
- Obrecht-Pflumio, S.; Dirheimer, G. In vitro DNA and dGMP adducts formation caused by Ochratoxin A. Chem. Biol. Interact. 2000, 127, 29–44. [Google Scholar] [CrossRef]
- Obrecht-Pflumio, S.; Dirheimer, G. Horseradish peroxidase mediates DNA and deoxyguanosine 3′-monophosphate adduct formation in the presence of Ochratoxin A. Arch. Toxicol. 2001, 75, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Castegnaro, M. Further arguments in favour of direct covalent binding of Ochratoxin A (OTA) after metabolic biotransformation. Food Addit. Contam. 2005, 22, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Gautier, J.; Holzhaeuser, D.; Markovic, J.; Gremaud, E.; Schilter, B.; Turesky, R. Oxidative damage and stress response from Ochratoxin A exposure in rats. Free Radic. Biol. Med. 2001, 30, 1089–1098. [Google Scholar] [CrossRef]
- Petkova-Bocharova, T.; El Adlouni, C.; Faucet, V.; Pfohl-Leszkowicz, A.; Mantle, P. Analysis for DNA adducts, Ochratoxin A content and enzymes expression in kidneys of pigs exposed to mild experimental chronic ochratoxicosis. Facta Univ. Ser. Med. Biol. 2003, 10, 111–115. [Google Scholar]
- Schwartz, G. Hypothesis: Does OTA cause induces testicular cancer. Cancer Causes Control 2002, 13, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Faucet-Marquis, V.; Jennings-Gee, J.; Miller, M.; Manderville, R.; Pfohl-Leszkowicz, A. Prenatal exposure to Ochratoxin A causes DNA adducts in the testes of newborn mice. Scand. J. Urol. Nephrol. Suppl. 2007, 1, 217–220. [Google Scholar]
- Schwartz, G.; Manderville, R.; Pfohl-Leszkowicz, A. Response to comments of Peter G. Mantle. Toxins 2010, 2, 2337–2339. [Google Scholar] [CrossRef]
- Mantle, P.; Faucet-Marquis, V.; Manderville, R.; Squillaci, B.; Pfohl-Leszkowicz, A. Structures of covalent adducts between DNA and Ochratoxin A: A new factor in debate about genotoxicity and human risk assessment. Chem. Res. Toxicol. 2010, 23, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Molinié, A.; Pfohl-Leszkowicz, A. Toxic effects of co-contamination of Ochratoxin A and citrinin. Drug Metab. Rev. 2003, 35 (Suppl. 1), 77. [Google Scholar]
- Pfohl-Leszkowicz, A.; Molinié, A.; Tozlovanu, M.; Manderville, R.A. Combined toxic effects of Ochratoxin A and citrinin, in vitro and in vivo. In Food Contaminats Mycotoxins & Food Allergen; Siantar, D.P., Trucksess, M.W., Scott, P.M., Herman, E.M., Eds.; ACS Publication: Washington, DC, USA, 2008; Volume 1001, pp. 56–80. [Google Scholar]
- Mally, A.; Zepnik, H.; Wanek, P.; Eder, E.; Dingley, K.; Ihmels, H.; Völkel, W.; Dekant, W. Ochratoxin A: Lack of formation of covalent DNA adducts. Chem. Res. Toxicol. 2004, 17, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.; Hard, G. Chemically induced renal tubule tumors in the laboratory rat and mouse: Review of the NCI/NTP database and categorization of renal carcinogens based on mechanistic information. Crit. Rev. Toxicol. 2004, 34, 211–299. [Google Scholar] [CrossRef] [PubMed]
- Mantle, P.; Kulinskaya, E.; Nestler, S. Renal tumourigenesis in male rats in response to chronic dietary Ochratoxin A. Food Addit. Contam. 2005, 22, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Mantle, P.; Kulinskaya, E. Lifetime, low-dose Ochratoxin A dietary study on renal carcinogenesis in male Fischer rats. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk. Assess. 2010, 27, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Manderville, R.A. Case for the genotoxicity of Ochratoxin A by bioactivation and covalent DNA adduction. Chem. Res. Toxicol. 2005, 18, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Manderville, R. An update on direct genotoxicity as a molecular mechanism of Ochratoxin A carcinogenicity. Chem. Res. Toxicol. 2012, 25, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Zeljezic, D.; Domijan, A.; Peraica, M. DNA damage by Ochratoxin A in rat kidney assessed by the alkaline comet assay. Braz. J. Med. Biol. Res. 2006, 39, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Odell, E.; Mantle, P. DNA ploidy distribution in renal tumours induced in male rats by dietary Ochratoxin A. Exp. Toxicol. Pathol. 2007, 59, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Palma, N.; Cinelli, S.; Sapora, O.; Wilson, S.; Dogliotti, E. Ochratoxin A-induced mutagenesis in mammalian cells is consistent with the production of oxidative stress. Chem. Res. Toxicol. 2007, 20, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Delatour, T.; Mally, A.; Richoz, J.; Özden, S.; Dekant, W.; Ihmels, H.; Otto, D.; Gasparutto, D.; Marin-Kuan, M.; Schilter, B.; et al. Absence of 2′-deoxyguanosine-carbon 8-bound Ochratoxin A adduct in rat kidney DNA monitored by isotope dilution LC-MS/MS. Mol. Nutr. Food Res. 2008, 52, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Manderville, R.; Pfohl-Leszkowicz, A. Bioactivation and DNA adduction as a rationale for Ochratoxin A carcinogenesis. World Mycotoxin J. 2008, 1, 357–367. [Google Scholar] [CrossRef]
- Mally, A.; Dekant, W. Mycotoxins and the kidney: Modes of action for renal tumor formation by Ochratoxin A in rodents. Mol. Nutr. Food Res. 2009, 53, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S. Studies on carcinogenic and toxic effects of Ochratoxin A in chicks. Toxins 2010, 2, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Hibi, D.; Suzuki, Y.; Ishii, Y.; Jin, M.; Watanabe, M.; Sugita-Konishi, Y.; Yanai, T.; Nohmi, T.; Nishikawa, A.; Umemura, T. Site-specific in vivo mutagenicity in the kidney of gpt delta rats given a carcinogenic dose of Ochratoxin A. Toxicol. Sci. 2011, 122, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Akman, S.; Adams, M.; Case, D.; Park, G.; Manderville, R. Mutagenicity of Ochratoxin A and its hydroquinone metabolite in the SupF gene of the mutation reporter plasmid Ps189. Toxins 2012, 4, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, S.; Yendamuri, S.; Schimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphotic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, S.; Yendamuri, S.; Schimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Stachurska, A.; Ciesla, M.; Kozakowska, M.; Wolffram, S.; Boesch-Saadatmandi, Ch.; Rimbach, G.; Jozkowicz, A.; Dulak, J.; Loboda, A. Cross-talk between microRNAs, nuclear factor E2-related factor 2, and heme oxygenase-1 in ochratoxin A-induced toxic effects in renal proximal tubular epithelial cells. Mol. Nutr. Food Res. 2013, 57, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Hennemeier, I.; Humpf, H.U.; Gekle, M.; Schwerdt, G. Role of microRNA-29b in the ochratoxin A-induced enhanced collagen formation in human kidney cells. Toxicology 2014, 324, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arias, C.; Benitez-Trinidad, A.; Sordo, M.; Robledo-Marenco, L.; Medina-Díaz, I.; Barrón-Vivanco, B.; Marín, S.; Sanchis, V.; Ramos, A.; Rojas-García, A. Low doses of Ochratoxin A induce micronucleus formation and delay DNA repair in human lymphocytes. Food. Chem. Toxicol. 2014, 74, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yu, T.; Zhu, L.; Gao, J.; He, X.; Huang, K.; Luo, Y.; Xu, W. Ochratoxin A induces rat renal carcinogenicity with limited induction of oxidative stress responses. Toxicol. Appl. Pharmacol. 2014, 280, 543–549. [Google Scholar] [CrossRef] [PubMed]
- IARC. Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Man: Some Naturally Occurring Substances; IARC: Lyon, France, 1976; Volume 10, pp. 191–197. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Humans: Some Food Additives, Feed Additives and Naturally Occurring Substances; IARC: Lyon, France, 1983; Volume 31, pp. 191–206. [Google Scholar]
- IARC. Available online: http://monographs.iarc.fr/ENG/Preamble/CurrentPreamble.pdf (accessed on 10 April 2016).
- Degen, G. Tools for investigating workplace-related risks from mycotoxin exposure. World Mycotoxin J. 2011, 4, 315–327. [Google Scholar] [CrossRef]
- Hult, K.; Plestina, R.; Habazin-Novak, V.; Radic, B.; Ceovic, S. Ochratoxin A in human blood and Balkan Endemic Nephropathy. Arch Toxicol. 1982, 51, 313–321. [Google Scholar] [CrossRef]
- Baldwin, T.; Riley, R.; Zitomer, N.; Voss, K.; Coulombe, R., Jr.; Pestka, J.; Williams, D.; Glenn, A. The current state of mycotoxin biomarker development in humans and animals and the potential for application to plant systems. World Mycotoxin J. 2011, 4, 257–270. [Google Scholar] [CrossRef]
- Turner, P.; Flannery, B.; Isitt, C.; Ali, M.; Pestka, J. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr. Res. Rev. 2012, 25, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Pena, A.; Lino, C. Human Ochratoxin A biomarkers—from exposure to effect. Crit. Rev. Toxicol. 2011, 41, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Ruiz, M.; Manyes, L.; Juan-García, A. Blood, breast milk and urine: Potential biomarkers of exposure and estimated daily intake of Ochratoxin A: A review. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk. Assess. 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hult, K.; Hokby, E.; Gatenbeck, S.; Plestina, R.; Ceovic, S. Ochratoxin A and Balkan endemic nephropathy. IV. Occurrence of Ochratoxin A in humans. Chem. Rundschau 1979, 35, 32–33. [Google Scholar]
