Immunological Cross-Reactivity and Neutralisation of European Viper Venoms with the Monospecific Vipera berus Antivenom ViperaTAb
Abstract
:1. Introduction
2. Results and Discussion
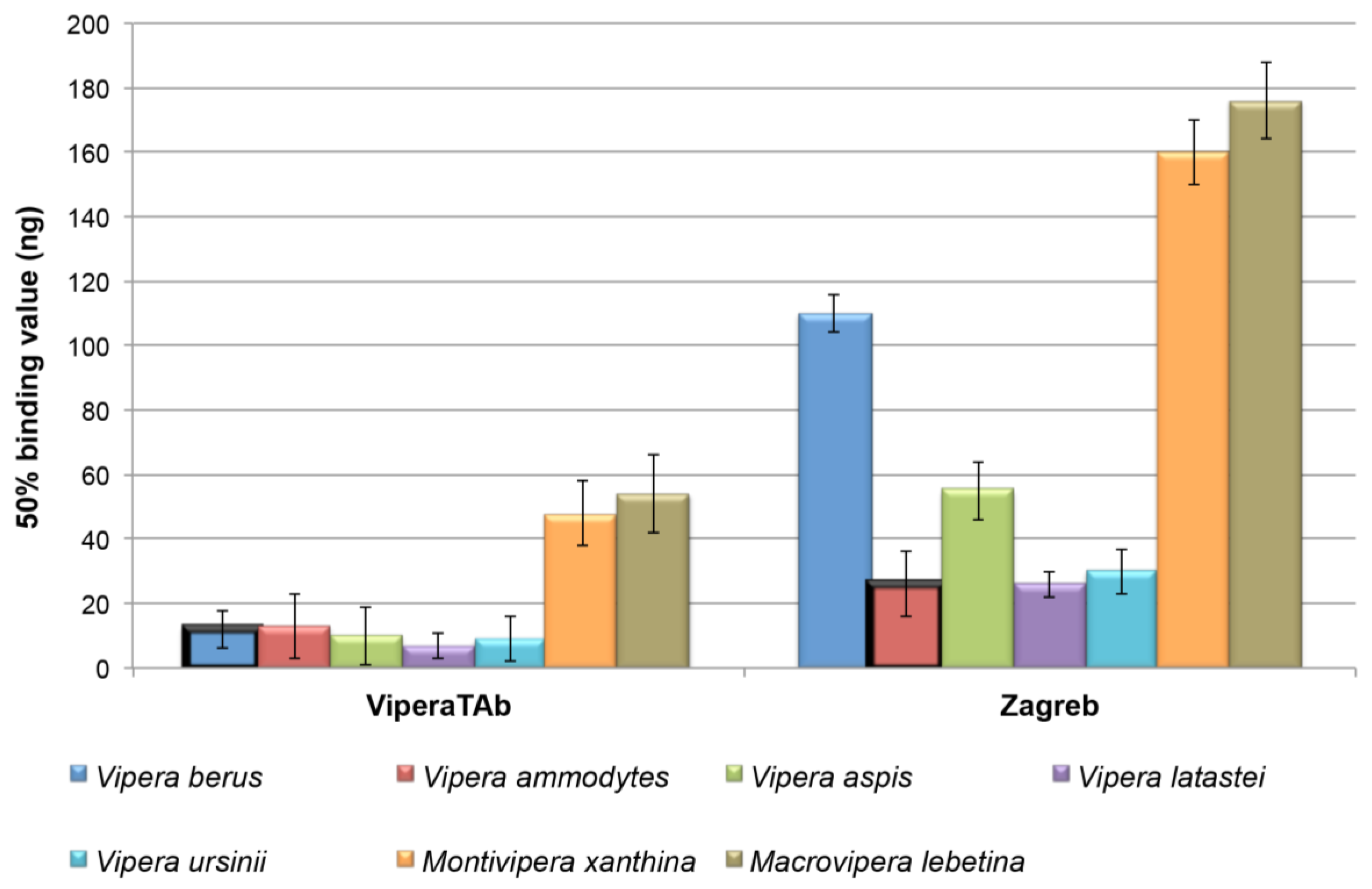
2.1. Immunological Cross-Reactivity Using ELISA
2.2. Immunological Cross-Reactivity Using Immunoblotting

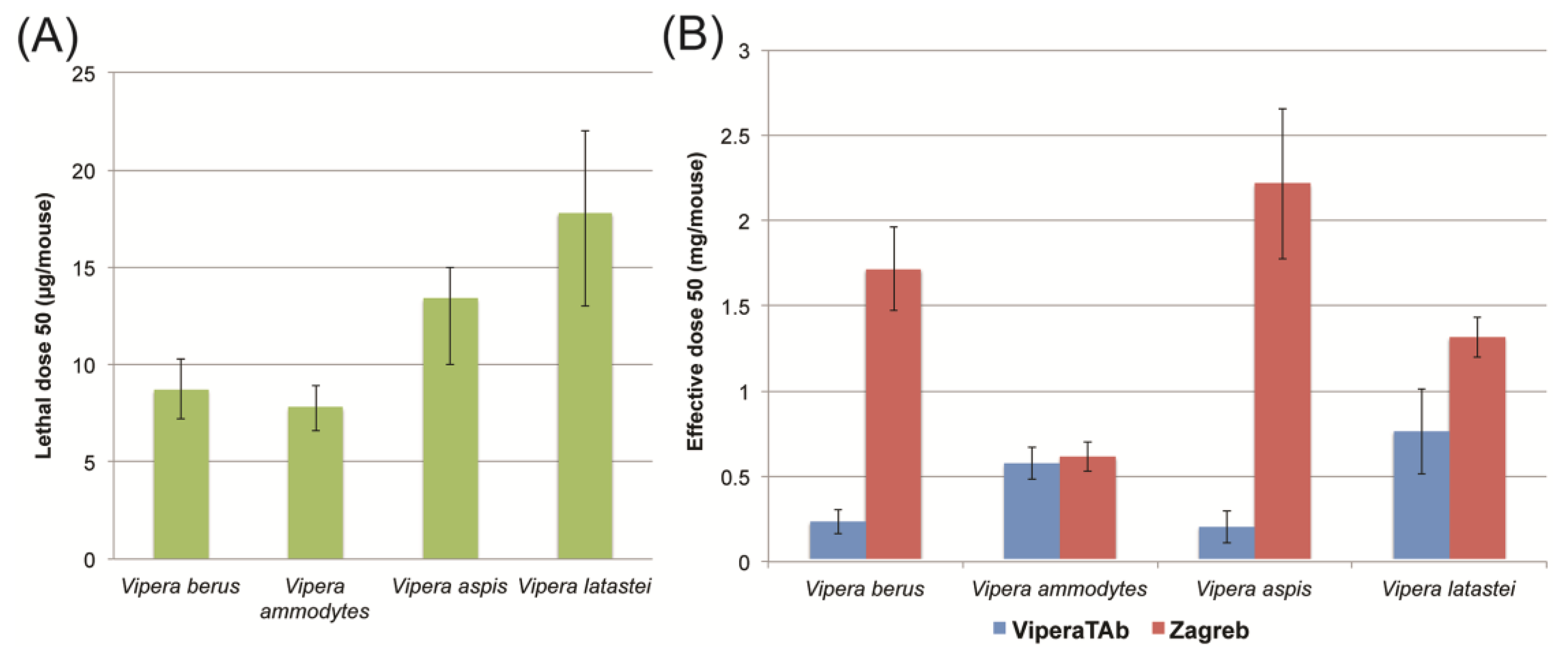
2.3. In Vivo Neutralisation of Venom Lethality
| Venom | Pharmacopoeial minimum specification | ViperaTAb® antivenom |
|---|---|---|
| Vipera berus | 50 | 597 |
| Vipera ammodytes | 100 | 222 |
| Vipera aspis | 100 | 690 |
| Vipera latastei | N/A | 176 |
2.4. Discussion
3. Experimental Section
3.1. Materials
3.2. ELISA
3.3. SDS-PAGE and Immunoblotting
3.4. In Vivo Venom Neutralisation (ED50)
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modeling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Epidemiology of snakebites in Europe: A systematic review of the literature. Toxicon 2012, 59, 86–99. [Google Scholar] [CrossRef]
- Persson, H.; Irestedt, B. A study of 136 cases of adder bite treated in Swedish hospitals during one year. Acta Med. Scand. 1981, 210, 433–439. [Google Scholar] [CrossRef]
- Karlson-Stiber, C.; Salmonson, H.; Persson, H. A nationwide study of Vipera berus bites during one year—Epidemiology and morbidity of 231 cases. Clin. Toxicol. 2006, 44, 25–30. [Google Scholar] [CrossRef]
- Boels, D.; Hamel, J.F.; Deguigne, M.; Harry, P. European viper envenomings: Assessment of Viperfav and other symptomatic treatments. Clin. Toxicol. 2012, 50, 189–196. [Google Scholar] [CrossRef]
- Gerrard, M.; Pugh, R. An adder bite with unusual consequences. Practitioner 1982, 226, 527–528. [Google Scholar]
- González, D. Clinical aspects of bites by viper in Spain. Toxicon 1982, 20, 349–353. [Google Scholar] [CrossRef]
- Cederholm, I.; Lennmarken, C. Vipera berus bites in children—Experience of early antivenom treatment. Acta Paediatrica Scandinavica 1987, 76, 682–684. [Google Scholar] [CrossRef]
