A P-Glycoprotein Is Linked to Resistance to the Bacillus thuringiensis Cry3Aa Toxin in a Leaf Beetle
Abstract
:1. Introduction
2. Results
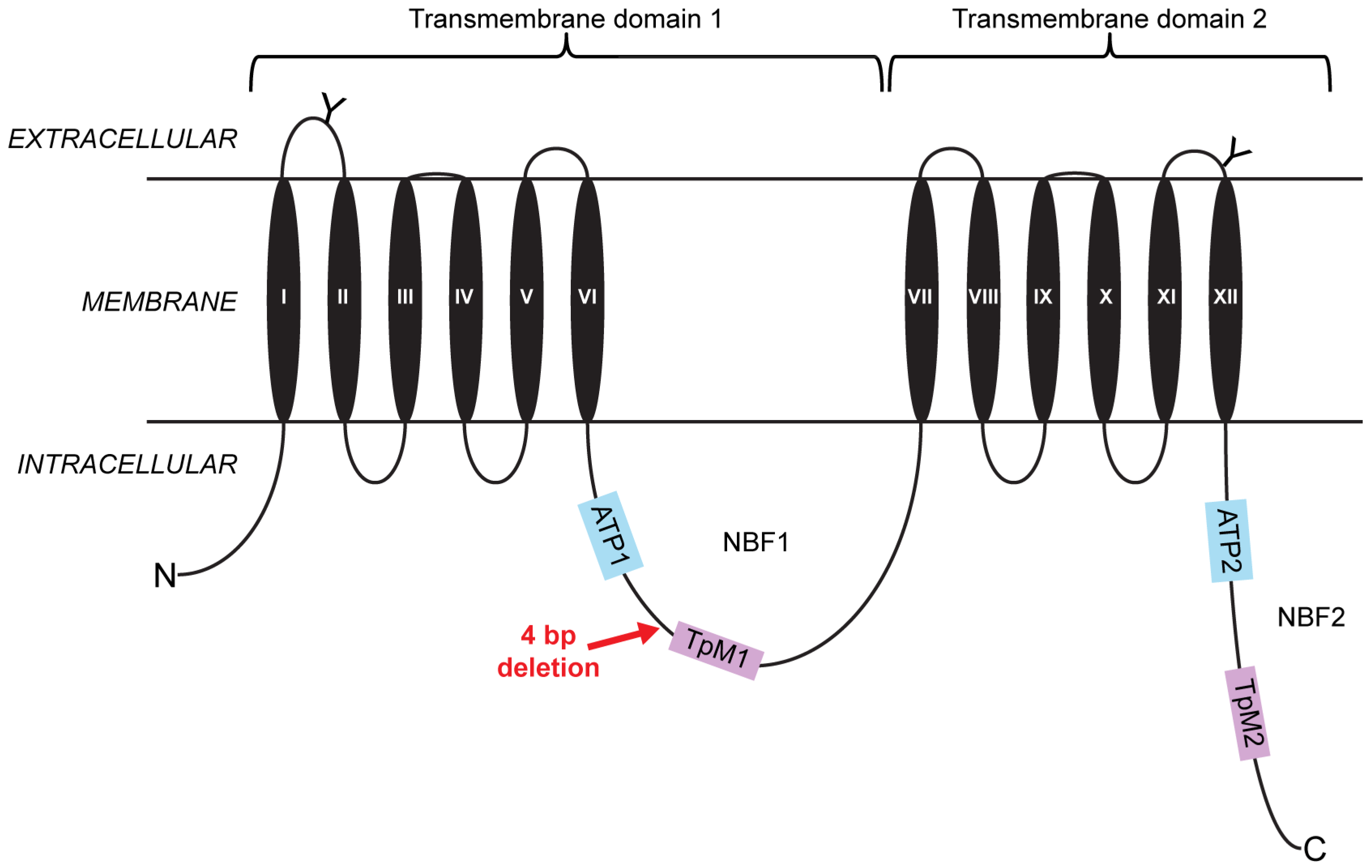
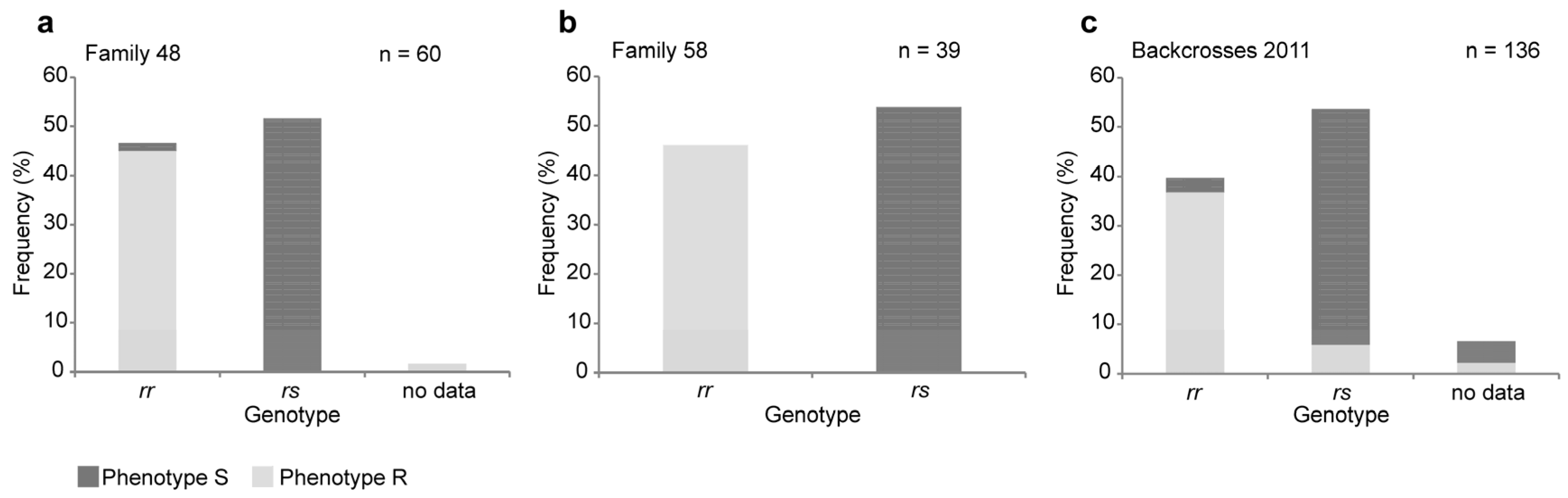
2.1. A Four-Base-Pair Deletion in CtABCB1 Is Genetically Linked to Cry3Aa Resistance
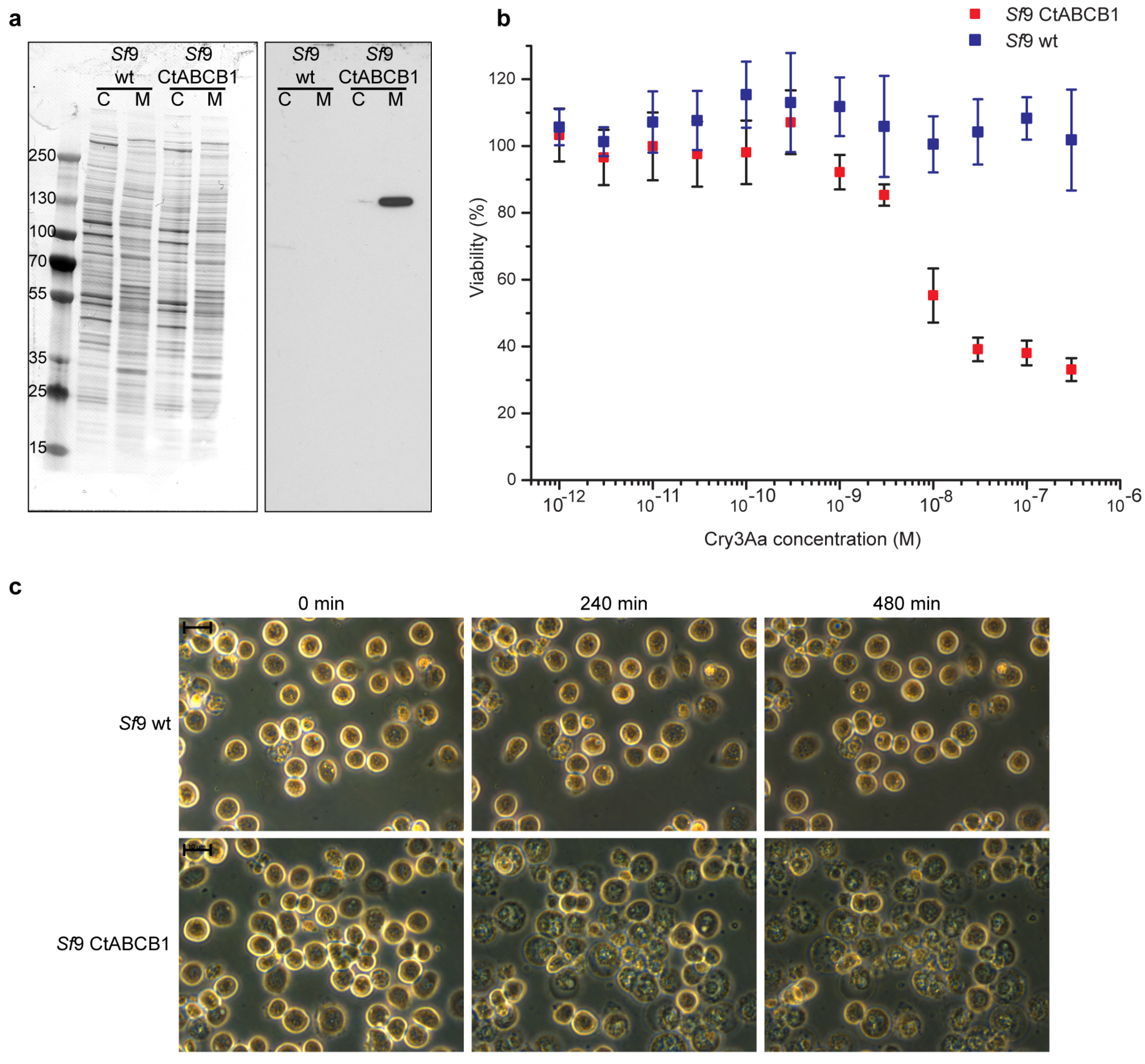
2.2. Lepidopteran Insect Cells Expressing CtABCB1 Are Susceptible to Cry3Aa
3. Discussion
4. Materials and Methods
4.1. Insect Rearing and Genetic Crosses
4.2. Genotyping of the Crosses
4.3. Expression of CtABCB1 in Sf9 Cells
4.4. Western Blotting
4.5. Toxin Preparation, Viability Assays and Morphological Changes
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.M.; Zhao, J.-Z.; Roush, R.T. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 2002, 47, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.; Petzold-Maxwell, J.L.; Keweshan, R.S.; Dunbar, M.W. Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Clifton, E.H.; Dunbar, M.W.; Hoffmann, A.M.; Ingber, D.A.; Keweshan, R.S. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proc. Natl. Acad. Sci. USA 2014, 111, 5141–5146. [Google Scholar] [CrossRef] [PubMed]
- Génissel, A.; Augustin, S.; Courtin, C.; Pilate, G.; Lorme, P.; Bourguet, D. Initial frequency of alleles conferring resistance to Bacillus thuringiensis poplar in a field population of Chrysomela tremulae. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Courtin, C.; Rejasse, A.; Lorme, P.; Genissel, A.; Bourguet, D. Genetics of resistance to transgenic Bacillus thuringiensis poplars in Chrysomela tremulae (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2004, 97, 1058–1064. [Google Scholar] [CrossRef]
- Pauchet, Y.; Wilkinson, P.; van Munster, M.; Augustin, S.; Pauron, D.; ffrench-Constant, R.H. Pyrosequencing of the midgut transcriptome of the poplar leaf beetle Chrysomela tremulae reveals new gene families in Coleoptera. Insect Biochem. Mol. Biol. 2009, 39, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Adang, M.J.; Crickmore, N.; Jurat-Fuentes, J.L. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. In Advances in Insect Physiology; Dhadialla, T.S., Gill, S.S., Eds.; Academic Press: Oxford, UK, 2014; Volume 47, pp. 39–87. [Google Scholar]