- Scott, P. Biomarkers of human exposure to Ochratoxin A. Food Addit. Contam. 2005, 22, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Brereton, P.; MacDonald, S. Assessment of dietary exposure to Ochratoxin A in the UK using a duplicate diet approach and analysis of urine and plasma samples. Food Addit. Contam. 2001, 18, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Castegnaro, M.; Canadas, D.; Vrabcheva, T.; Petkova-Bocharova, T.; Chernozemsky, I.; Pfohl-Leszkowicz, A. Balkan Endemic Nephropathy: Role of ochratoxins A through biomarkers. Mol. Nutr. Food Res. 2006b, 50, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Tozlovanu, M.; Stepanovic, J.; Stefanovic, V.; Manderville, R.; Castegnaro, M. Comparative genotoxicity of Ochratoxin A and aristolochic acid in human kidney cells: Interpretation of ongoing analyses of food, blood, urine and kidney tissue from Serbia. Coll. Antropol. Suppl. 2006, 30, 1–17. [Google Scholar]
- Muñoz, K.; Blaszkewicz, M.; Campos, V.; Vega, M.; Degen, G. Exposure of infants to Ochratoxin A with breast milk. Arch. Toxicol. 2014, 88, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Blaszkewicz, M.; Manirujjaman, M.; Perveen, R.; Nahid, A.; Mahmood, S.; Rahman, M.; Hossain, K.; Degen, G. Biomonitoring of Ochratoxin A in blood plasma and exposure assessment of adult students in Bangladesh. Mol. Nutr. Food Res. 2014, 58, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Hald, B. Ochratoxin A in human blood in European countries. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Bartsch, H., Eds.; IARC Scientific Publications: Lyon, France, 1991; Volume 115, pp. 159–164. [Google Scholar]
- Bauer, J.; Gareis, M. Ochratoxin A in the food chain (Ger). J. Vet. Med. 1987, B34, 613–627. [Google Scholar] [CrossRef]
- Petkova-Bocharova, T.; Chernozemsky, I.; Castegnaro, M. Ochratoxin A in human blood in relation to Balkan Endemic Nephropathy and urinary system tumours in Bulgaria. Food Addit. Contam. 1988, 5, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Petkova-Bocharova, T.; Castegnaro, M. Ochratoxin A in human blood in relation to endemic nephropathy and urinary tract tumors in Bulgaria. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Bartsch, H., Eds.; IARC Scientific Publications: Lyon, France, 1991; Volume 115, pp. 159–164. [Google Scholar]
- Golinski, P.; Grabatkiewicz-Szczenasna, J.; Chelkowski, J.; Hult, K.; Kostecki, M. Possible sources of Ochratoxin A in human blood. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Bartsch, H., Eds.; IARC Scientific Publications: Lyon, France, 1991; Volume 115, pp. 153–157. [Google Scholar]
- Fuchs, R.; Radic, B.; Ceovic, S.; Sostaric, B.; Hult, K. Human Exposure to Ochratoxin A; IARC Sci Publ.: Lyon, France, 1991; Volume 115, pp. 131–134. [Google Scholar]
- Hadlok, R.M.; Wagner, G. Vorkommen von Ochratoxin A beim menschen in Deutschland. Fleischwirtsch 1993, 73, 1079–1080. [Google Scholar]
- Fukal, L.; Reisnerova, H. Monitoring of aflatoxins and Ochratoxin A in Czechoslovak human sera by immunoassay. Bull. Environ. Contam. Toxicol. 1990, 44, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Creppy, E.E.; Betbeder, A.M.; Gharbi, A.; Counord, J.; Castegnaro, M.; Bartsch, H.; Moncharmont, P.; Fouillet, B.; Chambon, P.; Dirheimer, G. Human Ochratoxicosis in France; IARC Sci. Publ.: Lyon, France, 1991; Volume 115, pp. 131–134. [Google Scholar]
- Ruprich, J.; Ostry, V. Health risk assessment of the mycotoxin Ochratoxin A to humans: Czech Republic–Brno–1991/92. Cent. Eur. J. Public Health 1993, 1, 86–93. [Google Scholar] [PubMed]
- Creppy, E.E.; Castegnaro, M.; Grosse, Y.; Meriaux, J.; Moncharmont, P.; Waller, C. Etude de l´ochratoxicose humaine dans trois regions de France: Alsace, Aquitaine, et region Rhone—Alpes. In Human Ochratoxicosis and Its Pathologies; Creppy, E.E., Castegnaro, M., Dirheimer, G., Eds.; Colloque INSERM/John Libbey Eurotext: London, UK, 1993; Volume 231, pp. 147–158. [Google Scholar]
- Benford, D.; Boyle, C.; Dekant, W.; Fuchs, R.; Gaylor, D.W.; Hard, G.; McGregor, D.B.; Pitt, J.I.; Plestina, R.; Shepard, G.; et al. Ochratoxin A. In Safety Evaluation of Certain Mycotoxins in Food, Proceedings of the 56th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Geneva, 6–15 February, 2001; World Health Organization: Geneva, Switzerland, 2001; Volume 47, pp. 281–387. [Google Scholar]
- Breitholtz-Emanuelsson, A.; Minervini, F.; Hult, K.; Visconti, A. Ochratoxin A in human serum samples collected in southern Italy from healthy individuals and individuals suffering from different kidney disorders. Nat. Toxins 1994, 2, 366–370. [Google Scholar] [PubMed]
- Kovacs, F.; Sandor, G.; Vanyi, A.; Domany, S.; Zomborszky-Kovacs, M. Detection of Ochratoxin A in human blood and colostrum. Acta Vet. Hung. 1995, 43, 393–400. [Google Scholar] [PubMed]
- Palli, D.; Miraglia, M.; Saieva, C.; Masala, G.; Cava, E.; Colatosti, M.; Corsi, A.M.; Russo, A.; Brera, C. Serum levels of Ochratoxin A in healthy adults in Tuscany: Correlation with individual characteristics and between repeat measurements. Cancer Epidemiol. Biomark. Prev. 1999, 8, 265–269. [Google Scholar]
- Solti, L.; Salamon, F.; Barna-Vetro, I.; Gyongyosi, A.; Szabo, E.; Wolfling, A. Ochratoxin A content of human sera determined by a sensitive ELISA. J. Anal. Toxicol. 1997, 21, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Cerna, M.; Severa, J.; Jergeova, Z. Ochratoxin A—Toxicological importance, exposure of humans and health risk. Hygiena 1998, 43, 49–62. [Google Scholar]
- Malir, F.; Brndiar, M.; Roubal, T.; Severa, J.; Fixa, P.; Kacerovsky, J.; Zahradnik, J.; Osterreicher, J.; Knizek, J.; Cerna, M. A Study of the accumulation of Ochratoxin A in patients with Chronic Renal Insufficiency (CHRI) in the Czech Republic. Mycotoxin Res. 2001, 17, 39–44. [Google Scholar]
- Malir, F.; Roubal, T.; Brndiar, M.; Osterreicher, J.; Severa, J.; Knizek, J.; Kacerovsky, J.; Tmejova, M.; Betbeder, A.; Baudrimont, I.; et al. Ochratoxin A in the Czech Republic. Toxin Rev. 2001b, 20, 261–274. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Grosse, Y.; Roubal, T.; Skarkova, J.; Ruprich, J. Monitoring the mycotoxins in food and their biomarkers in the Czech Republic. Mol. Nutr. Food Res. 2006, 50, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.; Lopez de Cerain, A.; Gonzalez-Peñas, E.; Bello, J.; Betbeder, A.; Creppy, E. Exposure to Ochratoxin A in Europe: Comparison with a region of northern Spain. Toxin Rev. 1998, 17, 479–491. [Google Scholar] [CrossRef]
- Perez de Obanos, A.; Lopez de Cerain, A.; Jimenez, A.M.; Gonzales-Penas, E.; Bello, J. Ochratoxin A in human plasma: New data of exposition in Spain. Rev Toxicol. 2001, 18, 19–23. [Google Scholar]
- Tapai, K.; Teren, J.; Mesterhazy, A. Ochratoxin A in the sera of blood donors and ill persons. Cereal Res. Commun. 1997, 25, 307–308. [Google Scholar]
- Domijan, A.M.; Peraica, M.; Fuchs, R.; Lucic, A.; Radic, B.; Balija, M.; Bosanac, I.; Grgicevic, D. Ochratoxin A in blood of healthy population in Zagreb. Arh. Hig. Rada Toksikol. 1999, 50, 263–271. [Google Scholar] [PubMed]
- Peraica, M.; Domijan, A.; Fuchs, R.; Lucić, A.; Radić, B. The Occurrence of Ochratoxin A in blood in general population of Croatia. Toxicol. Lett. 1999, 110, 105–112. [Google Scholar] [CrossRef]
- Peraica, M.; Domijan, A.; Matašin, M.; Lucić, A.; Radić, B.; Delaš, F.; Horvat, M.; Bosanac, I.; Balija, M.; Grgičević, D. Variations of Ochratoxin A concentration in the blood of healthy populations in some Croatian cities. Arch. Toxicol. 2001, 75, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Thuvander, A.; Paulsen, J.; Axberg, K.; Johansson, N.; Vidnes, A.; Enghardt-Barbieri, H.; Trygg, K.; Lund-Larsen, K.; Jahrl, S.; Widenfalk, A.; et al. Levels of Ochratoxin A in blood from Norwegian and Swedish blood donors and their possible correlation with food consumption. Food. Chem. Toxicol. 2001, 39, 1145–1151. [Google Scholar] [CrossRef]
- Rösner, H.; Rohrmann, B.; Peiker, G. Ochratoxin A in human serum. Arch. Lebensm. 2000, 51, 104–107. [Google Scholar]
- MacDonald, S.J.; Langton, S.; Brereton, P.A. Assessment of human exposure to Ochratoxin A in the UK-relationship between dietary intake and plasma and urine levels. In Mycotoxins and Phycotoxins in Perspective at the Turn of the Millennium; De Koe, W.J., Samson, R.A., van Egmond, H.P., Gilbert, J., Sabino, M., Eds.; IUPAC: Wageningen, The Netherlands, 2001; Volume 1, pp. 181–188. [Google Scholar]
