Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review
Abstract
:1. Introduction
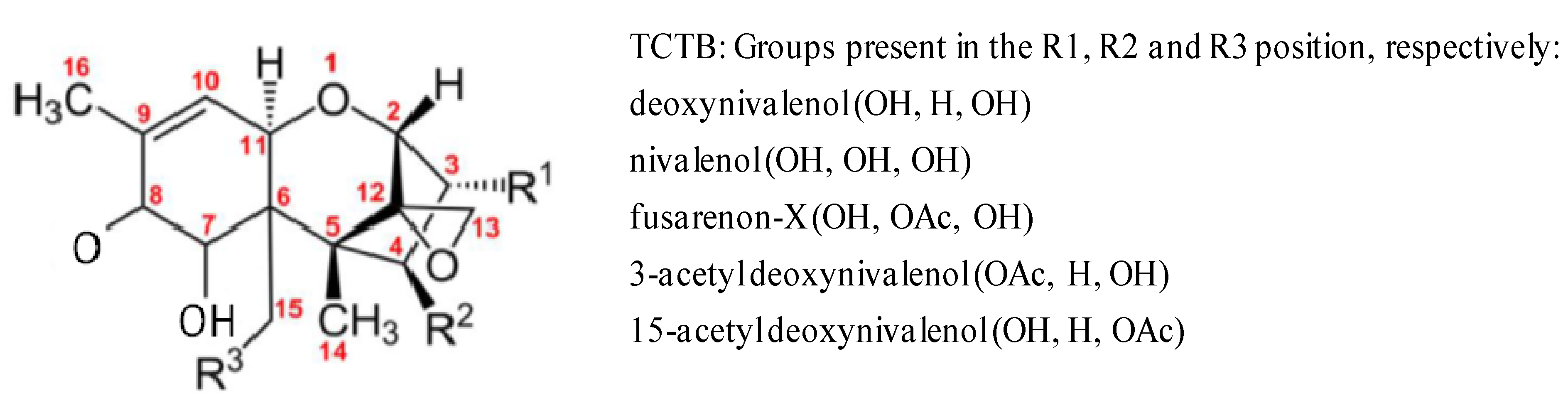
2. DON and Other TCTB Reduce Growth
3. DON and Other TCTB Affect Nutrient Absorption
3.1. Humans
3.2. Rodents
| Toxin | Animal species | Concentration and duration of exposure | Effects on nutrients absorption | References |
|---|---|---|---|---|
| DON | Human HT-29 cell line (in vitro) | 10 µM 48 h | Inhibition of D glucose/D galactose transporters | [32] |
| Inhibition of D-fructose transporter Inhibition of the active L-serine transporter | ||||
| Inhibition of active and passive L-serine transport | ||||
| Increase in palmitate transport | ||||
| DON | Mouse (in vivo) | 10 mg/kg feed 6- weeks | Reduced weight gain | [33] |
| Decreased transfer of glucose | ||||
| Decreased jejunal transfer and tissue accumulation of 5-methyltetrahydro folic acid | ||||
| Poultry (ex vivo) | 33 µM 30 min | Inhibition of jejunal Na+-amino acid co-transport | [34] | |
| Poultry (ex vivo) | 33 µM 30 and 45 min | Decrease in jejunal glucose uptake | [35] | |
| NIV | Poultry (ex vivo) | 33 µM 30 min | Decrease in jejunal glucose uptake | [31] |
| 15-ADON | Poultry (ex vivo) | 33 µM 30 min | Decrease in jejunal glucose uptake | [31] |
| FUS-X | Poultry (ex vivo) | 33 µM 30 min | No obvious effect | [31] |
3.3. Farm Animals
4. DON and Other TCTB Induce Intestinal Lesions
| Toxin | Animal species | Concentration and duration of exposure | Intestinal Lesions | References |
|---|---|---|---|---|
| Repeated exposure to TCTB (dietary or gavage) | ||||
| DON | Pig | 0.75–4.2 mg/kg 3–5 weeks | Edema and congestion | [42,43,44] |
| 0.7–5.8 mg/kg 4 weeks | Slight to moderate inflammation and congestion of intestinal mucosa. Slight to moderate degeneration of lymphoid cells in Peyer’s patches and in lymph nodes | [45] | ||
| 4 mg/kg | Corrugations in the fundic region (stomach) | [46] | ||
| 2–3 mg/kg 4 weeks | Corrugations in jejunum | [47] | ||
| 2.8 mg/kg | Multifocal atrophy and villus fusion, Apical necrosis of villi, | [48,49] | ||
| 5 weeks | Cytoplasmatic vacuolation of enterocytes, Edema of lamina propria Decrease in villus height Decrease in the number of goblet cells in the jejunum and the ileum | |||
| Rat | 10 mg/kg 4 weeks | Alteration in villus architecture of the jejunum (increased villus fusion and shorter villus length). Increased apoptosis score in jejunal epithelial cells in association with higher number of mitotic cells and crypt fission | [50] | |
| DON 15-ADON | Pig | DON 2.3 mg/kg | Reduction in villus height greater in presence of DON + 15-ADON compared to DON | [51] |
| DON 1.2 mg/kg+ 15-ADON 0.9 mg/kg 4 weeks | Histological scores of the jejunum lower in animals fed DON + 15-ADON compared to DON | |||
| Ex vivo short-term exposure to TCTB | ||||
| DON 3-ADON 15-ADON | Pig explants | 10 µM 4 h | Flattened and coalescent villi Lyses of enterocytes Interstitial edema and apoptosis 15-ADON >> DON = 3-ADON | [51] |
4.1. Humans
4.2. Rodents
4.3. Pigs
5. DON and Other TCTB Alter Intestinal Barrier Function
5.1. Effects on Cell Proliferation and Differentiation
5.1.1. Effects on Cell Growth
| Toxin | Animal species | Concentration and duration of exposure | Effects on barrier function | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| In vivo approach | ||||||||||
| DON | Mouse |
acute exposure 25 mg/kg bw (one gavage) | Increase in 4 kDa dextran permeability Effect on the distribution pattern of claudin 1, 3 and 3 tight junction proteins in small intestine | [70] | ||||||
| Rat |
chronic exposure 2 mg/kg feed 28 days | Decrease in transepithelial electrical resistance (TEER) Increase in 4 kDa dextran permeability | [50] | |||||||
| In vitro approach: intestinal epithelial cell lines | ||||||||||
| DON | Human HT-29 cell line |
2 to 50 µM 24 h 10 µM 0–24 h | Dose dependent inhibition of cell viability (IC50 = 10 µmol/L) Time dependent: Increase in total DNA damage Increase in p53 protein level Increase in caspase-3 activity | [68] | ||||||
| Human Caco-2 cell line |
84 µM 24 h | Decreased survival rate of 40% | [64] | |||||||
| Human HT-29 cell line |
0.13 to 0.7 µM 6 to 15 d | Decrease in brush border enzyme activity Decrease in protein content Decrease in transepithelial electrical resistance (TEER) Increase in lucifer yellow permeability | [62] | |||||||