- Vachon, V.; Laprade, R.; Schwartz, J.L. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: A critical review. J. Invertebr. Pathol. 2012, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Munster, M.; le Gleuher, M.; Pauchet, Y.; Augustin, S.; Courtin, C.; Amichot, M.; Ffrench-Constant, R.H.; Pauron, D. Molecular characterization of three genes encoding aminopeptidases n in the poplar leaf beetle Chrysomela tremulae. Insect Mol. Biol. 2011, 20, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.W.; Badenes-Pérez, F.R.; Morrison, A.; Vogel, H.; Crickmore, N.; Kain, W.; Wang, P.; Heckel, D.G.; Jiggins, C.D. Parallel evolution of Bacillus thuringiensis toxin resistance in Lepidoptera. Genetics 2011, 189, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Gahan, L.J.; Pauchet, Y.; Vogel, H.; Heckel, D.G. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Mahon, R.J.; Heckel, D.G.; Walsh, T.K.; Downes, S.; James, W.; Lee, S.-F.; Reineke, A.; Williams, A.K.; Gordon, K.H.J. Insect resistance to Bacillus thuringiensis toxin Cry2Ab is conferred by mutations in an ABC transporter subfamily a protein. PLoS Genet. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, T.; Liu, C.; Heckel, D.G.; Li, X.; Tabashnik, B.E.; Wu, K. Mis-splicing of the abcc2 gene linked with Bt toxin resistance in Helicoverpa armigera. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Strauss, A.S.; Wang, D.; Stock, M.; Gretscher, R.R.; Groth, M.; Boland, W.; Burse, A. Tissue-specific transcript profiling for ABC transporters in the sequestering larvae of the phytophagous leaf beetle Chrysomela populi. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Flagel, L.E.; Swarup, S.; Chen, M.; Bauer, C.; Wanjugi, H.; Carroll, M.; Hill, P.; Tuscan, M.; Bansal, R.; Flannagan, R.; et al. Genetic markers for western corn rootworm resistance to Bt toxin. G3 2015, 5, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Jakka, S.R.K.; Shrestha, R.B.; Gassmann, A.J. Broad-spectrum resistance to Bacillus thuringiensis toxins by western corn rootworm (Diabrotica virgifera virgifera). Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, A.; Heckel, D.G.; Pauchet, Y. Three toxins, two receptors, one mechanism: Mode of action of Cry1A toxins from Bacillus thuringiensis in Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 76, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Miyamoto, K.; Noda, H.; Jurat-Fuentes, J.L.; Yoshizawa, Y.; Endo, H.; Sato, R. The ATP-binding cassette transporter subfamily C member 2 in Bombyx mori larvae is a functional receptor for Cry toxins from Bacillus thuringiensis. FEBS J. 2013, 280, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Fabrick, J.; Oppert, C.; Lorenzen, M.D.; Morris, K.; Oppert, B.; Jurat-Fuentes, J.L. A novel Tenebrio molitor cadherin is a functional receptor for Bacillus thuringiensis Cry3Aa toxin. J. Biol. Chem. 2009, 284, 18401–18410. