Cloning and Characterization of a Unique Cytotoxic Protein Parasporin-5 Produced by Bacillus thuringiensis A1100 Strain
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of a Cytotoxic Crystal Protein from B. thuringiensis Strain A1100
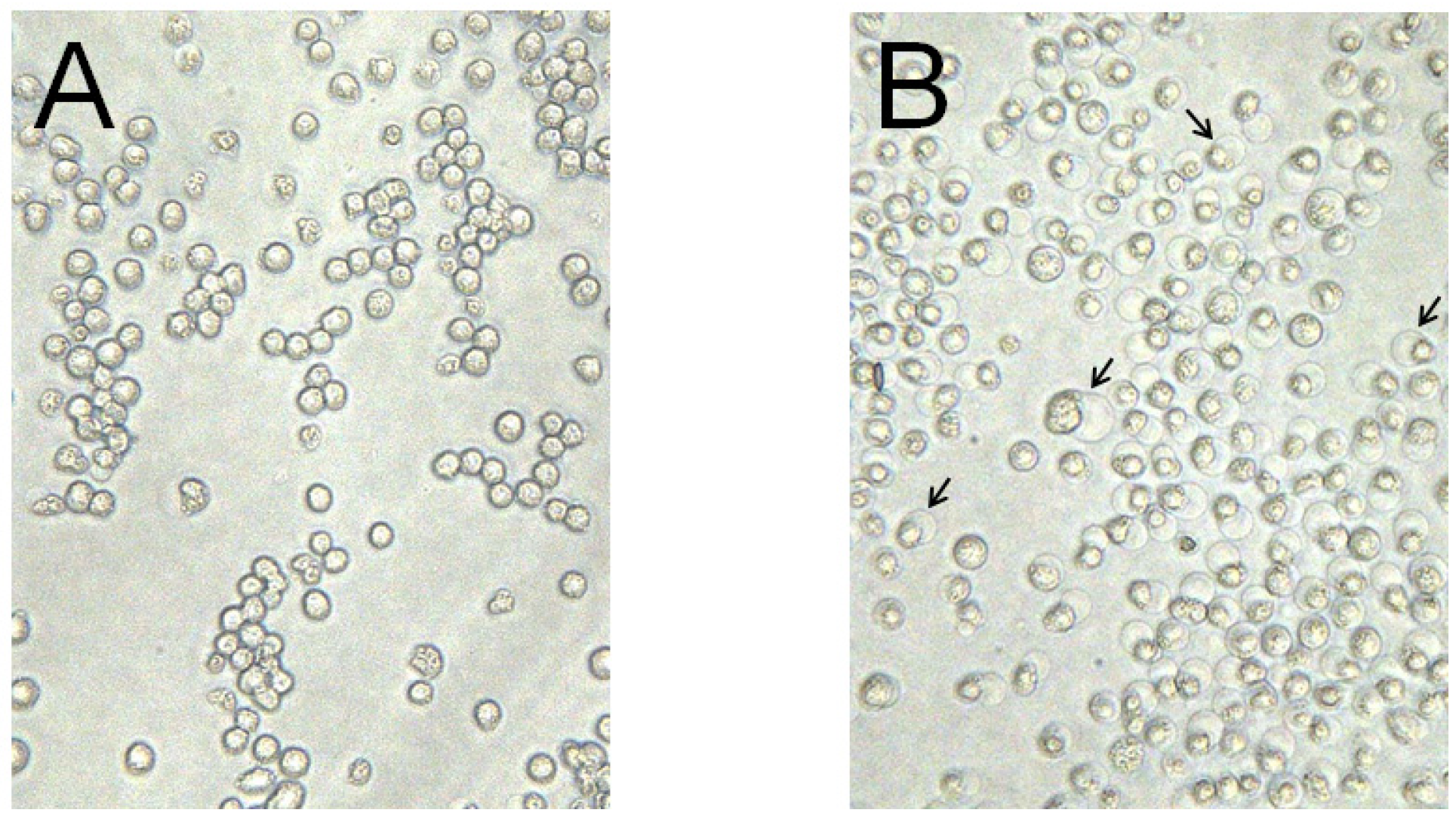

2.2. Genetic Characteristics of the Cytotoxic Protein

2.3. Preparation of the Recombinant PS5 Protein

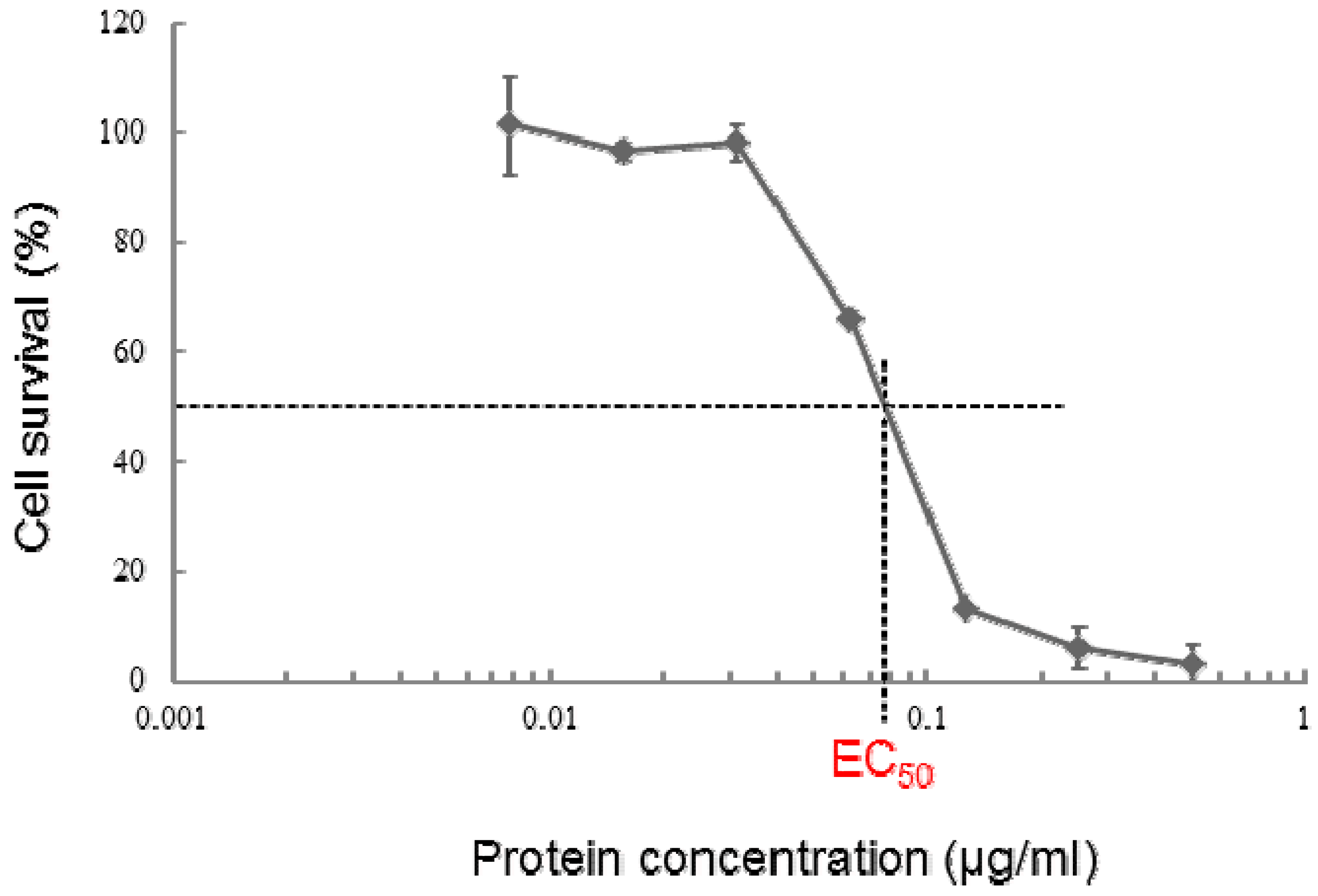
2.4. Cytotoxic Activity of PS5 against Various Mammalian Cells
| Cell line | Origin | EC50 (μg/mL) |
|---|---|---|
| MOLT-4 | leukemic T cell, human | 0.075 |
| CACO-2 | colon cancer, human | 0.30 |
| Jurkat | leukemic T cell, human | 0.124 |
| A549 | lung cancer, human | 4.41 |
| HepG2 | hepatocyte cancer, human | 0.049 |
| TCS | uterus cervix cancer, human | 0.046 |
| HL60 | promyelocytic leukemia cell, human | 1.079 |
| K562 | myelogenous leukemia cell, human | 4.249 |
| U937 | DE-4 lymphoma cell, human | >10 |
| HeLa | uterus cervix cancer, human | 0.080 |
| COS7 | kidney cell, monkey | 0.045 |
| NIH3T3 | embryo cell, NIH Swiss mouse | 0.321 |
| Vero | kidney cell, monkey | 0.050 |
