Pueraria mirifica Exerts Estrogenic Effects in the Mammary Gland and Uterus and Promotes Mammary Carcinogenesis in Donryu Rats
Abstract
:1. Introduction
2. Results
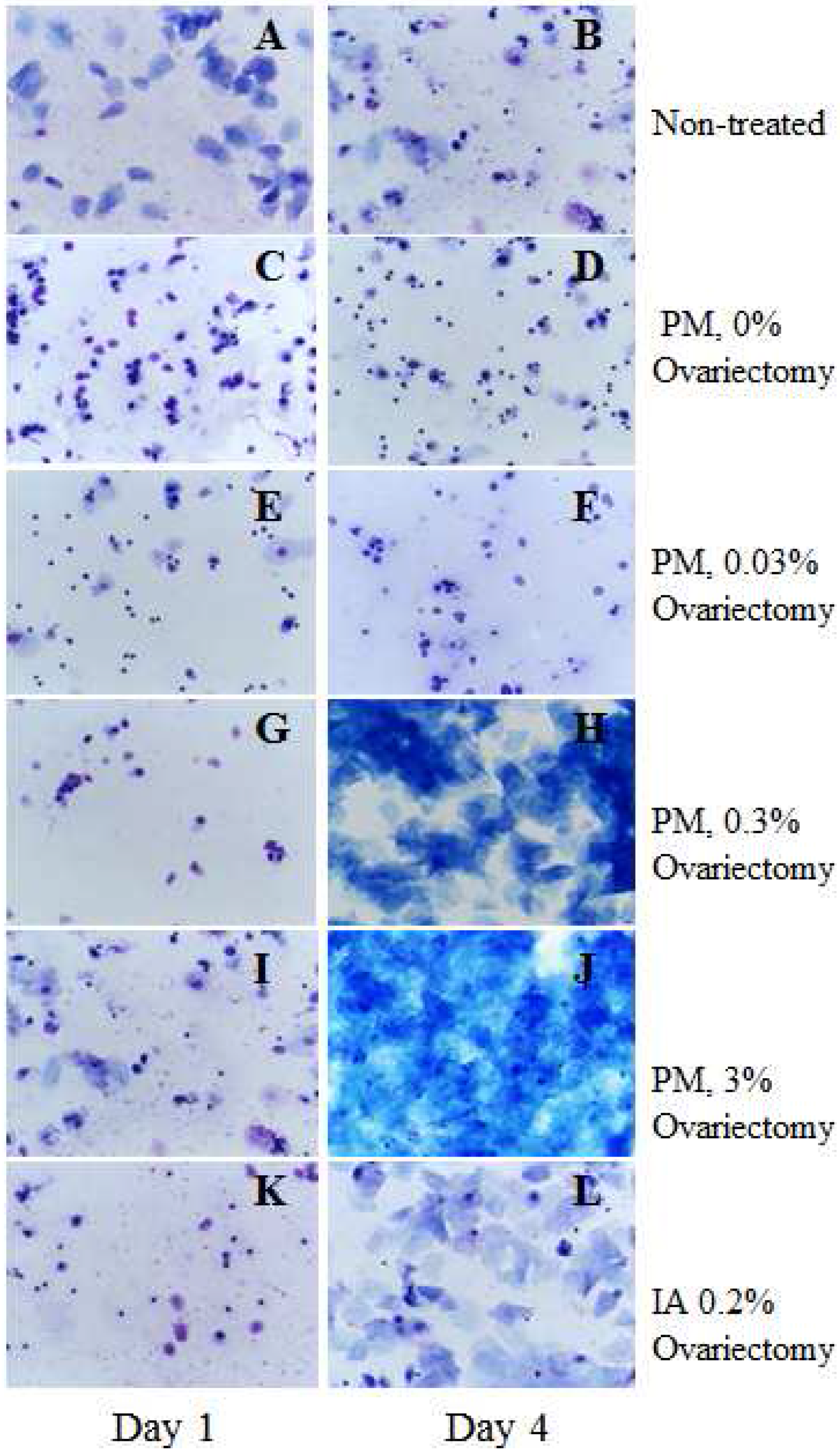
2.1. Estrogenic Effect of Test Compounds (Short-Term Experiment 1)
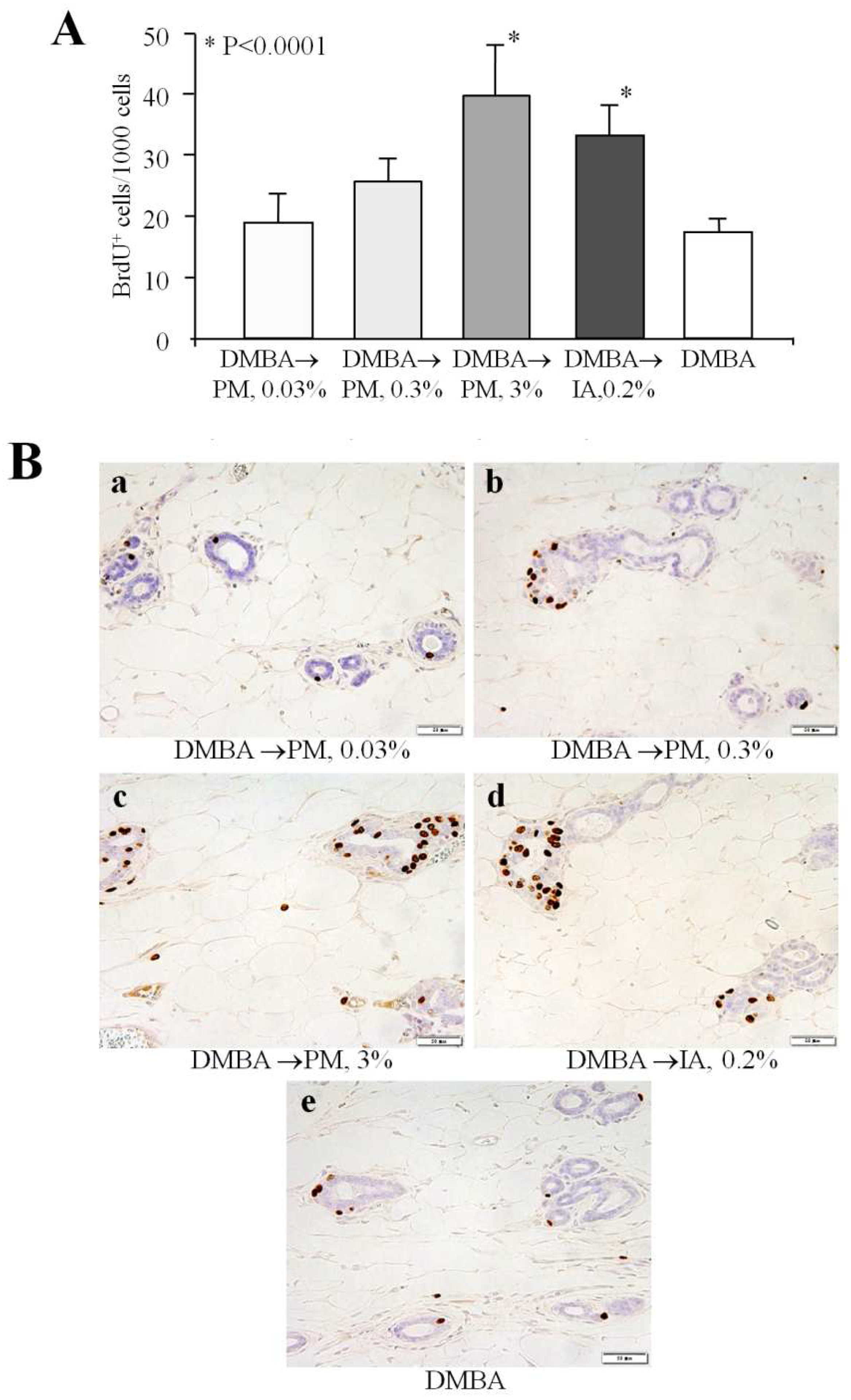
2.2. Cell Proliferation in the Mammary Gland (Short-Term Experiment 2)
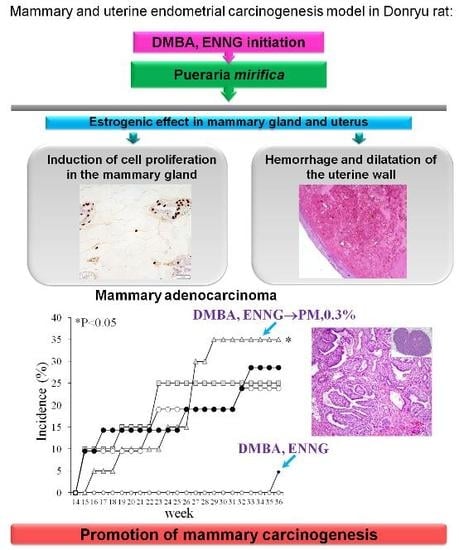
2.3. Long-Term Study (Experiment 3)
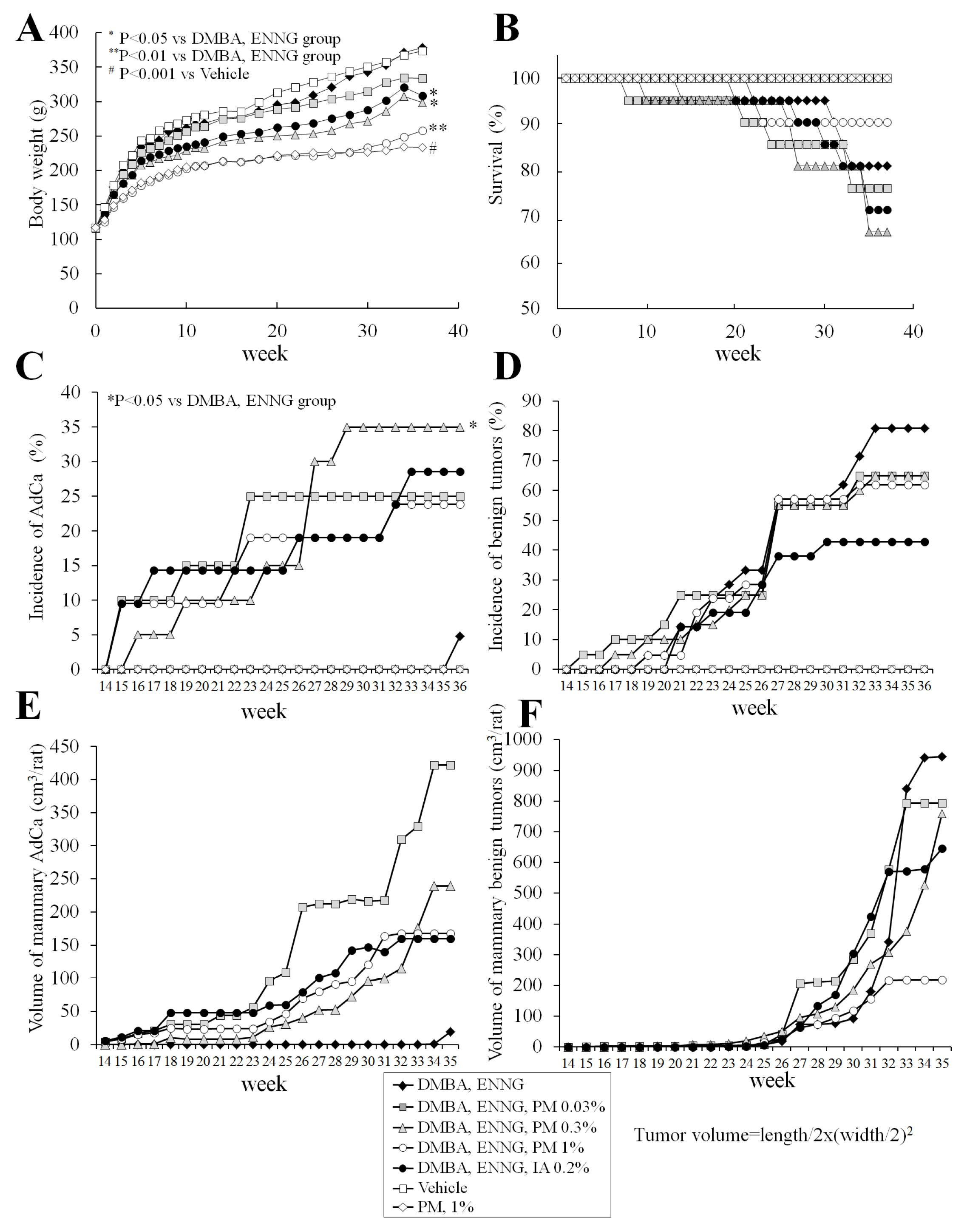
2.3.1. Body, Organ Weights, Food, and Water Consumption
2.3.2. Survival
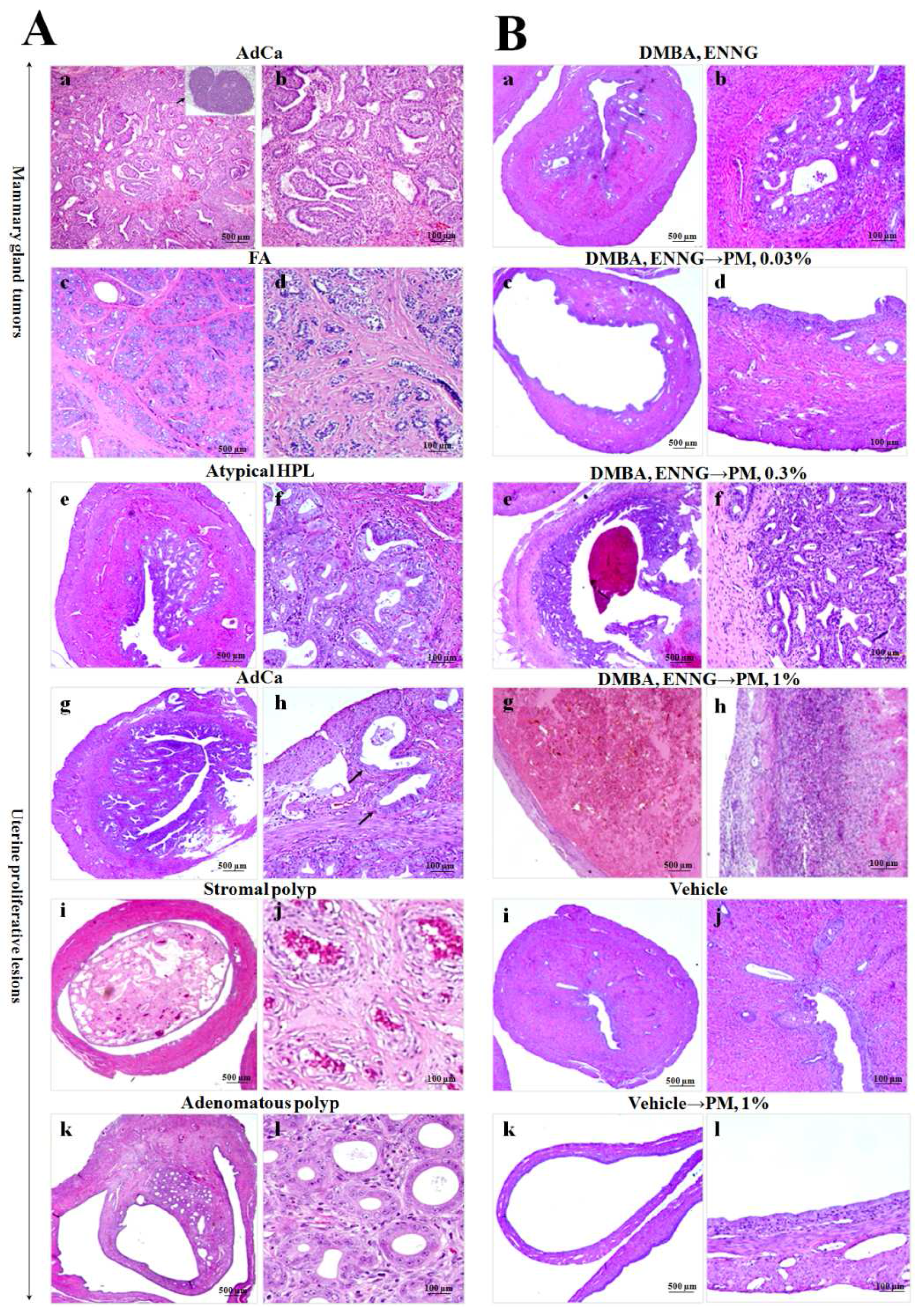
2.3.3. Histopathological Analysis of Mammary Glands
2.3.4. Histopathological Analysis of Uteri
2.3.5. Blood Hematology and Biochemistry
3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Test Compounds
4.3. Animals
4.4. Short-Term Experiment 1
4.5. Short-Term Experiment 2
4.6. Long-Term Experiment (Experiment 3)
4.7. BrdU Immunohistochemistry
4.8. Blood Hematology and Biochemistry
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Subcharoen, P. Thai traditional medicine in the new millennium. J. Med. Assoc. Thail. 2004, 87 (Suppl. S4), S52–S57. [Google Scholar]
- Ingham, J.L.; Tabara, S.; Pope, G.S. Chemical Components and Pharmacology of the Rejuvenating Plant Pueraria mirifica; Taylor and Fransis: London, UK, 2002. [Google Scholar]
- Malaivijitnond, S. Medical applications of phytoestrogens from the Thai herb Pueraria mirifica. Front. Med. 2012, 6, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Cain, J.C. Miroestrol: An oestrogen from the plant Pueraria mirifica. Nature 1960, 188, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, S.; Kumamoto, T.; Ishikawa, T.; Takashi, M.; Higuchi, Y.; Chaichantipyuth, C.; Chansakaow, S. Quantitative