Subacute Microcystin-LR Exposure Alters the Metabolism of Thyroid Hormones in Juvenile Zebrafish (Danio Rerio)
Abstract
:1. Introduction
2. Results
2.1. Whole-Body Thyroid Hormone Levels
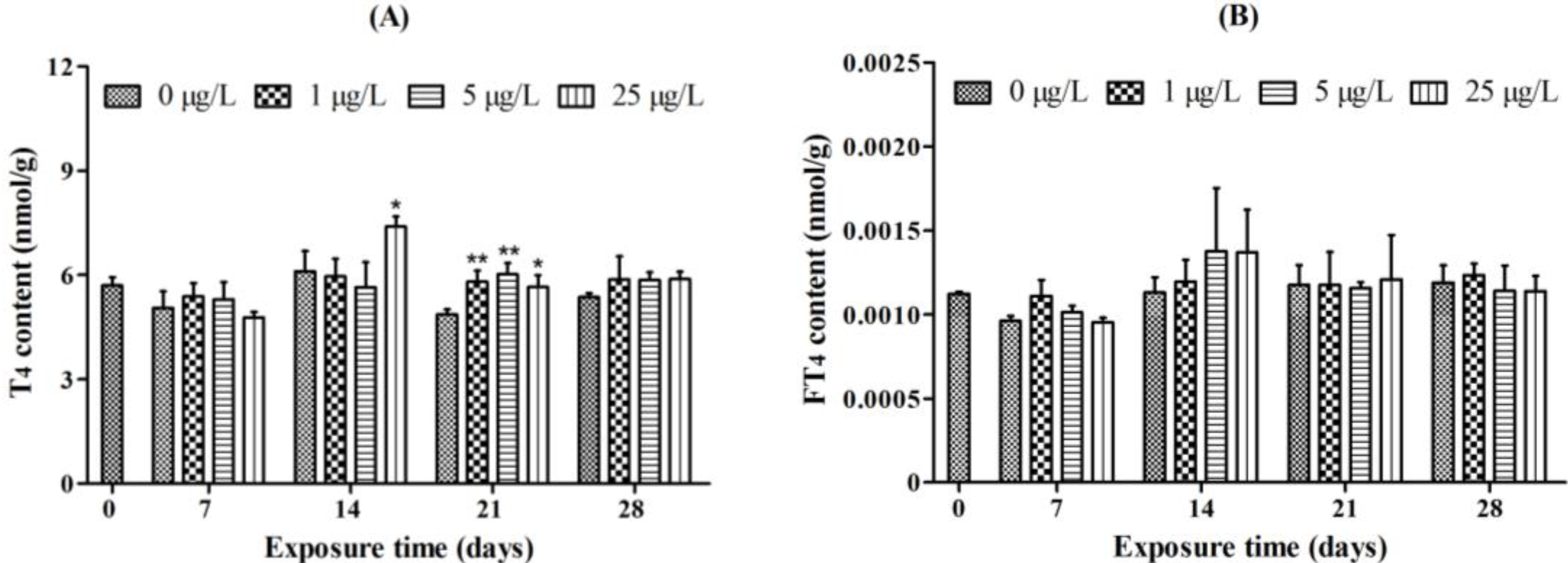
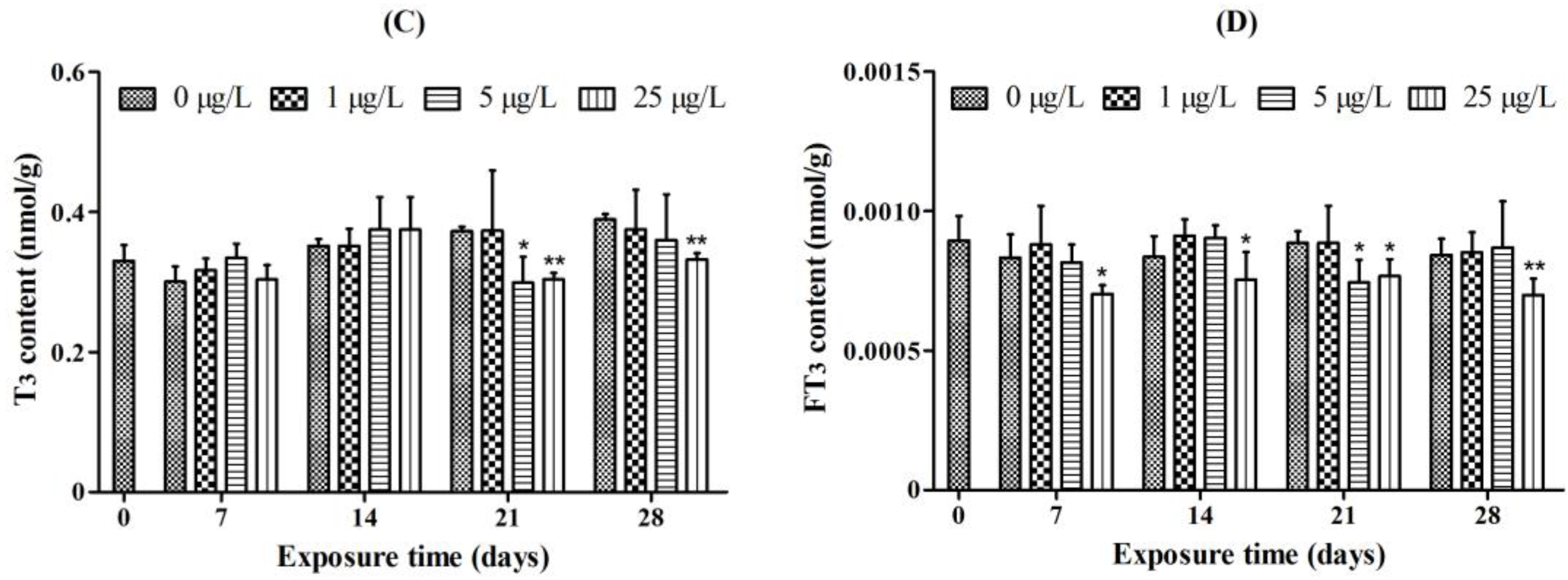
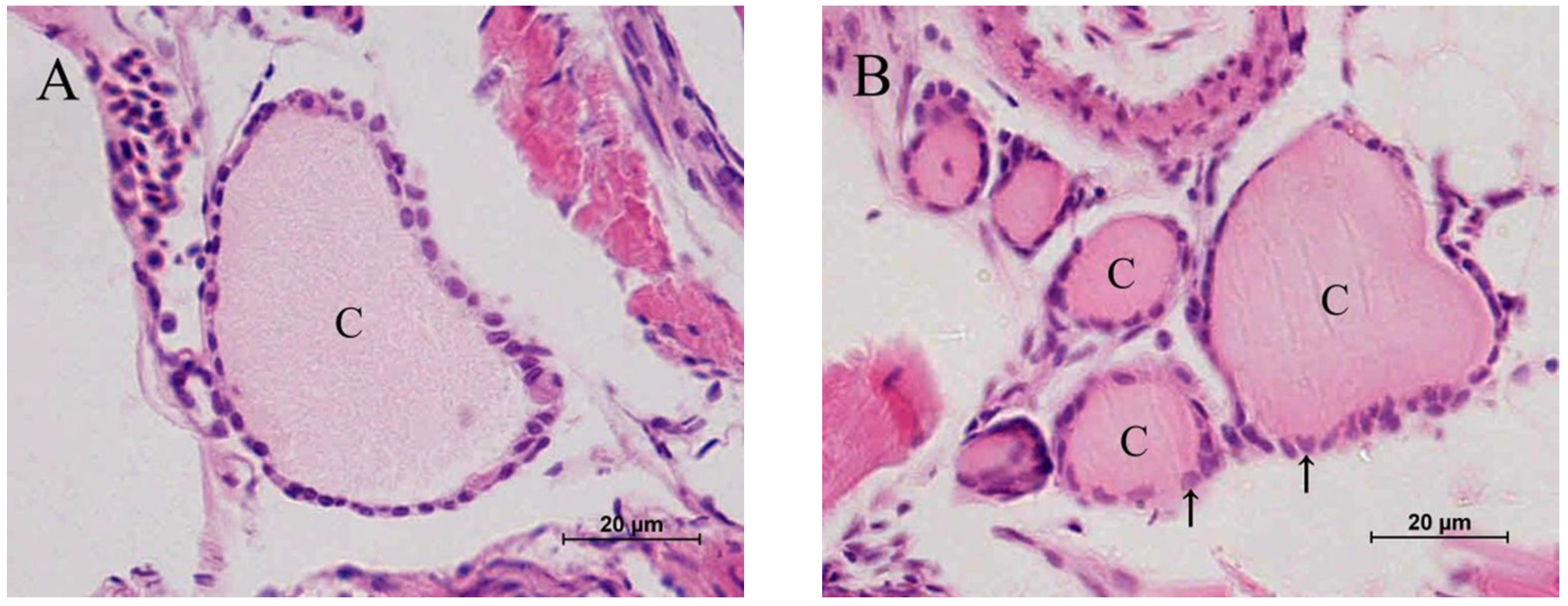
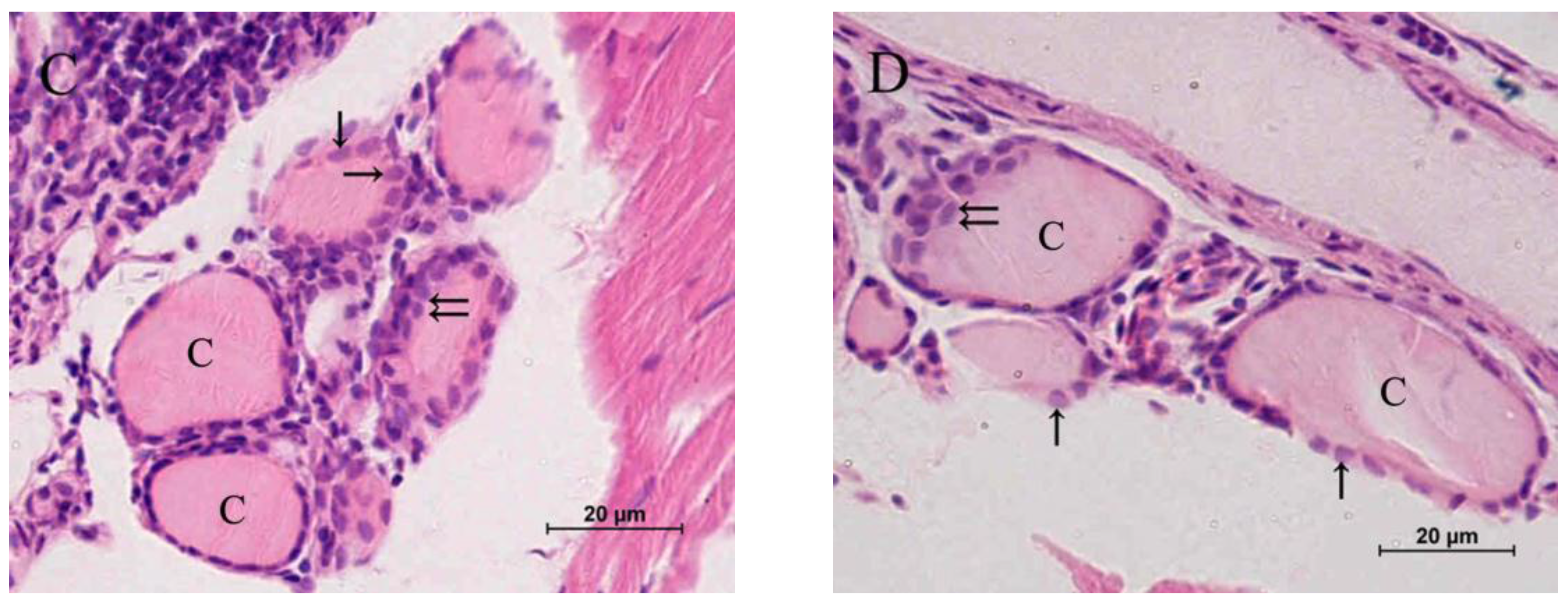
2.2. Histopathology
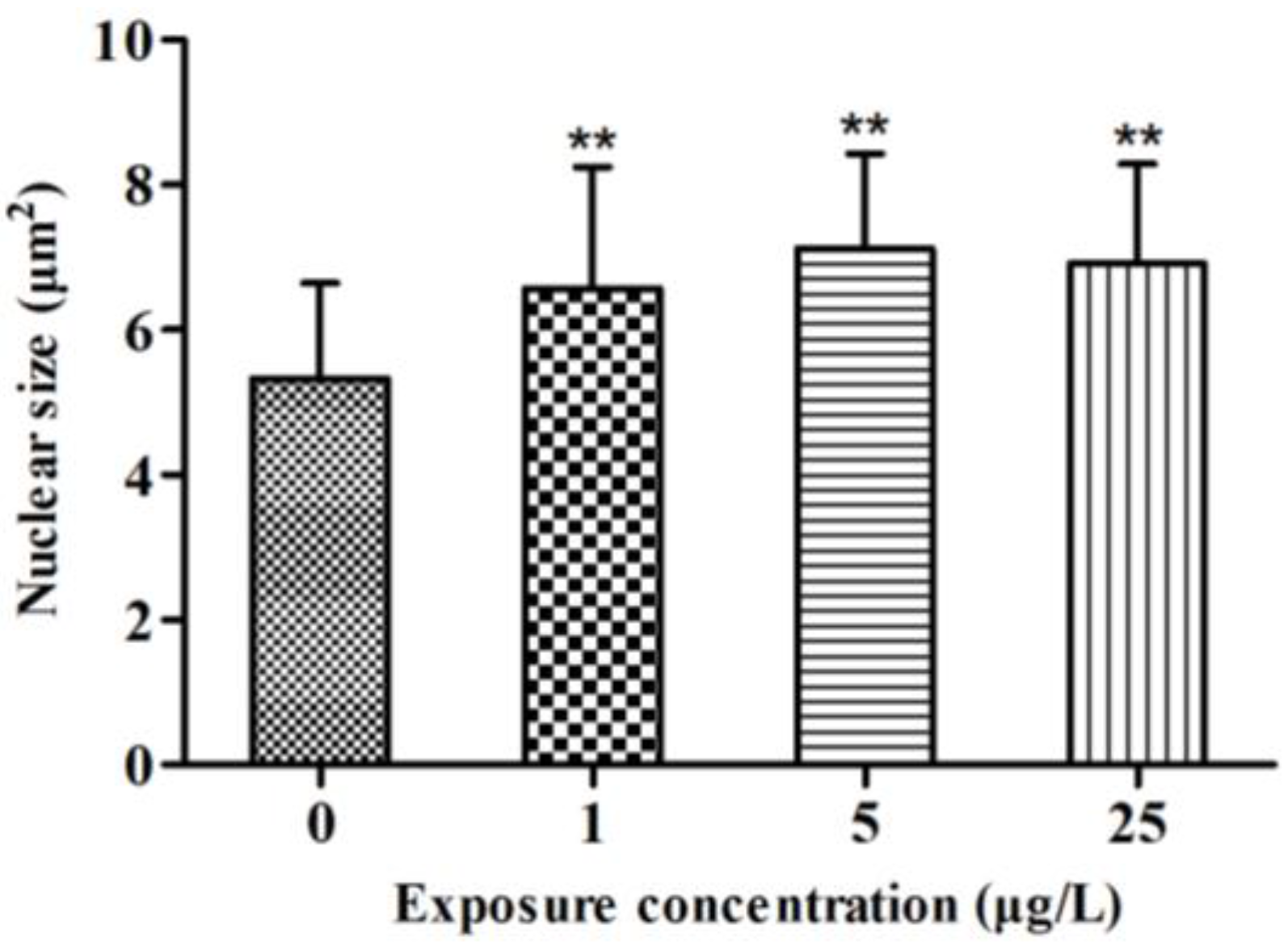
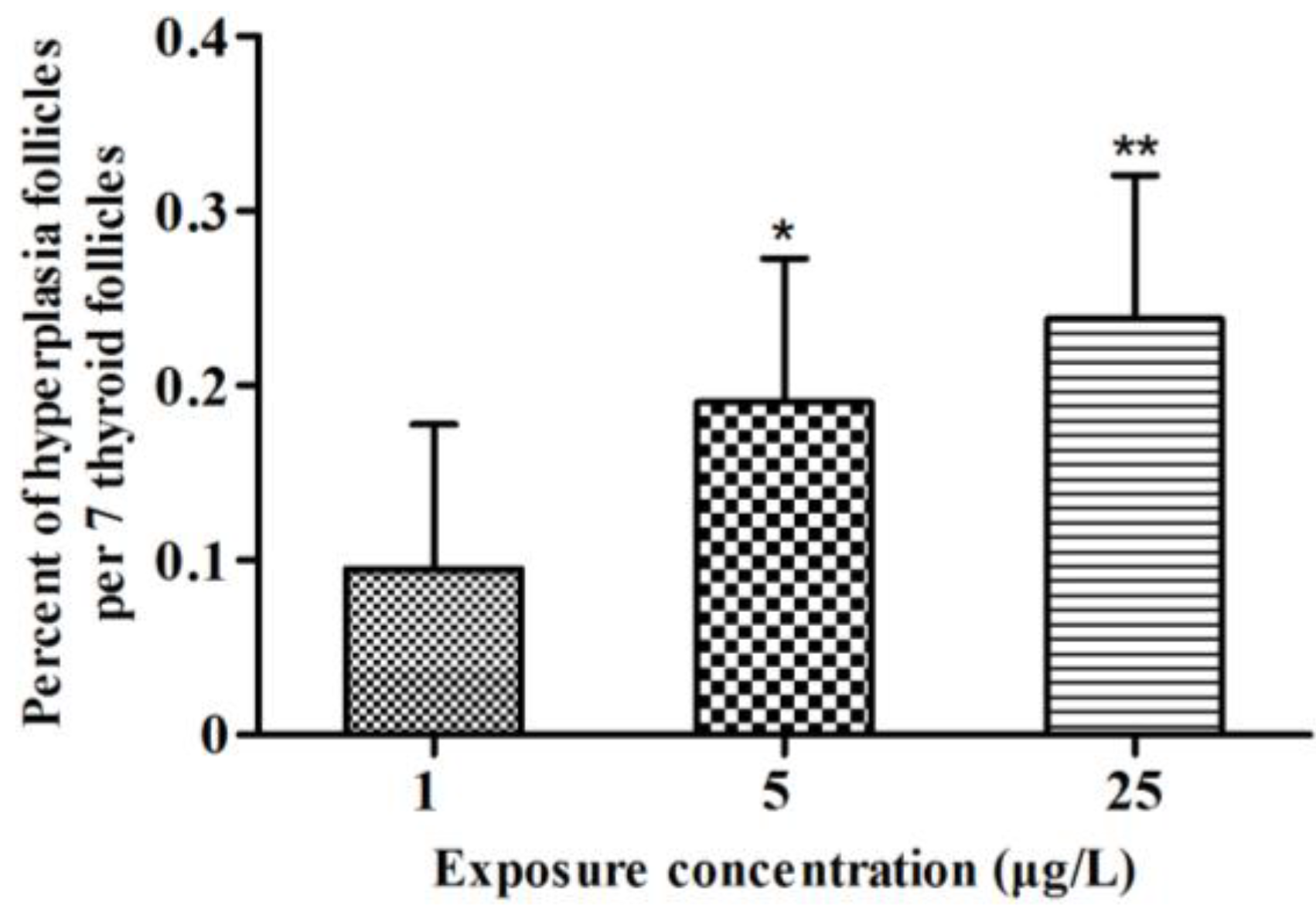
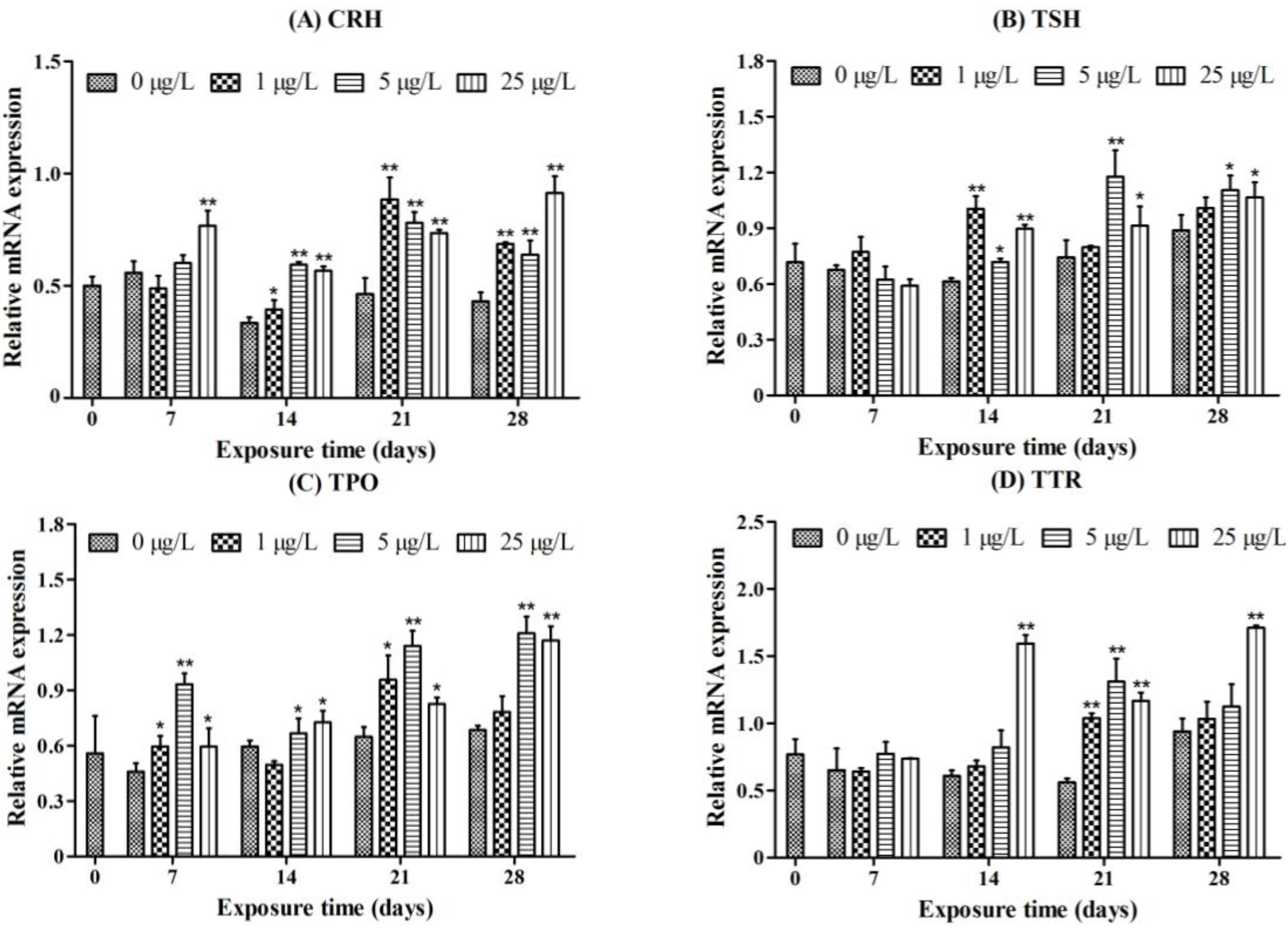
2.3. Gene Transcription Profile
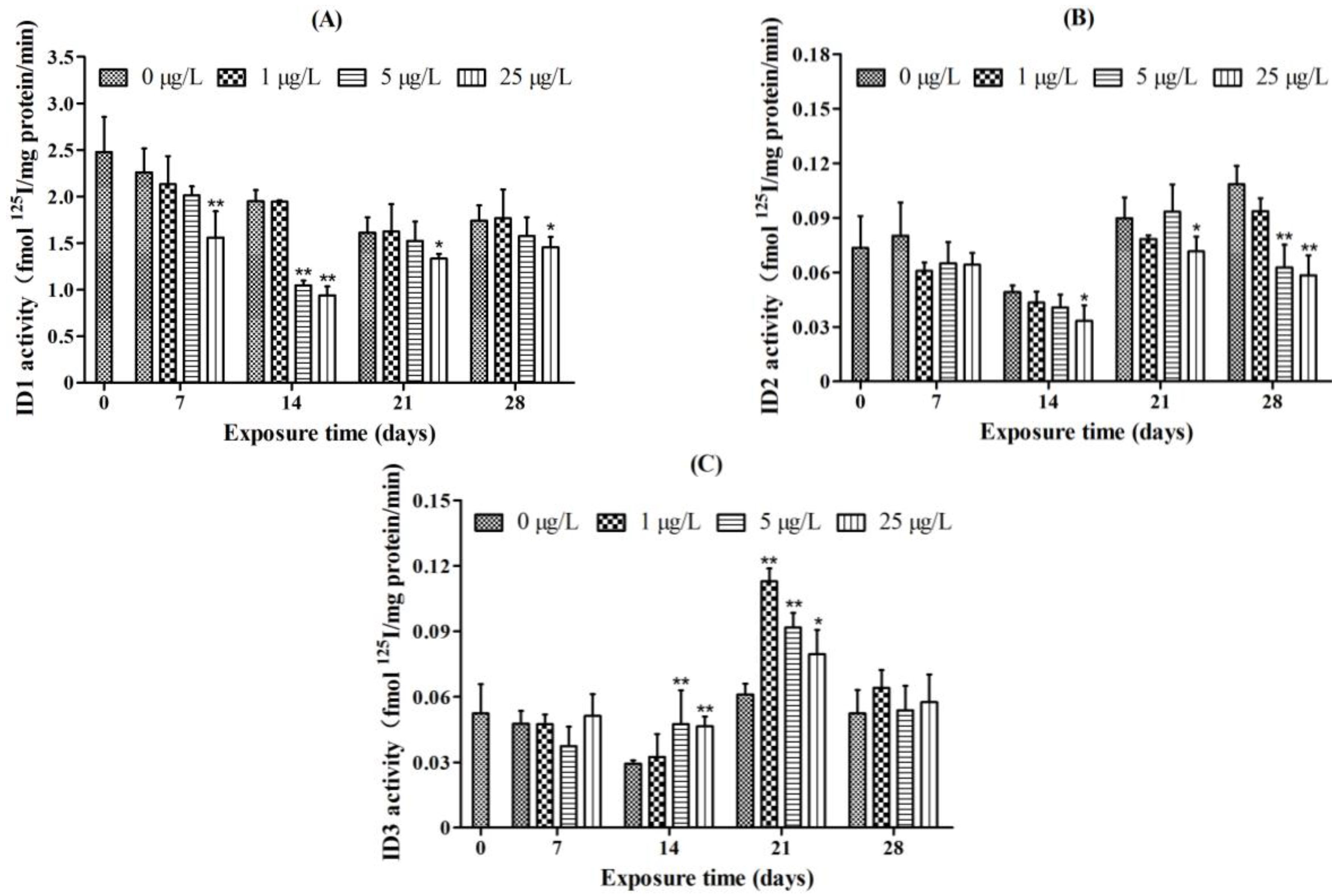
2.4. Iodothyronine Deiodinase Activities
3. Discussion
4. Materials and Methods
4.1. Chemicals and Fish
4.2. Experimental Design
| Items | MC-LR concentrations (μg/L) | |||
|---|---|---|---|---|
| Target doses of MC-LR | control | 1.0 | 5.0 | 25.0 |
| Measured MC-LR in solutions | 0.00 | 0.88 ± 0.06 | 4.30 ± 0.35 | 23.29 ± 0.79 |
4.3. Thyroid Hormone Extraction and Measurement
4.4. Histology
4.5. Gene Expression
4.5.1. RNA Extraction and Reverse Transcription
| Gene | Sequence of the primers (5ʹ→3ʹ) | Genbank accession No. |
|---|---|---|
| GAPDH | F: CTGGTGACCCGTGCTGCTT | NM001115114 |
| R: TTTGCCGCCTTCTGCCTTA | ||
| CRH | F: TTCGGGAAGTAACCACAAGC | NM001007379 |
| R: CTGCACTCTATTCGCCTTCC | ||
| TSH | F: GCAGATCCTCACTTCACCTACC | AY135147 |
| R: GCACAGGTTTGGAGCATCTCA | ||
| TPO | F: GCGCTTGGAACACAGTATCA | EU267076 |
| R: CTTCAGCACCAAACCCAAAT | ||
| TTR | F: CGGGTGGAGTTTGACACTTT | BC081488 |
| R: GCTCAGAAGGAGAGCCAGTG |
4.5.2. Real-Time PCR
4.6. Deiodinase Activity Assays
