Tetrodotoxin and Its Analogues in the Pufferfish Arothron hispidus and A. nigropunctatus from the Solomon Islands: A Comparison of Their Toxin Profiles with the Same Species from Okinawa, Japan
Abstract
:1. Introduction
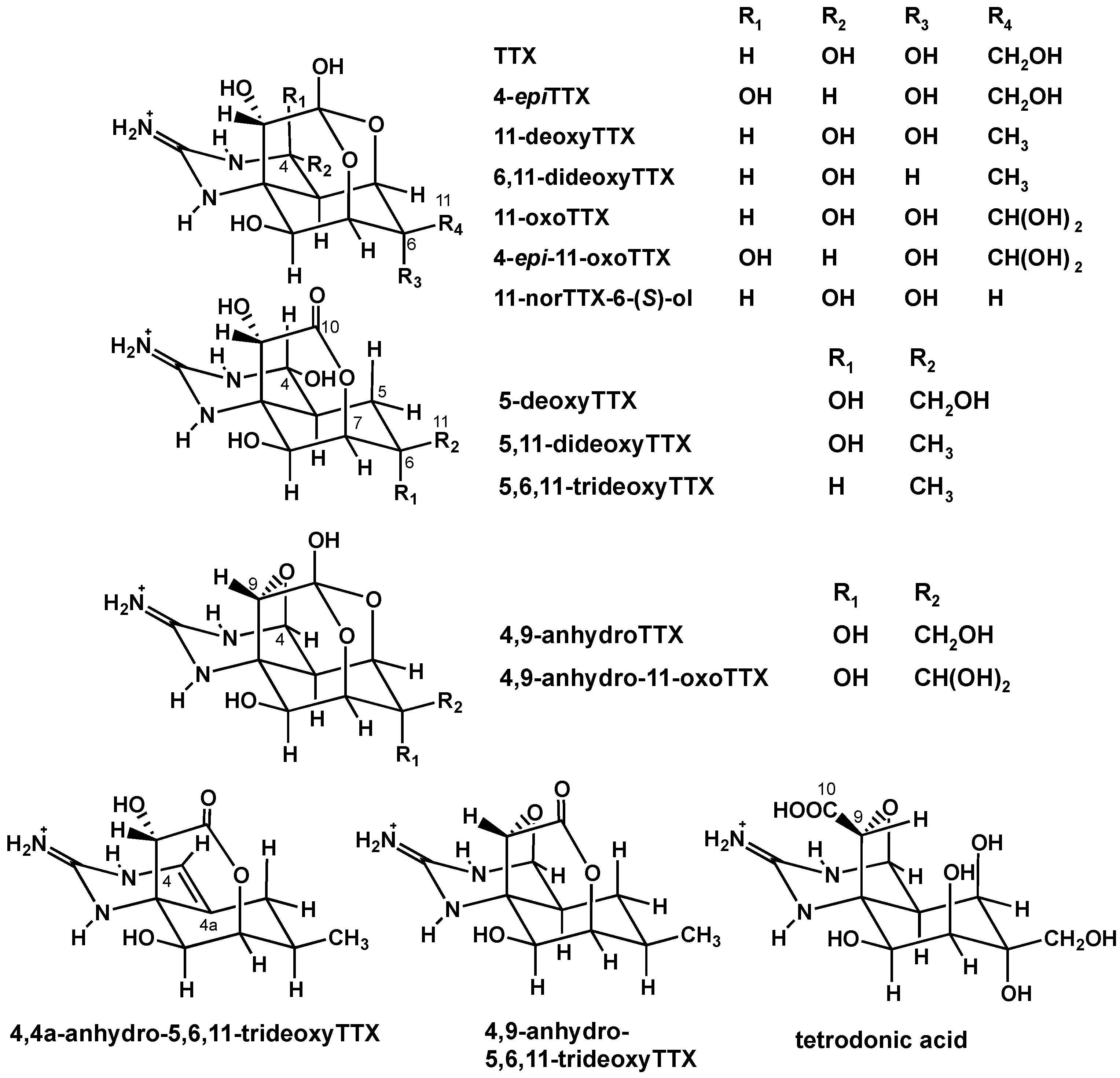
2. Results
2.1. Analysis of TTX and Its Analogues in the Pufferfish from the Solomon Islands
2.1.1. Preliminary Analysis of TTX and Its Analogues in the Skin of A. hispidus from the Solomon Islands using LC-FLD
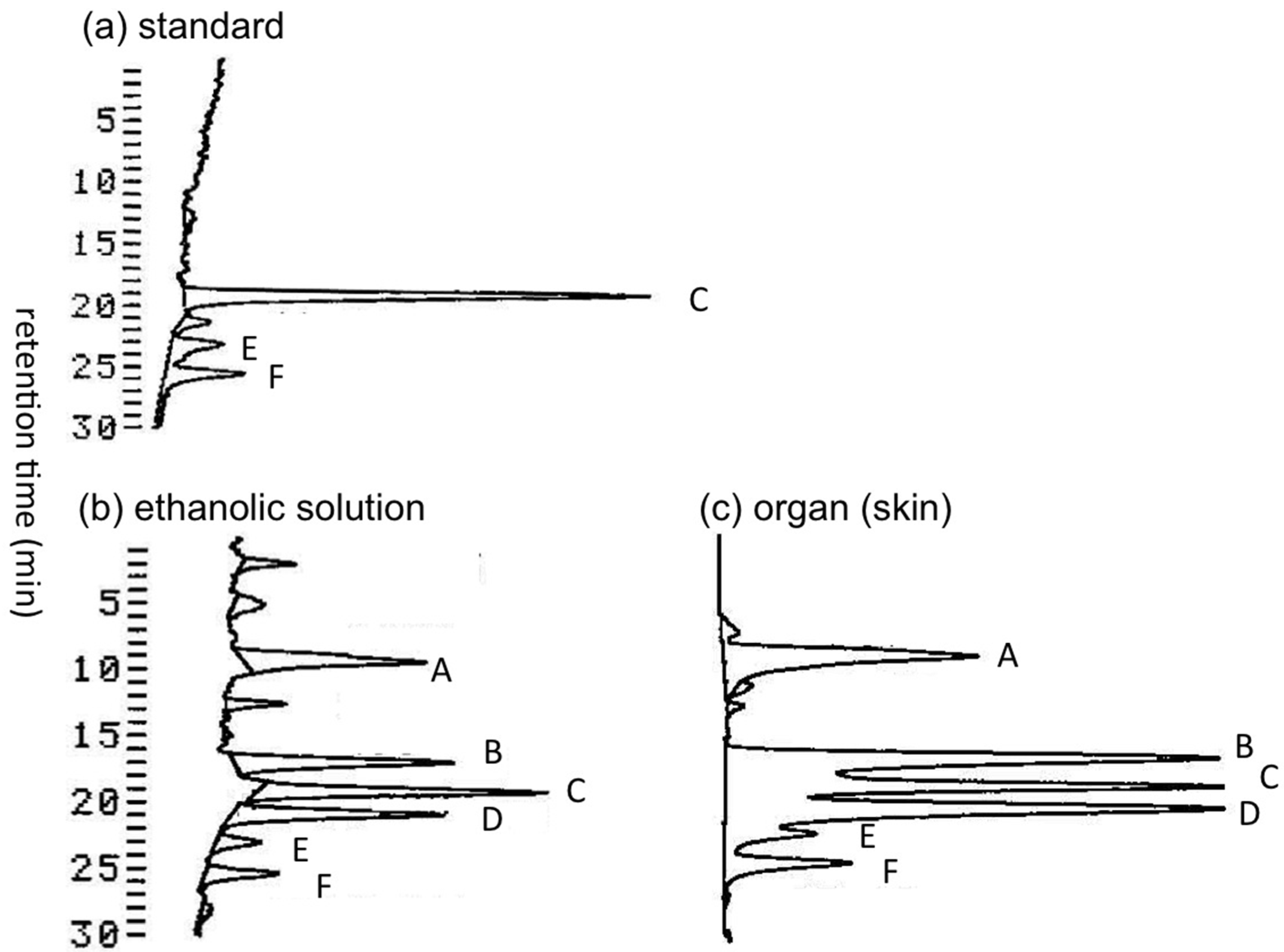
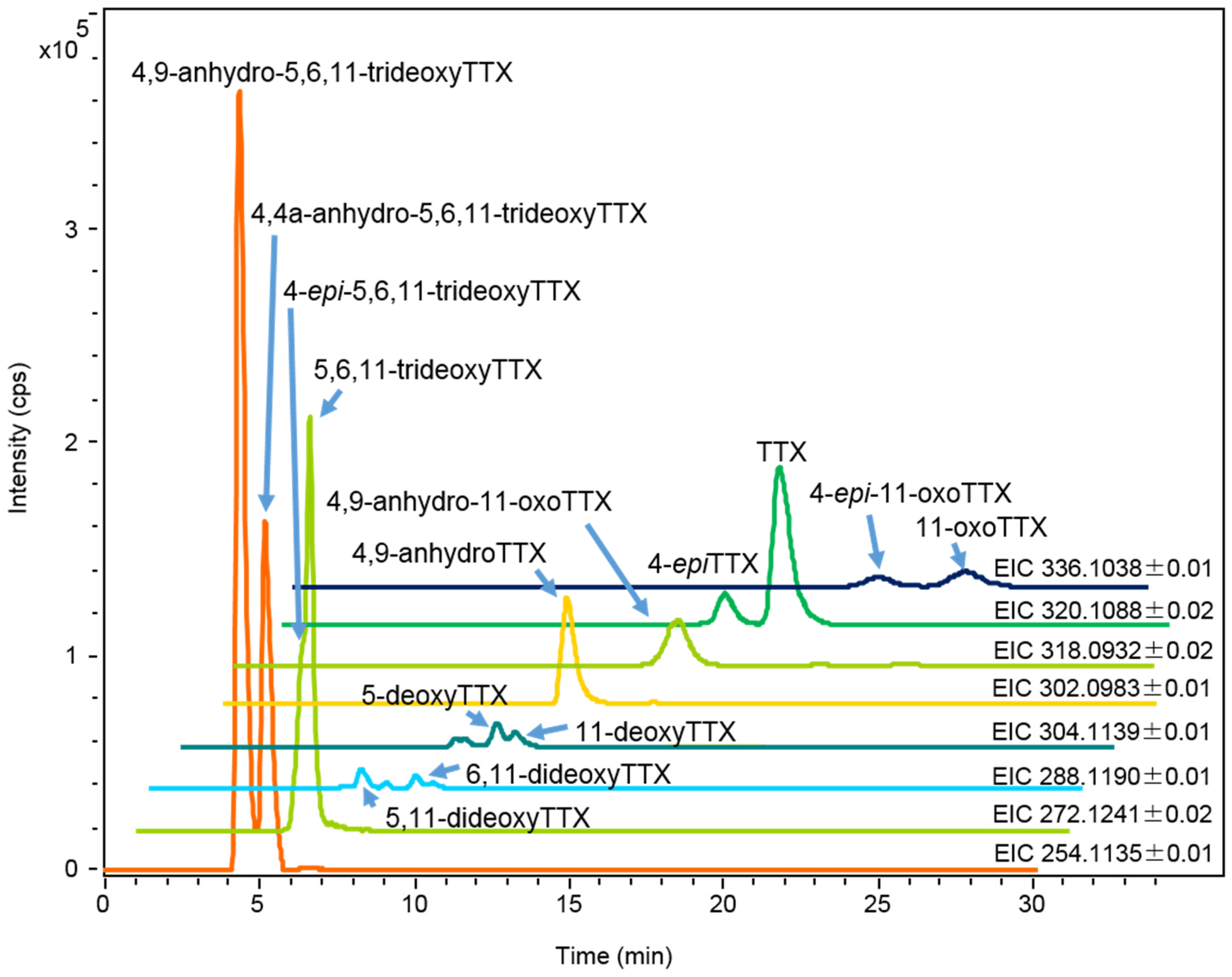
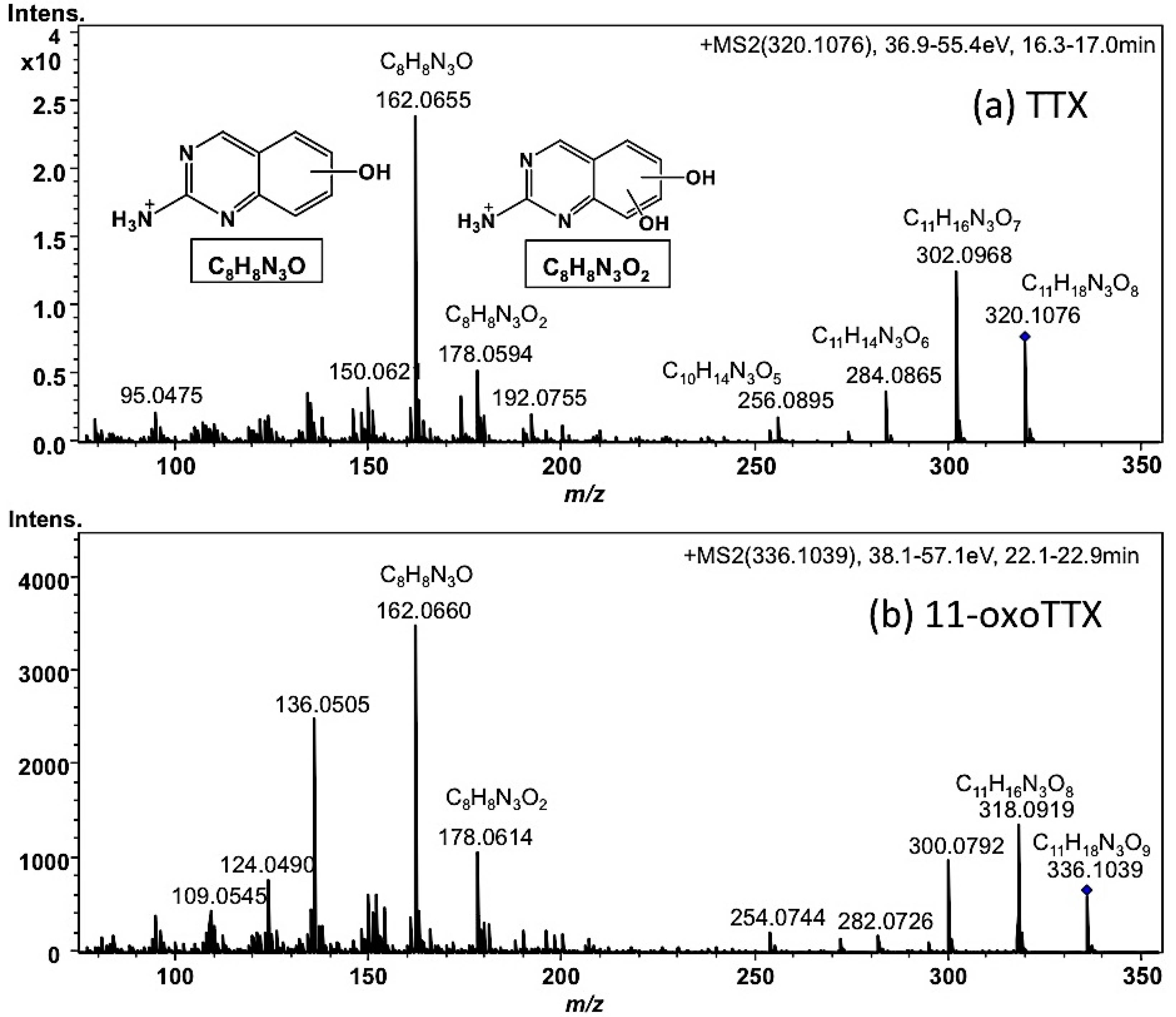
2.1.2. Characterization and Quantitation of TTX and Its Analogues Using High Resolution-LC-MS
2.1.3. Estimation of the Concentrations of TTX and Its Analogues in Each Organ of A. hispidus, A. nigropunctatus, and D. holocanthus from the Solomon Islands
| Organ | Skin | Liver | Ovary | Testis | Stomach | Intestine | Flesh |
|---|---|---|---|---|---|---|---|
| n = 3 | n = 3 | n = 1 | n = 2 | n = 3 | n = 2 | n = 1 | |
| TTX | 7.20–51.0 | 0.03–7.99 | 1.89 | <LOD, 11.7 | 0.58–9.45 | 1.95, 5.45 | 0.07 |
| 4-epiTTX | 1.80–14.8 | <LOD-1.29 | 0.23 | <LOD, 1.93 | <LOD-1.52 | <LOD, 0.27 | <LOD |
| 4,9-anhydroTTX | 3.60–26.0 | <LOD-4.00 | <LOD | <LOD, 6.63 | <LOD-4.93 | <LOD, <LOQ(0.02) | <LOD |
| 11-deoxyTTX | 0.10–4.32 | <LOD-0.67 | <LOD | <LOD, 1.17 | <LOD-0.66 | <LOD, 0.06 | <LOD |
| 5-deoxyTTX | <LOD-5.51 | <LOD-0.21 | <LOD | <LOD, 0.35 | <LOD-0.16 | 0.10, 0.10 | <LOD |
| 6,11-dideoxyTTX | <LOD-1.47 | <LOD-0.08 | <LOD | <LOD | <LOD-0.06 | <LOD | <LOD |
| 5,11-dideoxyTTX | <LOD-3.23 | <LOD-0.14 | <LOD | <LOD | <LOD-0.56 | <LOD, 0.16 | <LOD |
| 5,6,11-trideoxyTTX | 1.47–84.3 | <LOD-16.4 | 10.1 | <LOD, 14.6 | <LOD-19.5 | 5.54, 7.20 | 0.05 |
| 4,4a-anhydro-5,6,11-trideoxyTTX | 1.36–70.1 | <LOD-10.4 | 6.74 | <LOD, 8.99 | 7.34–7.92 | 4.93, 5.36 | 0.11 |
| 4,9-anhydro-5,6,11-trideoxyTTX | 2.06–133.2 | 0.34–12.2 | 7.94 | <LOD, 9.15 | 7.71–21.6 | 5.04, 10.7 | 0.11 |
| 11-norTTX-6(S)-ol | <LOD-2.23 | <LOD-0.51 | 0.04 | <LOD, 1.02 | <LOD-0.74 | <LOD, 0.07 | <LOD |
| 11-oxoTTX | 0.32–7.07 | <LOD-0.35 | <LOD | <LOD, 0.41 | <LOD-0.31 | < LOQ(0.05), 1.68 | <LOD |
| 4-epi-11-oxoTTX | <LOQ(0.06)-3.53 | <LOD-<LOQ(0.06) | <LOD | <LOD, <LOQ(0.09) | <LOD-<LOQ(0.13) | <LOD, 0.26 | <LOD |
| 4,9-anhydro-11-oxoTTX | <LOD-13.7 | <LOD-0.18 | 0.22 | <LOD, 0.58 | <LOQ(0.08)-0.41 | 0.21, 1.72 | <LOD |
| Organ | Skin | Liver | Ovary | Testis | Stomach | Intestine |
|---|---|---|---|---|---|---|
| n = 3 | n = 3 | n = 2 | n = 1 | n = 2 | n = 1 | |
| TTX | 8.51–28.7 | <LOD-19.7 | 1.14, 19.7 | <LOD | 1.57, 21.9 | 0.38 |
| 4-epiTTX | 1.61–6.68 | <LOD-3.11 | 0.28, 3.16 | <LOD | 0.41, 3.04 | 0.05 |
| 4,9-anhydroTTX | 5.15–15.9 | <LOD-10.8 | 0.91, 8.60 | <LOD | <LOD, 11.0 | 0.19 |
| 11-deoxyTTX | <LOD-0.83 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 5-deoxyTTX | <LOD-0.14 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 6,11-dideoxyTTX | <LOD-0.10 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 5,11-dideoxyTTX | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 5,6,11-trideoxyTTX | 3.99–13.0 | <LOD-10.7 | 1.12, 6.14 | <LOD | 1.25, 9.17 | 0.55 |
