Abstract
Snakebite envenoming represents a neglected tropical disease that has a heavy public health impact worldwide, mostly affecting poor people involved in agricultural activities in Africa, Asia, Latin America and Oceania. A key issue that complicates the treatment of snakebite envenomings is the poor availability of the only validated treatment for this disease, antivenoms. Antivenoms can be an efficacious treatment for snakebite envenoming, provided they are safe, effective, affordable, accessible and administered appropriately. The shortage of antivenoms in various regions, particularly in Sub-Saharan Africa and some parts of Asia, can be significantly alleviated by optimizing the use of current antivenoms and by the generation of novel polyspecific antivenoms having a wide spectrum of efficacy. Complementing preclinical testing of antivenom efficacy using in vivo and in vitro functional neutralization assays, developments in venomics and antivenomics are likely to revolutionize the design and preclinical assessment of antivenoms by being able to test new antivenom preparations and to predict their paraspecific neutralization to the level of species-specific toxins.
1. Introduction: Snakebite Envenoming and the Challenge of Generating Effective Antivenoms
Snakebite envenoming is largely a neglected threat to public health that affects some of the world’s poorest rural communities, mainly those involved in subsistence farming and livestock grazing activities in tropical and subtropical regions of Africa, Asia, Latin America and Oceania. An estimated 5.5 million people are bitten by snakes each year, resulting in about 400,000 amputations and over 100,000 deaths; however, the true scale of this “disease of poverty” may be much greater than these hospital-based statistics, since many affected people do not attend health facilities [1,2,3,4]. Persistent underestimation of their true morbidity and mortality has made snakebites the most neglected of all of the World Health Organization (WHO)’s “neglected tropical diseases”, downgrading its public health importance. Moreover, the WHO recently moved snakebite envenoming to the category of “neglected tropical condition”, further reducing its relevance and thereby affecting the global awareness of the magnitude of this pathology. Strategies to address this neglect should include the improvement of affordable, effective antivenoms [3,5,6,7,8], the timely administration of which remains the only specific treatment for envenoming [9], more than a century after the development of the first serum antivenimeux by Calmette [10,11,12] and Phisalix and Bertrand [13,14] simultaneously, but independently, in 1894. In addition, the struggle against snakebite envenoming should include other aspects, such as improved statistics on morbidity and mortality, adequate access and distribution of safe and effective antivenoms in the public health system, training of health staff in the effective management of this disease and promotion of preventive campaigns at the local community level, among other goals. This series of tasks demand, in turn, the involvement of a large spectrum of stakeholders in diverse fields.
More than 45 commercial or government antivenom producers exist around the world [15]. However, the lack of financial incentives in a technology that has remained relatively unchanged for the better part of the second half of the 20th Century, along with dwindling markets and a lack of leadership from global public health organizations, have made antivenom production a field of limited improvements and very little innovation [7]. Snake antivenoms became scarce or non-existent as poor commercial incentives forced some manufacturers to leave the market and others to downscale production or increase the price, leading to a decline in the availability and accessibility for these life-saving antidotes to the millions of rural poor most at risk from snakebites in low and middle-income countries [3]. Furthermore, many manufacturing laboratories in public institutions have suffered a lack of investment and renewal of their technological platforms, together with deficient training of their staff, thereby affecting the quality and quantity of their antivenoms. To raise the awareness of public health authorities on the relevance of the snakebite problem and to ensure supplies of effective antivenoms in deficitary parts of the world, several initiatives have emerged in the last decade. In 2008, members of the international toxinology community established the Global Snakebite Initiative [16,17]. Some of the goals of this organization include advocacy on the seriousness of this health problem, together with the promotion of research initiatives to improve epidemiological and clinical knowledge of envenomings and initiatives to improve training of health staff and antivenom quality control and accessibility in some regions of the world [2,5,18,19]. The GSI strategy has also the goal of coupling modern proteomics, immunological, pharmacological and molecular biological techniques to the quest for improved therapeutics and the understanding of the underlying pathophysiology and clinical manifestations of snakebite envenomings [4,20,21,22,23].
A key technical issue concerning the generation of new antidotes for snakebite envenoming is the design of improved immunization mixtures in such a way that the resulting antivenoms are effective against most venoms of the medically-relevant snake species within the geographical range where these antivenoms are intended to be used. This purpose is not trivial given the well-documented occurrence of venom variability at the genus, species, subspecies, population and individual levels [24]. The variability of venom composition may endow snakes with the capability to adapt to different ecological niches. This is clearly evident for highly adaptable snake species of a wide geographical distribution in which allopatric venom variation may result in variable clinical presentations of envenomings [23,25]. In addition, variation in venom composition is dictated by different genomic and postgenomic mechanisms [26,27], and phenotypic venom variation across conspecific populations often involves ontogenetic shifts in venom protein expression [23]. Furthermore, venom is evolutionarily a highly labile trait, even among very closely-related taxa [28,29], preventing the prediction of venom composition and toxic activity based on phylogenetic distance. For instance, species within the genus Crotalus express either Type I (high levels of metalloprotease and low toxicity) or Type II (low metalloprotease, high toxicity) venoms, which result in completely different envenomings from a pathophysiological standpoint, and these venom phenotypes exhibit no phylogenetic relationship [30]. Furthermore, the finding of different evolutionary solutions within arboreal Bothriechis taxa for the same trophic purpose [31] (Figure 1) strengthens the view that phylogeny cannot be invoked as the sole criterion for species selection for antivenom production.
The occurrence of ontogenetic, geographic and individual intraspecific venom variability highlights the necessity of using pooled venoms as a representative sample for antivenom manufacture, and a thorough study of clinical, epidemiological, immunological, proteomic and toxicological information may contribute to the design of the venom mixtures for immunization. These methodological approaches include classical biochemical and state-of-the-art proteomic analysis of venoms, the study of the toxicological profile of venom effects using in vivo and in vitro tests, and the investigation of the immunological cross-reactivity of antivenoms against homologous and heterologous venoms. Knowledge on the paraspecificity of antivenoms is not only of applied importance to optimize the production strategy of a novel antivenom, but also for predicting the full clinical range of existing antivenoms against homologous and heterologous venoms. To this end, a platform has been developed to explore the neutralizing ability and immunological cross-reactivity of antivenoms through a combination of methodologies that will be briefly discussed.