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Park, Y.; Adang, M.J. Cadherin adCad1 in Alphitobius diaperinus larvae is a receptor of Cry3Bb toxin from Bacillus thuringiensis. Insect Biochem. Mol. Biol. 2014, 45, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Convents, D.; Van Damme, J.; Boets, A.; Van Rie, J.; Ellar, D.J. Intramolecular proteolytic cleavage of Bacillus thuringiensis Cry3A delta-endotoxin may facilitate its coleopteran toxicity. J. Invertebr. Pathol. 1997, 70, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Rausell, C.; Ochoa-Campuzano, C.; Martinez-Ramirez, A.C.; Bravo, A.; Real, M.D. A membrane associated metalloprotease cleaves Cry3Aa Bacillus thuringiensis toxin reducing pore formation in Colorado potato beetle brush border membrane vesicles. Biochim. Biophys. Acta 2007, 1768, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Whalon, M.E.; Miller, D.L.; Hollingworth, R.M.; Grafius, E.J.; Miller, J.R. Selection of a Colorado potato beetle (Coleoptera, Chrysomelidae) strain resistant to Bacillus thuringiensis. J. Econ. Entomol. 1993, 86, 226–233. [Google Scholar] [CrossRef]
- Wierenga, J.M.; Norris, D.L.; Whalon, M.E. Stage-specific mortality of Colorado potato beetle (Coleoptera: Chrysomelidae) feeding on transgenic potatoes. J. Econ. Entomol. 1996, 89, 1047–1052. [Google Scholar] [CrossRef]
- Federici, B.A.; Bauer, L.S. Cyt1Aa protein of Bacillus thuringiensis is toxic to the cottonwood leaf beetle, Chrysomela scripta, and suppresses high levels of resistance to Cry3Aa. Appl. Environ. Microbiol. 1998, 64, 4368–4371. [Google Scholar] [PubMed]
- Wenes, A.L.; Bourguet, D.; Andow, D.A.; Courtin, C.; Carre, G.; Lorme, P.; Sanchez, L.; Augustin, S. Frequency and fitness cost of resistance to Bacillus thuringiensis in Chrysomela tremulae (Coleoptera: Chrysomelidae). Heredity 2006, 97, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zukoff, S.N.; Ostlie, K.R.; Potter, B.; Meihls, L.N.; Zukoff, A.L.; French, L.; Ellersieck, M.R.; French, B.W.; Hibbard, B.E. Multiple assays indicate varying levels of cross resistance in Cry3Bb1-selected field populations of the western corn rootworm to mCry3A, eCry3.1Ab, and Cry34/35Ab1. J. Econ. Entomol. 