| CHO-K1 | ovary cell, chinese hamster | 0.571 |
| Sawano | uterus cancer, human | 0.065 |
| MRC-5 | normal embryonic lung fibroblast, human | 0.273 |
| UtSMC | normal uterus, human | 0.223 |
| HC | normal hepatocyte, human | >10 |
3. Experimental Section
3.1. Bacterial Strains, Culture Media, and Preparation of Parasporal Inclusion Proteins
3.2. Purification of Toxin Protein from Parasporal Inclusions
3.3. Mammalian Cells and Cell Culture
3.4. Cytotoxicity Assay
3.5. SDS-PAGE and Protein Determination
3.6. Mass Spectrometric Analysis
3.7. N-Terminal and Internal Amino Acid Sequencing
3.8. DNA Manipulation and PCR Experiments
3.9. Gene Cloning and Sequencing
3.10. Plasmid Construction for Expression of the Recombinant Protein
 ACCTGGTAAAGGCGATT-3′ (underlined bases indicate the XhoI site, and the double-underlined bases indicate the TAA stop codon). The PCR products were ligated into the expression vector pET32b between the NdeI and XhoI sites. The plasmids were designated as pET-33k and pET-30k, respectively. E. coli XL1-Blue cells were transformed with the plasmids and plated onto Luria-Bertani (LB) agar containing ampicillin (100 μg/mL).