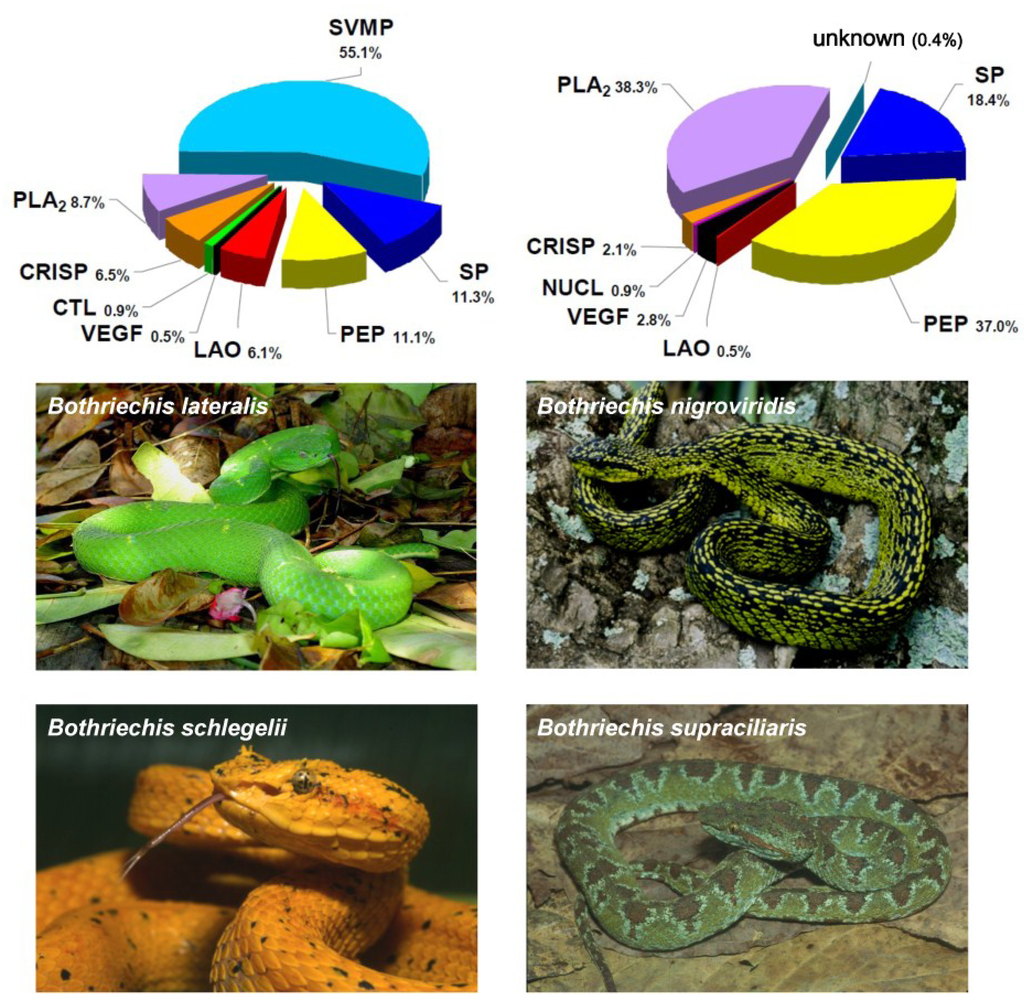
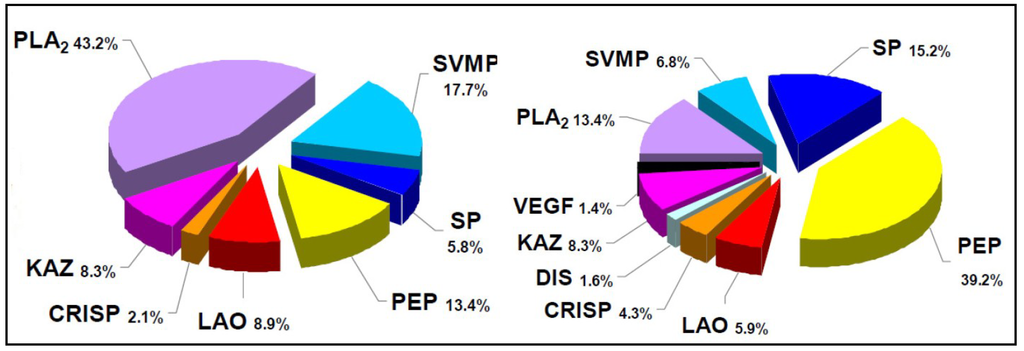
Figure 1.
Highly divergent toxin compositions in phylogenetically-close snake taxa. Venom components of four Bothriechis species that inhabit Costa Rica were assigned to protein families, and their abundances were estimated, by using the “snake venomics” analytical strategy. As shown in the corresponding pie charts summarizing protein family abundances (%), the venom of Bothriechis lateralis is dominated by metalloproteinases, whereas small peptides of the vasoactive type are predominant in the venom of B. supraciliaris. On the other hand, B. schlegelii venom contains the highest proportion of phospholipases A2, while B. nigroviridis venom completely lacks metalloproteinases and presents a high percentage of an unusual phospholipase A2 recently characterized as a crotoxin-like complex, found for the first time in a non-rattlesnake New World pit viper. In addition, a novel protein type for snake venoms (Kazal-type proteinase inhibitor-like) was found in B. schlegelii and B. supraciliaris, but not in the other two species of this genus. Protein family abbreviations correspond to: SVMP, metalloproteinase; PLA2, phospholipase A2; SP, serine proteinase; CRISP, cysteine-rich secretory proteins; CTL, C-type lectins/lectin-like; VEGF, vascular endothelial growth factor; LAO, L-amino acid oxidase; NUCL, nucleotidase; DIS, disintegrin; PEP, small peptide; KAZ, Kazal-type proteinase inhibitor-like. Data adapted from Lomonte et al. [32].
2. Biochemical and Toxinological Toolbox for the Preclinical Assessment of Antivenom Efficacy
The analysis of the ability of an antivenom to neutralize the most relevant toxic activities of the snake venoms for which it was designed is a preclinical requisite before it can go into clinical trials and is approved for medical use. Simple experimental protocols have been developed to assess the ability of antivenoms to neutralize the most relevant toxic effects of snake venoms [22,33,34,35,36,37]. The most widely-used protocol is based on the incubation of a fixed dose of venom and variable dilutions of antivenom, followed by the injection of aliquots of the mixtures in the corresponding assay systems [22,33]. Another experimental platform, which is not regularly used, but which is relevant when testing antivenoms of variable pharmacokinetic profiles, is based on the injection of venom, followed by the administration of antivenom by the intravenous route. This approach does not involve the mixture of venom and antivenom before injection and, consequently, reproduces more closely the actual dynamics of therapy in the clinical setting.
Lethality is the single most important effect to be tested when analyzing venom toxicity and its neutralization by antivenoms. For the lethality neutralization assay, a “challenge dose”, which usually corresponds to 3 to 6 LD50s, depending on the laboratory, is mixed with various dilutions of the antivenom, and the mixtures are incubated (generally for 30 min at 37 °C). Control samples include venom incubated with saline solution instead of antivenom. The mixtures are then injected in mice, either by the intravenous or the intraperitoneal routes, and deaths occurring during a predefined time span (24 h or 48 h) are recorded. Neutralization is expressed as the median effective dose (ED50), i.e., the antivenom/venom ratio in which 50% of the injected mice survive. Depending on the laboratory, ED50 is expressed in various ways, i.e., milligrams of venom neutralized per milligrams of antivenom proteins, milligrams of venom neutralized by milliliters of antivenom, milliliters of antivenom required to neutralize one milligram of venom or the number of LD50s of venom neutralized per milliliter antivenom. The assessment of the ability of antivenoms to neutralize lethality is routinely performed in the manufacturers’ quality control laboratories and by national regulatory agencies, as part of regular analyses of the antivenoms being manufactured, purchased and distributed. Unfortunately, some countries rely mostly on data reported by the manufacturers, rather than on control exerted by regulatory agencies. It is necessary to strengthen, through workshops and other activities, the national capacities to perform quality control of antivenoms worldwide.