| Human Caco-2 cell line |
30 µM 48 h | Decrease in TEER Decrease in claudin-4 tight junction proteins Increase in 4 kDa dextran permeability | [71] | |||||||
| Human Caco-2 cell line |
10 µM 12 h | Increase in E coli K12 translocation | [72] | |||||||
| Human Caco-2 cell line |
1.7 to 17 µM 24 h | Decrease in claudin-4 tight junction proteins | [73] | |||||||
| DON | Porcine IPEC-1 cell line |
10 to 50 µM 48 h | Decrease in TEER Decrease in claudin-3 and 4 tight junction proteins Increase in 4 kDa dextran permeability Increase in E coli 28C translocation | [71] | ||||||
| DON 3-ADON 15-ADON | Porcine IPEC-1 cell line | 10 to 30 µM | Decrease in TEER and increase in 4 kDa dextran permeability 15-ADON >> DON > 3-ADON | [51] | ||||||
| 24 to 48 h | Decrease in claudin-3 and -4 tight junction proteins expression 15-ADON >> DON = 3-ADON | |||||||||
| DON | Porcine IPEC-J2 cell line | 2.5 to 10 µM 24 h | Decrease in cell viability Increase of lactate dehydrogenase release Decrease in ATP content | [67] | ||||||
| Ex vivo approaches | ||||||||||
| DON | Porcine tissue (Ussing chamber) | 20 to 50 µM 2 h | Increase in 4 kDa dextran permeability | [71] | ||||||
| Porcine tissue (jejunal explants) | 1 to 10 µM 4 h | Shortened and coalescent villi, lysis of enterocytes, edema | [48] | |||||||
5.1.2. Effects on Cell Differentiation
5.2. Effects on Barrier Functions
5.2.1. Effects on TEER
5.2.2. Effects on Intestinal Permeability
5.2.3. Effects on Bacterial Translocation
5.2.4. Mode of Action
6. Genotoxic Effects of DON and Other TCTB
7. DON and Other TCTB Modulate the Intestinal Immune Response
7.1. DON and Other TCTB Induce Intestinal Inflammation
7.1.1. Modulation of the Cytokine Production in Intestinal Tissue by TCTB
| Toxin | Species/model | Concentration and duration exposure | Cytokine modulation | References |
|---|---|---|---|---|
| In vitro approach: intestinal epithelial cell lines | ||||
| DON | Human intestine 407 and Caco-2 cell lines | 0–3.3 µM 12 h [94] | ↗ IL-8 | [72,80,99] |
| 0–10 µM, 12 h [64] | ||||
| 0–16.9 µM, 48 h [66] | ||||
| Porcine IPEC-J2 cell line | 0.5 µM, 48 h | ↗ IL-1b, IL-6, IL-8, | [100] | |
| 2 µM, 48 h | ↘ IL-1a, MCP1 | |||
| ↗ IL-1a, IL-1b, IL-6, IL-8, TNFa, MCP1 | ||||
| Human intestine 407 cell line | 24 h pre-exposure to LPS endotoxin | ↘ IL-8 | [101] | |
| 1.7 µM, 12 h | ||||
| Ex vivo and in vivo approaches | ||||
| DON | Porcine jejunal explants (ex vivo) | 10 µM, 24 h | ↗ IL-21, IL-22, IL-23 | [102] |
| ↘ FoxP3, RALDH1 | ||||
| Porcine intestinal loops (in vivo) | 0–3.3 µM, 6 h | ↗ IL-1b, IL-8, MCP1, IL-6 | [87] | |
| Broiler chickens (in vivo) | 10 mg DON/kg, 35 d | ↘ IL-1β, IFN-g, TGFBR1 | [103] | |
| → TNF-α, IL-8, NF-κβ, | ||||
7.1.2. Modulation of the Cytokine Production in Intestinal Epithelial Cells by TCTB
7.2. DON and TCTB May Interfere with the Intestinal Homeostasis
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oswald, I.P.; Marin, D.E.; Bouhet, S.; Pinton, P.; Taranu, I.; Accensi, F. Immunotoxicological risk of mycotoxins for domestic animals. Food Addit. Contam. 2005, 22, 354–360. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Chu, F.S. Mycotoxins-Occurrence and Toxic Effect. Encyclopedia of Human Nutrition; Sadler, M., Strain, J.J., Caballero, B., Eds.; Academic Press: New York, NY, USA, 1998; pp. 858–869. [Google Scholar]
- Larsen, J.C.; Hunt, J.; Perrin, I.; Ruckenbauer, P. Workshop on trichothecenes with a focus on DON: Summary report. Toxicol. Lett. 2004, 153, 1–22. [Google Scholar] [CrossRef]
- Sugita-Konishi, Y.; Park, B.J.; Kobayashi-Hattori, K.; Tanaka, T.; Chonan, T.; Yoshikawa, K.; Kumagai, S. Effect of cooking process on the deoxynivalenol content and its subsequent cytotoxicity in wheat products. Biosci. Biotechnol. Biochem. 2006, 70, 1764–1768. [Google Scholar] [CrossRef]
- Canady, R.; Coker, R.; Rgan, S.; Krska, R.; Kuiper-Goodman, T.; Olsen, M.; Pestka, J.; Resnik, S.; Schlatter, J. Deoxynivalenol. Safety evaluation of certain mycotoxins in food. WHO Food Addit. Ser. 2001, 47, 420–555. [Google Scholar]