- Parvinen, T.; Alanen, M.; Iisalo, E. Tappaako kyy? Duodecim 1987, 103, 707–713. [Google Scholar]
- Kjellstrom, B.T. Acute pancreatitis after snake bite. Case report. Acta Chirurgica Scandinavica 1989, 155, 291–292. [Google Scholar]
- Warrell, D.A. Treatment of bites by adders and exotic venomous snakes. BMJ 2005, 331, 1244–1247. [Google Scholar] [CrossRef]
- Lukšić, B.; Bradarić, N.; Prgomet, S. Venomous snakebites in Southern Croatia. Coll. Antropol. 2006, 30, 191–197. [Google Scholar]
- Ferquel, E.; de Haro, L.; Jan, V.; Guillemin, I.; Jourdain, S.; Teynié, A.; d’Alater, J.; Choumet, V. Reappraisal of Vipera aspis Venom Neurotoxicity. PLoS One 2007, 2, e1194. [Google Scholar] [CrossRef]
- Malina, T.; Krecsak, L.; Warrell, D.A. Neurotoxicity and hypertension following European adder (Vipera berus berus) bites in Hungary: Case report and review. QJM 2008, 101, 801–806. [Google Scholar] [CrossRef]
- Smith, D.C.; Reddi, K.R.; Laing, G.; Theakston, R.G.; Landon, J. An affinity purified ovine antivenom for the treatment of Vipera berus envenoming. Toxicon 1992, 30, 865–871. [Google Scholar]
- Casewell, N.R.; Cook, D.A.; Wagstaff, S.C.; Nasidi, A.; Durfa, N.; Wüster, W.; Harrison, R.A. Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific antivenom. PLoS Negl. Trop. Dis. 2010, 4, e851. [Google Scholar] [CrossRef]
- Smith, T.W.; Lloyd, B.L.; Spicer, N.; Haber, E. Immunogenicity and kinetics of distribution and elimination of sheep digoxin-specific IgG and Fab fragments in the rabbit and baboon. Clin. Exp. Immunol. 1979, 36, 384–396. [Google Scholar]
- Gutiérrez, J.M.; León, G.; Lomonte, B. Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin. Pharmacokinet. 2003, 42, 721–741. [Google Scholar] [CrossRef]
- Lavonas, E.J.; Schaeffer, T.H.; Kokko, J.; Mlynarchek, S.L.; Bogdan, G.M. Crotaline Fab antivenom appears to be effective in cases of severe North American pit viper envenomation: An integrative review. BMC Emerg. Med. 2009, 9, 13. [Google Scholar]