The neutralization of venom lethality is, and will remain, the gold standard in the preclinical testing of antivenom efficacy. However, the study of the biochemical and toxicological complexity of snake venoms has shown clearly that, besides lethality, the venoms of many species induce additional toxic activities that play key roles in the pathophysiology of human envenoming. For example, envenomings by viperid snakes in many regions around the world are characterized by complex local tissue damage (myonecrosis, dermonecrosis, edema, hemorrhage, blistering) and by systemic disturbances (hemorrhage, coagulopathy, cardiovascular shock, acute kidney injury). Therefore, a more detailed analysis of the preclinical efficacy of antivenoms should encompass, in addition to lethality, the neutralization of these other clinically-relevant effects [33,34,35]. A series of simple in vivo and in vitro laboratory assays has been developed for the quantitative assessment of hemorrhagic, myotoxic, dermonecrotic, coagulant and defibrinogenating activities, among others [36,38,39]. Thus, although the routine quality control of antivenoms involves the neutralization of lethal activity, when a new antivenom is being developed or when an existing antivenom is introduced to a new geographical setting, a comprehensive preclinical analysis of neutralizing efficacy should be performed against the most relevant toxic effects of the most important snake venoms in that particular region.
The single most important effect in elapid snake venoms is neurotoxicity. Presynaptically-acting PLA2s disrupt the integrity of nerve terminal plasma membrane, and post-synaptically-acting 3FTxs bind with strong affinity to the nicotinic cholinergic receptor at the motor end-plate, hence causing flaccid paralysis, which may result in paralysis of respiratory muscles and death [40]. The preclinical efficacy of antivenoms against these venoms can be assessed by the neutralization of lethal effect (the ED50 test), since the end result of neurotoxicity is death. Alternatively, neurotoxic activity can be assessed by using ex vivo nerve-muscle preparations, such as the phrenic-nerve and biventer-cervicis preparations. Venoms of a number of spitting cobras in Africa and Asia induce predominantly a local necrotizing effect in humans [40,41]. In these cases, antivenoms should be assessed for their capacity to neutralize lethality and dermonecrosis [42]. Sea snake venoms may induce, in addition to a neurotoxic effect, a systemic myotoxic action, which might result in rhabdomyolysis. Hence, antivenoms should be tested for the neutralization of lethal and myotoxic activities [22]. Venoms of some land Australian elapids induce neurotoxicity, myotoxicity and coagulopathy; consequently, preclinical evaluation of antivenoms should include the neutralization of these effects [43]. On the other hand, the preclinical assessment of antivenoms against viperid venoms should include the analysis of the lethal, hemorrhagic, myotoxic, coagulant and defibrinogenating activities of venoms [20]. A brief description of some of these methodologies follows.
- (1)
- Hemorrhagic activity: The most widely-used method is based on the intradermal injection of venom solutions, followed, several hours later, by the measurement of the area of the hemorrhagic spot in the inner side of the skin [36,44,45]. Venom activity is expressed as the minimum hemorrhagic dose (MHD), which corresponds to the dose of venom that induces a hemorrhagic halo of 10 mm in diameter. More recently, the analysis of systemic, i.e., pulmonary, hemorrhage has been introduced. Mice are injected i.v. with venom, and one hour later, the animals are sacrificed and the thoracic cavity exposed for observation of hemorrhagic spots on the surface of the lungs. The minimum pulmonary hemorrhagic dose (MPHD) corresponds to the lowest amount of venom that induces hemorrhagic spots in the lungs of all mice injected [46].
- (2)
- Myotoxic activity: Venom-induced skeletal muscle necrosis can be assessed by histological examination of muscle tissue injected with venom. Mice receiving an intramuscular injection of venom solution, for example in the gastrocnemius muscle, are sacrificed 24 h later, and the injected muscle is processed for histological analysis. The number of necrotic cells and the total number of muscle cells are quantified by microscopic assessment, and the myotoxic effect is expressed as the necrotic index, i.e., the ratio of necrotic muscle fibers to total muscle fibers [47]. Venom activity can be expressed as the dose inducing a necrotic index of 0.5. Since histological analysis is time consuming and not all laboratories have facilities for tissue processing for histology, a convenient alternative for the histological analysis is the quantification of the activity of the plasmatic enzyme, creatine kinase (CK), which is released from muscle fibers when the plasma membrane of muscle cells is disrupted. Mice are injected intramuscularly, as described, and a blood sample is collected usually 3 h after injection. After separation of plasma by centrifugation, the plasma CK activity is quantified by using commercial kits. The minimum myotoxic dose (MMD) is defined as the dose of venom that increases the plasma CK activity four times as compared to mice injected with saline solution [48].
- (3)
- Dermonecrotic activity: This effect is assessed in either rats or mice by carrying out intradermal injections of venom solutions, followed by the measurement of the necrotic area in the inner side of the skin 72 h after venom injection [36].
- (4)
- Coagulant activity: In vitro coagulant activity of venoms is assessed by the addition of various doses of venom to samples of citrated human plasma, obtained from healthy donors, followed by the determination of clotting time. Activity is expressed as the minimum coagulant dose (MCD), defined as the dose of venom that induces clotting in 60 seconds [36,39]. For assessing the thrombin-like activity of venoms, a similar test is performed on fibrinogen solutions instead of plasma [36].
- (5)
- Defibrinogenating activity: This is assessed in rats or mice by intravenous injection of venom solutions. After a defined period of time, a blood sample is collected, placed in a glass tube and incubated at room temperature for observation of clot formation. Activity is expressed as the minimum defibrinogenating dose (MDD), defined as the dose of venom that induces incoagulability, i.e., blood remains unclottable, in all animals injected [36,39].
- (6)
- Other tests: Assays for the determination of other toxic activities, such as edema-forming activity, ex vivo neurotoxic activity and thrombocytopenic effect, have been described [49,50,51]. The analysis of the neutralization of venom enzyme activities, including neutralization of proteinase, phospholipase A2 and hyaluronidase activities, has been also investigated [41,52,53]. Similarly to the neutralization of lethality, for the analysis of the neutralization of venom enzyme activities, various mixtures of venom:antivenom incubated for 30 min at 37 °C are tested, and neutralization of venom activity is expressed as ED50, i.e., the antivenom/venom ratio in which the effect of venom is neutralized by 50% [54]. In the case of coagulant and defibrinogenating activities, neutralization is expressed as the effective dose (ED), defined as the antivenom/venom ratio at which the clotting time of plasma is prolonged three times when compared to plasma incubated with venom alone (for coagulant activity) or as the antivenom/venom ratio in which blood clots form in all animals injected (for defibrinogenating effect) [39].
3. Omics Toolbox for the Preclinical Assessment of Antivenom Efficacy: The Venomics-Antivenomics Platform
Research on venoms has been continuously enhanced by advances in technology. Progress in high-throughput “omics” methodologies and associated instrumentation has catalyzed an expansion of the scope of biological research from the biochemical analyses of just a few molecules at a time to the systematic and simultaneous study of whole genome sequences, transcriptomes and proteomes. Particularly, the last decade has witnessed the introduction of protocols and strategies for unravelling the toxin composition of venoms (“venomics”) in great detail, both directly (employing proteomics-centered approaches) [20,21,55,56,57] or indirectly (via cDNA library construction or next-generation high-throughput venom gland transcriptomics and bioinformatic analysis) [58,59] in a relatively rapid and cost-effective manner (reviewed in [23]). Proteomics-centered venomics requires homologous searchable databases to fully exploit its analytical capabilities. In addition, the combination of homologous venom proteomics and venom gland transcriptomics can yield complete, or nearly complete, locus-specific, assigned venom proteomes [60,61,62]. Such proteomics characterization of snake venoms offers a framework for rationalizing the bioactivities of venoms (Figure 2) and for clustering venoms based on within- and between-species shared traits and trends [23].