2016, 109, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J. Resistance to Bt maize by western corn rootworm: Insights from the laboratory and the field. Curr. Opin. Insect Sci. 2016, 15, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Rausell, C.; García-Robles, I.; Sánchez, J.; Muñóz-Garay, C.; Martínez-Ramírez, A.C.; Real, M.D.; Bravo, A. Role of toxin activation on binding and pore formation activity of the Bacillus thuringiensis Cry3 toxins in membranes of Leptinotarsa decemlineata (Say). Biochim. Biophys. Acta 2004, 1660, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Campuzano, C.; Real, M.D.; Martínez-Ramírez, A.C.; Bravo, A.; Rausell, C. An ADAM metalloprotease is a Cry3Aa Bacillus thuringiensis toxin receptor. Biochem. Biophys. Res. Commun. 2007, 362, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Loseva, O.; Ibrahim, M.; Candas, M.; Koller, C.N.; Bauer, L.S.; Bulla, L.A. Changes in protease activity and Cry3Aa toxin binding in the Colorado potato beetle: Implications for insect resistance to Bacillus thuringiensis toxins. Insect Biochem. Mol. Biol. 2002, 32, 567–577. [Google Scholar] [CrossRef]
- Sayed, A.; Nekl, E.R.; Siqueira, H.A.; Wang, H.C.; Ffrench-Constant, R.H.; Bagley, M.; Siegfried, B.D. A novel cadherin-like gene from western corn rootworm, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae), larval midgut tissue. Insect Mol. Biol. 2007, 16, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hua, G.; Jurat-Fuentes, J.L.; Abdullah, M.A.; Adang, M.J. Synergism of Bacillus thuringiensis toxins by a fragment of a toxin-binding cadherin. Proc. Natl. Acad. Sci. USA 2007, 104, 13901–13906. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Abdullah, M.A.F.; Taylor, M.D.; Rahman, K.; Adang, M.J. Enhancement of Bacillus thuringiensis Cry3Aa and Cry3Bb toxicities to coleopteran larvae by a toxin-binding fragment of an insect cadherin. Appl. Environ. Microbiol. 2009, 75, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hua, G.; Taylor, M.D.; Adang, M.J. A coleopteran cadherin fragment synergizes toxicity of Bacillus thuringiensis toxins Cry3Aa, Cry3Bb, and Cry8Ca against lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Invertebr. Pathol. 2014, 123, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Miyamoto, K.; Yamamoto, K.; Narukawa, J.; Kawai, S.; Sezutsu, H.; Kobayashi, I.; Uchino, K.; Tamura, T.; Mita, K.; et al. A single amino acid mutation in an ABC transporter causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2012, 109, E1591–E1598. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, J.H.; Endicott, J.A.; Juranka, P.F.; Henderson, G.; Sarangi, F.; Deuchars, K.L.; Ling, V. Homology between P-glycoprotein and a bacterial hemolysin transport protein suggests a model for multidrug resistance. Nature 1986, 324, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Pastan, I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993, 62, 385–427. [Google Scholar] [CrossRef] [PubMed]