ACCTGGTAAAGGCGATT-3′ (underlined bases indicate the XhoI site, and the double-underlined bases indicate the TAA stop codon). The PCR products were ligated into the expression vector pET32b between the NdeI and XhoI sites. The plasmids were designated as pET-33k and pET-30k, respectively. E. coli XL1-Blue cells were transformed with the plasmids and plated onto Luria-Bertani (LB) agar containing ampicillin (100 μg/mL). 3.11. Expression and Purification of Recombinant Toxin Proteins
3.12. Nucleotide Sequence Accession Number
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beegle, C.C.; Yamamoto, T. History of Bacillus thuringiensis Berliner research and development. Can. Entomol. 1992, 124, 587–616. [Google Scholar] [CrossRef]
- Thomas, W.E.; Ellar, D.J. Bacillus thuringiensis var. israelensis crystal δ-endotoxin: Effects on insect and mammalian cells in vitro and in vivo. J. Cell Sci. 1983, 60, 181–197. [Google Scholar]
- Ohba, M.; Aizawa, K.J. Distribution of Bacillus thuringiensis in soils of Japan. J. Invertebr. Pathol. 1986, 47, 12–20. [Google Scholar] [CrossRef]
- Meadows, M.P.; Ellis, D.J.; Butt, J.; Jarrett, P.; Burges, H.D. Distribution, frequency, and diversity of Bacillus thuringiensis in an animal feed mill. Appl. Environ. Microbiol. 1992, 58, 1344–1350. [Google Scholar]
- Mizuki, E.; Ohba, M.; Akao, T.; Yamashita, S.; Saitoh, H.; Park, Y.S. Unique activity associated with non-insecticidal Bacillus thuringiensis parasporal inclusions: In vitro cell-killing action on human cancer cells. J. Appl. Microbiol. 1999, 86, 477–486. [Google Scholar] [CrossRef]
- Mizuki, E.; Park, Y.S.; Saitoh, H.; Yamashita, S.; Akao, T.; Higuchi, K.; Ohba, M. Parasporin, a human leukemic cell-recognizing parasporal protein of Bacillus thuringiensis. Clin. Diagn. Lab. Immunol. 2000, 7, 625–634. [Google Scholar]
- Kim, H.S.; Yamashita, S.; Akao, T.; Saitoh, H.; Higuchi, K.; Park, Y.S.; Mizuki, E.; Ohba, M. In vitro cytotoxicity of non-cyt inclusion proteins of a Bacillus thuringiensis isolate against human cells, including cancer cells. J. Appl. Microbiol. 2000, 289, 16–23. [Google Scholar]
- Lee, D.; Katayama, H.; Akao, T.; Maeda, M.; Tanaka, R.; Yamashita, S.; Saitoh, H.; Mizuki, E.; Ohba, M. A 28 kDa protein of the Bacillus thuringiensis serovar shandongiensis isolate 89-T-34-22 induces a human leukemic cell-specific cytotoxicity. Biochim. Biophys. Acta 2001, 1547, 57–63. [Google Scholar]
- Katayama, H.; Yokota, H.; Akao, T.; Nakamura, O.; Ohba, M.; Mekada, E.; Mizuki, E. Parasporin-1, a novel cytotoxic protein to human cells from non-insecticidal parasporal inclusions of Bacillus thuringiensis. J. Biochem. 2005, 137, 17–25. [Google Scholar] [CrossRef]
- Ohba, M.; Mizuki, E.; Uemori, A. Parasporin, a new anticancer protein group from Bacillus thuringiensis. Anticancer Res. 2009, 29, 427–434. [Google Scholar]
- Wong, S.Y.R. Bacillus thuringiensis parasporal proteins and their effect on human cancer cells. IeJSME 2010, 4, 3–9. [Google Scholar]
- Ohba, M.; Mizuki, E.; Crickmore, N.; Coté, J.-C.; Nagamatsu, Y.; Kitada, S.; Sakai, H.; Harata, K.; Shin, T.; Okumura, S. Parasporin nomenclature. Available online: http://parasporin.fitc.pref.fukuoka.jp/ (accessed on 11 April 2014).