- Karlson-Stiber, C.; Persson, H. Antivenom treatment in Vipera berus envenoming—report of 30 cases. J. Int. Med. 1994, 235, 57–61. [Google Scholar] [CrossRef]
- Chippaux, J.-P.; Williams, V.; White, J. Snake venom variability: Methods of study, results and interpretation. Toxicon 1991, 29, 1279–1303. [Google Scholar] [CrossRef]
- Alape-Girón, A.; Sanz, L.; Escolano, J.; Flores-Díaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake Venomics of the Lancehead Pitviper Bothrops asper: Geographic, Individual, and Ontogenetic Variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar]
- Casewell, N.R.; Harrison, R.A.; Wüster, W.; Wagstaff, S.C. Comparative venom gland transcriptome surveys of the saw-scaled vipers (Viperidae: Echis) reveal substantial intra-family gene diversity and novel venom transcripts. BMC Genomics 2009, 10, 564. [Google Scholar]
- Williams, D.J.; Gutiérrez, J.M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paica, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteomics 2011, 74, 1735–1767. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wagstaff, S.C.; Wüster, W.; Cook, D.A.N.; Bolton, F.M.S.; King, S.I.; Pla, D.; Sanz, L.; Calvete, J.J.; Harrison, R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 9205–9210. [Google Scholar]
- Sanz, L.; Ayvazyan, N.; Calvete, J.J. Snake venomics of the Armenian mountain vipers Macrovipera lebetina obtusa and Vipera raddei. J. Proteomics 2008, 71, 198–209. [Google Scholar] [CrossRef]
- Bazaa, A.; Marrakchi, N.; El Ayeb, M.; Sanz, L.; Calvete, J.J. Snake venomics: Comparative analysis of the venom proteomes of the Tunisian snakes Cerastes cerastes, Cerastes vipera and Macrovipera lebetina. Proteomics 2005, 5, 4223–4235. [Google Scholar] [CrossRef]
- Ritonja, A.; Gubenšek, F. Ammodytoxin A, a highly lethal phospholipase A2 from Vipera ammodytes venom. Biochim. Biophys. Acta 1985, 828, 306–312. [Google Scholar] [CrossRef]