A specific question that has been addressed in recent years using a venomics approach, that is of relevance for the treatment of snakebites, concerns the occurrence and onset of geographic and ontogenetic shifts in toxin composition within Bothrops [63,64,65,66], Crotalus [25,67,68,69], Sistrurus [70,71], Lachesis [72,73] and Gloydius [74] genera. These studies highlight the concept that a species should be considered as a group of metapopulations and that within a species range, pedomorphic and ontogenetic venom phenotypes often occur in geographically-differentiated areas [23,75]. This knowledge is of fundamental importance for the selection of species and specimens for the manufacturing of improved therapeutic antivenoms. To aid in antivenom design and to assess the range of the possible clinical applications of current commercial or experimental monospecific and polyspecific antivenoms, the proteomics-centered protocol, dubbed “antivenomics”, was developed [76,77].
Antivenomics complements the in vitro and in vivo venom activity neutralization assays and traditional immunological methods, such as ELISA and western blot analyses, for assessing the preclinical neutralizing spectrum of antivenoms. Second generation antivenomics [77] is an affinity chromatography protocol to investigate the immuno-capturing ability of immobilized IgG, F(ab')2, or Fab antibody molecules followed by the proteomic identification of the venom components recovered in the retained and the non-bound fractions. The antivenomic analysis provides qualitative and also quantitative information on the sets of venom proteins presenting antivenom-recognized epitopes and those exhibiting impaired immunoreactivity. While comparing the levels of immune recognition gathered from antivenomics with the in vivo neutralization capacity of an antivenom is not straightforward, since both experiments involve radically different protocols, in our experience, even a moderate immunocapturing capability of ~20%–25% correlates with a good outcome in the in vivo neutralization tests. Assuming that the degree of immunorecognition of a toxin by the antivenom’s antibodies represents a measure of the capability of this antivenom to neutralize the toxic activity of that toxin, the antivenomics analysis may assist in assessing the range of clinical applications of current commercial or experimental antivenoms and in the development of improved antivenoms on an immunologically-sound basis. Antivenomics provides thus the grounds for rationalizing the paraspecificity of antivenoms and its capability to aid in the formulation of hypotheses as to how venom mixtures might be designed or re-designed for the manufacturing of improved therapeutic antivenoms. Despite its recent introduction, the usefulness and validity of antivenomics to complement the in vivo standard preclinical assays of neutralization of lethality and toxic activities by antivenoms has been documented in a number of investigations in recent years [22,23,42,62,73,78,79,80]. Figure 3 illustrates a practical example of this point.
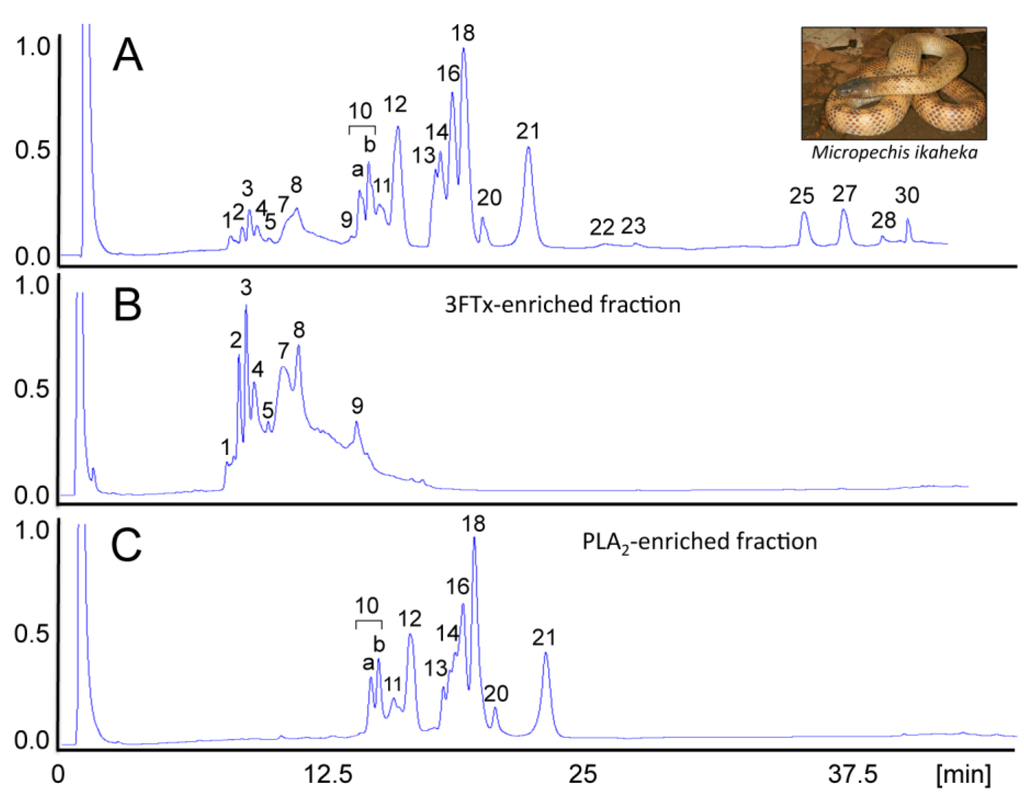
Figure 2.
Venomics complements neutralization assays. Major effects of envenomings by the New Guinea small-eyed snake, Micropechis ikaheka, a large and powerfully-built elapid endemic to Papua New Guinea and Indonesian West Papua province, include life-threatening post-synaptic neuromuscular blockade, resulting in respiratory paralysis, PLA2-mediated myotoxicity, hypotension and cardiovascular abnormalities. The venom proteome of M. ikaheka is dominated by at least 29 D49-phospholipases A2 (PLA2) and 14 short and long neurotoxins of the three-finger toxin (3FTx) family [62]. These protein classes represent, respectively, 80% and 9.2% of the total venom proteins. Reverse-phase HPLC allowed the fractionation of M. ikaheka venom (A) into 3FTx- (B) and PLA2-enriched (C) fractions. In vivo neutralization assays showed that PLA2 molecules represent the main myotoxic components of M. ikaheka venom. The estimated LD50 for mice of the reverse-phase-isolated 3FTx- (0.22 mg/kg) and PLA2- (1.62 mg/kg) enriched fractions, indicated that these two toxin classes contribute synergistically to venom lethality (0.62 mg/kg), with the 3FTxs playing a dominant role [62]. Reproduced with permission from reference [62]. Copyright 2014 Elsevier.