- Skaug, M.A. Levels of Ochratoxin A and IgG against conidia of Penicillium verrucosum in blood samples from healthy farm workers. Ann. Agric. Environ. Med. 2003, 10, 73–77. [Google Scholar] [CrossRef]
- Lino, C.; Baeta, M.; Henri, M.; Dinis, A.; Pena, A.; Silveira, M. Levels of Ochratoxin A in serum from urban and rural Portuguese populations and estimation of exposure degree. Food. Chem. Toxicol. 2008, 46, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Grajewski, J.; Jarzemski, P.; Twaruzek, M.; Kuzminska, K.; Trepala, M. The level of Ochratoxin A in patients after nephrectomy. Mycotoxin Res. 2007, 23, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Roubal, T.; Brndiar, M.; Kacerovsky, J.; Pacovsky, J.; Moravek, P.; Melichar, B.; Malirova, E.; Cerna, M. The monitoring mycotoxins and their potential impact on human health: Ochratoxin A. (in Czech). In Proceedings of the 4. Seminary with International Participation. Mykotoxíny 2008, Prague, Czech Republic, 9–10 October 2008.
- Medina, Á.; Mateo, E.; Roig, R.; Blanquer, A.; Jiménez, M. Ochratoxin A levels in the plasma of healthy blood donors from Valencia and estimation of exposure degree: Comparison with previous national Spanish data. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk. Assess. 2010, 27, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Coronel, M.; Sanchis, V.; Ramos, A.; Marin, S. Assessment of the exposure to Ochratoxin A in the province of Lleida, Spain. Food. Chem. Toxicol. 2009, 47, 2847–2852. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, K.; Blaszkewicz, M.; Degen, G. Simultaneous analysis of Ochratoxin A and its major metabolite ochratoxin alpha in plasma and urine for an advanced biomonitoring of the mycotoxin. J. Chromatogr. B Biomed. Appl. 2010, 878, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Coronel, M.; Sanchis, V.; Ramos, A.; Marin, S. Ochratoxin A in adult population of Lleida, Spain: Presence in blood plasma and consumption in different regions and seasons. Food. Chem. Toxicol. 2011a, 49, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, R.; Bertuzzi, T.; Rossi, F.; Rastelli, S.; Mulazzi, A.; Capraro, J.; de Curtis, A.; Iacoviello, L.; Pietri, A. Plasma Ochratoxin A levels, food consumption, and risk biomarkers of a representative sample of men and women from the Molise region in Italy. Eur. J. Nutr. 2011, 51, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Dofkova, M.; Roubal, T.; Dvorak, V.; Dohnal, V. Ochratoxin A levels in blood serum of Czech women in the first trimester of pregnancy and its correspondence with dietary intake of the mycotoxin contaminant. Biomarkers 2013, 18, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Dohnal, V.; Dvořák, V.; Malíř, F.; Ostrý, V.; Roubal, T. A comparison of ELISA and HPLC methods for determination of Ochratoxin A in human blood serum in the Czech Republic. Food. Chem. Toxicol. 2013, 62, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Khalef, A.; Zidane, C.; Charef, A.; Gharbi, A.; Tadjerouna, M.; Betbeder, A.M.; Creppy, E.E. Ochratoxicoses humaines en Algerie. In Human Ochratoxicosis and Its Pathologies; Creppy, E., Castegnaro, M., Dirheimer, G., Eds.; John Libbey Eurotext: Paris, France, 1993; Volume 231, pp. 123–127. [Google Scholar]
- Maaroufi, K.; Achour, A.; Betbeder, A.; Hammami, M.; Ellouz, F.; Creppy, E.; Bacha, H. Foodstuffs and human blood contamination by the mycotoxin Ochratoxin A: Correlation with chronic interstitial nephropathy in Tunisia. Arch. Toxicol. 1995, 69, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Jonsyn, F. Intake of aflatoxins and ochratoxins by infants in Sierra Leone: Possible effects on the general health of these hildren. J. Nutr. Environ. Med. 1999, 9, 15–22. [Google Scholar] [CrossRef]
- Filali, A.; Betbeder, A.; Baudrimont, I.; Benayada, A.; Soulaymani, R.; Creppy, E. Ochratoxin A in human plasma in Morocco: A preliminary survey. Hum. Exp. Toxicol. 2002, 21, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.; Hassen, W.; Achour, A.; Skhiri, H.; Maaroufi, K.; Ellouz, F.; Creppy, E.; Bacha, H. Ochratoxin A and human chronic nephropathy in Tunisia: Is the situation endemic? Hum. Exp. Toxicol. 2003, 22, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hassen, W.; Abid, S.; Achour, A.; Creppy, E.; Bacha, H. Ochratoxin A and β2-microglobulinuria in healthy individuals and in Chronic Interstitial Nephropathy patients in the centre of Tunisia: A hot spot of Ochratoxin A exposure. Toxicology 2004, 199, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Sangare-Tigori, B.; Moukha, S.; Kouadio, J.; Dano, D.; Betbeder, A.; Achour, A.; Creppy, E. Ochratoxin A in human blood in Abidjan, Côte D'ivoire. Toxicon 2006, 47, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Hmaissia Khlifa, K.; Ghali, R.; Mezigh, C.; Aouni, Z.; Ghorbel, H.; Harrzallah, K.; Machgoul, S.; Hedhili, A. Serum levels of Ochratoxin A in healthy subjects and in nephropathic patients in Tunisia. Ann. Biol. Clin. (Paris) 2008, 66, 631–636. [Google Scholar] [PubMed]
- Karima, H.; Ridha, G.; Zied, A.; Chekib, M.; Salem, M.; Abderrazek, H. Estimation of Ochratoxin A in human blood of healthy Tunisian population. Exp. Toxicol. Pathol. 2010, 62, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Zaied, C.; Bouaziz, C.; Azizi, I.; Bensassi, F.; Chour, A.; Bacha, H.; Abid, S. Presence of Ochratoxin A in Tunisian blood nephropathy patients. Exposure level to OTA. Exp. Toxicol. Pathol. 2011, 63, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Hmaissia Khlifa, K.; Ghali, R.; Mazigh, C.; Aouni, Z.; Machgoul, S.; Hedhili, A. Ochratoxin A levels in human serum and foods from nephropathy patients in Tunisia: Where are you now? Exp. Toxicol. Pathol. 2012, 64, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Maki, S.; Lin, J.; Furuya, M.; Sugiura, Y.; Kawamura, O. A 4-year study of plasma Ochratoxin A in a selected population in Tokyo by immunoassay and immunoaffinity column-linked HPLC. Food. Chem. Toxicol. 1998, 36, 445–449. [Google Scholar] [CrossRef]
- Assaf, H.; Betbeder, A.; Creppy, E.; Pallardy, M.; Azouri, H. Ochratoxin A levels in human plasma and foods in Lebanon. Hum. Exp. Toxicol. 2004, 23, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Beg, A.; Blaszkewicz, M.; Degen, G.; Golka, K. Ochratoxin A blood concentration in healthy subjects and bladder cancer cases from Pakistan. Toxicol. Lett. 2006, 164, S280. [Google Scholar] [CrossRef]
- Özçelik, N.; Koşar, A.; Soysal, D. Ochratoxin A in human serum samples collected in Isparta-Turkey from healthy Individuals and individuals suffering from different urinary disorders. Toxicol. Lett. 2001, 121, 9–13. [Google Scholar] [CrossRef]
- Erkekoğlu, P.; Sabuncuoğlu, S.; Aydın, S.; Şahin, G.; Giray, B. Determination of seasonal variations in serum Ochratoxin A levels in healthy population living in some regions of Turkey by Enzyme-Linked Immunosorbent assay. Toxicon 2010, 55, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Sabuncuoglu, S.; Erkekoglu, P.; Aydin, S.; Şahin, G.; Kocer-Gumusel, B. The effects of season and gender on the serum aflatoxins and Ochratoxin A levels of healthy adult subjects from the Central Anatolia Region, Turkey. Eur. J. Nutr. 2014, 54, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Kuiper-Goodman, T.; Ominski, K.; Marquardt, R.R.; Malcom, S.; McMullen, E.; Lombaert, G.A.; Morton, T. Estimating human exposure to Ochratoxin A in Canada. In Human Ochratoxicosis and Its Pathologies; Creppy, E.E., Castegnaro, M., Dirheimer, G., Eds.; INSERM/John Libbey Eurotext: Montrouge, France, 1993; pp. 167–174. [Google Scholar]
- Scott, P.; Kanhere, S.; Lau, B.; Levvis, D.; Hayward, S.; Ryan, J.; Kuiper-Goodman, T. Survey of Canadian human blood plasma for Ochratoxin A. Food Addit. Contam. 1998, 15, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, K.; Vega, M.; Rios, G.; Muñoz, S.; Madariaga, R. Preliminary study of Ochratoxin A in human plasma in agricultural zones of Chile and its relation to food consumption. Food. Chem. Toxicol. 2006, 44, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.M.; Guerrero, F.A.; Chaves, J.A. Ochratoxin A in human plasma and coffee from Costa Rica by ELISA. Arch. Latinoam. Nutr. 2007, 57, 168–172. [Google Scholar] [PubMed]
- Pacin, A.; Ciancio Bovier, E.; Motta, E.; Resnik, S.; Villa, D.; Olsen, M. Survey of Argentinean human plasma for Ochratoxin A. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk. Assess. 2008, 25, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Castegnaro, M.; Maru, V.; Petkova-Bocharova, T.; Nikolov, I.; Bartsch, H. Concentrations of Ochratoxin A in the urine of endemic nephropathy patients and controls in Bulgaria: Lack of detection of 4-hydroxyochratoxin A. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Bartsch, H., Eds.; IARC Scientific Publications: Lyon, France, 1991; Volume 115, pp. 165–169. [Google Scholar]