| 4,4a-anhydro-5,6,11-trideoxyTTX | 2.39–8.44 | 0.02–10.1 | 0.92, 5.89 | <LOD | 1.32, 8.63 | 0.53 |
| 4,9-anhydro-5,6,11-trideoxyTTX | 3.90–11.1 | <LOD-11.0 | 1.07, 6.73 | <LOD | 1.31, 8.94 | 0.54 |
| 11-norTTX-6(S)-ol | 0.10–0.84 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 11-oxoTTX | 0.16–0.67 | <LOD-0.77 | <LOD, 0.56 | <LOD | <LOQ(0.05), 0.80 | <LOD |
| 4-epi-11-oxoTTX | <LOQ(0.09)-0.50 | <LOD-0.27 | <LOD, 0.22 | <LOD | <LOD, 0.26 | <LOD |
| 4,9-anhydro-11-oxo-TTX | 0.33–1.49 | <LOD-1.11 | <LOD, 0.66 | <LOD | 0.19, 0.93 | <LOQ(0.07) |
2.2. The concentrations of TTX and Its Analogues in Each Organ of A. hispidus and A. nigropunctatus From Okinawa, Japan
| Organ | Skin | Liver | Ovary | Testis | Stomach | Intestine | Flesh | Spleen |
|---|---|---|---|---|---|---|---|---|
| n = 3 | n = 3 | n = 1 | n = 2 | n = 3 | n = 3 | n = 3 | n = 3 | |
| TTX | 4.26–12.7 | 0.17–0.55 | 0.37 | 1.08, 1.11 | <LOD-2.44 | 1.61-2.05 | 0.23–0.61 | 0.15–2.09 |
| 4-epiTTX | 0.40-2.75 | <LOD-0.03 | 0.04 | 0.07, 0.17 | <LOD-0.52 | 0.10-0.36 | <LOD-0.04 | <LOD-0.25 |
| 4,9-anhydroTTX | 2.22–8.84 | <LOD-0.26 | 0.17 | 0.46, 0.52 | <LOD-1.74 | 0.59–1.36 | 0.04–0.18 | <LOD-0.74 |
| 11-deoxyTTX | 1.34–2.12 | <LOD | <LOD | 0.04, 0.18 | <LOD-0.21 | <LOD-0.32 | <LOD | <LOD-0.11 |
| 5-deoxyTTX | 0.73–1.19 | <LOD | <LOD | 0.11, 0.16 | <LOD-0.05 | <LOD-0.31 | <LOD | <LOD |
| 6,11-dideoxyTTX | 0.12–0.31 | <LOD | <LOD | <LOD, 0.05 | <LOD | <LOD-<LOQ(0.02) | <LOD | <LOD |
| 5,11-dideoxyTTX | 0.11–0.52 | <LOD | <LOD | <LOD, 0.03 | <LOD | <LOD-<LOQ(0.02) | <LOD | <LOD |
| 5,6,11-trideoxyTTX | 1.09–6.02 | < 0.06 | 0.18 | < 0.20, 0.56 | < 0.10-0.19 | 0.21–0.36 | 0.03–0.23 | <LOD-0.10 |
| 4,4a-anhydro-5,6,11-trideoxyTTX | 1.91–18.6 | 0.31–0.46 | 0.33 | 0.24, 0.67 | < 0.14–0.80 | 0.42–0.73 | 0.07–0.31 | 0.03–0.55 |
| 4,9-anhydro-5,6,11-trideoxyTTX | 7.36–46.7 | 0.44–1.66 | 1.11 | 1.70, 3.79 | < 0.46–2.23 | 1.77–2.80 | 0.21–0.45 | 0.15–1.10 |
| 11-norTTX-6(S)-ol | 0.27–0.92 | <LOD | <LOD | 0.04, 0.06 | <LOD-0.18 | 0.08–0.12 | <LOD-0.05 | <LOD-0.12 |
| 11-oxoTTX | <LOQ(0.07)-0.20 | <LOD | <LOD | <LOD, <LOQ(0.12) | <LOD | <LOD- <LOQ(0.05) | <LOD | <LOD |
| 4-epi-11-oxoTTX | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 4,9-anhydro-11-oxoTTX | <LOQ(0.12)-0.15 | <LOD | <LOD | <LOD, <LOQ(0.07) | <LOD-< LOQ(0.08) | <LOD- <LOQ(0.09) | <LOD | <LOD |
| Organ | Skin | Liver | Ovary | Testis | Stomach | Intestine | Flesh | Spleen |
|---|---|---|---|---|---|---|---|---|
| n = 4 | n = 4 | n = 1 | n = 3 | n = 4 | n = 4 | n = 4 | n = 4 | |
| TTX | 12.6–255 | 0.29–7.46 | 1.02 | 0.26–10.1 | 0.57–25.5 | 1.22–4.51 | 0.19–4.26 | 0.85–4.19 |
| 4-epiTTX | 1.19–27.9 | <LOD-0.49 | 0.09 | <LOD-2.64 | 0.07–2.05 | 0.07–0.35 | <LOD-0.92 | 0.18–0.62 |