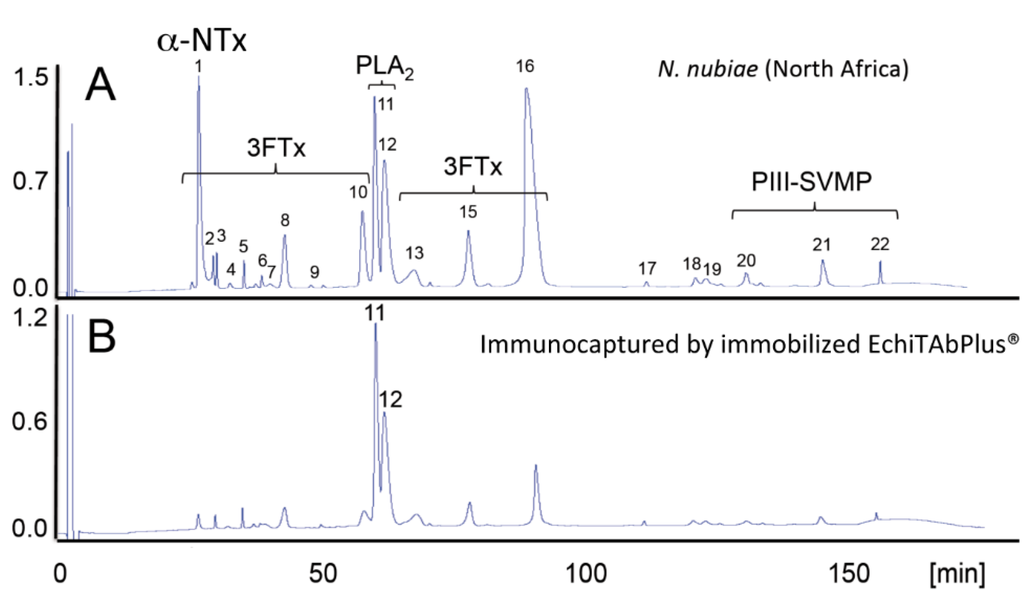
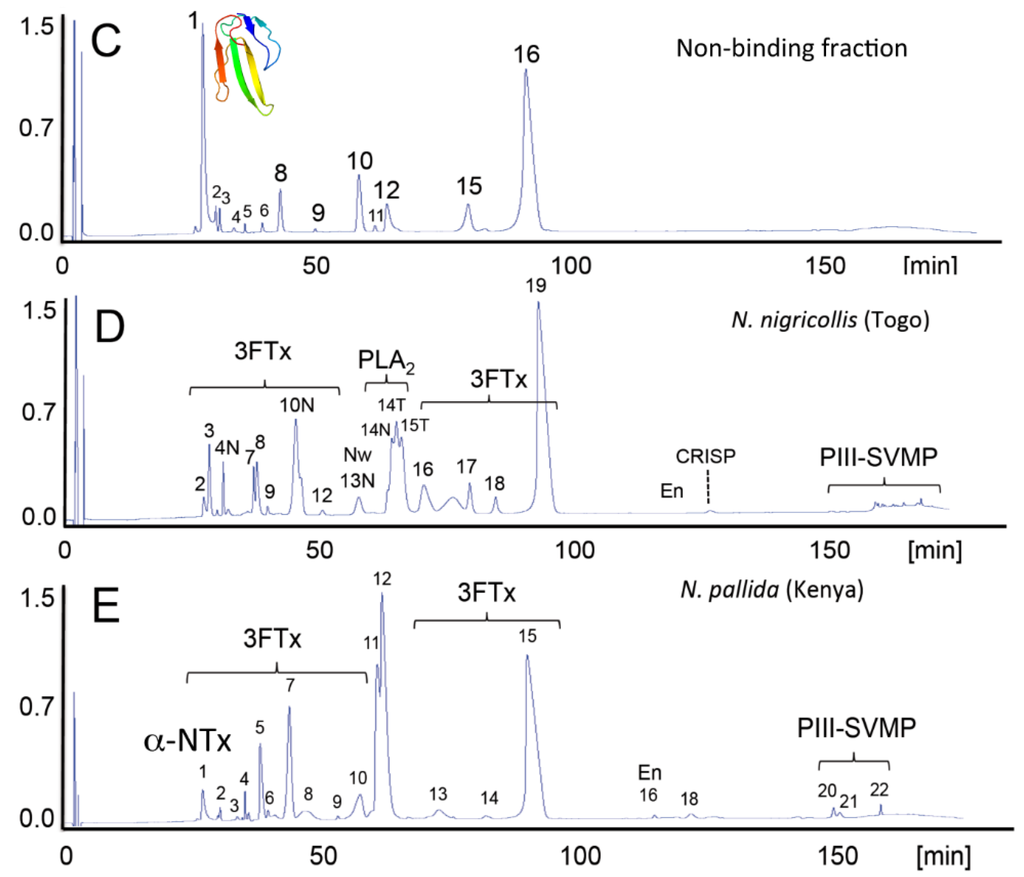
Figure 3.
Antivenomics complements neutralization assays. The capability of the Costa Rican antivenom, EchiTAb-Plus-ICP® [81], to reverse the effects of the venoms of African spitting cobras was investigated by neutralization tests [42,77]. EchiTAb-Plus-ICP® neutralized the PLA2 and dermonecrotic activities of all of the venoms of African Naja snakes sampled. Lethality induced by venoms of the black-necked spitting cobra (N. nigricollis), the Mozambique spitting cobra (N. mossambica) and the red spitting cobra (N. pallida) was eliminated, but did not prevent the lethal effect of the venoms of the Katian spitting cobra (N. katiensis) and the Nubian spitting cobra (N. nubiae). Antivenomics analysis showed that the antivenom immunocaptured PLA2 molecules (Peaks 11 and 12 (B) and, to a lesser extent, a 3FTx eluted in Peak 16 (B), but had impaired the capability of the antivenom to immunodeplete a high abundance type-1 α-neurotoxin (α-NTx) of N. nubiae ((A) and (C); Peak 1, 12.6% of the total venom proteome) and N. katiensis (4.4%) venoms correlated with the pre-clinical inability of EchiTAb-Plus-ICP® antivenom to neutralize the lethality of N. nubiae and N. katiensis venoms. Strikingly, although this lethal α-neurotoxin was originally purified “from the venom of N. nigricollis collected in Ethiopia in 1961” [82], a recent proteomics survey failed to find α-neurotoxin (6,786.7 Da, SwissProt Accession Code P01426) in N. nigricollis venoms [42] (D). African spitting cobras have had a long history of taxonomic uncertainty. Relevant for rationalizing the “α-toxin paradox”, N. nigricollis pallida was elevated to full species status by Branch [83] and Hughes [84]. This was later supported by Wüster and Broadley [85], who, in addition, described a new species, N. nubiae, in populations previously considered to belong to the Katian spitting cobra. α-Neurotoxin is expressed in venoms of N. pallida and N. nubiae (reverse-phase HPLC Peak 1, in (A) and (E), respectively), but is virtually absent from the other African spitting Naja venoms investigated. Thus, the failure of the EchiTAb-Plus-ICP® antivenom to neutralize the lethal activity of the Nubian and Katian spitting cobra venoms may be due to the absence of α-neurotoxin epitopes in the N. nigricollis venom (D) employed in the immunization mixture to generate this antivenom. The “α-neurotoxin paradox”, resolved by combining neutralization assays and antivenomics, underpins the importance of getting the taxonomy right for the development of a strategy for the improvement of antivenoms.