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Kondo, F.; Ito, Y.; Oka, H.; Yamada, S.; Tsuji, K.; Imokawa, M.; Niimi, Y.; Harada, K.-I.; Ueno, Y.; Miyazaki, Y. Determination of microcystins in lake water using reusable immunoaffinity column. Toxicon 2002, 40, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Luckas, B.; Dahlmann, J.; Erler, K.; Gerdts, G.; Wasmund, N.; Hummert, C.; Hansen, P. Overview of key phytoplankton toxins and their recent occurrence in the north and baltic seas. Environ. Toxicol. 2005, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zimba, P.V.; Khoo, L.; Gaunt, P.S.; Brittain, S.; Carmichael, W.W. Confirmation of catfish, Ictalurus punctatus (Rafinesque), mortality from microcystis toxins. J. Fish Dis. 2001, 24, 41–47. [Google Scholar] [CrossRef]
- Jiang, J.; Gu, X.; Song, R.; Wang, X.; Yang, L. Microcystin-LR induced oxidative stress and ultrastructural alterations in mesophyll cells of submerged macrophyte Vallisneria natans (Lour.) Hara. J. Hazard. Mater. 2011, 190, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Malbrouck, C.; Kestemont, P. Effects of microcystins on fish. Environ. Toxicol. Chem. 2006, 25, 72–86. [Google Scholar] [PubMed]
- Zhao, M.; Xie, S.; Zhu, X.; Yang, Y.; Gan, L.; Song, L. Effect of inclusion of blue-green algae meal on growth and accumulation of microcystins in gibel carp (Carassius auratus gibelio). J. Appl. Ichthyol. 2006, 22, 72–78. [Google Scholar] [CrossRef]
- Ding, X.-S.; Li, X.-Y.; Duan, H.-Y.; Chung, I.-K.; Lee, J. Toxic effects of Microcystis cell extracts on the reproductive system of male mice. Toxicon 2006, 48, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.S.; Guha, S. Microcystin toxicity in a freshwater fish, Heteropneustes fossilis (Bloch). Curr. Sci. 2006, 91, 1261–1271. [Google Scholar]