analysis of miroestrol and kwakhurin for standardisation of Thai miracle herb ‘Kwao Keur’ (Pueraria mirifica) and establishment of simple isolation procedure for highly estrogenic miroestrol and deoxymiroestrol. Nat. Prod. Res. 2013, 27, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Chansakaow, S.; Ishikawa, T.; Seki, H.; Sekine, K.; Okada, M.; Chaichantipyuth, C. Identification of deoxymiroestrol as the actual rejuvenating principle of “Kwao Keur”, Pueraria mirifica. The known miroestrol may be an artifact. J. Nat. Prod. 2000, 63, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Pope, G.S.; Grundy, H.M.; Jones, H.E.H.; Tait, S.A.S. The oestrogenic substance (miroestrol) from the tuberous roots of Pueraria mirifica. J. Endocrinol. 1958, 17, XV–XVI. [Google Scholar]
- Chansakaow, S.; Ishikawa, T.; Sekine, K.; Okada, M.; Higuchi, Y.; Kudo, M.; Chaichantipyuth, C. Isoflavonoids from Pueraria mirifica and their estrogenic activity. Planta Med. 2000, 66, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Tiyasatkulkovit, W.; Charoenphandhu, N.; Wongdee, K.; Thongbunchoo, J.; Krishnamra, N.; Malaivijitnond, S. Upregulation of osteoblastic differentiation marker mRNA expression in osteoblast-like UMR106 cells by puerarin and phytoestrogens from Pueraria mirifica. Phytomedicine 2012, 19, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Tiyasatkulkovit, W.; Malaivijitnond, S.; Charoenphandhu, N.; Havill, L.M.; Ford, A.L.; VandeBerg, J.L. Pueraria mirifica extract and puerarin enhance proliferation and expression of alkaline phosphatase and type I collagen in primary baboon osteoblasts. Phytomedicine 2014, 21, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Suthon, S.; Jaroenporn, S.; Charoenphandhu, N.; Suntornsaratoon, P.; Malaivijitnond, S. Anti-osteoporotic