4. Concluding Remarks
The shortage of antivenoms in various parts of the planet can be in part counteracted through the improved deployment of currently existing antivenoms, but also through the design and production of next-generation polyspecific antivenoms of a wide neutralizing spectrum. Venomics-guided uncovering of evolutionary convergent compositional and immunological trends between homologous and heterologous snake venoms may help in the design of such broad specificity antidotes. For this purpose, the choice of immunization mixture cannot be based on phylogenetic distance, but on a deep understanding of the intraspecific venom variation of the medically-important snakes across the geographical range where the antivenom will be deployed. A deep understanding of the venom toxin profile and the identification of divergent and convergent trends along the evolutionary history of venoms have significant implications for selecting the most appropriate species and specimens for manufacturing antivenoms exhibiting an improved therapeutic scope. Antivenomics represents a knowledge-based approach to help in designing improved venom-based immunogen mixtures and to predict paraspecific neutralization by antivenom preparations to the level of species-specific toxins. Accumulating evidence shows the potential of the combination of antivenomics and neutralization assays for analyzing at the molecular level the preclinical efficacy of antivenoms against homologous and heterologous venoms.
Acknowledgments
Funding for the projects described in this paper was provided by Grants BFU2007–61563 and BFU2010–17373 from the Ministerios de Educación y Ciencia and Ciencia é Innovación, Madrid, joint programme Fundación Costa Rica-USA/Consejo Superior de Investigaciones Científicas (CRUSA-CSIC) (2007CR0004 and 2009CR0021) and Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo (CYTED) (206AC0281). Travelling between Spain and Costa Rica was financed by Acciones Integradas 2006CR0010 between CSIC and the University of Costa Rica (UCR).
Author Contributions
All of the authors contribute to writing the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modeling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Gutiérrez, J.M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteomics 2011, 74, 1735–1767. [Google Scholar] [CrossRef]
- Warrell, D.A.; Gutiérrez, J.M.; Calvete, J.J.; Williams, D. New approaches and technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J. Med. Res. 2013, 138, 38–59. [Google Scholar] [PubMed]
- Gutiérrez, J.M.; Williams, D.J.; Fan, H.W.; Warrell, D.A. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon 2010, 56, 1223–1235. [Google Scholar]
- Gutiérrez, J.M.; León, G.; Burnouf, T. Antivenoms for the treatment of snakebite envenomings: The road ahead. Biologicals 2011, 39, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Cook, D.A.; Renjifo, C.; Casewell, N.R.; Currier, R.B.; Wagstaff, S.C. Research strategies to improve snakebite treatment: Challenges and progress. J. Proteomics 2011, 74, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M. Improving antivenom availability and accessibility: Science, technology, and beyond. Toxicon 2012, 60, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, D.G.; Theakston, R.D.G. Snake antivenoms. J. Toxicol. Clin. Toxicol. 2003, 41, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Calmette, A. L'immunisation artificielle des animaux contre le venin des serpents, et la thérapeutic expérimentale des morsures venimeuses. Compte. Rendus Soc. Biol. 1894, 46, 120–124. [Google Scholar]
- Calmette, A. Contribution à l'étude du venin des serpents. Immunisation des animaux et traitement de l'envenimation. Ann. l'Institut Pasteur 1894, 8, 275–291. (In French) [Google Scholar]
- Hawgood, B.J. Doctor Albert Calmette 1863–1933: Founder of antivenomous serotherapy and of antituberculous BCG vaccination. Toxicon 1999, 37, 1241–1258. [Google Scholar] [CrossRef] [PubMed]
- Phisalix, C.; Bertrand, G. Sur la propriété antitoxique du sang des animaux vaccinée contre le venin de vipére. Compte. Rendus Soc. Biol. 1894, 46, 111–113. [Google Scholar]
- Phisalix, C.; Bertrand, G. Propriétés antitoxique du sang des animaux vaccineé contre le venin de vipère. Contribution à l'étude du mécanisme de la vaccination contre ce venin. Arch. Physiol. 1894, 6, 611–619. (In French) [Google Scholar]
- World Health Organization: Venomous snakes and antivenoms search interface. Available online: http://apps.who.int/bloodproducts/snakeantivenoms/database (accessed on 11 December 2014).
- Global Snakebite Initiative. Available online: http://www.snakebiteinitiative.org (accessed on 11 December 2014).
- Williams, D.J.; Gutiérrez, J.M.; Harrison, R.; Warrell, D.A.; White, J.; Winkel, K.D.; Gopalakrishnakone, P.; Global Snakebite Initiative Working Group; International Society on Toxinology. The Global Snakebite Initiative: an antidote for snakebite. Lancet 2010, 375, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Warrell, D.A.; Williams, D.J.; Jensen, S.; Brown, N.; Calvete, J.J.; Harrison, R.A.; Global Snakebite Initiative. The need for full integration of snakebite envenoming within a global strategy to combat the neglected tropical diseases: the way forward. PLoS Negl. Trop. Dis. 2013, 7, e2162. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Burnouf, T.; Harrison, R.A.; Calvete, J.J.; Kuch, U.; Warrell, D.A.; Williams, D.J.; Global Snakebite Initiative. A multicomponent strategy to improve the availability of antivenom for treating snakebite envenoming. Bull. World Health Organ. 2014, 92, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, D.; Arni, R.K.; Betzel, C. Proteome analysis of snake venom toxins: pharmacological insights. Expert Rev. Proteomics 2008, 5, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Proteomic tools against the neglected pathology of snakebite envenoming. Expert Rev. Proteomics 2011, 8, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Solano, G.; Pla, D.; Herrera, M.; Segura, A.; Villalta, M.; Vargas, M.; Sanz, L.; Lomonte, B.; Calvete, J.J.; León, G. Assessing the preclinical efficacy of antivenoms: From the lethality neutralization assay to antivenomics. Toxicon 2013, 69, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Snake venomics: from the inventory of toxins to biology. Toxicon 2013, 75, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P.; Williams, V.; White, J. Snake venom variability: Methods of study, results and interpretation. Toxicon 1991, 29, 1279–1303. [Google Scholar] [CrossRef] [PubMed]
- Massey, D.J.; Calvete, J.J.; Sánchez, E.E.; Sanz, L.; Richards, K.; Curtis, R.; Boesen, K. Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona. J. Proteomics 2012, 75, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Durban, J.; Pérez, A.; Sanz, L.; Gómez, A.; Bonilla, F.; Rodríguez, S.; Chacón, D.; Sasa, M.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Integrated “omics” profiling indicates that miRNAs are modulators of the ontogenetic venom composition shift in the Central American rattlesnake, Crotalus simus simus. BMC Genomics 2013, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wagstaff, S.C.; Wüster, W.; Cook, D.A.; Bolton, F.M.; King, S.I.; Pla, D.; Sanz, L.; Calvete, J.J.; Harrison, R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 9205–9210. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, H.L.; Sanz, L.; Sovic, M.G.; Calvete, J.J. Phylogeny-based comparative analysis of venom proteome variation in a clade of rattlesnakes (Sistrurus sp.). PLoS One 2013, 8, e67220. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Tsai, W.C.; Ureña-Diaz, J.M.; Sanz, L.; Mora-Obando, D.; Sánchez, E.E.; Fry, B.G.; Gutiérrez, J.M.; Gibbs, H.L.; Sovic, M.G.; Calvete, J.J. Venomics of New World pit vipers: Genus-wide comparisons of venom proteomes across Agkistrodon. J. Proteomics 2014, 96, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P. Venom composition in rattlesnakes: Trends and biological significance. In The Biology of Rattlesnakes; Hayes, H.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 495–510. [Google Scholar]
- Fernández, J.; Lomonte, B.; Sanz, L.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics of Bothriechis nigroviridis reveals extreme variability among palm pitviper venoms: different evolutionary solutions for the same trophic purpose. J. Proteome Res. 2009, 9, 4234–4241. [Google Scholar] [CrossRef]
- Lomonte, B.; Fernández, J.; Sanz, L.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Venomous snakes of Costa Rica: biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics. J. Proteomics 2014, 105, 323–339. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. Available online: www.who.int/bloodproducts/snakeantivenoms (accessed on 11 December 2014).