- Bhat, R.V.; Beedu, S.R.; Ramakrishna, Y.; Munshi, K.L. Outbreak of trichothecene mycotoxicosis associated with consumption of mould-damaged wheat production in Kashmir Valley, India. Lancet 1989, 1, 35–37. [Google Scholar]
- Luo, Y. Fusarium toxins contamination of cereals in China. In Proceedings of the 7th International IUPAC Symposium on Mycotoxins and Phycotoxins, Tokyo, Japan, 16–19 August 1988; Aibara, K., Kumagai, S., Ohtsubo, K., Yoshizawa, T., Eds.; Japanese Association of Mycotoxicology: Tokyo, Japan, 1988; pp. 97–98. [Google Scholar]
- Food and Drugs Administration. Guidance for Industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products used for Animal Feed. FDA: Rockville, MD, USA. Available online: http://www.fda.gov/downloads/Food/GuidanceRegulation/UCM217558.pdf (accessed on 15 May 2014).
- European-Union. Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:229:0007:0009:EN:PDF (accessed on 15 May 2014).
- European-Union. COUNCIL REGULATION (EC) No 463/2005 of 16 March 2005 terminating the partial interim review of the anti-dumping measures applicable to imports of certain tube or pipe fittings, of iron or steel, originating, inter alia, in Thailand. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R0463&from=EN (accessed on 15 May 2014).
- Bouhet, S.; Oswald, I.P. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet. Immunol. Immunopathol. 2005, 108, 199–209. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Pitman, R.S.; Blumberg, R.S. First line of defense: The role of the intestinal epithelium as an active component of the mucosal immune system. J. Gastroenterol. 2000, 35, 805–814. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Nejdfors, P.; Ekelund, M.; Jeppsson, B.; Westrom, B.R. Mucosal in vitro permeability in the intestinal tract of the pig, the rat, and man: Species- and region-related differences. Scand. J. Gastroenterol. 2000, 35, 501–507. [Google Scholar]
- Rothkotter, H.J.; Sowa, E.; Pabst, R. The pig as a model of developmental immunology. Hum. Exp. Toxicol. 2002, 21, 533–536. [Google Scholar] [CrossRef]
- Flannery, B.M.; Wu, W.; Pestka, J.J. Characterization of deoxynivalenol-induced anorexia using mouse bioassay. Food Chem. Toxicol. 2011, 49, 1863–1869. [Google Scholar] [CrossRef]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar]
- Wu, W.; Flannery, B.M.; Sugita-Konishi, Y.; Watanabe, M.; Zhang, H.; Pestka, J.J. Comparison of murine anorectic responses to the 8-ketotrichothecenes 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X and nivalenol. Food Chem. Toxicol. 2012, 50, 2056–2061. [Google Scholar] [CrossRef]
- Fioramonti, J.; Dupuy, C.; Dupuy, J.; Bueno, L. The mycotoxin, deoxynivalenol, delays gastric emptying through serotonin-3 receptors in rodents. J. Pharmacol. Exp. Ther. 1993, 266, 1255–1260. [Google Scholar]
- Girish, C.K.; MacDonald, E.J.; Scheinin, M.; Smith, T.K. Effects of feedborne fusarium mycotoxins on brain regional neurochemistry of turkeys. Poult. Sci. 2008, 87, 1295–1302. [Google Scholar]
- Li, Y. Sensory signal transduction in the vagal primary afferent neurons. Curr. Med. Chem. 2007, 14, 2554–2563. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Amuzie, C.J.; Shinozuka, J.; Pestka, J.J. Induction of suppressors of cytokine signaling by the trichothecene deoxynivalenol in the mouse. Toxicol. Sci. 2009, 111, 277–287. [Google Scholar] [CrossRef]
- Flannery, B.M.; Clark, E.S.; Pestka, J.J. Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY. Toxicol. Sci. 2012, 130, 289–297. [Google Scholar] [CrossRef]
- Gaige, S.; Bonnet, M.; Tardivel, C.; Pinton, P.; Trouslard, J.; Jean, A.; Guzylack, L.; Troadec, J.; Dallaporta, M. c-Fos immunoreactivity in the pig brain following deoxynivalenol intoxication: Focus on NUCB2/nesfatin-1 expressing neurons. NeuroToxicology 2013, 34, 135–149. [Google Scholar] [CrossRef]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Mounien, L.; Trouslard, J.; et al. Central inflammation and sickness-like behavior induced by the food contaminant deoxynivalenol: A PGE2-independent mechanism. Toxicol. Sci. 2011, 124, 179–191. [Google Scholar] [CrossRef]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Wanaverbecq, N.; Mounien, L.; et al. The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS One 2011, 6, e26134. [Google Scholar] [CrossRef]