- Broehan, G.; Kroeger, T.; Lorenzen, M.; Merzendorfer, H. Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum. BMC Genom. 2013, 14, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Buss, D.; Callaghan, A. Interaction of pesticides with P-glycoprotein and other ABC proteins: A survey of the possible importance to insecticide, herbicide and fungicide resistance. Pestic. Biochem. Physiol. 2008, 90, 141–153. [Google Scholar] [CrossRef]
- Oswald, K.J.; French, B.W.; Nielson, C.; Bagley, M. Assessment of fitness costs in Cry3Bb1-resistant and susceptible western corn rootworm (Coleoptera: Chrysomelidae) laboratory colonies. J. Appl. Entomol. 2012, 136, 730–740. [Google Scholar] [CrossRef]
- Hoffmann, A.M.; French, B.W.; Hellmich, R.L.; Lauter, N.; Gassmann, A.J. Fitness costs of resistance to Cry3Bb1 maize by western corn rootworm. J. Appl. Entomol. 2015, 139, 403–415. [Google Scholar] [CrossRef]
- Heckel, D.G. Learning the ABCs of Bt: ABC transporters and insect resistance to Bacillus thuringiensis provide clues to a crucial step in toxin mode of action. Pestic. Biochem. Physiol. 2012, 104, 103–110. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.T.; Liu, K.Y.; Zhang, D.D.; Gong, L.L.; He, F.; Soberón, M.; Bravo, A.; Tabashnik, B.E.; Wu, K.M. Resistance to Bacillus thuringiensis mediated by an ABC transporter mutation increases susceptibility to toxins from other bacteria in an invasive insect. PLoS Pathog. 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; McLachlin, J.R.; Brown, M.R.; Adang, M.J. Expression of a glycosyl phosphatidylinositol-linked Manduca sexta aminopeptidase N in insect cells. Protein Express Purif. 1999, 17, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Agrawal, N.; Selvapandiyan, A.; Sivakumar, S.; Ahmad, S.; Bhatnagar, R.K. Recombinantly expressed isoenzymic aminopeptidases from Helicoverpa armigera (american cotton bollworm) midgut display differential interaction with closely related Bacillus thuringiensis insecticidal proteins. Biochem. J. 2003, 370, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.M.; Newcomb, R.D. Binding of Bacillus thuringiensis delta-endotoxins Cry1Ac and Cry1Ba to a 120-kda aminopeptidase-N of Epiphyas postvittana purified from both brush border membrane vesicles and baculovirus-infected Sf9 cells. Insect Biochem. Mol. Biol. 2000, 30, 1069–1078. [Google Scholar] [CrossRef]