effects of Pueraria candollei var. mirifica on bone mineral density and histomorphometry in estrogen-deficient rats. J. Nat. Med. 2016, 70, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Trisomboon, H.; Malaivijitnond, S.; Suzuki, J.; Hamada, Y.; Watanabe, G.; Taya, K. Long-term treatment effects of Pueraria mirifica phytoestrogens on parathyroid hormone and calcium levels in aged menopausal cynomolgus monkeys. J. Reprod. Dev. 2004, 50, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Cherdshewasart, W.; Panriansaen, R.; Picha, P. Pretreatment with phytoestrogen-rich plant decreases breast tumor incidence and exhibits lower profile of mammary ERalpha and ERbeta. Maturitas 2007, 58, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Lucki, N.C.; Sewer, M.B. Genistein stimulates MCF-7 breast cancer cell growth by inducing acid ceramidase (ASAH1) gene expression. J. Biol. Chem. 2011, 286, 19399–19409. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, A.; Tago, Y.; Yoshida, M.; Sokuza, Y.; Wei, M.; Fukushima, S.; Wanibuchi, H. Hormonally active doses of isoflavone aglycones promote mammary and endometrial carcinogenesis and alter the molecular tumor environment in Donryu rats. Toxicol. Sci. 2012, 126, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.C.; Soares da Costa, C.A.; Armada, L.; de Paula Lopes Gonzalez, G.; dos Santos Ribeiro, M.; de Sousa dos Santos, A.; de Carvalho, J.J.; do Nascimento Saba, C.C. Fat tissue morphology of long-term sex steroid deficiency and estrogen treatment in female rats. Fertil. Steril. 2011, 95, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.J.; Wood, C.E. Soy isoflavones, estrogen therapy, and breast cancer risk: Analysis and commentary. Nutr. J. 2008, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, W.; Jarry, H.; Seidlova-Wuttke, D. Isoflavones-safe food additives or dangerous drugs? Ageing Res. Rev. 2007, 6, 150–188. [Google Scholar] [CrossRef] [PubMed]