- Yamashita, S.; Katayama, H.; Saitoh, H.; Akao, T.; Park, Y.S.; Mizuki, E.; Ohba, M.; Ito, A. Typical three-domain Cry proteins of Bacillus thuringiensis strain A1462 exhibit cytocidal activity on limited human cancer cells. J. Biochem. 2005, 138, 663–672. [Google Scholar] [CrossRef]
- Nagamatsu, Y.; Okamura, S.; Saitou, H.; Akao, T.; Mizuki, E. Three Cry toxins in two types from Bacillus thuringiensis strain M019 preferentially kill human hepatocyte cancer and uterus cervix cancer cells. Biosci. Biotechnol. Biochem. 2010, 74, 494–498. [Google Scholar] [CrossRef]
- Akiba, T.; Abe, Y.; Kitada, S.; Kusaka, Y.; Ito, A.; Ichimatsu, T.; Katayama, H.; Akao, T.; Higuchi, K.; Mizuki, E.; et al. Crystal structure of the parasporin-2 Bacillus thuringiensis toxin that recognizes cancer cells. J. Mol. Biol. 2009, 386, 121–133. [Google Scholar] [CrossRef]
- Ito, A.; Sasaguri, Y.; Kitada, S.; Kusaka, Y.; Kuwano, K.; Masutomi, K.; Mizuki, E.; Akao, T.; Ohba, M. A Bacillus thuringiensis crystal protein with selective cytocidal action to human cells. J. Biol. Chem. 2004, 279, 21282–21286. [Google Scholar] [CrossRef]
- Abe, Y.; Shimada, H.; Kitada, S. Raft-targeting and oligomerization of parasporin-2, a Bacillus thuringiensis crystal protein with antitumour activity. J. Biochem. 2008, 143, 269–275. [Google Scholar] [CrossRef]
- Okumura, S.; Saitoh, H.; Ishikawa, T.; Wasano, N.; Yamashita, S.; Kusumoto, K.; Akao, T.; Mizuki, E.; Ohba, M.; Inouye, K. Identification of a novel cytotoxic protein, Cry45Aa, from Bacillus thuringiensis A1470 and its selective cytotoxic activity against various mammalian cell lines. J. Agric. Food Chem. 2005, 53, 6313–6318. [Google Scholar] [CrossRef]
- Okumura, S.; Saitoh, H.; Ishikawa, T.; Inouye, K.; Mizuki, E. Mode of action of parasporin-4, a cytocidal protein from Bacillus thuringiensis. Biochim. Biophys. Acta 2011, 1808, 1476–1482. [Google Scholar]
- Crickmore, N.; Baum, J.; Bravo, A.; Lereclus, D.; Narva, K.; Sampson, K.; Schnepf, E.; Sun, M.; Zeigler, D.R. Bacillus thuringiensis toxin nomenclature. Available online: http://www.btnomenclature.info/ (accessed on 11 April 2014).