| 4,9-anhydroTTX | 5.39–96.6 | <LOD-2.75 | 0.50 | 0.13–6.13 | 0.24–9.65 | 0.52–2.41 | <LOD-1.82 | 0.85–2.26 |
| 11-deoxyTTX | 1.25–14.9 | <LOD-0.54 | 0.10 | 0.02–3.13 | 0.06–2.19 | 0.15–0.65 | <LOD-0.88 | 0.10–0.76 |
| 5-deoxyTTX | <LOD-4.03 | <LOD-0.23 | 0.09 | 0.10–3.80 | <LOD-5.41 | <LOD-0.54 | <LOD-0.62 | <LOD-0.80 |
| 6,11-dideoxyTTX | 0.40–1.77 | <LOD-0.07 | 0.05 | <LOD-1.31 | 0.02–0.84 | <LOD-0.27 | <LOD | <LOD-0.43 |
| 5,11-dideoxyTTX | 0.52–2.92 | <LOD-0.06 | <LOQ(0.02) | 0.10–0.94 | <LOD-1.03 | <LOD-0.12 | <LOD-0.18 | <LOD-0.14 |
| 5,6,11-trideoxyTTX | 15.3–275 | <LOD -3.69 | 0.39 | 0.61–22.3 | 0.28–25.2 | <LOD-8.40 | 0.04–3.92 | 0.74–10.0 |
| 4,4a-anhydro-5,6,11-tri-deoxyTTX | 6.81–178 | 0.34–2.69 | 0.56 | 0.45–15.3 | 0.39–10.2 | 0.74–5.31 | 0.34–3.32 | 1.01–5.69 |
| 4,9-anhydro-5,6,11-trideoxyTTX | 43–438 | 0.30–8.28 | 3.48 | 2.05–26.9 | 1.42–77.0 | 0.93–11.0 | 0.70–4.79 | 3.74–10.5 |
| 11-norTTX-6(S)-ol | 0.11–9.72 | <LOD-0.30 | <LOD | <LOD-0.32 | <LOD-0.11 | <LOD-0.19 | <LOD-0.11 | <LOD-0.15 |
| 11-oxoTTX | 3.94–42.4 | <LOQ(0.05)-0.84 | <LOQ(0.12) | <LOQ(0.07)-0.82 | <LOQ(0.05)-9.98 | <LOQ(0.12)-0.53 | <LOD-<LOQ(0.11) | 0.12–0.46 |
| 4-epi-11-oxoTTX | 0.51–6.54 | <LOD-0.16 | <LOD | <LOD-0.16 | <LOD-1.81 | <LOD-<LOQ(0.10) | <LOD | 0.05–0.16 |
| 4,9-anhydro-11-oxoTTX | 2.57–25.0 | <LOQ(0.04)-0.5 | 0.15 | < LOQ(0.05)-0.69 | <LOQ(0.11)-4.15 | 0.20–0.41 | <LOD-0.35 | 0.09–0.53 |
2.3. Analysis of Saxitoxin (STX) and Its Analogues in A. hispidus and A. nigropunctatus from the Solomon Islands and Okinawa, Japan
3. Discussion
4. Experimental Section
4.1. Standards and Reagents
4.2. Sampling and Test Materials
4.3. Preliminary Analysis of TTXs in the Skin of A. hispidus from the Solomon Islands Using LC-FLD for TTXs
4.4. Sample Preparation for HR-LC-MS Analysis
4.4.1. From the Ethanolic Solution Used to Soak the Pufferfish Organs from the Solomon Islands
4.4.2. From the Organs of Solomon Islands Pufferfish Soaked in Ethanolic Solution, and from the Frozen Organs of Pufferfish from Okinawa, Japan
4.5. LC-FLD for TTXs
4.6. High Resolution LC-MS and LC-MS/MS for TTXs
4.7. High Resolution LC-MS for STXs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yokoo, A. Chemical studies on tetrodotoxin Rept. III. Isolation of spheroidine. J. Chem. Soc. Japan 1950, 71, 591–592. [Google Scholar]
- Noguchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Harada, T. Occurrence of tetrodotoxin in the Japanese ivory shell Babylonia japonica. Bull. Japan. Soc. Sci. Fish. 1981, 47, 901–913. [Google Scholar] [CrossRef]
- Yasumura, D.; Oshima, Y.; Yasumoto, T.; Alcala, A.C.; Alcala, L.C. Tetrodotoxin and paralytic shellfish toxins in Philippine crabs. Agric. Biol. Chem. 1986, 50, 593–598. [Google Scholar] [CrossRef]
- Maruyama, J.; Noguchi, T.; Narita, H.; Jeon, J.K.; Otsuka, M.; Hashimoto, K. Occurrence of tetrodotoxin in a starfish, Astropecten scoparius. Agric. Biol. Chem. 1985, 49, 3069–3070. [Google Scholar] [CrossRef]