- Ostry, V.; Skarkova, J.; Kavrik, R.; Ruprich, J. An occurrence of Ochratoxin A and aflatoxin M1 biomarkers in human urine. In Proceedings of the 32th Mycotoxin Workshop, Lyngby, Denmark, 14–16 June, 2010.
- Ostry, V.; Skarkova, J.; Malir, F.; Ruprich, J. An occurrence of Ochratoxin A, a biomarker of dietary exposure, in human biological materials. In Proceedings of the 6. Seminary with International Participation. Mykotoxíny 2010, Prague, VSCHT, 14–15 October 2010.
- Warth, B.; Sulyok, M.; Berthiller, F.; Schuhmacher, R.; Krska, R. New insights into the human metabolism of the fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol. Lett. 2013, 220, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Sulyok, M.; Krska, R. LC-MS/MS-based multibiomarker approaches for the assessment of human exposure to mycotoxins. Anal. Bioanal. Chem. 2013, 405, 5687–5695. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Sulyok, M.; Fruhmann, P.; Mikula, H.; Berthiller, F.; Schuhmacher, R.; Hametner, C.; Abia, W.; Adam, G.; Fröhlich, J.; et al. Development and validation of a rapid multi-biomarker liquid chromatography/tandem mass spectrometry method to assess human exposure to mycotoxins. Rapid Commun. Mass Spectrom. 2012, 26, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Ediage, N.E.; Diana Di Mavungu, J.; Song, S.; Sioen, I.; de Saeger, S. Multimycotoxin analysis in urines to assess infant exposure: A case study in Cameroon. Environ. Int. 2013, 57–58, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Jonsyn, F.E. Seasonal variation in exposure frequency and concentration levels of aflatoxins and ochratoxins in urine samples of boys and girls. Mycopathologia 2000, 152, 35–40. [Google Scholar] [CrossRef]
- Domijan, A.; Peraica, M.; Miletić-Medved, M.; Lucić, A.; Fuchs, R. Two different clean-up procedures for liquid chromatographic determination of Ochratoxin A in urine. J. Chromatogr. B Biomed. Appl. 2003, 798, 317–321. [Google Scholar] [CrossRef]
- Fazekas, B.; Tar, A.; Kovács, M. Ochratoxin A content of urine samples of healthy humans in Hungary. Acta Vet. Hung. 2005, 53, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Seifrtová, M.; Lino, C.; Silveira, I.; Solich, P. Estimation of Ochratoxin A in Portuguese population: New data on the occurrence in human urine by high performance liquid chromatography with fluorescence detection. Food. Chem. Toxicol. 2006, 44, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Manique, R.; Pena, A.; Lino, C.; Moltó, J.; Mañes, J. Ochratoxin A in the morning and afternoon portions of urine from Coimbra and Valencian populations. Toxicon 2008, 51, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Bento, J.; Pena, A.; Lino, C. Ochratoxin A exposure assessment of the inhabitants of Lisbon during winter 2007/2008 through bread and urine analysis. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk. Assess. 2009, 26, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.; Peraica, M.; Markov, K.; Fuchs, R. Urine Ochratoxin A and sphinganine/sphingosine ratio in residents of the Endemic nephropathy area in Croatia. Arch. Ind. Hyg. Toxicol. 2009, 60, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Bento, J.; Pena, A.; Lino, C.; Delerue-Matos, C.; Oliva-Teles, T.; Morais, S.; Correia, M.; Oliveira, M.; Alves, M.; et al. Monitoring of Ochratoxin A exposure of the Portuguese population through a nationwide urine survey—Winter 2007. Sci. Total Environ. 2010, 408, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, C.; Ulker, O.; Basaran, A.; Ozkaya, S.; Karakaya, A. Estimation of Ochratoxin A in some Turkish populations: An analysis in urine as a simple, sensitive and reliable biomarker. Food. Chem. Toxicol. 2010, 48, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Coronel, M.; Marin, S.; Tarragó, M.; Cano-Sancho, G.; Ramos, A.; Sanchis, V. Ochratoxin A and its metabolite Ochratoxin Alpha in urine and assessment of the exposure of inhabitants of Lleida, Spain. Food. Chem. Toxicol. 2011b, 49, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Rubert, J.; Soriano, J.; Mañes, J.; Soler, C. Rapid mycotoxin analysis in human urine: A pilot study. Food. Chem. Toxicol. 2011, 49, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Gambacorta, L.; Lattanzio, V.; Powers, S.; Visconti, A. Simultaneous LC–MS/MS determination of aflatoxin M1, Ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, Α and Β-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal. Bioanal. Chem. 2011, 401, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, B.; Nanayakkara, S.; Harada, K.; Hitomi, T.; Chandrajith, R.; Karunaratne, U.; Abeysekera, T.; Koizumi, A. Mycotoxin detection in urine samples from patients with chronic kidney disease of uncertain etiology in Sri Lanka. Bull. Environ. Contam. Toxicol. 2011, 87, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Alves, M.; Pena, A.; Lino, C. Determinants of Ochratoxin A exposure—A One year follow-up study of urine levels. Int. J. Hyg. Environ. Health 2012, 215, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Klapec, T.; Šarkanj, B.; Banjari, I.; Strelec, I. Urinary Ochratoxin A and ochratoxin alpha in pregnant women. Food. Chem. Toxicol. 2012, 50, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Abia, W.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.; Njobeh, P.; Turner, P.; Kouanfack, C.; Eyongetah, M.; Dutton, M.; et al. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food. Chem. Toxicol. 2013, 62, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.; Burger, H.; Gambacorta, L.; Gong, Y.; Krska, R.; Rheeder, J.; Solfrizzo, M.; Srey, C.; Sulyok, M.; Visconti, A.; et al. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food. Chem. Toxicol. 2013, 62, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Gambacorta, L.; Visconti, A. Assessment of multi-mycotoxin exposure in Southern Italy by urinary multi-biomarker determination. Toxins 2014, 6, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Lino, C.; Pena, A. Ochratoxin A in food and urine: A nationwide Portuguese two-year study. World Mycotoxin J. 2015, 8, 121–132. [Google Scholar] [CrossRef]
- Gerding, J.; Ali, N.; Schwartzbord, J.; Cramer, B.; Brown, D.; Degen, G.; Humpf, H. A Comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 2015, 31, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Gareis, M.; Martlbauer, E.; Bauer, J.; Gedek, B. Determination of Ochratoxin A in human milk. Proc. Jpn. Assoc. Mycotoxicol. 1988, 61–62. [Google Scholar] [CrossRef]
- Breitholtz-Emanuelsson, A.; Olsen, M.; Oskarsson, A.; Palminger, I.; Hult, K. Ochratoxin A in cow’s milk and in human milk with corresponding human blood samples. J. AOAC Int. 1993, 76, 842–846. [Google Scholar] [PubMed]
- Micco, C.; Ambruzzi, M.A.; Miraglia, M.; Brera, C.; Onori, R.; Benelli, I. Contamination of human milk with Ochratoxin A. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours; Castegnaro, M., Plestina, R., Dirheimer, G., Chernozemsky, I.N., Bartsch, H., Eds.; IARC Scientific Publications: Lyon, France, 1991; Volume 115, pp. 105–108. [Google Scholar]
- Skaug, M.; Helland, I.; Solvoll, K.; Saugstad, O. Presence of Ochratoxin A in human milk in relation to dietary intake. Food Addit. Contam. 2001, 18, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Micco, C.; Miraglia, M.; Brera, C.; Corneli, S.; Ambruzzi, A. Evaluation of Ochratoxin A level in human milk in Italy. Food Addit. Contam. 1995, 12, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, M.; De Dominicis, A.; Brera, C.; Corneli, S.; Cava, E.; Menghetti, E.; Miraglia, E. Ochratoxin A levels in human milk and related food samples: An exposure assessment. Nat. Toxins 1995, 3, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Skaug, M.A.; Størmer, F.C.; Saugstad, O.D. Ochratoxin A: A naturally occurring mycotoxin found in human milk samples from Norway. Acta Paediatr. 1998, 87, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Turconi, G.; Guarcello, M.; Livieri, C.; Comizzoli, S.; Maccarini, L.; Castellazzi, A.; Pietri, A.; Piva, G.; Roggi, C. Evaluation of xenobiotics in human milk and ingestion by the newborn. Eur. J. Nutr. 2004, 43, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Postupolski, J.; Karlowski, K.; Kubik, P. Ochratoxin A and foetal blood and in maternal milk. Rocz. Panstw. Zakl. Hig. 2006, 57, 23–30. [Google Scholar] [PubMed]
- Galvano, F.; Pietri, A.; Bertuzzi, T.; Gagliardi, L.; Ciotti, S.; Luisi, S.; Bognanno, M.; la Fauci, L.; Iacopino, A.; Nigro, F.; et al. Maternal dietary habits and mycotoxin occurrence in human mature milk. Mol. Nutr. Food Res. 2008, 52, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.; Jakusova, L.; Cajdova, J.; Hudeckova, H. Results of the first studies of occurrence of Ochratoxin A in human milk in Slovakia. Bratisl. Lek. Listy 2008, 109, 276–278. [Google Scholar] [PubMed]