- Trisomboon, H.; Malaivijitnond, S.; Watanabe, G.; Taya, K. Estrogenic effects of Pueraria mirifica on the menstrual cycle and hormone-related ovarian functions in cyclic female cynomolgus monkeys. J. Pharmacol. Sci. 2004, 94, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Trisomboon, H.; Malaivijitnond, S.; Watanabe, G.; Taya, K. Ovulation block by Pueraria mirifica: A study of its endocrinological effect in female monkeys. Endocrine 2005, 26, 33–39. [Google Scholar] [CrossRef]
- Trisomboon, H.; Malaivijitnond, S.; Watanabe, G.; Cherdshewasart, W.; Taya, K. The estrogenic effect of Pueraria mirifica on gonadotrophin levels in aged monkeys. Endocrine 2006, 29, 129–134. [Google Scholar] [CrossRef]
- Trisomboon, H.; Malaivijitnond, S.; Cherdshewasart, W.; Watanabe, G.; Taya, K. Assessment of urinary gonadotropin and steroid hormone profiles of female cynomolgus monkeys after treatment with Pueraria mirifica. J. Reprod. Dev. 2007, 53, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Jaroenporn, S.; Malaivijitnond, S.; Wattanasirmkit, K.; Watanabe, G.; Taya, K.; Cherdshewasart, W. Assessment of fertility and reproductive toxicity in adult female mice after long-term exposure to Pueraria mirifica herb. J. Reprod. Dev. 2007, 53, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Malaivijitnond, S.; Kiatthaipipat, P.; Cherdshewasart, W.; Watanabe, G.; Taya, K. Different effects of Pueraria mirifica, a herb containing phytoestrogens, on LH and FSH secretion in gonadectomized female and male rats. J. Pharmacol. Sci. 2004, 96, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Malaivijitnond, S.; Chansri, K.; Kijkuokul, P.; Urasopon, N.; Cherdshewasart, W. Using vaginal cytology to assess the estrogenic activity of phytoestrogen-rich herb. J. Ethnopharmacol. 