- Li, L.; Xie, P.; Chen, J. In vivo studies on toxin accumulation in liver and ultrastructural changes of hepatocytes of the phytoplanktivorous bighead carp i.p.-injected with extracted microcystins. Toxicon 2005, 46, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.J.; Dietrich, D.R. Pathological and biochemical characterization of microcystin-induced hepatopancreas and kidney damage in carp (Cyprinus carpio). Toxicol. Appl. Pharmacol. 2000, 164, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Malbrouck, C.; Trausch, G.; Devos, P.; Kestemont, P. Hepatic accumulation and effects of microcystin-LR on juvenile goldfish Carassius auratus L. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2003, 135, 39–48. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, P.; Li, D.; Shi, Z. Hematological and plasma biochemical responses of crucian carp (Carassius auratus) to intraperitoneal injection of extracted microcystins with the possible mechanisms of anemia. Toxicon 2007, 49, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, P.; Zhang, X. Changes in plasma thyroid hormones and cortisol levels in crucian carp (Carassius auratus) exposed to the extracted microcystins. Chemosphere 2008, 74, 13–18. [Google Scholar] [PubMed]
- Yan, W.; Zhou, Y.; Yang, J.; Li, S.; Hu, D.; Wang, J.; Chen, J.; Li, G. Waterborne exposure to microcystin-LR alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis in zebrafish larvae. Chemosphere 2012, 87, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.D.; Henry, T.B.; Twiner, M.J.; Gouffon, J.S.; McPherson, J.T.; Boyer, G.L.; Sayler, G.S.; Wilhelm, S.W. Global gene expression profiling in larval zebrafish exposed to microcystin-LR and microcystis reveals endocrine disrupting effects of cyanobacteria. Environ. Sci. Technol. 2011, 45, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, J.; Xie, P.; Jiang, Y.; Wu, L.; Zhang, X. Protein expression profiling in the zebrafish (Danio rerio) embryos exposed to the microcystin-LR. Proteomics 2011, 11, 2003–2018. [Google Scholar] [CrossRef] [PubMed]