- Sheumack, D.D.; Howden, M.E.H.; Spence, I.; Quinn, R.J. Maculotoxin: A Neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin. Science 1978, 199, 188–189. [Google Scholar] [CrossRef] [PubMed]
- McNabb, P.; Selwood, A.I.; Munday, R.; Wood, S.A.; Taylor, D.I.; MacKenzie, A.L.; van Ginkel, R.; Rhodes, L.L.; Cornelisen, C.; Heasman, K.; et al. Detection of tetrodotoxin from the grey sided-gilled sea slug-Pleurobranchaea maculata, and associated dog neurotoxicosis on beaches adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon 2010, 56, 466–473. [Google Scholar] [CrossRef] [PubMed]
- McNabb, P.S.; Taylor, D.I.; Ogilvie, S.C.; Wilkinson, L.; Anderson, A.; Hamon, D.; Wood, S.A.; Peake, B.M. First detection of tetrodotoxin in the bivalve Paphies australis by liquid chromatography coupled to triple quadrupole mass spectrometry with and without precolumn reaction. J. AOAC Int. 2014, 97, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. Euro Surveill. 2015, 20. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21009 (accessed on 22 August 2015). [Google Scholar]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First Detection of tetrodotoxin in Greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Furman, F.A. Pharmacological studies on tetrodotoxin, a potent neurotoxin. J. Pharmacol. Exp. Ther. 1963, 140, 31–40. [Google Scholar] [PubMed]
- Moore, J.; Anderson, N.; Narahashi, T. Tetrodoixin blocking early conductance channel on sodium. Fed. Proc. Am. Soc. Exp. Biol. 1966, 25, 569–572. [Google Scholar]
- Yasumoto, T.; Yasumura, D.; Yotsu, M.; Michishita, T.; Endo, A.; Kotaki, Y. Bacterial production of tetrodotoxin and anhydrotetrodotoxin. Agric. Biol. Chem. 1986, 50, 793–795. [Google Scholar] [CrossRef]
- Noguchi, T.; Jeon, J.K.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of axantihd crab, Atergatis floridus. J. Biochem. 1986, 99, 311–314. [Google Scholar] [PubMed]
- Hanifin, C.T. The chemical and evolutionary ecology of tetrodotoxin (TTX) toxicity in terrestrial tertebrates. Mar. Drugs 2010, 8, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Ritson-Williams, R.; Yotsu-Yamashita, M.; Paul, V. Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc. Natl. Acad. Sci. USA 2006, 103, 3176–3179. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M. Chemistry of puffer fish toxin. J. Toxicol. Toxin Rev. 2001, 20, 51–66. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Abe, Y.; Kudo, Y.; Ritson-Williams, R.; Paul, V.J.; Konoki, K.; Cho, Y.; Adachi, M.; Imazu, T.; Nishikawa, T.; et al. First identification of 5,11-dideoxytetrodotoxin in marine animals, and characterization of major fragment ions of tetrodotoxin and its analogs by high resolution ESI-MS/MS. Mar. Drugs 2013, 11, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Yamashita, Y.; Mebs, D.; Cho, Y.; Konoki, K.; Yasumoto, T.; Yotsu-Yamashita, M. C5–C10 directly bonded tetrodotoxin analogues: possible biosynthetic precursors of tetrodotoxin from newts. Angew. Chem. Int. Ed. 2014, 53, 14546–14549. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Yotsu, M.; Murata, M.; Naoki, H. New tetrodotoxin analogue from the newt Cynops ensicauda. J. Am. Chem. Soc. 1988, 110, 2344–2345. [Google Scholar] [CrossRef]