- Andow, D.A.; Pueppke, S.G.; Schaafsma, A.W.; Gassmann, A.J.; Sappington, T.W.; Meinkei, L.J.; Mitche, P.D.; Hurley, T.M.; Hellmich, R.L.; Porterl, R.P. Early detection and mitigation of resistance to Bt maize by western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2016, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Gould, F. Delaying corn rootworm resistance to Bt corn. J. Econ. Entomol. 2012, 105, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Chen, M.; Zhang, X.F.; Luan, H.H.; Tian, Y.C.; Su, X.H. Expression of Bt-Cry3A in transgenic Populus alba × P. glandulosa and its effects on target and non-target pests and the arthropod community. Transgenic Res. 2011, 20, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Génissel, A.; Leple, J.C.; Millet, N.; Augustin, S.; Jouanin, L.; Pilate, G. High tolerance against Chrysomela tremulae of transgenic poplar plants expressing a synthetic Cry3Aa gene from Bacillus thuringiensis ssp. tenebrionis. Mol. Breed. 2003, 11, 103–110. [Google Scholar] [CrossRef]
- Martínez-Torres, D.; Chandre, F.; Williamson, M.S.; Darriet, F.; Bergé, J.B.; Devonshire, A.L.; Guillet, P.; Pasteur, N.; Pauron, D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol. 1998, 7, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, F.J. Biometry: The principles and practice of statistics in biological research. 1969. Available online: http://imb-biblio.u-bourgogne.fr/Record.htm?record=293212401149&idlist=1 (accessed on 25 November 2016).
- Genotyping datasets. Available online: https://www.ice.mpg.de/downloads/ent-group/ypauchet-datasets_s2_s3_s4.zip (accessed on 25 November 2016).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauchet, Y.; Bretschneider, A.; Augustin, S.; Heckel, D.G. A P-Glycoprotein Is Linked to Resistance to the Bacillus thuringiensis Cry3Aa Toxin in a Leaf Beetle. Toxins 2016, 8, 362. https://doi.org/10.3390/toxins8120362
Pauchet Y, Bretschneider A, Augustin S, Heckel DG. A P-Glycoprotein Is Linked to Resistance to the Bacillus thuringiensis Cry3Aa Toxin in a Leaf Beetle. Toxins. 2016; 8(12):362. https://doi.org/10.3390/toxins8120362
Chicago/Turabian StylePauchet, Yannick, Anne Bretschneider, Sylvie Augustin, and David G. Heckel. 2016. "A P-Glycoprotein Is Linked to Resistance to the Bacillus thuringiensis Cry3Aa Toxin in a Leaf Beetle" Toxins 8, no. 12: 362. https://doi.org/10.3390/toxins8120362
APA StylePauchet, Y., Bretschneider, A., Augustin, S., & Heckel, D. G. (2016). A P-Glycoprotein Is Linked to Resistance to the Bacillus thuringiensis Cry3Aa Toxin in a Leaf Beetle. Toxins, 8(12), 362. https://doi.org/10.3390/toxins8120362