- Biasucci, G.; Calabrese, G.; di Giuseppe, R.; Carrara, G.; Colombo, F.; Mandelli, B.; Maj, M.; Bertuzzi, T.; Pietri, A.; Rossi, F. The presence of Ochratoxin A in cord serum and in human milk and its correspondence with maternal dietary habits. Eur. J. Nutr. 2011, 50, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, K.; Wollin, K.; Kalhoff, H.; Degen, G. Zum vorkommen des mykotoxins Ochratoxin A in muttermilchproben aus Deutschland. Gesundheitswesen 2013, 75, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Jonsyn, F.; Maxwell, S.; Hendrickse, R. Ochratoxin A and aflatoxins in breast milk samples from Sierra Leone. Mycopathologia 1995, 131, 121–126. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Soher, E.; Neamat-Allah, A. Human exposure to mycotoxins in Egypt. Mycotoxin Res. 2002, 18, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Sheashaa, H.; Fattah, M.; Ibrahim, A.; Gaber, O.; Sobh, M. Study of Ochratoxin A as an environmental risk that causes renal injury in breast-fed Egyptian infants. Pediatr. Nephrol. 2006, 21, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, E.; El-Nezami, H.S.; Ahoka, J.T.; Donohe, D.D. The evaluation of Ochratoxin A in breast milk in Victoria (Australia). Rev. Med. Vet. 1998, 149, 709. [Google Scholar]
- Gürbay, A.; Girgin, G.; Sabuncuoğlu, S.; Şahin, G.; Yurdakök, M.; Yiğit, Ş.; Tekinalp, G. Ochratoxin A: Is it present in human breast milk samples obtained mothers from Ankara, Turkey. Toxicol. Lett. 2009, 189, 232. [Google Scholar] [CrossRef]
- Afshar, P.; Shokrzadeh, M.; Kalhori, S.; Babaee, Z.; Saeedi Saravi, S. Occurrence of Ochratoxin A and aflatoxin M1 in human breast milk in Sari, Iran. Food Control 2013, 31, 525–529. [Google Scholar] [CrossRef]
- Dehghan, P.; Pakshir, K.; Rafiei, H.; Chadeganipour, M.; Akbari, M. Prevalence of Ochratoxin A in human milk in the Khorrambid Town, Fars province, South of Iran. Jundishapur J. Microbiol. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Navas, S.; Sabino, M.; Rodriguez-Amaya, D. Aflatoxin M1 and Ochratoxin A in a human milk bank in the city of São Paulo, Brazil. Food Addit. Contam. 2005, 22, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, K.; Campos, V.; Blaszkewicz, M.; Vega, M.; Alvarez, A.; Neira, J.; Degen, G. Exposure of neonates to Ochratoxin A: First biomonitoring results in human milk (colostrum) from Chile. Mycotoxin Res. 2010, 26, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; da Silva, J.; Caldas, E. Simultaneous analysis of aflatoxins B1, B2, G1, G2, M1 and Ochratoxin A in breast milk by high-performance liquid chromatography/fluorescence after liquid–liquid extraction with low temperature purification (LLE–LTP). J. Chromatogr. A 2013, 1304, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Iha, M.; Barbosa, C.; Heck, A.; Trucksess, M. Aflatoxin M1 and Ochratoxin A in human milk in Ribeirão Preto-SP, Brazil. Food Control 2014, 40, 310–313. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T.; Hilts, C.; Billiard, S.; Kiparissis, Y.; Richard, I.; Hayward, S. Health risk assessment of Ochratoxin A for all age-sex strata in a market economy. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk. Assess. 2010, 27, 212–240. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Roubal, T. Ochratoxin A exposure biomarkers in the Czech Republic and comparison with foreign countries. Biomarkers 2012, 17, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Gekle, M.; Silbernagl, S. Ochratoxin A and kidney. In Proceedings of the 16th Mycotoxin Workshop Stuttgart-Hohenheim, Hohenheim, Germany, 16–18 May 1994.
- Ostry, V.; Malir, F.; Roubal, T.; Skarkova, J.; Ruprich, J.; Cerna, M.; Creppy, E. Monitoring of mycotoxin biomarkers in the Czech Republic. Mycotoxin Res. 2005, 21, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Park, D.L.; Troxell, T.C.U.S. Perspective on Mycotoxin Regulatory Issues. In Mycotoxins and Food Safety; Vries, J.W., de Trucksess, M.W., Jackson, L.S., Eds.; Kluwer Academic/ Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Worldwide Regulations for Mycotoxins in Food and Feed in 2003, FAO 2003. p. 27. Available online: http://www.fao.org/docrep/007/y5499e/y5499e00.htm#Contents (accessed on 12 April 2016).
- Van Egmond, H.P. Worldwide regulation for oxhratoxin A. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumors; Castegnaro, M., Plestina, R., Dirheimer, G., Eds.; IARC Sci. Publ.: Lyon, Frace, 1991; Volume 115, pp. 331–336. [Google Scholar]
- Duarte, S.; Lino, C.; Pena, A. Mycotoxin food and feed regulation and the specific case of Ochratoxin A: A review of the worldwide status. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk. Assess. 2010, 27, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Fan, Y.; Jia, Y.; Sun, L.; Ma, S.; Ji, C.; Ma, Q.; Zhang, J. Occurrence of mycotoxins in feed ingredients and complete feeds obtained from the Beijing region of China. J. Anim. Sci. Biotechnol. 2014, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Codex Allimentarius. General Standard for Contaminants and Toxins in Food and Feed, CODEX STAN 193–1995, 1995–2015.
- Codex Allimentarius. Code of Practice for the Prevention and Reduction of Mycotoxin Contamination in Cereals Including Annexes on Ochratoxin A, Zearalenone, Fumonisins and Tricothecenes, CAC/RCP 51-2003, 2003–2014.
- Codex Allimentarius. Code of Practice for the Prevention and Reduction of Ochratoxin A Contamination in Wine, CAC/RCP 63-2007, 2007.
- Codex Allimentarius. Code of Practice for the Prevention and Reduction of Ochratoxin A Contamination in Coffee, CAC/RCP 69-2009, 2009.
- Codex Allimentarius. Code of Practice for the Prevention and Reduction of Ochratoxin A Contamination in Cocoa, CAC/RCP 72-2013, 2013.
- Food and Agriculture Organization/World Health Organisation. 2007–2014. Available online: http://www.fao.org/fao-whocodexalimentarius/standards/list-of-standards/en (accessed on 12 April 2016).
- European Union. Commission Regulation (EC) No 472/2002 of 12 March 2002 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). Off. J. Eur. Union 2002, L 75, 18–20. [Google Scholar]
- European Union. Commission Regulation (EC) No 466/2001 of 8 March 2001 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). Off. J. Eur. Union 2001, L 77, 1–13. [Google Scholar]
- European Union. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Verstraete, F. Decision-making process and overview of recent and future European Union legislation on mycotoxins in food and feed. In The Mycotoxin Factbook: Food & Feed Topics; Barug, D., Bhatnagar, D., Egmond, H.P., van Kamp, J.W., van der Osenbruggen, W.A., Visconti, A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006. [Google Scholar]
- European Unio. Commission Recommendation No 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, Ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding, (Text with EEA relevance). Off. J. Eur. Union 2006, C 229, 7–9. [Google Scholar]
- Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A.; Tortello, M.L. (Eds.) Academic Press: San Diego, CA, USA, 2014.
- Dohlman, E. Mycotoxin Hazards and Regulations. Impacts on Food and Animal Feed Crop Trade. In International Trade and Food Safety; Buzby, J., Ed.; U.S. Dept. of Agriculture, Economic Research Service: Washington, DC, USA, 2003; pp. 97–108. [Google Scholar]
- Wu, F. A tale of two commodities: How EU mycotoxin regulations have hurt, or helped, food industries. In Proceedings of the Fourth Conference, Cincinnati, OH, USA, November 6–8, 2006.
- Van Egmond, H.; Schothorst, R.; Jonker, M. Regulations relating to mycotoxins in food. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kὄszegi, T.; Poór, M. Ochratoxin A: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Tozlovanu, M. Evaluation du Risque de Contamination Alimentaire en Mycotoxines Néphrotoxiques et Cancérogènes (nOtamment l’Ochratoxine A): Validation de Biomarqueurs D’exposition et d’Effet. Ph.D. Thesis, Undergraduate, Institut National Polytechnique de Toulouse, France, 2008. [Google Scholar]
- Woo, C.S.J.; El-Nezami, H. Maternal-fetal cancer risk assessment of Ochratoxin A during pregnancy. Toxins 2016, 8, 87. [Google Scholar] [CrossRef] [PubMed]
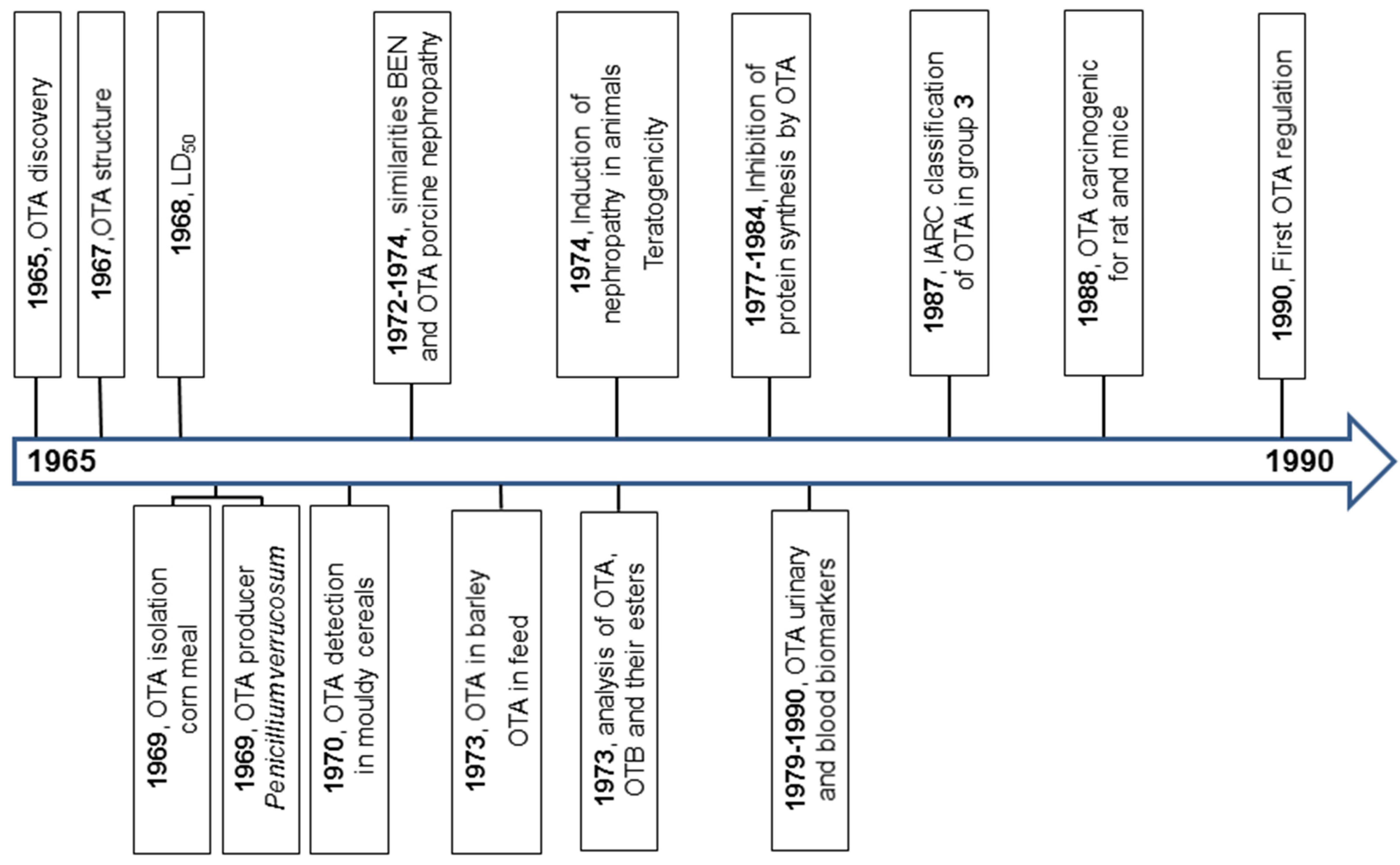


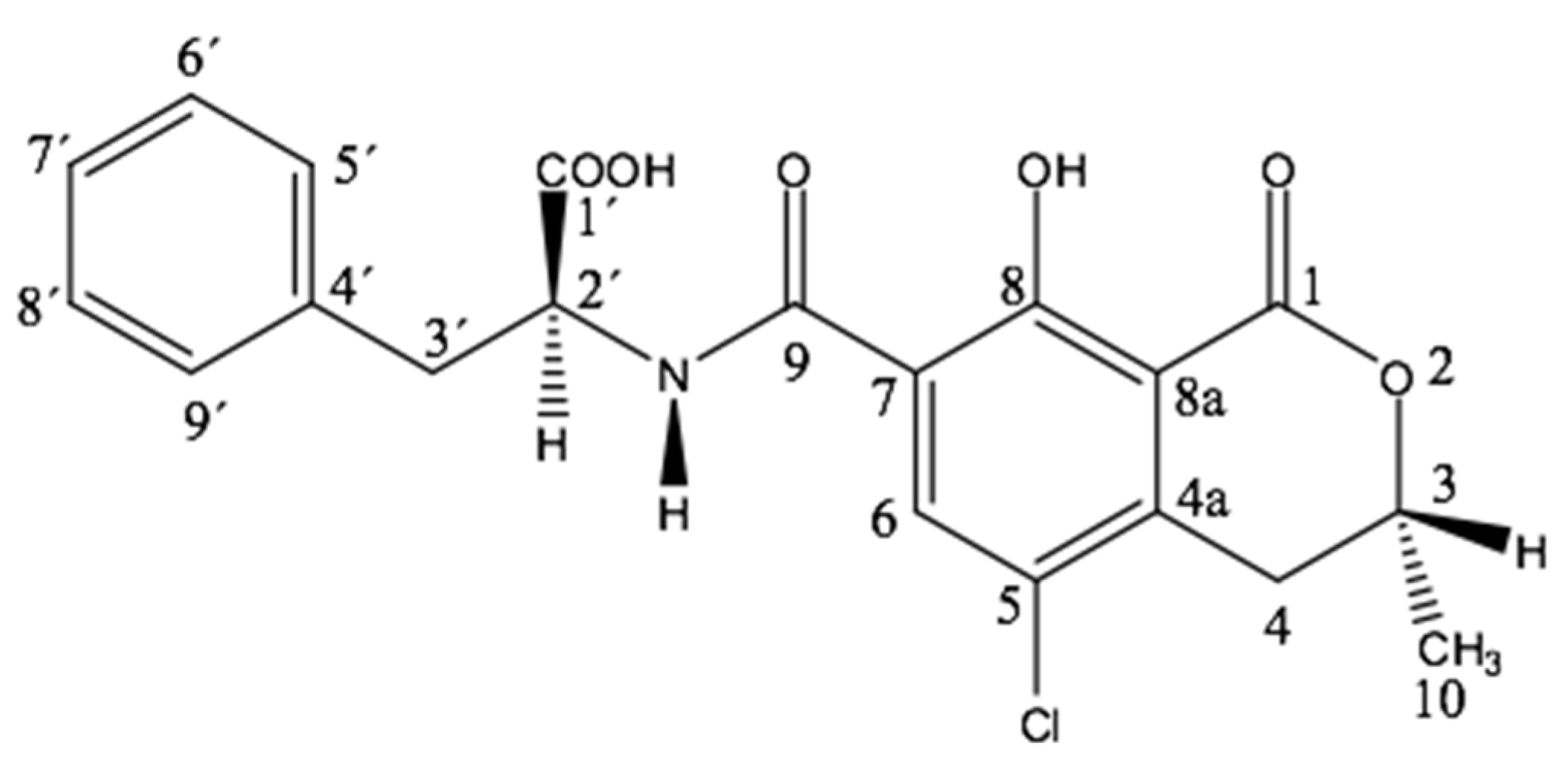
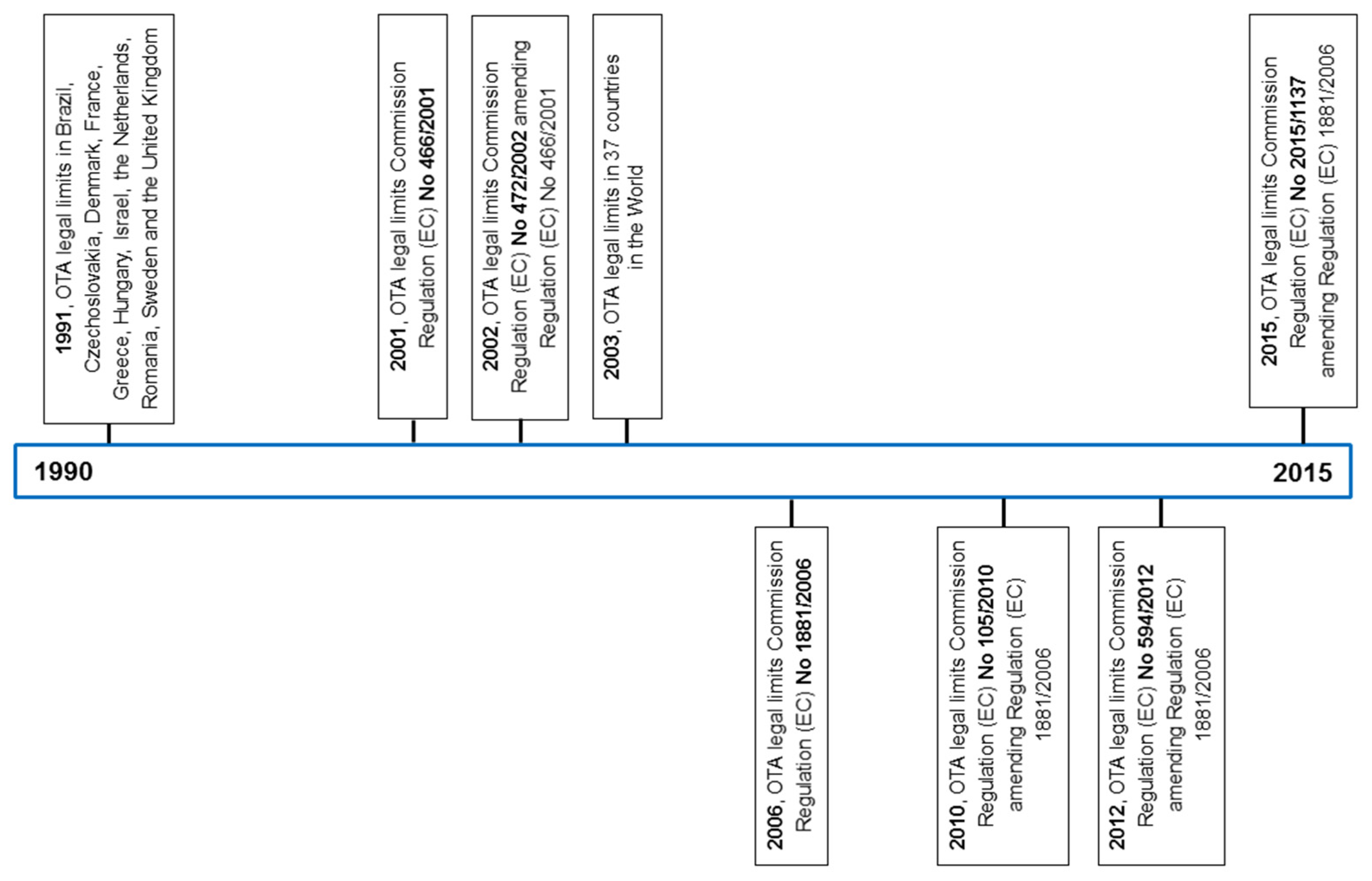
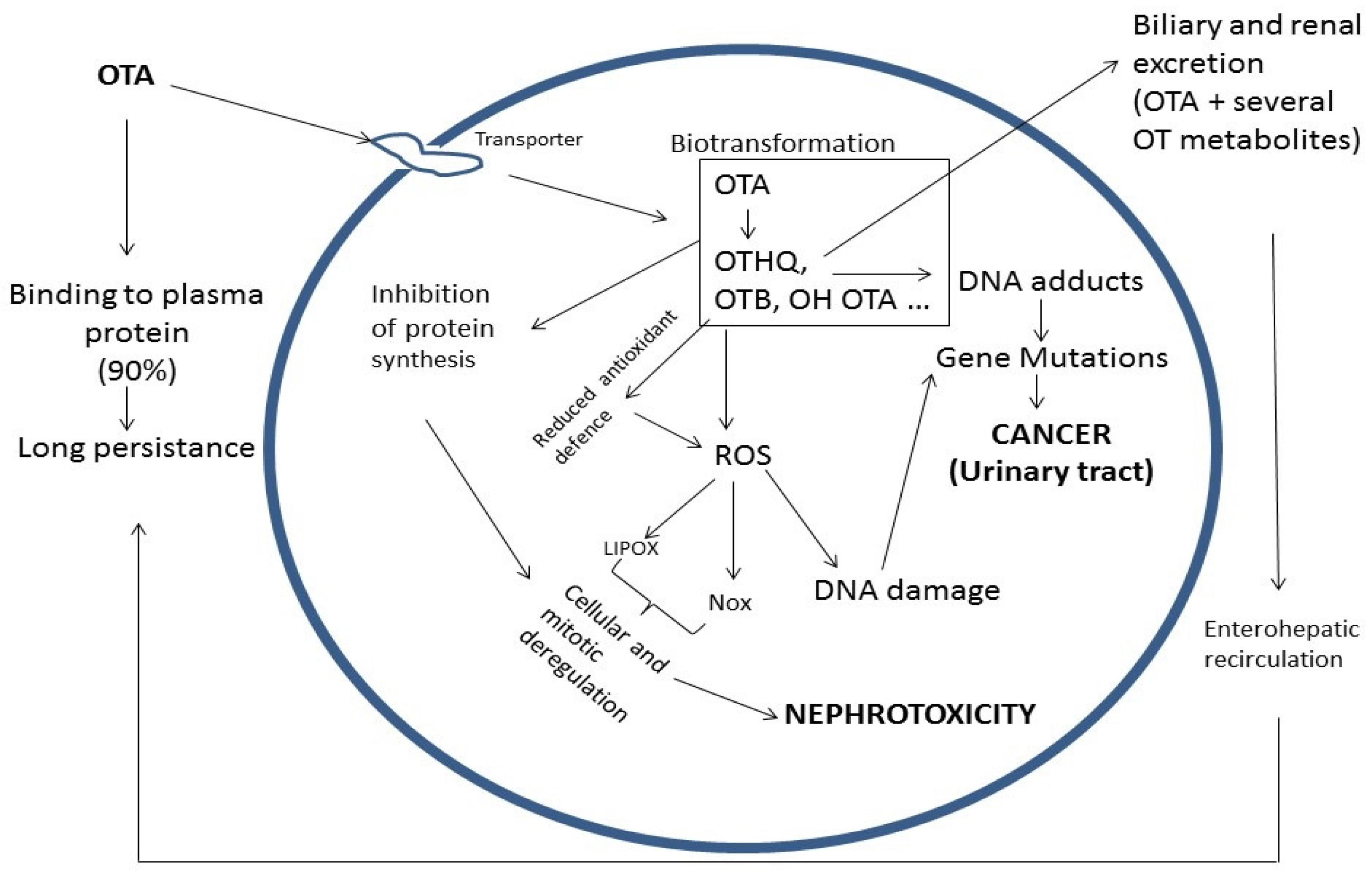
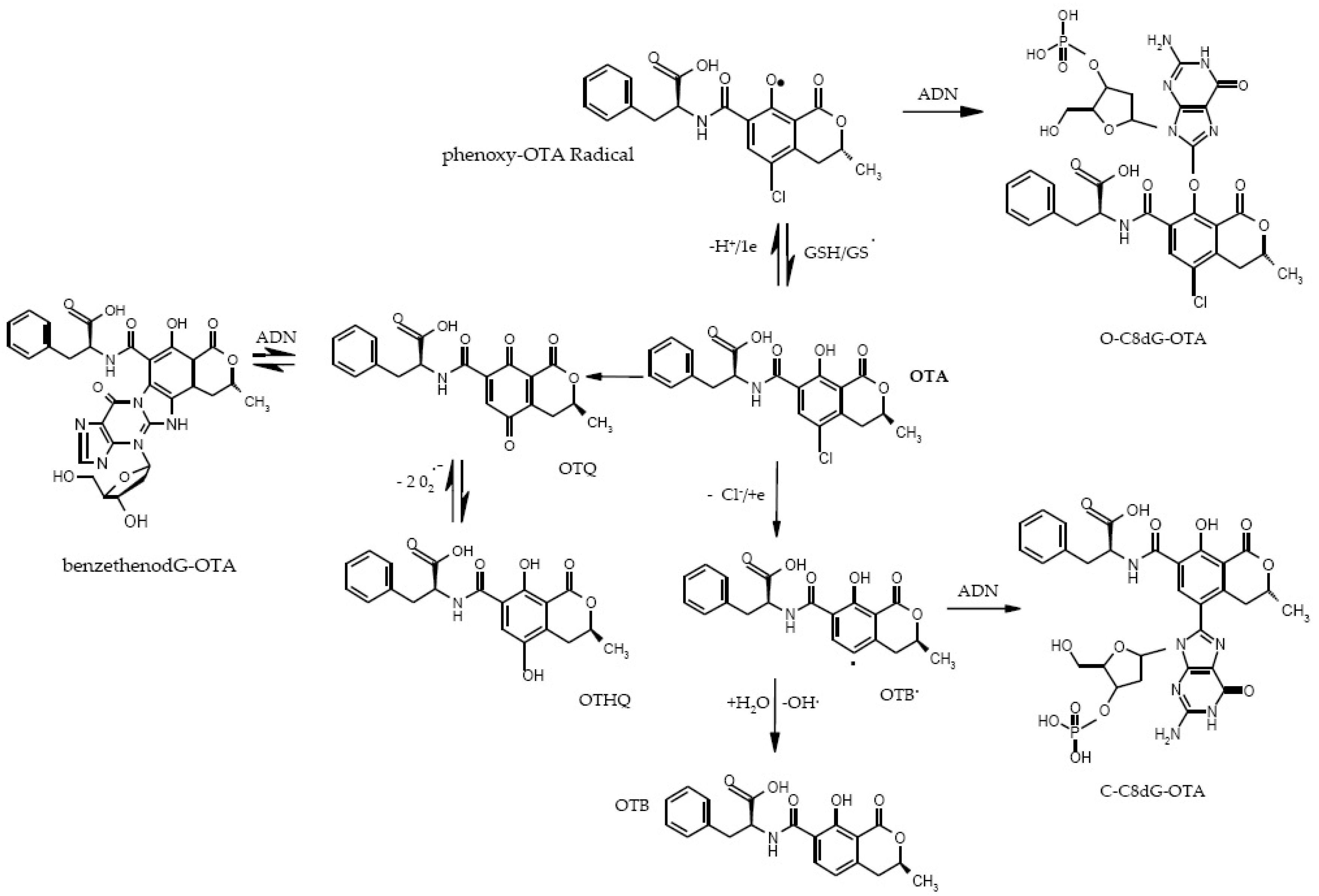
| Genera | Section | Species | Foodstuffs (Examples) | Year of Discovery |
|---|---|---|---|---|
| Aspergillus | Circumdati | A. ochraceus G. Wilh. | Soya bean, nuts, red pepper, cereals, green coffee beans | 1965 |
| A. steynii Frisvad & Samson | Coffee beans | 2004 | ||
| A. westerdijkiae Frisvad & Samson | Coffee beans | 2004 | ||