- Theakston, R.D.G. Characterization of venoms and standardization of antivenoms. In Natural Toxins. Animal, Plant and Microbial; Harris, J.B., Ed.; Clarendon Press: Oxford, UK, 1986; pp. 287–303. [Google Scholar]
- Gutiérrez, J.M.; Rojas, G.; Bogarín, G.; Lomonte, B. Evaluation of the neutralizing ability of antivenoms for the treatment of snakebite envenoming in Central America. In Envenomings and Their Treatments; Bon, C., Goyffon, M., Eds.; Fondation Marcel Mérieux: Lyon, France, 1996; pp. 223–231. [Google Scholar]
- Theakston, R.D.G.; Reid, H.A. Development of simple standard assay procedures for the characterization of snake venom. Bull. World Health Org. 1983, 61, 949–956. [Google Scholar] [PubMed]
- Theakston, R.D.G.; Warrell, D.A.; Griffiths, E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon 2003, 41, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Arroyo, O.; Bolaños, R. Mionecrosis, hemorragia y edema inducidos por el veneno de Bothrops asper en ratón blanco. Toxicon 1980, 18, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Gené, J.A.; Roy, A.; Rojas, G.; Gutiérrez, J.M.; Cerdas, L. Comparative study on coagulant, defibrinating, fibrinolytic and fibrinogenolytic activities of Costa Rican crotaline snake venoms and their neutralization by a polyvalent antivenom. Toxicon 1989, 27, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snakebite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Clinical toxicology of snakebite in Africa and the Middle East/Arabian Peninsula. In Handbook of Clinical Toxicology of Animal Venoms and Poisons; Meier, J., White, J., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 433–492. [Google Scholar]
- Petras, D.; Sanz, L.; Segura, A.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; León, G.; Warrell, D.A.; Theakston, R.D.G.; Harrison, R.A.; et al. Snake venomics of African spitting cobras: Toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP® antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Segura, A.; Herrera, M.; Villalta, M.; Estrada, R.; Cerdas, M.; Paiva, O.; Matainaho, T.; Jensen, S.D.; Winkel, K.D.; et al. Preclinical evaluation of caprylic acid-fractionated IgG antivenom for the treatment of Taipan (Oxyuranus scutellatus) envenoming in Papua New Guinea. PLoS Negl. Trop. Dis. 2011, 5, e1144. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Kondo, S.; Ikezawa, I.; Murata, R.; Ohsaka, A. Studies of the quantitative method for the determination of hemorrhagic activity of Habu snake venom. Japan J. Med. Sci. Biol. 1960, 13, 43–51. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Gené, J.A.; Rojas, G.; Cerdas, L. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon 1985, 23, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Núñez, J.; Moura da Silva, A.M.; Rucavado, A.; Theakston, R.D.G.; Gutiérrez, J.M. Pulmonary hemorrhage induced by jararhagin, a metalloproteinase from Bothrops jararaca venom. Toxicol. Appl. Pharmacol. 2003, 193, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.P.; Zamunér, S.R.; Zuliani, J.P.; Fernandes, C.M.; Cruz-Hofling, M.A.; Fernandes, I.; Chaves, F.; Gutiérrez, J.M. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve 2003, 28, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.; Quesada, L.; Arce, V.; Lomonte, B.; Rojas, G.; Gutiérrez, J.M. Neutralization of four Peruvian Bothrops sp snake venoms by polyvalent antivenoms produced in Perú and Costa Rica: Preclinical assessment. Acta Trop. 2005, 93, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rojas, G.; Lomonte, B.; Gené, J.A.; Cerdas, L. Comparative study of the edema-forming activity of Costa Rican snake venoms and its neutralization by a polyvalent antivenom. Comp. Biochem. Physiol. 1986, 85, 171–175. [Google Scholar] [CrossRef]
- Barfaraz, A.; Harvey, A.L. The use of the chick biventer cervicis preparation to assess the protective activity of six international reference antivenoms on the neuromuscular effects of snake venoms in vitro. Toxicon 1994, 32, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Soto, M.; Escalante, T.; Loría, G.D.; Arni, R.D.; Gutiérrez, J.M. Thrombocytopenia and platelet hypoaggregation induced by Bothrops asper snake venom. Toxins involved and their contribution to metalloproteinase-induced pulmonary hemorrhage. Thromb. Haemost. 2005, 94, 123–131. [Google Scholar] [PubMed]
- Gutiérrez, J.; Sanz, L.; Escolano, J.; Fernández, J.; Lomonte, B.; Angulo, Y.; Rucavado, A.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics of the Lesser Antillean pit vipers Bothrops caribbaeus and Bothrops lanceolatus. Correlation with toxicological activities and immunoreactivity of a heterologous antivenom. J. Proteome Res. 2008, 7, 4396–4408. [Google Scholar] [CrossRef] [PubMed]
- Gené, J.A.; Gómez, M.; Gutiérrez, J.M.; Cerdas, L. Neutralization of hyaluronidase and indirect hemolytic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon 1985, 23, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rojas, G.; Lomonte, B.; Gené, J.A.; Chaves, F.; Alvarado, J.; Rojas, E. Standardization of assays for testing the neutralizing ability of antivenoms. Toxicon 1990, 28, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Juárez, P.; Sanz, L. Snake venomics. Strategy and applications. J. Mass Spectrom. 2007, 42, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M.T. Exploring snake venom proteomes: multifaceted analyses for complex toxin mixtures. Proteomics 2008, 8, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Next-generation snake venomics: protein-locus resolution through venom proteome decomplexation. Exp. Rev. Proteomics 2014, 11, 315–329. [Google Scholar] [CrossRef]
- Rokyta, D.R.; Lemmon, A.R.; Margres, M.J.; Aronow, K. The venom-gland transcriptome of the eastern diamondback rattlesnake (Crotalus adamanteus). BMC Genomics 2012, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Margres, M.J.; Aronow, K.; Loyacano, J.; Rokyta, D.R. The venom-gland transcriptome of the eastern coral snake (Micrurus fulvius) reveals high venom complexity in the intragenomic evolution of venoms. BMC Genomics 2013, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- Margres, M.J.; McGivern, J.J.; Wray, K.P.; Seavy, M.; Calvin, K.; Rokyta, D.R. Linking the transcriptome and proteome to characterize the venom of the eastern diamondback rattlesnake (Crotalus adamanteus). J. Proteomics 2014, 96, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, S.C.; Sanz, L.; Juárez, P.; Harrison, R.A.; Calvete, J.J. Combined snake venomics and venom gland transcriptomic analysis of the ocellated carpet viper, Echis ocellatus. J. Proteomics 2009, 71, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Paiva, O.; Pla, D.; Wright, C.E.; Beutler, M.; Sanz, L.; Gutiérrez, J.M.; Williams, D.J.; Calvete, J.J. Combined venom gland cDNA sequencing and venomics of the New Guinea small-eyed snake, Micropechis ikaheka. J. Proteomics 2014, 110, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Alape-Girón, A.; Sanz, L.; Escolano, J.; Flores-Díaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake venomics of the lancehead pitviper Bothrops asper. Geographic, individual and ontogenetic variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Pérez, A.; Borges, A.; Vargas, A.M.; Lomonte, B.; Angulo, Y.; Gutiérrez, J.M.; Chalkidis, H.M.; Mourão, R.H.; et al. Snake population venomics and antivenomics of Bothrops atrox: paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J. Proteomics 2011, 74, 510–527. [Google Scholar] [CrossRef]