2006, 107, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Cherdshewasart, W.; Kitsamai, Y.; Malaivijitnond, S. Evaluation of the estrogenic activity of the wild Pueraria mirifica by vaginal cornification assay. J. Reprod. Dev. 2007, 53, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Cherdshewasart, W.; Sriwatcharakul, S.; Malaivijitnond, S. Variance of estrogenic activity of the phytoestrogen-rich plant. Maturitas 2008, 61, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Gomuttapong, S.; Pewphong, R.; Choeisiri, S.; Jaroenporn, S.; Malaivijitnond, S. Testing of the estrogenic activity and toxicity of Stephania venosa herb in ovariectomized rats. Toxicol. Mech. Methods 2012, 22, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Lamartiniere, C.A.; Moore, J.B.; Brown, N.M.; Thompson, R.; Hardin, M.J.; Barnes, S. Genistein suppresses mammary cancer in rats. Carcinogenesis 1995, 16, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Freireich, E.J.; Gehan, E.A.; Rall, D.P.; Schmidt, L.H.; Skipper, H.E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966, 50, 219–244. [Google Scholar] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, T.; Takegawa, K.; Takeuchi, M.; Maekawa, A. Effects of reproduction on spontaneous development of endometrial adenocarcinomas and mammary tumors in Donryu rats. Jpn. J. Cancer Res. 2000, 91, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.; Solanas, M.; Escrich, E. Histopathologic characterization of mammary neoplastic lesions induced with 7,12 dimethylbenz(alpha)anthracene in the rat: A comparative analysis with human breast tumors. Arch. Pathol. Lab. Med. 2002, 126, 915–927. [Google Scholar] [PubMed]