- Jang, J.H.; Yotsu-Yamashita, M. 6,11-DideoxyTTX from the puffer fish, Fugu pardalis. Toxicon 2007, 50, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Yamagishi, Y.; Yasumoto, T. 5,6,11-Trideoxytetrodotoxin from the puffer fish, Fugu poecilonotus. Tetrahedron Lett. 1995, 36, 9329–9332. [Google Scholar] [CrossRef]
- Khora, S.S.; Yasumoto, T. Isolation of 11-oxotetrodotoxin from the puffer Arothron nigropunctatus. Tetrahedron Lett. 1989, 30, 4393–4394. [Google Scholar] [CrossRef]
- Wu, B.Q.; Yang, L.; Kao, C.Y.; Levinson, S.R.; Yotsu-Yamashita, M.; Yasumoto, T. 11-Oxo-tetrodotoxin and a specifically labelled 3H-tetrodotoxin. Toxicon 1996, 34, 407–416. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Sugimoto, A.; Takai, A.; Yasumoto, T. Effects of specific modifications of several hydroxyls of tetrodotoxin on its affinity to rat brain membrane. J. Pharmacol. Exp. Ther. 1999, 289, 1688–1696. [Google Scholar] [PubMed]
- Brillantes, S.; Samosorn, W.; Faknoi, S.; Oshima, Y. Toxicity of puffers landed and marketed in Thailand. Fish. Sci. 2003, 69, 1224–1230. [Google Scholar] [CrossRef]
- Islam, Q.T.; Razzak, M.A.; Islam, M.A.; Bari, M.I.; Basher, A.; Chowdhury, F.R.; Sayeduzzaman, A.B.M.; Ahasan, H.A.M.N.; Faiz, M.A.; Arakawa, O.; et al. Puffer fish poisoning in Bangladesh: clinical and toxicological results from large out breaks in 2008. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.K.; Tsui, S.H.; Tong, H.K. A case series of puffer fish poisoning, Hong Kong. J. Emerg. Med. 2007, 14, 215–220. [Google Scholar]
- Yong, Y.S.; Quek, L.S.; Lim, E.K.; Ngo, A. A case report of puffer fish poisoning in Singapore. Case Rep. Med. 2013, 206971. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Onuki, K.; Arakawa, O. Tetrodotoxin poisoning due to pufferfish and gastropods, and their intoxication mechanism. Toxicology 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Bentur, Y.; Ashkar, J.; Lurie, Y.; Levy, Y.; Azzam, Z.S.; Litmanovich, M.; Golik, M.; Gurevych, B.; Golani, D.; Eisenman, A. Lessepsian migration and tetrodotoxin poisoning due to Lagocephalus sceleratus in the eastern Mediterranean. Toxicon 2008, 52, 964–968. [Google Scholar] [CrossRef]
- Kheifets, J.; Rozhavsky, B.; Girsh, S.Z.; Marianna, R.; Soroksky, A. Severe tetrodotoxin poisoning after consumption of Lagocephalus sceleratus (pufferfish, fugu) fished in Mediterranean Sea, treated with cholinesterase inhibitor. Case Rep. Crit. Care 2012, 2012, 782507. [Google Scholar]
- Cohen, N.J.; Deeds, J.R.; Wong, E.S.; Hanner, R.H.; Yancy, H.F.; White, K.D.; Thompson, T.M.; Wahl, M.; Pham, T.D.; Guichard, F.M.; et al. Public health response to puffer fish (tetrodotoxin) poisoning from mislabeled product. J. Food Prot. 2009, 72, 810–817. [Google Scholar] [PubMed]