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Rosado, C.J.; Kondos, S.; Bull, T.E.; Kuiper, M.J.; Law, R.H.; Buckle, A.M.; Voskoboinik, I.; Bird, P.I.; Trapani, J.A.; Whisstock, J.C.; et al. The MACPF/CDC family of pore-forming toxins. Cell. Microbiol. 2008, 10, 1765–1774. [Google Scholar] [CrossRef]
- Brown, K.L.; Whiteley, H.R. Molecular characterization of two novel crystal protein genes from Bacillus thuringiensis subsp. thompsoni. J. Bacteriol. 1992, 174, 549–557. [Google Scholar]
- Kim, H.S.; Saitoh, H.; Yamashita, S.; Akao, T.; Park, Y.S.; Maeda, M.; Tanaka, R.; Mizuki, E.; Ohba, M. Cloning and characterization of two novel crystal protein genes from a Bacillus thuringiensis serovar dakota strain. Curr. Microbiol. 2003, 46, 33–38. [Google Scholar] [CrossRef]
- Huang, D.F.; Zhang, J.; Song, F.P.; Lang, Z.H. Microbial control and biotechnology research on Bacillus thuringiensis in China. J. Invertebr. Pathol. 2007, 95, 175–180. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Q.; Xia, L.; Ding, X.; Hu, Q.; Federici, B.A.; Park, H.W. Identification and characterization of three previously undescribed crystal proteins from Bacillus thuringiensis subsp. jegathesan. Appl. Environ. Microbiol. 2013, 79, 3364–3370. [Google Scholar] [CrossRef]
- Amimoto, K.; Sasaki, Y.; Fukuyama, S.; Tamura, Y. Genetic variation and cross-reactivity of Clostridium septicum alpha-toxin. Vet. Microbiol. 2006, 114, 51–59. [Google Scholar] [CrossRef]
- Moniatte, M.; van der Goot, F.G.; Buckley, J.T.; Pattus, F.; Dorsselaer, V.A. Characterisation of the heptameric pore-forming complex of the Aeromonas toxin aerolysin using MALDI-TOF mass spectrometry. FEBS Lett. 1996, 384, 269–272. [Google Scholar] [CrossRef]
- Nagahama, M.; Hara, H.; Fernandez-Miyakawa, M.; Itohayashi, Y.; Sakurai, J. Oligomerization of Clostridium perfringens ε-toxin is dependent upon membrane fluidity in liposomes. Biochemistry 2006, 45, 296–302. [Google Scholar] [CrossRef]
- Okumura, S.; Saitoh, H.; Wasano, N.; Katayama, H.; Higuchi, K.; Mizuki, E.; Inouye, K. Efficient solubilization, activation, and purification of recombinant Cry45Aa of Bacillus thuringiensis expressed as inclusion bodies in Escherichia coli. Protein Expr. Purif. 2006, 47, 144–151. [Google Scholar] [CrossRef]
- Kitada, S.; Abe, Y.; Maeda, T.; Shimada, H. Parasporin-2 requires GPI-anchored proteins for the efficient cytocidal action to human hepatoma cells. Toxicology 2009, 264, 80–88. [Google Scholar] [CrossRef]
- Hang'ombe, M.B.; Mukamoto, M.; Kohda, T.; Sugimoto, N.; Kozaki, S. Cytotoxicity of Clostridium septicum α-toxin: Its oligomerization in detergent resistant membranes of mammalian cells. Microb. Pathog. 2004, 37, 279–286. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Current Protocols in Molecular Biology; Ausubel, F.M.; Bret, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. (Eds.) John Wiley & Sons, Inc.: New York, NY, USA, 1994; Chapter 2.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ekino, K.; Okumura, S.; Ishikawa, T.; Kitada, S.; Saitoh, H.; Akao, T.; Oka, T.; Nomura, Y.; Ohba, M.; Shin, T.; et al. Cloning and Characterization of a Unique Cytotoxic Protein Parasporin-5 Produced by Bacillus thuringiensis A1100 Strain. Toxins 2014, 6, 1882-1895. https://doi.org/10.3390/toxins6061882
Ekino K, Okumura S, Ishikawa T, Kitada S, Saitoh H, Akao T, Oka T, Nomura Y, Ohba M, Shin T, et al. Cloning and Characterization of a Unique Cytotoxic Protein Parasporin-5 Produced by Bacillus thuringiensis A1100 Strain. Toxins. 2014; 6(6):1882-1895. https://doi.org/10.3390/toxins6061882
Chicago/Turabian StyleEkino, Keisuke, Shiro Okumura, Tomoyuki Ishikawa, Sakae Kitada, Hiroyuki Saitoh, Tetsuyuki Akao, Takuji Oka, Yoshiyuki Nomura, Michio Ohba, Takashi Shin, and et al. 2014. "Cloning and Characterization of a Unique Cytotoxic Protein Parasporin-5 Produced by Bacillus thuringiensis A1100 Strain" Toxins 6, no. 6: 1882-1895. https://doi.org/10.3390/toxins6061882
APA StyleEkino, K., Okumura, S., Ishikawa, T., Kitada, S., Saitoh, H., Akao, T., Oka, T., Nomura, Y., Ohba, M., Shin, T., & Mizuki, E. (2014). Cloning and Characterization of a Unique Cytotoxic Protein Parasporin-5 Produced by Bacillus thuringiensis A1100 Strain. Toxins, 6(6), 1882-1895. https://doi.org/10.3390/toxins6061882