- Zelanis, A.; Tashima, A.K.; Pinto, A.F.; Leme, A.F.; Stuginski, D.R.; Furtado, M.F.; Sherman, N.E.; Ho, P.L.; Fox, J.W.; Serrano, S.M. Bothrops jararaca venom proteome rearrangement upon neonate to adult transition. Proteomics 2011, 11, 4218–4228. [Google Scholar] [CrossRef] [PubMed]
- Antunes, T.C.; Yamashita, K.M.; Barbaro, K.C.; Saiki, M.; Santoro, M.L. Comparative analysis of newborn and adult Bothrops jararaca snake venoms. Toxicon 2010, 56, 1443–1458. [Google Scholar] [CrossRef]
- Boldrini-França, J.; Corrêa-Netto, C.; Silva, M.M.S.; Rodrigues, R.S.; De La Torre, P.; Pérez, A.; Soares, A.M.; Zingali, R.B.; Nogueira, R.A.; Rodrigues, V.M.; et al. Snake venomics and antivenomics of Crotalus durissus subspecies from Brazil: Assessment of geographic variation and its implication on snakebite management. J. Proteomics 2010, 73, 1758–1776. [Google Scholar]
- Calvete, J.J.; Sanz, L.; Cid, P.; De La Torre, P.; Flores-Díaz, M.; Dos Santos, M.C.; Borges, A.; Bremo, A.; Angulo, Y.; Lomonte, B.; et al. Snake venomics of the Central American rattlesnake Crotalus simus and the South American Crotalus durissus complex points to neurotoxicity as an adaptive paedomorphic trend along Crotalus dispersal in South America. J. Proteome Res. 2010, 9, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Undheim, E.A.; Scheib, H.; Gren, E.C.; Cochran, C.; Person, C.E.; Koludarov, I.; Kelln, W.; Hayes, W.K.; King, G.F.; et al. Intraspecific venom variation in the medically significant Southern Pacific Rattlesnake (Crotalus oreganus helleri): Biodiscovery, clinical and evolutionary implications. J. Proteomics 2014, 99, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, H.L.; Chiucchi, J.E. Deconstructing a complex molecular phenotype: Population-level variation in individual venom proteins in Eastern Massasauga rattlesnakes (Sistrurus c. catenatus). J. Mol. Evol. 2011, 72, 383–397. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Sanz, L.; Chiucchi, J.E.; Farrell, T.M.; Calvete, J.J. Proteomic analysis of ontogenetic and diet-related changes in venom composition of juvenile and adult Dusky Pigmy rattlesnakes (Sistrurus miliarius barbouri). J. Proteomics 2011, 74, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, M.; Sanz, L.; Flores-Diaz, M.; Sasa, M.; Núñez, V.; Alape-Girón, A.; Calvete, J.J. Snake venomics across genus Lachesis. Ontogenetic changes in the venom composition of L. stenophrys and comparative proteomics of the venoms of adult L. melanocephala and L. acrochorda. J. Proteomics 2012, 77, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Sanz, L.; Molina-Sánchez, P.; Zorita, V.; Madrigal, M.; Flores-Díaz, M.; Alape-Girón, A.; Núñez, V.; Andrés, V.; Gutiérrez, J.M.; et al. Snake venomics of Lachesis muta rhombeata and genus-wide antivenomics assessment of the paraspecific immunoreactivity of two antivenoms evidence the high compositional and immunological conservation across Lachesis. J. Proteomics 2013, 89, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.F.; Wang, J.; He, Y.; Qu, Y.F.; Lin, L.H.; Ma, X.M.; Ji, X. Proteomic and biochemical analyses of short-tailed pit viper (Gloydius brevicaudus) venom: Age-related variation and composition-activity correlation. J. Proteomics 2014, 105, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Wooten, J.A.; Gibbs, H.L. Niche divergence and lineage diversification among closely related Sistrurus rattlesnakes. J. Evol. Biol. 2012, 25, 317–328. [Google Scholar] [CrossRef]
- Lomonte, B.; Escolano, J.; Fernández, J.; Sanz, L.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics and antivenomics of the arboreal neotropical pitvipers Bothriechis lateralis and Bothriechis schlegelii. J. Proteome Res. 2008, 7, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Gutiérrez, J.M.; Calvete, J.J. Second generation snake antivenomics: Comparing immunoaffinity and immunodepletion protocols. Toxicon 2012, 60, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Tsai, W.C.; Pla, D.; Solano, G.; Lomonte, B.; Sanz, L.; Angulo, Y.; Calvete, J.J. Preclinical assessment of a polyspecific antivenom against the venoms of Cerrophidion sasai, Porthidium nasutum and Porthidium ophryomegas: Insights from combined antivenomics and neutralization assays. Toxicon 2013, 64, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B.; Sanz, L.; Calvete, J.J.; Pla, D. Immunological profile of antivenoms: Preclinical analysis of the efficacy of a polyspecific antivenom through antivenomics and neutralization assays. J. Proteomics 2014, 105, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Paiva, O.K.; Sanz, L.; Beutler, M.; Wright, C.E.; Calvete, J.J.; Williams, D.J.; Gutiérrez, J.M. Preclinical efficacy of Australian antivenoms against the venom of the small-eyed snake, Micropechis ikaheka, from Papua New Guinea: An antivenomics and neutralization study. J. Proteomics 2014, 110, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rojas, E.; Quesada, L.; León, G.; Núñez, J.; Laing, G.D.; Sasa, M.; Renjifo, J.M.; Nasidi, A.; Warrell, D.A.; et al. Pan-African polyspecific antivenom produced by caprylic acid purification of horse IgG: An alternative to the antivenom crisis in Africa. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.; Eaker, D.L.; Porath, J. Purification of a neurotoxin from the venom of Naja nigricollis. Biochim. Biophys. Acta 1966, 127, 505–520. [Google Scholar]
- Branch, W.R. The venomous snakes of southern Africa. Part 2. Elapidae and Hydrophiidae. Snake 1979, 11, 199–225. [Google Scholar]
- Hughes, B. African snake faunas. Bonn Zool. Beitr. 1983, 34, 311–356. [Google Scholar]
- Wüster, W.; Broadley, D.G. A new species of spitting cobra from northeastern Africa (Serpentes: Elapidae: Naja). J. Zool. London 2003, 259, 345–359. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).