- Cole, J.B.; Heegaard, W.G.; Deeds, J.R.; McGrath, S.C.; Handy, S.M. Tetrodotoxin poisoning outbreak from imported dried puffer fish—Minneapolis, Minnesota, 2014. Morbidity Mortality Wkly Rep. 2015, 63, 1222–1225. [Google Scholar]
- Loison, G. Poisonous fish of the South Pacific. Noumea, New Caledonia: South Pacific commission. SPC Q. Bull. 1955, 5, 28–31. [Google Scholar]
- Landsberg, J.H.; Hall, S.; Johannessen, J.N.; White, K.D.; Conrad, S.M.; Abbott, J.P.; Flewelling, L.J.; Richardson, R.W.; Dickey, R.W.; Jester, E.L.; et al. Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Perspect. 2006, 114, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Arakawa, O.; Taniyama, S.; Nonaka, M.; Takatani, T.; Yamamori, K.; Fuchi, Y.; Noguchi, T. Occurrence of saxitoxins as a major toxin in the ovary of a marine puffer Arothron firmamentum. Toxicon 2004, 43, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Oshima, Y.; Yasumoto, T. Occurrence of saxitoxin in puffer fish. Toxicon 1984, 22, 381–385. [Google Scholar] [CrossRef]
- Sato, S.; Kodama, M.; Ogata, T.; Saitanu, K.; Furuya, M.; Hirayama, K.; Kakinuma, K. Saxitoxin as a toxic principle of a freshwater puffer, Tetraodon fangi, in Thailand. Toxicon 1997, 35, 137–140. [Google Scholar] [CrossRef]
- HP of Ministry of Health, Labour and Welfare of Japan. Available online: http://www.mhlw.go.jp/topics/syokuchu/poison/animal_01.html (accessed on 22 August 2015). (In Japanese)
- Nakamura, M.; Yasumoto, T. Tetrodotoxin derivatives in puffer fish. Toxicon 1985, 23, 271–276. [Google Scholar] [CrossRef]
- Yasumoto, T.; Michishita, T. Fluorometric determination of tetrodotoxin by high performance liquid chromatography. Agric. Biol. Chem. 1985, 49, 3077–3080. [Google Scholar] [CrossRef]
- Shoji, Y.; Yotsu-Yamashita, M.; Miyazawa, T.; Yasumoto, T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid Chromatography/ Mass Spectrometry, Tandem Mass Spectrometry, and Liquid Chromatography/Tandem Mass Spectrometry. Anal. Biochem. 2001, 290, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Jang, J.; Yotsu-Yamashita, M. Hydrophilic interaction liquid chromatography-electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006, 352, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Lee, J.S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackish water puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Jang, J.H.; Cho, Y.; Konoki, K. Optimization of simultaneous analysis of tetrodotoxin, 4-epitetrodotoxin, 4,9-anhydrotetrodotoxin, and 5,6,11-trideoxytetrodotoxin by hydrophilic interaction liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2011, 29, 61–64. [Google Scholar] [CrossRef]
- Kudo, Y.; Yasumoto, T.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Isolation and structural determination of the first 8-epi-type tetrodotoxin analogs from the newt, Cynops ensicauda popei, and comparison of tetrodotoxin analogs profiles of this newt and the puffer fish, Fugu poecilonotus. Mar. Drugs 2012, 10, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Khora, S.S.; Isa, J.; Yasumoto, T. Toxicity of puffers from Okinawa, Japan. Nippon Suisan Gakkaishi 1991, 57, 163–167. [Google Scholar] [CrossRef]
