Plectasin, First Animal Toxin-Like Fungal Defensin Blocking Potassium Channels through Recognizing Channel Pore Region
Abstract
:1. Introduction
2. Results and Discussion
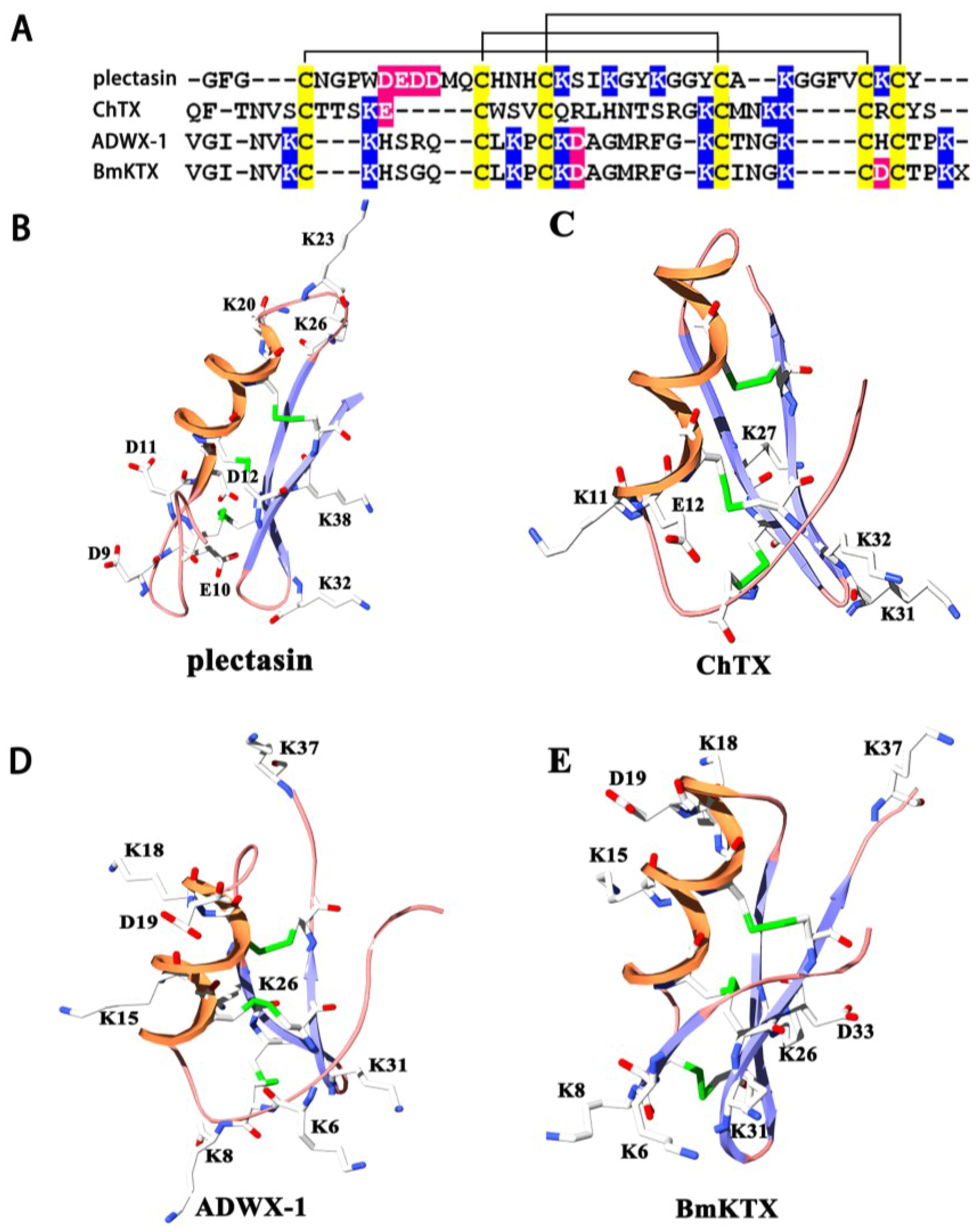
2.1. Structural Commonalities between Plectasin and Scorpion Toxins
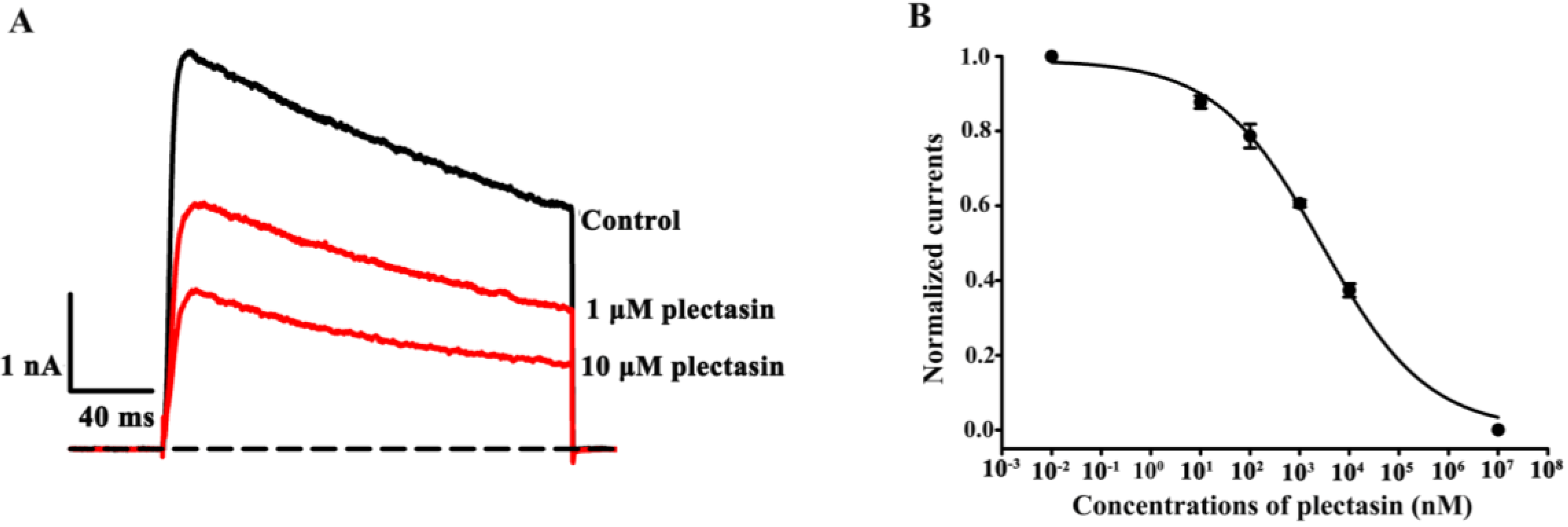
2.2. Plectasin Dose-Dependently Blocks Kv1.3 Channel Currents
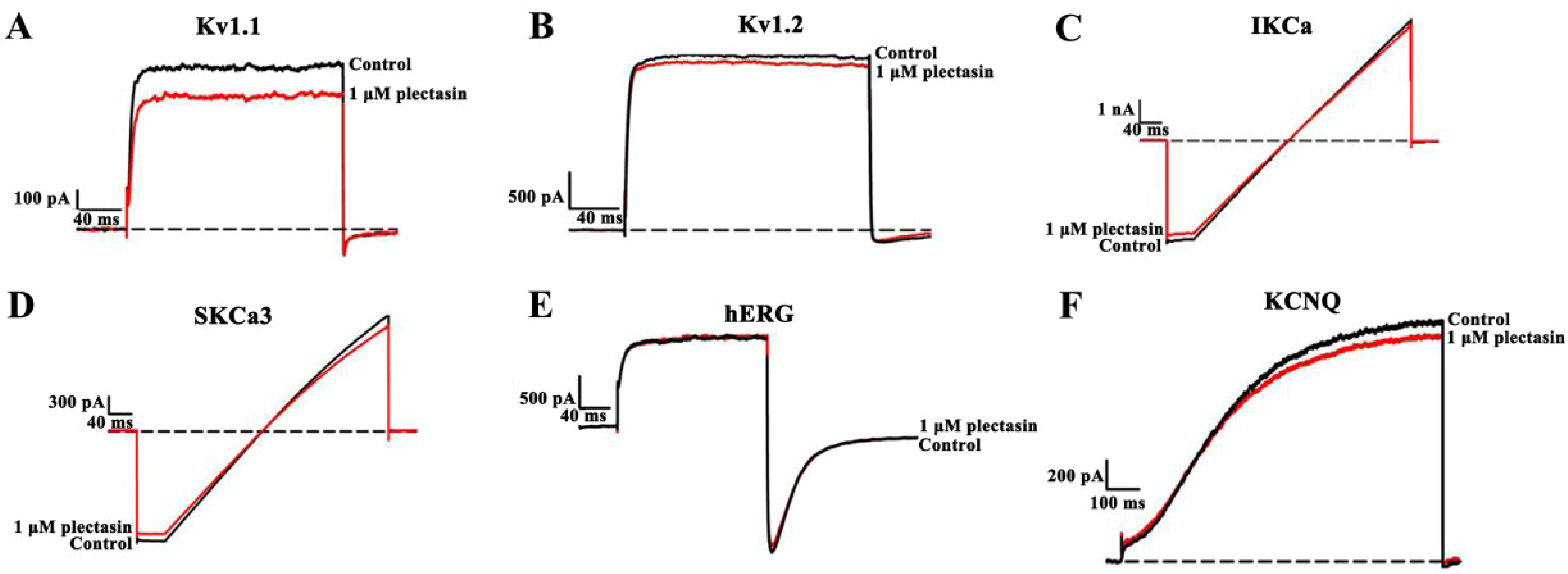
2.3. Plectasin Shows Much Weaker Activities on Other Potassium Channel Subtypes
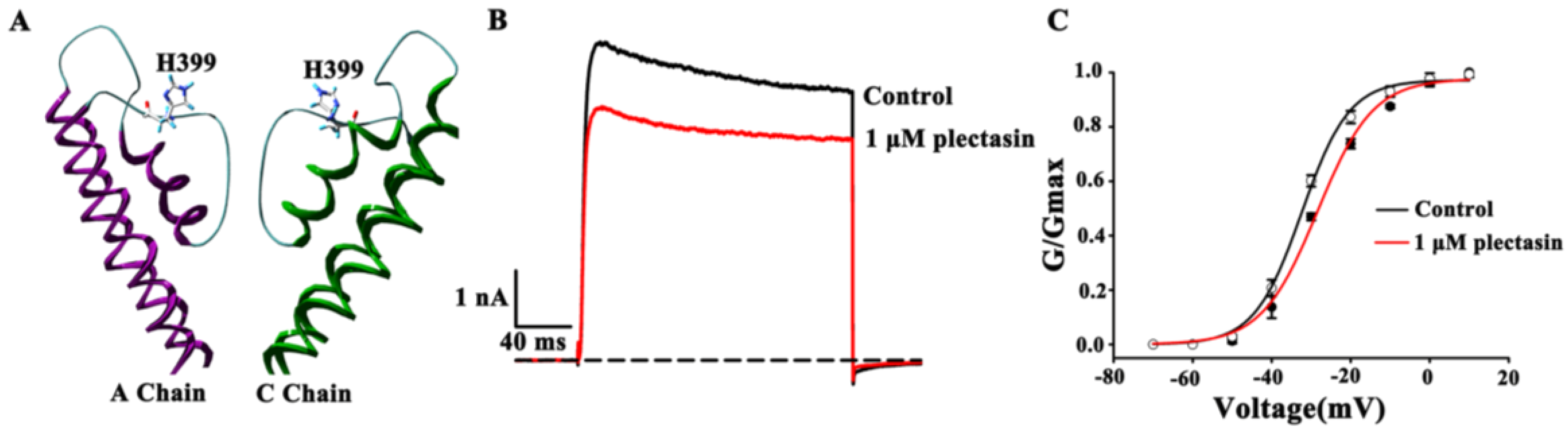
2.4. Kv1.3 Channel Pore Region Is Plectasin-Interacting Site
3. Materials and Methods
3.1. Peptides and Potassium Channels
3.2. Cell Culture
3.3. Electrophysiological Recordings
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shieh, C.C.; Coghlan, M.; Sullivan, J.P.; Gopalakrishnan, M. Potassium channels: Molecular defects, diseases, and therapeutic opportunities. Pharmacol. Rev. 2000, 52, 557–594. [Google Scholar] [PubMed]
- Mouhat, S.; Andreotti, N.; Jouirou, B.; Sabatier, J.M. Animal toxins acting on voltage-gated potassium channels. Curr. Pharm. Des. 2008, 14, 2503–2518. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Hu, Y.; Yi, H.; Yin, S.; Han, S.; Hu, J.; Chen, Z.; Yang, W.; Cao, Z.; de Waard, M.; et al. Two conserved arginine residues from the SK3 potassium channel outer vestibule control selectivity of recognition by scorpion toxins. J. Biol. Chem. 2013, 288, 12544–12553. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, W.H.; Han, S.; Peng, B.W.; Yin, J.; Wu, Y.L.; He, X.H.; Li, W.X. Selective inhibition of CCR7− effector memory T cell activation by a novel peptide targeting Kv1.3 channel in a rat experimental autoimmune encephalomyelitis model. J. Biol. Chem. 2012, 287, 29479–29494. [Google Scholar] [CrossRef] [PubMed]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R.; et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Kuyucak, S. Free energy simulations of binding of HsTx1 toxin to Kv1 potassium channels: The basis of Kv1.3/Kv1.1 selectivity. J. Phys. Chem. B 2014, 118, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wu, Y. Molecular mechanism of the sea anemone toxin ShK recognizing the Kv1.3 channel explored by docking and molecular dynamic simulations. J. Chem. Inf. Model. 2007, 47, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Robinson, A.; Gordon, D.; Chung, S.H. Modeling the binding of three toxins to the voltage-gated potassium channel (Kv1.3). Biophys. J. 2011, 101, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Kuyucak, S. Affinity and selectivity of ShK toxin for the Kv1 potassium channels from free energy simulations. J. Phys. Chem. B 2012, 116, 4812–4822. [Google Scholar] [CrossRef] [PubMed]
- Spelbrink, R.G.; Dilmac, N.; Allen, A.; Smith, T.J.; Shah, D.M.; Hockerman, G.H. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol. 2004, 135, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Cheung, R.C.; Wong, J.H.; Ye, X.J. Antimicrobial activity of defensins and defensin-like peptides with special emphasis on those from fungi and invertebrate animals. Curr. Protein Pept. Sci. 2013, 14, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Tassanakajon, A.; Somboonwiwat, K.; Amparyup, P. Sequence diversity and evolution of antimicrobial peptides in invertebrates. Dev. Comp. Immunol. 201 2015, 48, 324–341. [Google Scholar] [CrossRef]