- Kumagai, S.; Shimizu, T. Effects of fusarenon-X and T-2 toxin on intestinal absorption of monosaccharide in rats. Arch. Toxicol. 1988, 61, 489–495. [Google Scholar] [CrossRef]
- Awad, W.A.; Razzazi-Fazeli, E.; Bohm, J.; Zentek, J. Effects of B-trichothecenes on luminal glucose transport across the isolated jejunal epithelium of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2008, 92, 225–230. [Google Scholar] [CrossRef]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002, 132, 2723–2731. [Google Scholar]
- Hunder, G.; Schumann, K.; Strugala, G.; Gropp, J.; Fichtl, B.; Forth, W. Influence of subchronic exposure to low dietary deoxynivalenol, a trichothecene mycotoxin, on intestinal absorption of nutrients in mice. Food Chem. Toxicol. 1991, 29, 809–814. [Google Scholar] [CrossRef]
- Awad, W.A.; Rehman, H.; Bohm, J.; Razzazi-Fazeli, E.; Zentek, J. Effects of luminal deoxynivalenol and L-proline on electrophysiological parameters in the jejunums of laying hens. Poult. Sci. 2005, 84, 928–932. [Google Scholar] [CrossRef]
- Awad, W.A.; Aschenbach, J.R.; Setyabudi, F.M.; Razzazi-Fazeli, E.; Bohm, J.; Zentek, J. In vitro effects of deoxynivalenol on small intestinal D-glucose uptake and absorption of deoxynivalenol across the isolated jejunal epithelium of laying hens. Poult. Sci. 2007, 86, 15–20. [Google Scholar] [CrossRef]
- Awad, W.A.; Bohm, J.; Razzazi-Fazeli, E.; Hulan, H.W.; Zentek, J. Effects of deoxynivalenol on general performance and electrophysiological properties of intestinal mucosa of broiler chickens. Poult. Sci. 2004, 83, 1964–1972. [Google Scholar] [CrossRef]
- Awad, W.A.; Bohm, J.; Razzazi-Fazeli, E.; Zentek, J. In vitro effects of deoxynivalenol on electrical properties of intestinal mucosa of laying hens. Poult. Sci. 2005, 84, 921–927. [Google Scholar] [CrossRef]
- Yunus, A.W.; Blajet-Kosicka, A.; Kosicki, R.; Khan, M.Z.; Rehman, H.; Bohm, J. Deoxynivalenol as a contaminant of broiler feed: Intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poult. Sci. 2012, 91, 852–861. [Google Scholar] [CrossRef]
- Devreese, M.; Girgis, G.N.; Tran, S.T.; de Baere, S.; de Backer, P.; Croubels, S.; Smith, T.K. The effects of feed-borne Fusarium mycotoxins and glucomannan in turkey poults based on specific and non-specific parameters. Food Chem. Toxicol. 2014, 63, 69–75. [Google Scholar] [CrossRef]
- Dietrich, B.; Neuenschwander, S.; Bucher, B.; Wenk, C. Fusarium mycotoxin-contaminated wheat containing deoxynivalenol alters the gene expression in the liver and the jejunum of broilers. Animal 2013, 6, 278–291. [Google Scholar]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated mycotoxin Deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef]
- Pollmann, D.S.; Koch, B.A.; Seitz, L.M.; Mohr, H.E.; Kennedy, G.A. Deoxynivalenol-contaminated wheat in swine diets. J. Anim. Sci. 1985, 60, 239–247. [Google Scholar]
- Trenholm, H.L.; Hamilton, R.M.; Friend, D.W.; Thompson, B.K.; Hartin, K.E. Feeding trials with vomitoxin (deoxynivalenol)-contaminated wheat: Effects on swine, poultry, and dairy cattle. J. Am. Vet. Med. Assoc. 1984, 185, 527–531. [Google Scholar]
- Harvey, R.B.; Kubena, L.F.; Huff, W.E.; Corrier, D.E.; Clark, D.E.; Phillips, T.D. Effects of aflatoxin, deoxynivalenol, and their combinations in the diets of growing pigs. Am. J. Vet. Res. 1989, 50, 602–607. [Google Scholar]
- Cote, L.M.; Beasley, V.R.; Bratich, P.M.; Swanson, S.P.; Shivaprasad, H.L.; Buck, W.B. Sex-related reduced weight gains in growing swine fed diets containing deoxynivalenol. J. Anim. Sci. 1985, 61, 942–950. [Google Scholar]
- D’Mello, J.P.F. Antinutritional factors and mycotoxins. In Farm Animal Metabolism and Nutrition; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2000; pp. 383–403. [Google Scholar]
- Kolf-Clauw, M; Oswald, I.P. Toxalim, Research Centre in Food Toxicology, Toulouse, France. Effect of DON on the intestine in pig. 2013. [Google Scholar]
- Kolf-Clauw, M.; Castellote, J.; Joly, B.; Bourges-Abella, N.; Raymond-Letron, I.; Pinton, P.; Oswald, I.P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol. Vitro 2009, 23, 1580–1584. [Google Scholar] [CrossRef]
- Bracarense, A.P.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef]