- Nagaoka, T.; Takeuchi, M.; Onodera, H.; Matsushima, Y.; Ando-Lu, J.; Maekawa, A. Sequential observation of spontaneous endometrial adenocarcinoma development in Donryu rats. Toxicol. Pathol. 1994, 22, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, A.; Hagiwara, A.; Imai, N.; Wei, M.; Fukushima, S.; Wanibuchi, H. Induction of cell proliferation in the rat liver by the short-term administration of ethyl tertiary-butyl ether. J. Toxicol. Pathol. 2015, 28, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, A.; Ishii, N.; Fujioka, M.; Doi, K.; Gi, M.; Wanibuchi, H. Ethanol-extracted Brazilian propolis exerts protective effects on tumorigenesis in Wistar Hannover rats. PLoS ONE 2016, 11, e0158654. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kanamori, M.; Ohori, K.; Takeuchi, H. A new decision tree method for statistical analysis of quantitative data obtained in toxicity studies on rodent. Sangyo Eiseigaku Zasshi 2000, 42, 125–129. [Google Scholar] [PubMed]
| Treatment | No. Rats a | Fibroadenoma | Fibroma | Adenoma | AdCa | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mammary Glands | |||||||||
| Incidence (No. Rats (%)) | |||||||||
| DMBA, ENNG | 21 | 20 (95.2) | 7 (33.3) | 3 (14.3) | 1 (4.8) | ||||
| DMBA, ENNG → PM, 0.03% | 20 | 19 (95) | 8 (40) | 2 (10) | 6 (30) | ||||
| DMBA, ENNG → PM, 0.3% | 20 | 19 (95) | 8 (40) | 4 (20) | 7 (35) * | ||||
| DMBA, ENNG → PM 1% | 21 | 20 (95.2) | 5 (23.8) | 4 (19.1) | 6 (28.6) | ||||
| DMBA, ENNG → IA, 0.2% | 21 | 18 (85.7) | 3 (14.3) | 2 (9.5) | 6 (28.6) | ||||
| Vehicle | 5 | 1 (20) | 0 | 0 | 0 | ||||