- Yang, W.; Feng, J.; Xiang, F.; Xie, Z.; Zhang, G.; Sabatier, J.M.; Cao, Z.; Li, W.; Chen, Z.; Wu, Y. Endogenous animal toxin-like human beta-defensin 2 inhibits own K channels through interaction with channel extracellular pore region. Cell. Mol. Life Sci. 2014. [Google Scholar] [CrossRef]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sonksen, C.P.; Ludvigsen, S.; Raventos, D.; Buskov, S.; Christensen, B.; de Maria, L.; et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Brinch, K.S.; Sandberg, A.; Baudoux, P.; van Bambeke, F.; Tulkens, P.M.; Frimodt-Moller, N.; Hoiby, N.; Kristensen, H.H. Plectasin shows intracellular activity against Staphylococcus aureus in human THP-1 monocytes and in a mouse peritonitis model. Antimicrob. Agents Chemother. 2009, 53, 4801–4808. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Kruse, T.; Wimmer, R.; Wiedemann, I.; Sass, V.; Pag, U.; Jansen, A.; Nielsen, A.K.; Mygind, P.H.; Raventos, D.S.; et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science 2010, 328, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Gan, G.; Yi, H.; Chen, M.; Sun, L.; Li, W.; Wu, Y.; Ding, J. Structural basis for toxin resistance of beta4-associated calcium-activated potassium (BK) channels. J. Biol. Chem. 2008, 283, 24177–24184. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Yi, H.; Liu, H.; Cao, Z.; Wu, Y.; Li, W. Molecular Information of charybdotoxin blockade in the large conductance calcium-activated potassium channel. J. Chem. Inf. Model. 