- Teruya, N.; Oshiro, N.; Tamanaha, K. Toxicity of puffers from Okinawa. Available online: http://www.pref.okinawa.jp/site/hoken/eiken/syoho/documents/s40_93-98.pdf (accessed on 24 August 2015). (In Japanese)
- Campbell, S.; Harada, R.M.; DeFelice, S.V.; Bienfang, P.K.; Li, Q.X. Bacterial production of tetrodotoxin in the pufferfish Arothron hispidus. Nat. Product Res. Part A 2009, 17, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Sugimoto, A.; Terakawa, T.; Shoji, Y.; Miyazawa, T.; Yasumoto, T. Purification, characterization, and cDNA cloning of a novel soluble saxitoxin and tetrodotoxin binding protein from plasma of the pufferfish, Fugu pardalis. Eur. J. Biochem. 2001, 268, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Okoshi, N.; Watanabe, K.; Araki, N.; Yamaki, H.; Shoji, Y.; Terakawa, T. Localization of pufferfish saxitoxin and tetrodotoxin binding protein (PSTBP) in the tissues of the pufferfish, Takifugu pardalis, analyzed by immunohistochemical staining. Toxicon 2013, 72, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Lee, J.M.; Shiraishik, M.; Komatsu, S.; Miki, S.; Shimasaki, Y.; Mochioka, N.; Kusakabe, T.; Oshima, Y. Characterization and evolutionary analysis of tributyltin-binding protein and pufferfish saxitoxin and tetrodotoxin-binding protein genes in toxic anc nontoxic pufferfish. J. Evol. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Pires, O.R.; Sebbena, A.; Schwartza, E.F.; Bloch, C.J.; Rodrigo, A.V.; Morales, R.A.V.; Schwartza, C.A. The occurrence of 11-oxotetrodotoxin, a rare tetrodotoxin analogue, in the brachycephalidae frog Brachycephalus ephippium. Toxicon 2003, 42, 563–566. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Mebs, D. Occurrence of 11-oxotetrodotoxin in the red-spotted newt, Notophthalmus viridescens, and further studies on the levels of tetrodotoxin and its analogues in the newt efts. Toxicon 2003, 41, 893–897. [Google Scholar] [CrossRef]
- Kudo, Y.; Finn, J.; Fukushima, K.; Sakugawa, S.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Isolation of 6-deoxytetrodotoxin from the pufferfish, Takifugu pardalis, and a comparison of the effects of the C-6 and C-11 hydroxy groups of tetrodotoxin on its activity. J. Nat. Prod. 2014, 77, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, S.; Isami, Y.; Matsumoto, T.; Nagashima, Y.; Takatani, T.; Arakawa, O. Toxicity and toxin profile of tetrodotoxin detected in the scavenging gastropod Nassarius (Alectrion) glans. “Kinshibai”. Shokuhin Eiseigaku Zasshi 2009, 50, 22–28. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Arakawa, O.; Noguchi, T.; Shida, Y.; Onoue, Y. Occurrence of 11-oxotetrodotoxin and 11-nortetrodotoxin-6(R)-ol in a xanthid crab Atergatis floridus collected at Kojima, Ishigaki Island. Fish. Sci. 1994, 60, 769–771. [Google Scholar]