| Vehicle → PM, 1% | 6 | 1 (16.7) | 0 | 0 | 0 | ||||
| Multiplicity (No./Rat) | |||||||||
| DMBA, ENNG | 21 | 10.90 ± 4.93 d | 0.55 ± 0.89 b | 0.15 ± 0.37 | 0.05 ± 0.22 | ||||
| DMBA, ENNG → PM, 0.03% | 20 | 9.85 ± 5.39 | 0.40 ± 0.50 | 0.10 ± 0.31 | 0.40 ± 0.68 | ||||
| DMBA, ENNG → PM, 0.3% | 20 | 8.30 ± 4.93 | 0.50 ± 0.69 | 0.20 ± 0.41 | 0.45 ± 0.69 # | ||||
| DMBA, ENNG → PM 1% | 21 | 7.95 ± 4.26 | 0.29 ± 0.56 | 0.19 ± 0.40 | 0.33 ± 0.58 | ||||
| DMBA, ENNG → IA, 0.2% | 21 | 6.90 ± 5.02 * | 0.14 ± 0.36 | 0.10 ± 0.30 | 0.43 ± 0.75 | ||||
| Vehicle | 5 | 0.20 ± 0.45 | 0 | 0 | 0 | ||||
| Vehicle → PM, 1% | 6 | 0.17 ± 0.41 | 0 | 0 | 0 | ||||
| Uterus | Dilatation | Endometrial HPL | AdCa | Polyps | |||||
| Mild | Moderate | Severe | Total | S | A | ||||
| Incidence (No. Rats (%)) | |||||||||
| DMBA, ENNG | 21 | 8 (38.1) | 10 (47.6) | 5 (23.8) | 2 (9.5) | 17 (81.0) | 1 (4.8) | 6 (28.6) | 3 (14.3) |
| DMBA, ENNG → PM, 0.03% | 20 | 13 (65) | 16 (80) | 4 (20) | 1 (5) | 17 (85) | 3 (15) | 5 (25) | 2 (10) |
| DMBA, ENNG → PM, 0.3% | 20 | 19 (95) ** | 17 (85) | 7 (35) | 1 (5) | 19 (95) | 2 (10) | 11 (55) | 8 (40) |
| DMBA, ENNG → PM 1% | 21 | 19 (90.5) ** | 11(52.4) | 2 (9.5) | 2 (9.53) | 12 (57.1) | 0 (0) | 4 (19.0) | 5 (23.8) |
| DMBA, ENNG → IA, 0.2% | 21 | 19 (90.5) ** | 16 (76.2) | 5 (23.8) | 4 (19.0) | 20 (95.2) | 2 (9.5) | 9 (42.9) | 9 (42.9) |
| Vehicle | 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vehicle → PM, 1% | 6 | 6 (100) * | 3 (50.0) | 0 (0) | 0 (0) | 3 (50.0) | 0 (0) | 0 (0) | 0 (0) |
| Multiplicity (No./Rat) | |||||||||
| DMBA, ENNG | 21 | - | 0.67 ± 0.80 c | 0.24 ± 0.44 | 0.14 ± 0.48 | 1.10 ± 0.64 d | 0.05 ± 0.22 | 0.33 ± 0.58 | 0.14 ± 0.36 |
| DMBA, ENNG → PM, 0.03% | 20 | - | 1.25 ± 0.91 * | 0.24 ± 0.54 | 0.05 ± 0.22 | 1.55 ± 1.00 | 0.15 ± 0.37 | 0.25 ± 0.44 | 0.15 ± 0.47 |