- Zaccaroni, A.; Gamberoni, M.; Mandrioli, L.; Sirri, R.; Mordenti, O.; Scaravelli, D.; Sarli, G.; Parmeggiani, A. Thyroid hormones as a potential early biomarker of exposure to 4-nonylphenol in adult male shubunkins (Carassius auratus). Sci. Total Environ. 2009, 407, 3301–3306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; John-Alder, H.; Weis, J.; Weis, P. Endocrine disruption: Thyroid dysfunction in mummichogs (Fundulus heteroclitus) from a polluted habitat. Mar. Environ. Res. 2000, 50, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, L.; Yang, L.; Zhou, B. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat. Toxicol. 2012, 110–111, 141–148. [Google Scholar] [CrossRef] [PubMed]
- De Groef, B.; van der Geyten, S.; Darras, V.M.; Kühn, E.R. Role of corticotropin-releasing hormone as a thyrotropin-releasing factor in non-mammalian vertebrates. Gen. Comp. Endocrinol. 2006, 146, 62–68. [Google Scholar]
- Yu, L.; Deng, J.; Shi, X.; Liu, C.; Yu, K.; Zhou, B. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis of zebrafish larvae. Aquat. Toxicol. 2010, 97, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Morgado, I.; Santos, C.R.A.; Jacinto, R.; Power, D.M. Regulation of transthyretin by thyroid hormones in fish. Gen. Comp. Endocrinol. 2007, 152, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chang, J.; Zhao, Y.; Zhu, G. Changes of thyroid hormone levels and related gene expression in zebrafish on early life stage exposure to triadimefon. Environ. Toxicol. Pharmacol. 2011, 32, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.; Valverde, R.C. Thyroid hormone deiodination in fish. Thyroid 2005, 15, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zha, J.; Spear, P.A.; Li, Z.; Yang, L.; Wang, Z. Changes of thyroid hormone levels and related gene expression in Chinese rare minnow (Gobiocypris rarus) during 3-amino-1,2,4-triazole exposure and recovery. Aquat. Toxicol. 