- Payros, D; Oswald, I.P. Toxalim, Research Centre in Food Toxicology, Toulouse, France. Effect of DON on the intestine in rat. 2014. [Google Scholar]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junctions proteins and MAPKinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef]
- Awad, W.A.; Razzazi-Fazeli, E.; Bohm, J.; Zentek, J. Influence of deoxynivalenol on the D-glucose transport across the isolated epithelium of different intestinal segments of laying hens. J. Anim. Physiol. Anim. Nutr. 2007, 91, 175–180. [Google Scholar] [CrossRef]
- Zielonka, L.; Wisniewska, M.; Gajecka, M.; Obremski, K.; Gajecki, M. Influence of low doses of deoxynivalenol on histopathology of selected organs of pigs. Pol. J. Vet. Sci. 2009, 12, 89–95. [Google Scholar]
- Yoshizawa, T.; Yamashita, A.; Luo, Y. Fumonisin occurrence in corn from high- and low-risk areas for human esophageal cancer in China. Appl. Environ. Microbiol. 1994, 60, 1626–1629. [Google Scholar]
- Luo, Y.; Yoshizawa, T.; Katayama, T. Comparative study on the natural occurrence of Fusarium mycotoxins (trichothecenes and zearalenone) in corn and wheat from high- and low-risk areas for human esophageal cancer in China. Appl. Environ. Microbiol. 1990, 56, 3723–3726. [Google Scholar]
- Ohtsubo, K.; Ryu, J.C.; Nakamura, K.; Izumiyama, N.; Tanaka, T.; Yamamura, H.; Kobayashi, T.; Ueno, Y. Chronic toxicity of nivalenol in female mice: A 2-year feeding study with Fusarium nivale Fn 2B-moulded rice. Food Chem. Toxicol. 1989, 27, 591–598. [Google Scholar] [CrossRef]
- Basso, K.; Gomes, F.; Bracarense, A.P. Deoxynivanelol and fumonisin, alone or in combination, induce changes on intestinal junction complexes and in e-cadherin expression. Toxins 2013, 5, 2341–2352. [Google Scholar] [CrossRef]
- Ueno, Y. Trichothecenes: Chemical, Biological, and Toxicological Aspects. Trichothecenes; Ueno, Y., Ed.; Elsevier Press: Amsterdam, The Netherlands, 1983; pp. 135–146. [Google Scholar]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta 2011, 1813, 1619–1633. [Google Scholar] [CrossRef]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. 2008, 25, 1128–1140. [Google Scholar] [CrossRef]
- Booth, C.; Potten, C.S. Gut instincts: Thoughts on intestinal epithelial stem cells. J. Clin. Invest. 2000, 105, 1493–1499. [Google Scholar] [CrossRef]
- Kasuga, F.; Hara-Kudo, Y.; Saito, N.; Kumagai, S.; Sugita-Konishi, Y. In vitro effect of deoxynivalenol on the differentiation of human colonic cell lines Caco-2 and T84. Mycopathologia 1998, 142, 161–167. [Google Scholar] [CrossRef]
- Sergent, T.; Parys, M.; Garsou, S.; Pussemier, L.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol. Lett. 2006, 164, 167–176. [Google Scholar] [CrossRef]
- Instanes, C.; Hetland, G. Deoxynivalenol (DON) is toxic to human colonic, lung and monocytic cell lines, but does not increase the IgE response in a mouse model for allergy. Toxicology 2004, 204, 13–21. [Google Scholar] [CrossRef]
- Bony, S.; Carcelen, M.; Olivier, L.; Devaux, A. Genotoxicity assessment of deoxynivalenol in the Caco-2 cell line model using the Comet assay. Toxicol. Lett. 2006, 166, 67–76. [Google Scholar] [CrossRef]
- Bony, S.; Olivier-Loiseau, L.; Carcelen, M.; Devaux, A. Genotoxic potential associated with low levels of the Fusarium mycotoxins nivalenol and fusarenon X in a human intestinal cell line. Toxicol. Vitro 2007, 21, 457–465. [Google Scholar] [CrossRef]
- Awad, W.A.; Aschenbach, J.R.; Zentek, J. Cytotoxicity and metabolic stress induced by deoxynivalenol in the porcine intestinal IPEC-J2 cell line. J. Anim. Physiol. Anim. Nutr. 2012, 96, 709–716. [Google Scholar] [CrossRef]
- Bensassi, F.; El Golli-Bennour, E.; Abid-Essefi, S.; Bouaziz, C.; Hajlaoui, M.R.; Bacha, H. Pathway of deoxynivalenol-induced apoptosis in human colon carcinoma cells. Toxicology 2009, 264, 104–109. [Google Scholar] [CrossRef]
- Bianco, G.; Fontanella, B.; Severino, L.; Quaroni, A.; Autore, G.; Marzocco, S. Nivalenol and deoxynivalenol affect rat intestinal epithelial cells: A concentration related study. PLoS One 2012, 7, e52051. [Google Scholar]
- Akbari, P.; Braber, S.; Gremmels, H.; Koelink, P.J.; Verheijden, K.A.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol: A trigger for intestinal integrity breakdown. FASEB J. 2014, in press. [Google Scholar]