| Nigri | A. carbonarius (Bainier) Thom | Grapes, red pepper, coffee beans | 1996 | |
| A. foetidus Thom & Raper | Grapes | 1996 | ||
| A. lacticoffeatus Frisvad & Samson | Coffee beans | 2004 | ||
| A. niger Tiegh. | Grapes, peanuts | 1994 | ||
| A. sclerotioniger Frisvad & Samson | Coffee beans | 2004 | ||
| A. tubingensis Mosseray | Grapes | 2005 |
| Genera | Subgenus | Series | Species | Foodstuffs (Examples) | Year of Discovery |
|---|---|---|---|---|---|
| Penicillium | Penicillium | Verrucosa | P. verrucosum Dierckx | Cereals | 1969 |
| Verrucosa | P. nordicum Dragoni & Marino | Dry ham, salami | 2001 |
| Metabolites | Abbreviations | MW | R1 | R2 | R3 | R4 | R5 | R6 | References |
|---|---|---|---|---|---|---|---|---|---|
| Ochratoxin A | OTA | 403 | Phe | Cl | H | H | H | OH | [3,4] |
| Ochratoxin B | OTB | 370 | Phe | H | H | H | H | OH | [51] |
| Ochratoxin C | OTC | 431 | Phe Ethyl ester | Cl | H | H | H | OH | [52] |
| Ochratoxin α | OTα | 256 | OH | Cl | H | H | H | OH | [53] |
| Ochratoxin β | OTβ | 223 | OH | H | H | H | H | OH | [54] |
| 4R-hydroxy Ochratoxin A | 4R-OHOA | 419 | Phe | Cl | H | OH | H | OH | [55] |
| 4S-hydroxy Ochratoxin A | 4S-OHOA | 419 | Phe | Cl | OH | H | H | OH | [55] |
| 10-hydroxy Ochratoxin A | 10-OHOA | 419 | Phe | Cl | H | H | OH | OH | [56] |
| Ochratoxin A open lactone | OP-OA | 421 | Phe | Cl | H | H | - | OH | [57] |
| Ochratoxin B open lactone | OP-OB | 388 | Phe | H | H | H | - | OH | [57] |
| Ochratoxin α open lactone | OP-OTα | 274 | OH | Cl | H | H | - | OH | [57] |
| Ochratoxin β open lactone | OP-OTβ | 241 | OH | H | H | H | - | OH | [57] |
| Ochratoxin A quinone | OTQ | 383 | Phe | O | H | H | H | O | [58] |
| Ochratoxin A hydroquinone | OTHQ | 385 | Phe | OH | H | H | H | OH | [58] |
| OTHQ decarboxylated | DC-OTHQ | 366 | Decarboxylated Phe | OH | H | H | H | OH | [43] |
| Conjugate Ochratoxin A quinone–glutathion | OTQ-Glutathion | 689 | Phe | O | H | H | H | O | [59] |
| Conjugate Ochratoxin A–acyl hexose | Acyl-hexose-OTA | 565 | Phe acyl hexose | Cl | H | H | H | OH | [60] |
| Conjugate Ochratoxin A–acyl pentose | Acyl-pentose OTA | 535 | Phe acyl pentose | Cl | H | H | H | OH | [60] |
| Ochratoxin A methyl ester | OTA-Me | 417 | Phe methyl ester | Cl | H | H | H | OH | [57] |
| Ochratoxin B methyl ester | OTB-Me | 384 | Phe methyl ester | H | H | H | H | OH | [57] |
| Ochratoxin B ethyl ester | OTB-Et | 398 | Phe ethyl ester | H | H | H | H | OH | [57] |
| 4R-hydroxy Ochratoxin A methyl ester | 4R-OHOA-Me | 433 | Phe methyl ester | Cl | H | OH | H | OH | [57] |
| 10-hydroxy Ochratoxin A methyl ester | 10-OHOA-Me | 433 | Phe methyl ester | Cl | H | H | OH | OH | [57] |
| Ethylamide Ochratoxin A | OE-OA | 430 | Phe ethyl amide | Cl | H | H | H | OH | [61] |
| Ochratoxin A decarboxylated | DC-OA | 359 | Phe decarboxylated | Cl | H | H | H | OH | [61] |
| Ochratoxin A O-methyl | OM-OA | 417 | Phe | Cl | H | H | H | OCH3 | [61] |
| d-Ochratoxin A | d-OA | 403 | d-Phe | Cl | H | H | H | OH | [61] |
| Ochratoxin α ester methyl | M-Oα | 270 | OCH3 | Cl | H | H | H | OH | [61] |
| Tyrosine Ochratoxin A | OTA-Tyrosine | 419 | Tyrosine | Cl | H | H | H | OH | [62] |
| Method | Year | Biological Material | Limit of Detection (LOD) | References |
|---|---|---|---|---|
| TLC | 1973 | barley | 12 ng/g | [68] |
| TLC | 1973 | other commodities | 3–5 ng/g | [69] |
| spectrophotometry | 1976 | barley, pigs kidney, human blood (confirmation by carboxypeptidase A) | 1–4 ng/g | [70] |
| HPLC-UVD | 1979 | cereals | 1–5 ng/g | [71] |
| HPLC-FLD | 1980 | food and feed | 5 ng/g | [72] |
| HPLC-FLD | 1980 | (confirmation by boron trifuoride methanol) | [73] | |
| HPLC-FLD | 1981 | feed | 1 ng/g | [74] |
| RIA | 1975 | - | 20 ng/g | [75] |
| ELISA | 1981 | food, feed, biological fluids | 25 pg/assay | [76] |
| LC-MS | 1987 | barley | 0.5 ng/g | [77] |
| ion–pair HPLC | 1991 | human plasma | 0.02 ng/mL | [78] |
| GC-MS | 1992 | food | <0.1 ng/g | [79] |
| HPLC-FLD | 1992 | corn, barley, kidney | 0.2 | [80] |
| ELISA | 1993 | human sera | 10 pg/mL | [81] |
| IAC coupled with Fluorometer | 1997 | liquid food matrices | pg/mL | [82] |
| LC-ESI-MS/MS | 1998 | food (coffee) | 20 pg/on column | [83] |
| LC-ESI-MS/MS | 1999 | pig kidney, rye flour | 0.02 ng/g | [84] |
| HPLC-FLD Confirmation carboxypeptidase | 2003 | Blood, urine | 0.1 ng/mL (blood); 4 ng/mL (urine) | [85] |
| HPLC-FLD Confirmations with carboxypeptidase + LC-MS/MS | 2004 | Breakfast cereal | 0.05 ng/g | [86] |
| PFIA | 2004 | barley | 3 ng/mL | [87] |
| DNA aptamer | 2008 | wheat | 2 ng/g | [88] |
| LC-MS/MS | 2010 | urine | 0.001–0.045 ng/mL | [89] |
| ICP-MS | 2010 | wine | 0.003 ng/mL | [90] |
| LC-MS/MS | 2012 | urine | OTA: 0.03 ng/mL | [91] |
| flow electrochemical aptasensor with aptamer | 2013 | beer | 0.05 ng/mL | [92] |
| UHPLC-FLR (LC-ESI-MS/MS) | 2014 | ginger | OTA: 0.1 ng/g; (0.005–0.2 ng/g) | [93] |
| LC-MS/MS | 2015 | dried blood spots | 0.2 pg/on column | [94] |
| ELISA | 2012 | - | 1.2 ng/g | [95] |
| Metal enhanced fluorescence | 2014 | Food/drinks (milk, juice) | 0.5 µg/kg | [96] |
| Electroluminescence/Biosensor | 2015 | corn | 0.02 pg/mL | [97] |
| Molecular imprinting | 2015 | Beer/wine | 1.7 µg/L | [98] |
| PCR | 2015 | wine | 19 nM | [99] |
| Date of Case | Country | Foodstuffs | OTA (ng/g) |
|---|---|---|---|
| 16/01/2015 | Finland | Pumpkin seeds from China | 19 |
| 22/01/2015 | Germany | Dried figs from Spain | 124 |
| 03/03/2015 | Belgium | Wheat from Canada | 17 |
| 13/03/2015 | Netherlands | Pumpkin seeds from China | 29 |
| 13/03/2015 | France | Dried figs from Spain | 183 |
| 24/03/2015 | France | Wheat from Canada | 18 |
| 27/03/2015 | Switzerland | Ground mace from Sri Lanka | 42.5 |
| 12/05/2015 | France | Buckwheat flour from France | 40 |
| 04/06/2015 | Ireland | Liquorice root from Turkey | 433.5 |
| 10/06/2015 | Poland | Raisins from Turkey | 19.3 |
| 15/07/2015 | Slovak Republic | Raisins from Chile | 11.8 |
| 10/08/2015 | France | Rye flour from France | 12.9 |
| 12/08/2015 | Finland | Pumpkin seeds from China | 20000 |
| 13/08/2015 | Luxembourg | Dried red chili peppers from Thailand | 30.8 |
| 01/09/2015 | Romania | Sultanas from Turkey | 15.6 |
| 02/09/2015 | Belgium | Rye malt from France | 13.8 |
| 02/09/2015 | Belgium | Rye malt from France | 25.7 |
| 02/09/2015 | Belgium | Rye malt from France | 38.6 |
| 25/09/2015 | Croatia | Black pepper from Vietnam | 155 |
| 21/10/2015 | Malta | Soft oaty bars from Switzerland | 1.4 |
| 02/12/2015 | Belgium | Dried figs from Turkey | 14.4 |
| 08/12/2015 | Latvia | Chili from China | 40 |
| 11/12/2015 | Cyprus | Dried sultana raisins from Greece | 18.5 |
| 23/12/2015 | Belgium | Dried figs from Turkey | 27.8 |
| Date of Case | Country | Foodstuffs | OTA (ng/g) |
|---|---|---|---|
| 22/01/2015 | Poland | Raisins from Uzbekistan | 21.1 |
| 26/01/2015 | Netherlands | Dried figs from Turkey | 24 |
| 11/02/2015 | Germany | Raisins from Afghanistan | 11.8 |
| 19/02/2015 | Latvia | Raisins from Afghanistan | 61 |
| 26/02/2015 | Germany | Dried figs from Turkey | 17.4 |
| 13/03/2015 | Hungary | Raisins from Uzbekistan | 24.3 |
| 30/06/2015 | Croatia | Mixed spices from Kuwait | 45 |
| 21/07/2015 | United Kingdom | Red pepper powder from Ethiopia | 92.5 |
| 13/08/2015 | The Netherlands | Pistachios from the United States | 74 |
| 31/08/2015 | Germany | Berbere spice mix from Ethiopia | 85.3 |
| 07/09/2015 | The Netherlands | Red chili powder from India | 69 |
| 28/10/2015 | Poland | Red chili powder from India | 32.6 |
| 16/12/2015 | Germany | Red pepper spice mix from Ethiopia | 69.9 |
| Date of Case | Country | Foodstuffs | OTA (ng/g) |
|---|---|---|---|
| 13/01/2015 | Germany | Dried figs from Turkey | 69.9 |
| 16/01/2015 | Germany | Dried figs from Turkey | 45 |
| 16/02/2015 | Germany | Sun dried figs from Turkey | 86 |
| 17/02/2015 | Germany | Dried figs from Turkey | 32 |
| 02/06/2015 | Germany | Spice mix and paprika from Ethiopia | 139 |
| 24/07/2015 | Denmark | Organic raisins from Australia | 28 |
| 23/12/2015 | Germany | Dried figs from Turkey | 10.8 |
| Year | Nephrotoxicity Testing | References |
|---|---|---|
| 1972 | Balkan endemic nephropathy (BEN) has been suggested to be the result of fungal poisoning. The role of OTA in mycotoxicosis—BEN in humans and porcine nephropathy. | [156] |
| 1972 | In view of the similarities between BEN and OTA induced porcine nephropathy, it has been suggested that OTA may be involved in the etiology of BEN. | [157] |
| 1978 | OTA is potentially nephrotoxic in all species tested with the exception of adult ruminants. | [158] |
| 1987 | Findings of higher OTA levels in the serum of patients suffering from BEN, which is a subtype of tubulointerstitial nephritis, led to hypotheses about the association between the nephrotoxicity of OTA and the BEN and also the incidence of renal system tumors in the population of these Balkan regions. | [159] |