- Codex Alimentarius, CODEX STAN 292-2008. Available online: http://www.codexalimentarius.org/input/download/standards/11109/CXS_292e.pdf (accessed on 22 August 2015).
- EU Regulation 853/2004. Regulation (EC) no. 853/2004 of the European Parliament and of the Council of 29 April 2004. Laying down specific hygiene rules for food of animal origin. Off. J. Eur. Commun. 2004, L226, 22–82. [Google Scholar]
- U.S. Food and Drug Administration. National Shellfish Sanitation Program Guide for the Control of Molluscan Shellfish 2011 Revision. Available online: http://www.fda.gov/downloads/Food/GuidanceRegulation/FederalStateFoodPrograms/UCM350344.pdf (accessed on 22 August 2015).
- Veterinary Sanitation Division Director-General. Notification No. 30 of Veterinary Sanitation Division, from the Veterinary Sanitation Division Director-General, Veterinary Sanitation Division, Environmental Health Bureau, Ministry of Health, Labour and Welfare, 1980. Available online: http://www.mhlw.go.jp/topics/syokuchu/kanren/kanshi/s58_1202-2.html (accessed on 22 August 2015). (In Japanese)
- Oshima, Y. Postcolumn derivatization liquid chromatographic method for paralytic shellfish toxins. J. AOAC Int. 1995, 78, 528–532. [Google Scholar]
- Dell’Aversano, C.; Hess, P.; Quilliam, M.A. Hydrophilic interaction liquid chromatography-mass spectrometry for the analysis of paralytic shellfish poisoning (PSP) toxins. J. Chromatogr. A 2005, 1081, 190–201. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puilingi, C.G.; Kudo, Y.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Tetrodotoxin and Its Analogues in the Pufferfish Arothron hispidus and A. nigropunctatus from the Solomon Islands: A Comparison of Their Toxin Profiles with the Same Species from Okinawa, Japan. Toxins 2015, 7, 3436-3454. https://doi.org/10.3390/toxins7093436
Puilingi CG, Kudo Y, Cho Y, Konoki K, Yotsu-Yamashita M. Tetrodotoxin and Its Analogues in the Pufferfish Arothron hispidus and A. nigropunctatus from the Solomon Islands: A Comparison of Their Toxin Profiles with the Same Species from Okinawa, Japan. Toxins. 2015; 7(9):3436-3454. https://doi.org/10.3390/toxins7093436
Chicago/Turabian StylePuilingi, Clyde Gorapava, Yuta Kudo, Yuko Cho, Keiichi Konoki, and Mari Yotsu-Yamashita. 2015. "Tetrodotoxin and Its Analogues in the Pufferfish Arothron hispidus and A. nigropunctatus from the Solomon Islands: A Comparison of Their Toxin Profiles with the Same Species from Okinawa, Japan" Toxins 7, no. 9: 3436-3454. https://doi.org/10.3390/toxins7093436
APA StylePuilingi, C. G., Kudo, Y., Cho, Y., Konoki, K., & Yotsu-Yamashita, M. (2015). Tetrodotoxin and Its Analogues in the Pufferfish Arothron hispidus and A. nigropunctatus from the Solomon Islands: A Comparison of Their Toxin Profiles with the Same Species from Okinawa, Japan. Toxins, 7(9), 3436-3454. https://doi.org/10.3390/toxins7093436