- Pinton, P.; Nougayrede, J.P.; del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef]
- Maresca, M.; Yahi, N.; Younes-Sakr, L.; Boyron, M.; Caporiccio, B.; Fantini, J. Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: Stimulation of interleukin-8 secretion, potentiation of interleukin-1beta effect and increase in the transepithelial passage of commensal bacteria. Toxicol. Appl. Pharmacol. 2008, 228, 84–92. [Google Scholar] [CrossRef]
- Van De Walle, J.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar] [CrossRef]
- Grenier, B.; Oswald, I.P. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed–focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef]
- Awad, W.A.; Bohm, J.; Razzazi-Fazeli, E.; Ghareeb, K.; Zentek, J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult. Sci. 2006, 85, 974–979. [Google Scholar] [CrossRef]
- Madara, J.L. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 1998, 60, 143–159. [Google Scholar] [CrossRef]
- Harhaj, N.S.; Antonetti, D.A. Regulation of tight junctions and loss of barrier function in pathophysiology. Int. J. Biochem. Cell Biol. 2004, 36, 1206–1237. [Google Scholar] [CrossRef]
- Van De Walle, J.; Romier, B.; Larondelle, Y.; Schneider, Y.J. Influence of deoxynivalenol on NF-kappaB activation and IL-8 secretion in human intestinal Caco-2 cells. Toxicol. Lett. 2008, 177, 205–214. [Google Scholar]
- Diesing, A.K.; Nossol, C.; Danicke, S.; Walk, N.; Post, A.; Kahlert, S.; Rothkotter, H.J.; Kluess, J. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS One 2011, 6, e17472. [Google Scholar] [CrossRef]
- Gonzalez-Vallina, R.; Wang, H.; Zhan, R.; Berschneider, H.M.; Lee, R.M.; Davidson, N.O.; Black, D.D. Lipoprotein and apolipoprotein secretion by a newborn piglet intestinal cell line (IPEC-1). Am. J. Physiol. 1996, 271, G249–G259. [Google Scholar]
- Pinto, M.; Robine-Leon, S.; Appay, M. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Kadota, T.; Furusawa, H.; Hirano, S.; Tajima, O.; Kamata, Y.; Sugita-Konishi, Y. Comparative study of deoxynivalenol, 3-acetyldeoxynivalenol, and 15-acetyldeoxynivalenol on intestinal transport and IL-8 secretion in the human cell line Caco-2. Toxicol. Vitro 2013, 27, 1888–1895. [Google Scholar] [CrossRef]
- Barrett, K.E. Positive and negative regulation of chloride secretion in T84 cells. Am. J. Physiol. 1993, 265, C859–C868. [Google Scholar]
- Nossol, C.; Diesing, A.K.; Kahlert, S.; Kersten, S.; Kluess, J.; Ponsuksili, S.; Hartig, R.; Wimmers, K.; Danicke, S.; Rothkotter, H.J. Deoxynivalenol affects the composition of the basement membrane proteins and influences en route the migration of CD16 cells into the intestinal epithelium. Mycotoxin Res. 2013, 29, 245–254. [Google Scholar] [CrossRef]
- Vandenbroucke, V.; Croubels, S.; Martel, A.; Verbrugghe, E.; Goossens, J.; van Deun, K.; Boyen, F.; Thompson, A.; Shearer, N.; de Backer, P.; et al. The mycotoxin deoxynivalenol potentiates intestinal inflammation by salmonella typhimurium in porcine ileal loops. PLoS One 2011, 6, e23871. [Google Scholar] [CrossRef]
- Hara-Kudo, Y.; Sugita-Konoshi, Y.; Kasuga, F.; Kumagai, S. Effects of deoxynivalenol on Salmonella enteritidis infection. Mycotoxins 1996, 42, 51–55. [Google Scholar]
- Li, M.; Cuff, C.F.; Pestka, J.J. Modulation of murine host response to enteric reovirus infection by the trichothecene deoxynivalenol. Toxicol. Sci. 2005, 87, 134–145. [Google Scholar]
- Lowell, C.A. Src-family kinases: Rheostats of immune cell signaling. Mol. Immunol. 2004, 41, 631–643. [Google Scholar] [CrossRef]
- Zhou, H.R.; Jia, Q.; Pestka, J.J. Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase Hck. Toxicol. Sci. 2005, 85, 916–926. [Google Scholar] [CrossRef]
- Pinton, P.; Braicu, C.; Nougayrede, J.P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef]
- Diesing, A.K.; Nossol, C.; Ponsuksili, S.; Wimmers, K.; Kluess, J.; Walk, N.; Post, A.; Rothkotter, H.J.; Kahlert, S. Gene regulation of intestinal porcine epithelial cells ipec-j2 is dependent on the site of deoxynivalenol toxicological action. PLoS One 2012, 7, e34136. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Monographs on the evaluation of carcinogenic risks to humans. Available online: http://monographs.iarc.fr/ENG/Monographs/vol56/volume56.pdf (accessed on 15 May 2014).