| DMBA, ENNG → PM, 0.3% | 20 | - | 1.35 ± 0.81 * | 0.35 ± 0.49 | 0.05 ± 0.22 | 1.75 ± 0.79 * | 0.10 ± 0.31 | 0.95 ± 1.19 | 0.65 ± 1.09 |
| DMBA, ENNG → PM 1% | 21 | - | 0.57 ± 0.60 | 0.10 ± 0.30 | 0.14 ± 0.48 | 0.81 ± 0.81 | 0.00 ± 0.00 | 0.43 ± 1.33 | 0.33 ± 0.73 |
| DMBA, ENNG → IA, 0.2% | 21 | - | 1.00 ± 0.71 | 0.24 ± 0.44 | 0.19 ± 0.40 | 1.52 ± 0.60 * | 0.10 ± 0.30 | 0.57 ± 0.75 | 0.57 ± 0.75 * |
| Vehicle | 5 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vehicle → PM, 1% | 6 | - | 0.50 ± 0.55 | 0 | 0 | 0.50 ± 0.55 | 0 | 0 | 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakehashi, A.; Yoshida, M.; Tago, Y.; Ishii, N.; Okuno, T.; Gi, M.; Wanibuchi, H. Pueraria mirifica Exerts Estrogenic Effects in the Mammary Gland and Uterus and Promotes Mammary Carcinogenesis in Donryu Rats. Toxins 2016, 8, 275. https://doi.org/10.3390/toxins8110275
Kakehashi A, Yoshida M, Tago Y, Ishii N, Okuno T, Gi M, Wanibuchi H. Pueraria mirifica Exerts Estrogenic Effects in the Mammary Gland and Uterus and Promotes Mammary Carcinogenesis in Donryu Rats. Toxins. 2016; 8(11):275. https://doi.org/10.3390/toxins8110275
Chicago/Turabian StyleKakehashi, Anna, Midori Yoshida, Yoshiyuki Tago, Naomi Ishii, Takahiro Okuno, Min Gi, and Hideki Wanibuchi. 2016. "Pueraria mirifica Exerts Estrogenic Effects in the Mammary Gland and Uterus and Promotes Mammary Carcinogenesis in Donryu Rats" Toxins 8, no. 11: 275. https://doi.org/10.3390/toxins8110275
APA StyleKakehashi, A., Yoshida, M., Tago, Y., Ishii, N., Okuno, T., Gi, M., & Wanibuchi, H. (2016). Pueraria mirifica Exerts Estrogenic Effects in the Mammary Gland and Uterus and Promotes Mammary Carcinogenesis in Donryu Rats. Toxins, 8(11), 275. https://doi.org/10.3390/toxins8110275