2009, 49, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Lee, A.; Campbell, E.; Mackinnon, R. Structure of a pore-blocking toxin in complex with a eukaryotic voltage-dependent K+ channel. Elife 2013, 2, e00594. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yi, H.; Yin, S.J.; Chen, Z.Y.; Liu, H.; Cao, Z.J.; Wu, Y.L.; Li, W.X. Structural basis of a potent peptide inhibitor designed for Kv1.3 channel, a therapeutic target of autoimmune disease. J. Biol. Chem. 2008, 283, 19058–19065. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.J.; Jiang, L.; Yi, H.; Han, S.; Yang, D.W.; Liu, M.L.; Liu, H.; Cao, Z.J.; Wu, Y.L.; Li, W.X. Different residues in channel turret determining the selectivity of ADWX-1 inhibitor peptide between Kv1.1 and Kv1.3 channels. J. Proteome Res. 2008, 7, 4890–4897. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Hu, J.; Yang, W.; Sabatier, J.M.; de Waard, M.; Cao, Z.; Li, W.; Han, S.; Wu, Y. Unusual binding mode of scorpion toxin BmKTX onto potassium channels relies on its distribution of acidic residues. Biochem. Biophys. Res. Commun. 2014, 447, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Hu, Y.T.; Yang, W.S.; He, Y.W.; Feng, J.; Wang, B.; Zhao, R.M.; Ding, J.P.; Cao, Z.J.; Li, W.X.; et al. Hg1, novel peptide inhibitor specific for Kv1.3 channels from first scorpion Kunitz-type potassium channel toxin family. J. Biol. Chem. 2012, 287, 13813–13821. [Google Scholar] [CrossRef] [PubMed]