- Tsuda, S.; Kosaka, Y.; Murakami, M.; Matsuo, H.; Matsusaka, N.; Taniguchi, K.; Sasaki, Y.F. Detection of nivalenol genotoxicity in cultured cells and multiple mouse organs by the alkaline single-cell gel electrophoresis assay. Mutat. Res. 1998, 415, 191–200. [Google Scholar] [CrossRef]
- Stadnyk, A.W. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can. J. Gastroenterol. 2002, 16, 241–246. [Google Scholar]
- Rescigno, M.; di Sabatino, A. Dendritic cells in intestinal homeostasis and disease. J. Clin. Invest. 2009, 119, 2441–2450. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Y.; Xue, C.; Ma, J.; Xie, Q.; Wang, G.; Bi, Y.; Cao, Y. The combination of deoxynivalenol and zearalenone at permitted feed concentrations causes serious physiological effects in young pigs. J. Vet. Sci. 2008, 9, 39–44. [Google Scholar] [CrossRef]
- Moon, Y.; Yang, H.; Lee, S.H. Modulation of early growth response gene 1 and interleukin-8 expression by ribotoxin deoxynivalenol (vomitoxin) via ERK1/2 in human epithelial intestine 407 cells. Biochem. Biophys. Res. Commun. 2007, 362, 256–262. [Google Scholar] [CrossRef]
- Wan, L.Y.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of Fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef]
- Moon, Y.; Yang, H.; Park, S.H. Hypo-responsiveness of interleukin-8 production in human embryonic epithelial intestine 407 cells independent of NF-kappaB pathway: New lessons from endotoxin and ribotoxic deoxynivalenol. Toxicol. Appl. Pharmacol. 2008, 231, 94–102. [Google Scholar]
- Cano, P.M.; Seeboth, J.; Meurens, F.; Cognie, J.; Abrami, R.; Oswald, I.P.; Guzylack-Piriou, L. Deoxynivalenol as a new factor in the persistence of intestinal inflammatory diseases: An emerging hypothesis through possible modulation of Th17-mediated response. PLoS One 2013, 8, e53647. [Google Scholar]
- Ghareeb, K.; Awad, W.A.; Soodoi, C.; Sasgary, S.; Strasser, A.; Bohm, J. Effects of feed contaminant deoxynivalenol on plasma cytokines and mRNA expression of immune genes in the intestine of broiler chickens. PLoS One 2013, 8, e71492. [Google Scholar]
- Islam, Z.; Gray, J.S.; Pestka, J.J. p38 Mitogen-activated protein kinase mediates IL-8 induction by the ribotoxin deoxynivalenol in human monocytes. Toxicol. Appl. Pharmacol. 2006, 213, 235–244. [Google Scholar] [CrossRef]
- Mbandi, E.; Pestka, J.J. Deoxynivalenol and satratoxin G potentiate proinflammatory cytokine and macrophage inhibitory protein 2 induction by Listeria and Salmonella in the macrophage. J. Food Prot. 2006, 69, 1334–1339. [Google Scholar]
- Zhou, H.R.; Harkema, J.R.; Yan, D.; Pestka, J.J. Amplified proinflammatory cytokine expression and toxicity in mice coexposed to lipopolysaccharide and the trichothecene vomitoxin (deoxynivalenol). J. Toxicol. Environ. Health A 1999, 57, 115–136. [Google Scholar] [CrossRef]
- Van Leeuwen, P.A.; Boermeester, M.A.; Houdijk, A.P.; Ferwerda, C.C.; Cuesta, M.A.; Meyer, S.; Wesdorp, R.I. Clinical significance of translocation. Gut 1994, 35, S28–S34. [Google Scholar]
- Islam, L.N.; Nabi, A.H. Endotoxins of enteric pathogens modulate the functions of human neutrophils and lymphocytes. J. Biochem. Mol. Biol. 2003, 36, 565–571. [Google Scholar] [CrossRef]
- Islam, Z.; Pestka, J.J. Role of IL-1(beta) in endotoxin potentiation of deoxynivalenol-induced corticosterone response and leukocyte apoptosis in mice. Toxicol. Sci. 2003, 74, 93–102. [Google Scholar] [CrossRef]
- Azcona-Olivera, J.I.; Ouyang, Y.; Murtha, J.; Chu, F.S.; Pestka, J.J. Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): Relationship to toxin distribution and protein synthesis inhibition. Toxicol. Appl. Pharmacol. 1995, 133, 109–120. [Google Scholar] [CrossRef]
- Maheshwari, A.; Lacson, A.; Lu, W.; Fox, S.E.; Barleycorn, A.A.; Christensen, R.D.; Calhoun, D.A. Interleukin-8/CXCL8 forms an autocrine loop in fetal intestinal mucosa. Pediatr. Res. 2004, 56, 240–249. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Bambou, J.C.; Giraud, A.; Menard, S.; Begue, B.; Rakotobe, S.; Heyman, M.; Taddei, F.; Cerf-Bensussan, N.; Gaboriau-Routhiau, V. In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J. Biol. Chem. 2004, 279, 42984–42992. [Google Scholar] [CrossRef]
- Hopkins, S.J. The pathophysiological role of cytokines. Leg Med. Tokyo 2003, 5, S45–S57. [Google Scholar] [CrossRef]
- Do, K.H.; Choi, H.J.; Kim, J.; Park, S.H.; Kim, K.H.; Moon, Y. SOCS3 regulates BAFF in human enterocytes under ribosomal stress. J. Immunol. 2013, 190, 6501–6510. [Google Scholar] [CrossRef]
- Borka, K.; Kaliszky, P.; Szabo, E.; Lotz, G.; Kupcsulik, P.; Schaff, Z.; Kiss, A. Claudin expression in pancreatic endocrine tumors as compared with ductal adenocarcinomas. Virchows Arch. 2007, 450, 549–557. [Google Scholar] [CrossRef]