| 1991 | Nephropathy is primarily related to the mobilization of intracellular calcium. | [160] |
| 1992 | In terms of human pathologies, OTA is suspected to be the main etiological agent responsible for BEN and associated urinary tract tumors (UTT) in humans. | [161] |
| 1993 | Experimental studies on the nephrotoxicity of OTA both in vitro and in vivo have shown that OTA disturbs the intracellular metabolic processes (with subsequent apoptosis of the renal cells), renal hemodynamics, and—significantly and perhaps preponderantly—the functions of the proximal tubules (even after subchronic exposition). OTA causes the decrease of glomerular filtration and tubular resorption and affects all parts of the nephron and kidneys in toto. | [162,163,164,165,166,167,168] |
| 1993 | A case of acute nephrotoxicity in humans. | [169,170] |
| 1999 | OTA induces apoptosis in cultured human proximal tubule cells. | [171] |
| 2002–2005 | The kidney is the main target of OTA toxicity in all animal species tested. | [14,172] |
| 2002–2005 | OTA has been also implicated in the etiology of BEN, a chronic degenerative kidney disease, in kidney tumors in humans in certain regions of the Balkan Peninsula, and in chronic interstitial nephropathy (CIN) in Tunisia and other North African countries. | [14,148,150] |
| 2005 | Exposure to low OTA doses is responsible for nephrotoxicity; at nanomolar concentrations, OTA leads to specific changes of function and phenotype in renal cells. | [173] |
| 2007–2010 | Very low OTA concentrations administered for a prolonged time (up to 14 days) influence the cellular fate (cellular hypertrophy) in human proximal tubule; furthermore, they act not only in the target organ, e.g., in the kidney, but also in as yet unsuspected cells, such as fibroblasts; the same damage will likely occur in chronic exposure. | [174,175] |
| 2013 | Nephrotoxicity is a consequence of acute, sub-acute, and also chronic exposure to OTA. | [9] |
| 2014 | OTA inhibits the nuclear factor, erythroid 2-like 2 (Nrf2) oxidative stress response pathway. Nrf2 overexpression confers a survival advantage and is often associated with cancer cell survival. | [176] |
| 2015 | Dietary exposure to OTA represents a serious health issue including, e.g., human endemic nephropathies. | [50] |
| Year | Nephrotoxicity testing | References |
|---|---|---|
| 1978 | OTA induces renal and hepatic tumors in mice. | [177] |
| 1984 | OTA is carcinogenic for mice. | [178] |
| 1984 | CIT increases OTA carcinogenicity. | [179] |
| 1987 | OTA carcinogenicity to humans: OTA classified in Group 3 (not classifiable as to its carcinogenicity to humans). | [180] |
| 1989 | Male rats are more susceptible to renal tumors than female rats (NTP study). | [181] |
| 1989 | The genotoxicity of ochratoxin A is reviewed. | [35,182] |
| 1991 | OTA-DNA adducts: For the first time, OTA-DNA adducts are found in the kidney, liver, and spleen of mice. | [183] |
| 1993 | OTA is re-classified as a possibly carcinogenic to humans based on a great amount of evidence of carcinogenity in several animal studies of 2B in 1993. | [11] |
| 1993 | OTA-DNA adducts: Other studies take place in mice and rat tissues after acute and subchronic exposure, and in urinary tract tumors (UTT) of Bulgarian subjects. | [184,185,186] |
| 1993-2009 | OTA-DNA adducts are also detected in tissues of humans presumably exposed to OTA in several countries (Bulgaria, Serbia, Croatia, Germany, Belgium, France, Tunisia). | [16,17,185,187,188,189,190] |
| 1998-2002 | DNA adduction following chronic exposure (carcinogenic study) of rats to OTA first described; sex differences and dual mechanism—oxidative pathways and DNA adduction—are observed | [12,13,191] |
| 1998 | OTA-DNA adducts are observed in mother and progeny of mice fed OTA nine months after birth male mice develop cancer. | [192] |
| 2000–2001 | In vitro formation of dG-OTA adduct. | [193,194] |
| 2001–2002 | Other studies with radiolabeled OTA were unable to detect any DNA binding of OTA, but explanation of this discrepancy is given in depth by Pfohl-Leszkowicz and Castegnaro in 2005 [ 195] | [60,196] |
| 2003 | OTA-DNA adduct in pigs subchronically exposed to low doses of OTA. Relation with biotransformation. | [197] |
| 2002–2010 | OTA may be involved in testicular cancer. | [175,198,199,200,201] |
| 2003–2008 | CIT increases genotoxicity of OTA and modifies the metabolism of rats exposed to low doses for three weeks. | [202,203] |
| 2004 | Evidence for covalent DNA adduction by OTA following chronic exposure to OTA in rats (and subacute exposure in pigs). | [190] |
| 2004 | Another research group, using the highly sensitive accelerator of the mass spectrometry technique, does not detect DNA adducts after the administration of 14C-labeled OTA to rats. | [204] |
| 2004 | In 2004, a review of the NTP experimental rat tumor data for OTA also places OTA in the category of “chemicals inducing renal tumors through direct interaction of the parent compound or metabolite with renal DNA” based on histopathological evidence. | [205] |
| 2004–2010 | The long-term OTA studies confirm the incidence of tumors in rats; in male rats, these tumors are related to OTA dose | [205,206,207] |
| 2004–2012 | OTA is a direct genotoxic forming covalent DNA adducts in the kidney OTA can indeed react with DNA via a phenolic radical resulting in C8-deoxyguanosine adduct (synthetized and chemical identified by mass spectrum). | [175,190,201,207,208,209] |
| 2006 | Confirmation of OTA genotoxicity via measurement of comet in rat kidneys. | [210] |
| 2007 | Chronic exposure to low OTA doses can be much more damaging than acute exposure to a high dose. | [16] |
| 2007 | DNA diploidy in rat tumors is associated to genetic damage. | [211] |
| 2007 | OTA induces an increase of mutation at two loci—hypoxantine-guanine phophoribosyl transferase (HPRT) and thymidine kinase (TK). | [212] |
| 2008 | DNA adduct cannot be confirmed, but the explanation is given by Pfohl-Leszkowicz et al. (2009) [64] | [213] |
| 2008 | Correlation between biotransformation of OTA and direct covalent binding on DNA. | [214] |
| 2009 | It is found that the kidney DNA adduct pattern of BEN patients is similar to the kidney DNA adduct pattern of pigs living in the same farm and pigs co-exposed to OTA, fumonisins, and citrinin. | [17] |
| 2009 | A different proposal of mechanism for OTA-mediated renal carcinogenesis and threshold model for its risk assessment. | [215] |
| 2009–2010 | Identification by LC-MS/MS of these DNA adduct in rat tissues. | [64,201] |
| 2010 | OTA is carcinogenic for poultry. | [216] |
| 2011 | Induction of mutation only in medulla of rat kidney exposed to carcinogenic dose. | [217] |
| 2012 | Relation structure activity studies clearly indicate that OTHQ (ochratoxin hydroxyquinone) is responsible of direct genotoxicity, whereas some others are cytotoxic. | [65,209] |
| 2012 | OTA is activated to a species that is a directly genotoxic mutagen. OTHQ in presence of cysteine is also mutagenic. | [218] |
| A new approach to cancer represents miRNA. | [219,220] | |
| 2013 | The induction of miR-132 and miR-200c by OTA elevates reactive oxygen species (ROS) levels and profibrotic (profibrotic transforming growth factors β, TGFβ) expression. | [221] |
| 2014 | OTA has the potential to initiate or support the development of fibrotic kidney diseases by involving post-transcriptional regulation mechanisms comprising miR-29b. OTA reduces the impact of miR-29b and thus enhances collagen protein expression. | [222] |
| 2014 | A low dose of OTA induces micronuclei, and OTA delays the DNA repair kinetics. | [223] |
| 2014 | OTA increases proliferating cell nuclear antigen after 13 weeks in kidney and kidney damages. Limited oxidative stress. | [224] |
| 2015 | Dietary exposure to OTA represents a serious health issue, including urinary tract tumors in humans. | [50] |
| Country | Collecting Period | n+ (%) | OTA min–max (μg/L) | OTA Mean (μg/L) | Reference |
|---|---|---|---|---|---|
| Europe | |||||
| Former Yugoslavia | 1980 | 7.8 | max. 8.0 | 5.4 | [229,241] |
| Germany | 1977–1985 | 56.5 | 0.1–14.4 | 0.6 | [242] |
| Bulgaria | 1984,1986, 1989–1990 | 10 | - | 12.0 | [243,244] |
| Poland | 1983–1985 | 7.2 | 1–40 | 0.28 | [245] |
| Former Yugoslavia | 1981–1989 | 0-3.7 | max. 50.0 | - | [246] |
| Germany | 1988 | 68 | 0.1–8.4 | 0.75 | [247] |
| Sweden | 1989 | 12.8 | 0.3–7.0 | 0.20 | [78] |
| Czechoslovakia | 1990 | 21 | 0.5–12.0 | 0.37 | [248] |
| Denmark | 1990 | 54.2 | 0.1–13.2 | 1.8 | [241] |
| France | - | - | 0.1–6.0 (rural); 0.1–1.3 (urban) | - | [249] |
| Czechoslovakia | 1990–1991 | 40 | 0.5–19.4 | 0.63 | [250] |
| France | 1991–1992 | 18.1 | 0.1-161 | 0.4 | [251,252] |
| Italy | 1992 | 100 | 0.1–2.0 | 0.53 | [253] |