- Chi, V.; Pennington, M.W.; Norton, R.S.; Tarcha, E.J.; Londono, L.M.; Sims-Fahey, B.; Upadhyay, S.K.; Lakey, J.T.; Iadonato, S.; Wulff, H.; et al. Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases. Toxicon 2012, 59, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chung, S.H. Engineering a potent and specific blocker of voltage-gated potassium channel Kv1.3, a target for autoimmune diseases. Biochemistry 2012, 51, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhang, R.; Liu, Y.; Feng, J.; Wang, W.; Wu, Y.; Ding, J. Physiological role of Kv1.3 channel in T lymphocyte cell investigated quantitatively by kinetic modeling. PLoS One 2014, 9, e89975. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, F.; Xie, Z.; Feng, J.; Yang, W.; Cao, Z.; Li, W.; Chen, Z.; Wu, Y. Plectasin, First Animal Toxin-Like Fungal Defensin Blocking Potassium Channels through Recognizing Channel Pore Region. Toxins 2015, 7, 34-42. https://doi.org/10.3390/toxins7010034
Xiang F, Xie Z, Feng J, Yang W, Cao Z, Li W, Chen Z, Wu Y. Plectasin, First Animal Toxin-Like Fungal Defensin Blocking Potassium Channels through Recognizing Channel Pore Region. Toxins. 2015; 7(1):34-42. https://doi.org/10.3390/toxins7010034
Chicago/Turabian StyleXiang, Fang, Zili Xie, Jing Feng, Weishan Yang, Zhijian Cao, Wenxin Li, Zongyun Chen, and Yingliang Wu. 2015. "Plectasin, First Animal Toxin-Like Fungal Defensin Blocking Potassium Channels through Recognizing Channel Pore Region" Toxins 7, no. 1: 34-42. https://doi.org/10.3390/toxins7010034
APA StyleXiang, F., Xie, Z., Feng, J., Yang, W., Cao, Z., Li, W., Chen, Z., & Wu, Y. (2015). Plectasin, First Animal Toxin-Like Fungal Defensin Blocking Potassium Channels through Recognizing Channel Pore Region. Toxins, 7(1), 34-42. https://doi.org/10.3390/toxins7010034