2009, 92, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Sawatsubashi, S.; Yamauchi, K. Endocrine disrupting chemicals: Interference of thyroid hormone binding to transthyretins and to thyroid hormone receptors. Mol. Cell. Endocrinol. 2003, 199, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, R.; Wang, L.; Liu, J.; Yang, Y.; Zhou, C.; Liu, W.; Fu, Z. Effects of metolachlor on transcription of thyroid system-related genes in juvenile and adult japanese medaka (Oryzias latipes). Gen. Comp. Endocrinol. 2011, 170, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Picard-Aitken, M.; Fournier, H.; Pariseau, R.; Marcogliese, D.J.; Cyr, D.G. Thyroid disruption in walleye (Sander vitreus) exposed to environmental contaminants: Cloning and use of iodothyronine deiodinases as molecular biomarkers. Aquat. Toxicol. 2007, 83, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Mayer, I. Molecular biomarkers of endocrine disruption in small model fish. Mol. Cell. Endocrinol. 2008, 293, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Balasubramanian, R. Toxicological evaluation of microcystins in aquatic fish species: Current knowledge and future directions. Aquat. Toxicol. 2013, 142, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Fang, X.; Zhang, H.; Dai, J. The thyroid-disrupting effects of long-term perfluorononanoate exposure on zebrafish (Danio rerio). Ecotoxicology 2011, 20, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Eales, J.; Brown, S. Measurement and regulation of thyroidal status in teleost fish. Rev. Fish Biol. Fish. 1993, 3, 299–347. [Google Scholar] [CrossRef]