| Switzerland | 1992–1993 | 100 | 0.06–6.02 | ca. 0.4 | [105] |
| Hungary | 1995 | 51 | 0.2–12.9 | - | [254] |
| Italy | 1994–1996 | 97 | 0.1–57.2 | 0.56 | [255] |
| Hungary | 1995 | 82 | 0.2–10.0 | - | [256] |
| Czech Republic | 1994–2002 | 94.2 | 0.1–13.7 | 0.24 | [257,258,259,260] |
| Spain | 1996–1998 | 53.3 | 0.5–4.0 | 0.71 | [261] |
| Spain | 1996–1997 | 72 | 0.21–6.96 | 0.63 | [262] |
| Hungary | 1997 | 77 | 0.1–1.4 | - | [263] |
| Croatia | 1997–1998 | 59.4 | max. 15.9 | 0.30 | [264,265,266] |
| Sweden | 1997 | 100 | 0.01–0.48 | 0.21 | [145,267] |
| Norway | 1998 | 100 | 0.05–0.42 | 0.18 | [145,267] |
| Germany | 1999 | 98.1 | 0.06–2.03 | 0.27 | [268] |
| UK | 2000 | 100 | 0.4–3.11 | 1.09 | [145,269] |
| Norway | - | - | 0.02–5.53 | 0.40 | [270] |
| Bulgaria | - | 100 | max. 8.4 | 1.59 | [85] |
| Portugal | 2001–2002 | 100 | 0.14–2.49 | - | [271] |
| Poland | 2005 | 100 | 0.1–0.4 | 0.37 | [272] |
| Germany | 2005–2006 | 100 | 0.05–0.75 | 0.75 | [18] |
| Czech Republic | 2005 | 83.7 | 0.1–2.3 | 0.21 | [273] |
| Spain | 2008 | 100 | 0.15–5.71 | 1.09 | [274] |
| Spain | 2008 | 98.6 | 0.11–8.68 | 0.86 | [275] |
| Germany | 2008 | 100 | 0.19–0.29 | 0.25 | [276] |
| Spain | - | 100 | 0.06–10.92 | 0.8 | [277] |
| Italy | - | 99.1 | 0.03–2.92 | 0.23 | [278] |
| Czech Republic | 2012 | 96 | 0.1–0.35 | 0.15 | [279] |
| Czech Republic | 2012 | - | 0.37–1.13 | 0.17 | [280] |
| Africa | |||||
| Algeria | - | 66.9 | max. 9.0 | 2.8 | [281] |
| Tunisia | - | 62 | max. 3.2 | 1.22 | [149] |
| - | 66 | max. 2.3 | 1.1 | [282] | |
| Egypt | - | 2.9 | max. 0.91 | 0.08 | [151] |
| Sierra Leone | 1996 | 33 | max. 18.2 | - | [283] |
| Morocco | 2000 | 60 | 0.08–6.59 | 0.2 | [284] |
| 1991–2000 | 62-82 | 0.1–5.5 | 2.0 | [285] | |
| 1996, 1998 | 100 | 0.1–8.06 | 0.53 | [150] | |
| - | 71 | max. 7.5 | 2.6 | [286] | |
| Ivory Coast | 2001, 2004 | 34.9 | max. 11.62 | 0.58 | [287] |
| Tunisia | - | 28 | 0.12–3.4 | 0.49 | [288] |
| Tunisia | - | 52.3 | 0.11–6.1 | 0.77 | [289] |
| Tunisia | 2007–2009 | 49 | 1.7–8.5 | 3.3 | [290] |
| Tunisia | - | 34 | 0.12–1.5 | 0.22 | [291] |
| Asia | |||||
| Japan | 1992-1996 | 85 | max. 0.28 | 0.07 | [292] |
| Lebanon | 2001-2002 | 33 | max. 1.24 | 0.31 | [293] |
| Pakistan | - | 97 | max. 1.24 | 0.31 | [294] |
| Turkey | - | - | max. 1.43 | 0.44 | [295] |
| Turkey | 2008–winter | 76.7 | 0.03–0.89 | 0.14 | |
| 2007–summer | 97.5 | 0.03–1.50 | 0.31 | [296] | |
| Bangladesh | - | 100 | 0.2–6.63 | 0.85 | [240] |
| Turkey | –summer | 100 | 0.03–1.55 | 0.31 | |
| –winter | 83.3 | 0.05–1.12 | 0.5 | [297] | |
| The Americas | |||||
| Canada | 1991 | 38.3 | max. 9.0 | 1.29 | [298] |
| Canada | 1994* | 100 | max. 2.37 | 0.88 | [299] |
| Chile | 2004 | 54 | 0.4–2.75 | 0.44 | |
| (2 regions) | 91 | 0.4–2.12 | 0.77 | [300] | |
| Costa Rica | - | 95 | max. 1.91 | 0.62 | [301] |
| Argentina | 2004–2005 | 63.8 | 0.19–47.6 | 0.15 | |
| (2 regions) | 0.19–74.8 | 0.43 | [302] |
| Country | n | n+ % | Mean (ng/L) | Reference |
|---|---|---|---|---|
| Croatia | 35 | 94 | 239.0 | [311] |
| Hungary | 88 | 61 | 13.0 | [312] |
| Portugal | 60 | 70 | 27.0 | [313] |
| Portugal | 30 | 43 | 19.0 | [314] |
| Portugal | 43 | 72.1 | 26.0 | [315] |
| Croatia | 45 | 43 | 17.0 | [316] |
| Croatia | 45 | 18 | 7.0 | [316] |
| Portugal | 155 | 92 | 18.0 | [317] |
| Turkey | 233 | 90 | 14.3 * | [318] |
| Germany | 13 | 100 | 70.0 | [276] |
| South Korea | 12 | 100 | 31.0 | [89] |
| Spain | 72 | 12.5 | 237.0 | [319] |
| Spain | 27 | no stated | - | [320] |
| Italy | 10 | 100 | - | [321] |
| Sri Lanka | 31 | 93.5 | 20.0 ** | [322] |
| Portugal | 95 | 87.4 | 22.0 (winter) | [323] |
| Portugal | 95 | 81.1 | 16.0 (summer) | [323] |
| Croatia | 40 | 78.0 | 90.0 (before enzyme treatment) | [324] |
| Croatia | 40 | 58.0 | 130.0 after enzyme treatment) | [324] |
| Cameroon | 175 | 63 | 280.0 | [308] |
| Cameroon | 145: HIV positive | 17 | 80.0 | [325] |
| 30: HIV: sero-negative | 10 | 60.0 | ||
| South Africa | 53 | 98 | 41.0 | [326] |
| Cameroon | 220 | 32 | 200.0 | [309] |
| Italy | 52 | 100 | 144.0 | [327] |
| Chile | 39 | 30–433 *** 30–124 **** | [239] | |
| Portugal | 472 | 86.4 | 19.0 ***** | [328] |
| Germany | 30 | 15 | 40.0 | [329] |
| Haiti | 47 | 33 | 109.0 | [329] |
| Bangladesh | 72 | 76 | 203.0 | [329] |
| Country | n | n+ (%) | Range Positive Samples (ng/L) | References |
|---|---|---|---|---|
| European countries | ||||
| Germany | 36 | 11 | 17–30 | [330] |
| Italy | 50 | 18 | 1,200–6,600 | [332] |
| Sweden | 40 | 58 | 10–40 | [331] |
| Hungary | 92 | 41 | 200–7,200 | [255] |
| Switzerland | 40 | 10 | 5–14 | [105] |
| Italy | 111 | 20 | 100–12,000 | [334] |
| Italy | 4 | 75 | 8-540 | [335] |
| Norway | 115 | 33 | 10–130 | [336] |
| Norway | 80 | 21 | 10–182 | [333] |
| Italy | 231 | 86 | 10–57 | [337] |
| Poland | 13 | 38 | 6–17 | [338] |
| Italy | 82 | 74 | 5–405 | [339] |
| Slovakia | 76 | 30 | 2–60 | [340] |
| Italy | 57 | 78.9 | 1–75 | [341] |
| Germany | 90 | 60 | 10–100 | [342] |
| Africa | ||||
| Sierra Leone | 113 | 35 | 200–337,000 | [343] |
| Egypt | 120 | 36 | 5,000–45,000 | [344] |
| Egypt | 50 | 72 | 1,890 ± 980 * | [345] |
| Australia | 100 | 2 | 3,000–3,600 | [346] |
| Asia | ||||
| Turkey | 75 | 100 | 620–13,111 | [347] |
| Iran | 136 | 2.7 | 90–140 | [348] |
| Iran | 87 | 84 | 1.6–60 | [349] |
| The Americas | ||||
| Brazil | 50 | 4 | 10–20 | [350] |
| Chile | 11 | 100 | 44–184 | [351] |
| Brazil | 224 | 0 | [352] | |
| Chile | 50 | 80 | 10–186 | [239] |
| Brazil | 100 | 66 | 0.3–21 | [353] |
| Foodstuffs | Maximum levels (ng/g) |
|---|---|
| Cereals (including rice and buckwheat) and derived cereal products | 5 |
| Raw cereal grains (including raw rice and buckwheat) | 5 |
| All products derived from cereals (including processed cereal products and cereal grains intended for direct human consumption) | 3 |
| Dried vine fruit (currants, raisins and sultanas) | 10 |
| Green and roasted coffee and coffee products, wine, beer, grape juice, cocoa and cocoa products, and spices | - |
| Code | Foodstuffs | Maximum Levels (ng/g) |
|---|---|---|
| 2.2.1 | Unprocessed cereals | 5.0 |
| 2.2.2. | All products derived from unprocessed cereals, including processed cereal products and cereals intended for direct human consumption with the exception of foodstuffs listed in 2.2.9, 2.2.10, and 2.2.13 | 3.0 |
| 2.2.3 | Dried vine fruit (currants, raisins, and sultanas) | 10.0 |
| 2.2.4 | Roasted coffee beans and ground roasted coffee, excluding soluble coffee | 5.0 |
| 2.2.5 | Soluble coffee (instant coffee) | 10.0 |
| 2.2.6 | Wine (including sparkling wine, excluding liqueur wine and wine with an alcoholic strength of not less than 15 vol %) and fruit wine | 2.0 |
| 2.2.7 | Aromatized wine, aromatized wine-based drinks, and aromatized wine-product cocktails | 2.0 |
| 2.2.8 | Grape juice, concentrated grape juice as reconstituted, grape nectar, grape must and concentrated grape must as reconstituted, intended for direct human consumption | 2.0 |
| 2.2.9 | Processed cereal-based foods and baby foods for infants and young children | 0.50 |
| 2.2.10 | Dietary foods for special medical purposes intended specifically for infants | 0.50 |
| 2.2.11. | Spices, including dried spices | |
| Piper spp. (fruits thereof, including white and black pepper), Myristica fragrans (nutmeg), Zingiber officinale (ginger), Curcuma longa (turmeric) | 15 | |
| Capsicum spp. (dried fruits thereof, whole or ground, including chilies, chili powder, cayenne, and paprika) | 20 | |
| Mixtures of spices containing one of the abovementioned spices | 15 | |
| 2.2.12. | Liquorice (Glycyrrhiza glabra, Glycyrrhiza inflate and other species) | |
| 2.2.12.1. | Liquorice root, ingredient for herbal infusion | 20 |
| 2.2.12.2. | Liquorice extract for use in food in particular beverages and confectionary | 80 |
| 2.2.13. | Wheat gluten not sold directly to the consumer | 8.0 |
| Feed | Guidance Value in mg/kg Relative to Feedstuffs with a Moisture Content of 12% |
|---|---|
| Feed materials *—Cereals and cereal products ** | 0.25 |
| Complementary and complete feedstuffs | |
| —Complementary and complete feedstuffs for pigs | 0.05 |
| —Complementary and complete feedstuffs for poultry | 0.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. https://doi.org/10.3390/toxins8070191
Malir F, Ostry V, Pfohl-Leszkowicz A, Malir J, Toman J. Ochratoxin A: 50 Years of Research. Toxins. 2016; 8(7):191. https://doi.org/10.3390/toxins8070191
Chicago/Turabian StyleMalir, Frantisek, Vladimir Ostry, Annie Pfohl-Leszkowicz, Jan Malir, and Jakub Toman. 2016. "Ochratoxin A: 50 Years of Research" Toxins 8, no. 7: 191. https://doi.org/10.3390/toxins8070191
APA StyleMalir, F., Ostry, V., Pfohl-Leszkowicz, A., Malir, J., & Toman, J. (2016). Ochratoxin A: 50 Years of Research. Toxins, 8(7), 191. https://doi.org/10.3390/toxins8070191