- Eun, C.S.; Han, D.S.; Lee, S.H.; Paik, C.H.; Chung, Y.W.; Lee, J.; Hahm, J.S. Attenuation of colonic inflammation by PPARgamma in intestinal epithelial cells: Effect on Toll-like receptor pathway. Dig. Dis. Sci. 2006, 51, 693–697. [Google Scholar] [CrossRef]
- Oswald, I.P. Role of intestinal epithelial cells in the innate immune defence of the pig intestine. Vet. Res. 2006, 37, 359–368. [Google Scholar] [CrossRef]
- Denning, T.L.; Wang, Y.C.; Patel, S.R.; Williams, I.R.; Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007, 8, 1086–1094. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A.; Walsh, S.W.; Willey, A.; Yager, D.R.; Fowler, A.A., 3rd; Graham, M.F. Linoleic acid induces interleukin-8 production by Crohn’s human intestinal smooth muscle cells via arachidonic acid metabolites. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G528–G537. [Google Scholar] [CrossRef]
- Banks, C.; Bateman, A.; Payne, R.; Johnson, P.; Sheron, N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J. Pathol. 2003, 199, 28–35. [Google Scholar] [CrossRef]
- Georganas, C.; Liu, H.; Perlman, H.; Hoffmann, A.; Thimmapaya, B.; Pope, R.M. Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: The dominant role for NF-kappa B but not C/EBP beta or c-Jun. J. Immunol. 2000, 165, 7199–7206. [Google Scholar]
- Martin, H.M.; Campbell, B.J.; Hart, C.A.; Mpofu, C.; Nayar, M.; Singh, R.; Englyst, H.; Williams, H.F.; Rhodes, J.M. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology 2004, 127, 80–93. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol-induced IgA production and IgA nephropathy-aberrant mucosal immune response with systemic repercussions. Toxicol. Lett. 2003, 140–141, 287–295. [Google Scholar] [CrossRef]
- Beagley, K.W.; Eldridge, J.H.; Lee, F.; Kiyono, H.; Everson, M.P.; Koopman, W.J.; Hirano, T.; Kishimoto, T.; McGhee, J.R. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J. Exp. Med. 1989, 169, 2133–2148. [Google Scholar] [CrossRef]
- Shi, Y.; Pestka, J.J. Attenuation of mycotoxin-induced IgA nephropathy by eicosapentaenoic acid in the mouse: Dose response and relation to IL-6 expression. J. Nutr. Biochem. 2006, 17, 697–706. [Google Scholar] [CrossRef]
- Yan, D.; Zhou, H.R.; Brooks, K.H.; Pestka, J.J. Potential role for IL-5 and IL-6 in enhanced IgA secretion by Peyer’s patch cells isolated from mice acutely exposed to vomitoxin. Toxicology 1997, 122, 145–158. [Google Scholar] [CrossRef]
- Pinton, P.; Accensi, F.; Beauchamp, E.; Cossalter, A.M.; Callu, P.; Grosjean, F.; Oswald, I.P. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol. Lett. 2008, 177, 215–222. [Google Scholar] [CrossRef]
- Bouhet, S.; le Dorze, E.; Peres, S.; Fairbrother, J.M.; Oswald, I.P. Mycotoxin fumonisin B1 selectively down-regulates the basal IL-8 expression in pig intestine: in vivo and in vitro studies. Food Chem. Toxicol. 2006, 44, 1768–1773. [Google Scholar] [CrossRef]
- McLaughlin, J.; Padfield, P.J.; Burt, J.P.; O’Neill, C.A. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am. J. Physiol. Cell Physiol. 2004, 287, C1412–C1417. [Google Scholar] [CrossRef]
- Sergent, T.; Dupont, I.; Jassogne, C.; Ribonnet, L.; van der Heiden, E.; Scippo, M.L.; Muller, M.; McAlister, D.; Pussemier, L.; Larondelle, Y.; et al. CYP1A1 induction and CYP3A4 inhibition by the fungicide imazalil in the human intestinal Caco-2 cells-comparison with other conazole pesticides. Toxicol. Lett. 2009, 184, 159–168. [Google Scholar] [CrossRef]
- Maresca, M.; Fantini, J. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon 2010, 56, 282–294. [Google Scholar] [CrossRef]
- Wache, Y.J.; Valat, C.; Postollec, G.; Bougeard, S.; Burel, C.; Oswald, I.P.; Fravalo, P. Impact of deoxynivalenol on the intestinal microflora of pigs. Int. J. Mol. Sci. 2009, 10, 1–17. [Google Scholar]
- Sirot, V.; Fremy, J.M.; Leblanc, J.C. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food Chem. Toxicol. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pinton, P.; Oswald, I.P. Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins 2014, 6, 1615-1643. https://doi.org/10.3390/toxins6051615
Pinton P, Oswald IP. Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins. 2014; 6(5):1615-1643. https://doi.org/10.3390/toxins6051615
Chicago/Turabian StylePinton, Philippe, and Isabelle P. Oswald. 2014. "Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review" Toxins 6, no. 5: 1615-1643. https://doi.org/10.3390/toxins6051615
APA StylePinton, P., & Oswald, I. P. (2014). Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins, 6(5), 1615-1643. https://doi.org/10.3390/toxins6051615