- Noyes, P.D.; Hinton, D.E.; Stapleton, H.M. Accumulation and debromination of decabromodiphenyl ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicol. Sci. 2011, 122, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, J. Angiogenesis in the thyroid gland. J. Endocrinol. 2000, 166, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Patino, R.; Wainscott, M.R.; Cruz-Li, E.I.; Balakrishnan, S.; McMurry, C.; Blazer, V.S.; Anderson, T.A. Effects of ammonium perchlorate on the reproductive performance and thyroid follicle histology of zebrafish. Environ. Toxicol. Chem. 2003, 22, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Lema, S.C.; Dickey, J.T.; Schultz, I.R.; Swanson, P. Dietary exposure to 2,2',4,4'-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormoneson, P. Dietary exposure to 2,2',4,4'-tetrabromodipheny. Environ. Health Perspect. 2008, 116, 1694. [Google Scholar] [CrossRef] [PubMed]
- Chiamolera, M.I.; Wondisford, F.E. Minireview: Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology 2009, 150, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Yoshiura, Y.; Sohn, Y.C.; Munakata, A.; Kobayashi, M.; Aida, K. Molecular cloning of the cDNA encoding the β subunit of thyrotropin and regulation of its gene expression by thyroid hormones in the goldfish, Carassius auratus. Fish Physiol. Biochem. 1999, 21, 201–210. [Google Scholar] [CrossRef]
- Shi, X.; Liu, C.; Wu, G.; Zhou, B. Waterborne exposure to PFOS causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Chemosphere 2009, 77, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, Y.; Shan, Z.; Yang, L.; Wang, X.; Shi, L. Bioaccumulation, oxidative stress and HSP70 expression in Cyprinus carpio L. exposed to microcystin-LR under laboratory conditions. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2012, 155, 483–490. [Google Scholar] [CrossRef]