- Leonardi, A.; Gubenšek, F.; Križaj, I. Purification and characterization of two haemorrhagic metalloproteinases from the venom of the long-nosed viper, Vipera ammodytes ammodytes. Toxicon 2001, 40, 55–62. [Google Scholar] [CrossRef]
- Leonardi, A.; Fox, J.W.; Trampuš-Bakija, A.; Križaj, I. Two coagulation factor X activators from Vipera a. ammodytes venom with potential to treat patients with dysfunctional factors IXa or VIIa. Toxicon 2008, 52, 628–637. [Google Scholar]
- Bolton, F.M.S.; Casewell, N.R.; Al-Abdulla, I.; Landon, J. Production and assessment of ovine antisera for the manufacture of a veterinary adder antivenom. Vet. Rec. 2014, 174, 406. [Google Scholar] [CrossRef]
- Theakston, R.D.; Reid, H.A. Effectiveness of Zagreb antivenom against envenoming by the adder, Vipera berus. Lancet 1976, 2, 121–123. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Casewell, N.R.; Al-Abdulla, I.; Smith, D.; Coxon, R.; Landon, J. Immunological Cross-Reactivity and Neutralisation of European Viper Venoms with the Monospecific Vipera berus Antivenom ViperaTAb. Toxins 2014, 6, 2471-2482. https://doi.org/10.3390/toxins6082471
Casewell NR, Al-Abdulla I, Smith D, Coxon R, Landon J. Immunological Cross-Reactivity and Neutralisation of European Viper Venoms with the Monospecific Vipera berus Antivenom ViperaTAb. Toxins. 2014; 6(8):2471-2482. https://doi.org/10.3390/toxins6082471
Chicago/Turabian StyleCasewell, Nicholas R., Ibrahim Al-Abdulla, David Smith, Ruth Coxon, and John Landon. 2014. "Immunological Cross-Reactivity and Neutralisation of European Viper Venoms with the Monospecific Vipera berus Antivenom ViperaTAb" Toxins 6, no. 8: 2471-2482. https://doi.org/10.3390/toxins6082471
APA StyleCasewell, N. R., Al-Abdulla, I., Smith, D., Coxon, R., & Landon, J. (2014). Immunological Cross-Reactivity and Neutralisation of European Viper Venoms with the Monospecific Vipera berus Antivenom ViperaTAb. Toxins, 6(8), 2471-2482. https://doi.org/10.3390/toxins6082471