- Liu, F.J.; Wang, J.S.; Theodorakis, C.W. Thyrotoxicity of sodium arsenate, sodium perchlorate, and their mixture in zebrafish Danio rerio. Environ. Sci. Technol. 2006, 40, 3429–3436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, H.; Wang, W.; Ru, S. Exposure to monocrotophos pesticide causes disruption of the hypothalamic-pituitary-thyroid axis in adult male goldfish (Carassius auratus). Gen. Comp. Endocrinol. 2013, 193, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.M.; Rinchard, J.; Carr, J.A.; Theodorakis, C. Perchlorate affects thyroid function in eastern mosquitofish (Gambusia holbrooki) at environmentally relevant concentrations. Environ. Sci. Technol. 2005, 39, 5190–5195. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Mousavi, S.M.; Safahieh, A.; Ghatrami, E.R.; Zargham, D. Effects of 4-nonylphenol on balance of steroid and thyroid hormones in sexually immature male yellowfin seabream (Acanthopagrus latus). Environ. Toxicol. 2014, 29, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Seoka, M.; Miyashita, S.; Kumai, H.; Ohta, H. Characterization of transthyretin in the pacific bluefin tuna, Thunnus orientalis. Zoolog. Sci. 2006, 23, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Power, D.M. Identification of transthyretin in fish (Sparus aurata): cDNA cloning and characterisation. Endocrinology 1999, 140, 2430–2433. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-Q.; Zhao, G.-F.; Feng, M.; Wen, W.; Li, K.; Zhang, P.-W.; Peng, X.; Huo, W.-J.; Zhou, H.-D. Chronic exposure to pentachlorophenol alters thyroid hormones and thyroid hormone pathway mRNAs in zebrafish. Environ. Toxicol. Chem. 2014, 33, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Power, D.; Llewellyn, L.; Faustino, M.; Nowell, M.; Björnsson, B.T.; Einarsdottir, I.; Canario, A.; Sweeney, G. Thyroid hormones in growth and development of fish. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2001, 130, 447–459. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Van der Geyten, S.; Mol, K.; Pluymers, W.; Kühn, E.; Darras, V. Changes in plasma T3 during fasting/refeeding in tilapia (Oreochromis niloticus) are mainly regulated through changes in hepatic type II iodothyronine deiodinase. Fish Physiol. Biochem. 1998, 19, 135–143. [Google Scholar]
- Hotz, C.S.; Belonje, B.; Fitzpatrick, D.W.; L’abbé, M.R. A method for the determination of type I iodothyronine deiodinase activity in liver and kidney using 125I-labelled reverse triiodothyronine as a substrate. Clin. Biochem. 1996, 29, 451–456. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Tang, R.; Li, D.; Hu, Q.; Wang, Y. Subacute Microcystin-LR Exposure Alters the Metabolism of Thyroid Hormones in Juvenile Zebrafish (Danio Rerio). Toxins 2015, 7, 337-352. https://doi.org/10.3390/toxins7020337
Liu Z, Tang R, Li D, Hu Q, Wang Y. Subacute Microcystin-LR Exposure Alters the Metabolism of Thyroid Hormones in Juvenile Zebrafish (Danio Rerio). Toxins. 2015; 7(2):337-352. https://doi.org/10.3390/toxins7020337
Chicago/Turabian StyleLiu, Zidong, Rong Tang, Dapeng Li, Qing Hu, and Ying Wang. 2015. "Subacute Microcystin-LR Exposure Alters the Metabolism of Thyroid Hormones in Juvenile Zebrafish (Danio Rerio)" Toxins 7, no. 2: 337-352. https://doi.org/10.3390/toxins7020337
APA StyleLiu, Z., Tang, R., Li, D., Hu, Q., & Wang, Y. (2015). Subacute Microcystin-LR Exposure Alters the Metabolism of Thyroid Hormones in Juvenile Zebrafish (Danio Rerio). Toxins, 7(2), 337-352. https://doi.org/10.3390/toxins7020337







