Effects of Decreased Vitamin D and Accumulated Uremic Toxin on Human CYP3A4 Activity in Patients with End-Stage Renal Disease
Abstract
:1. Introduction
2. Results
2.1. Effect of Uremic Serum or Uremic Toxins on Testosterone 6β-Hydroxylation in HLMs
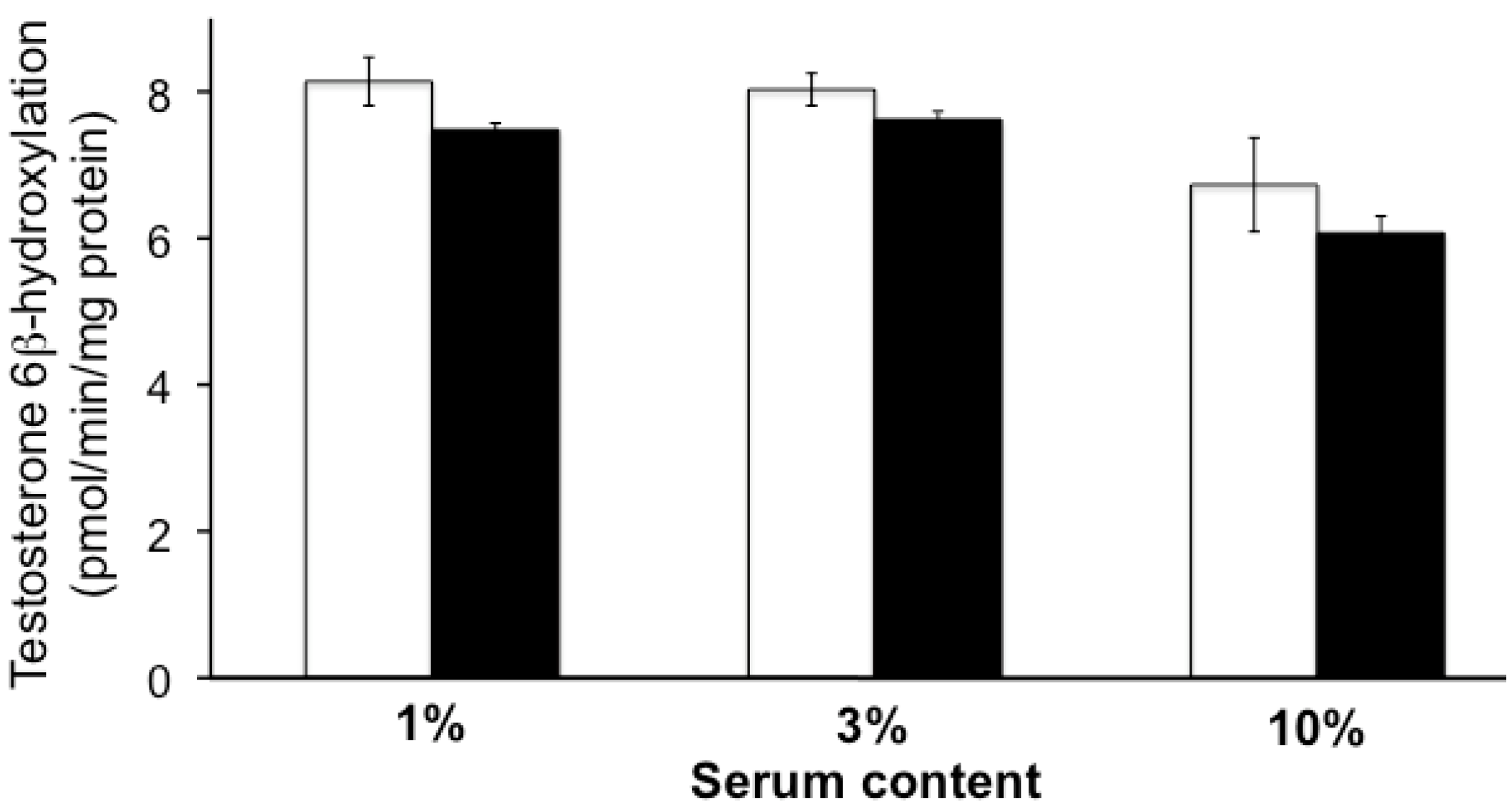
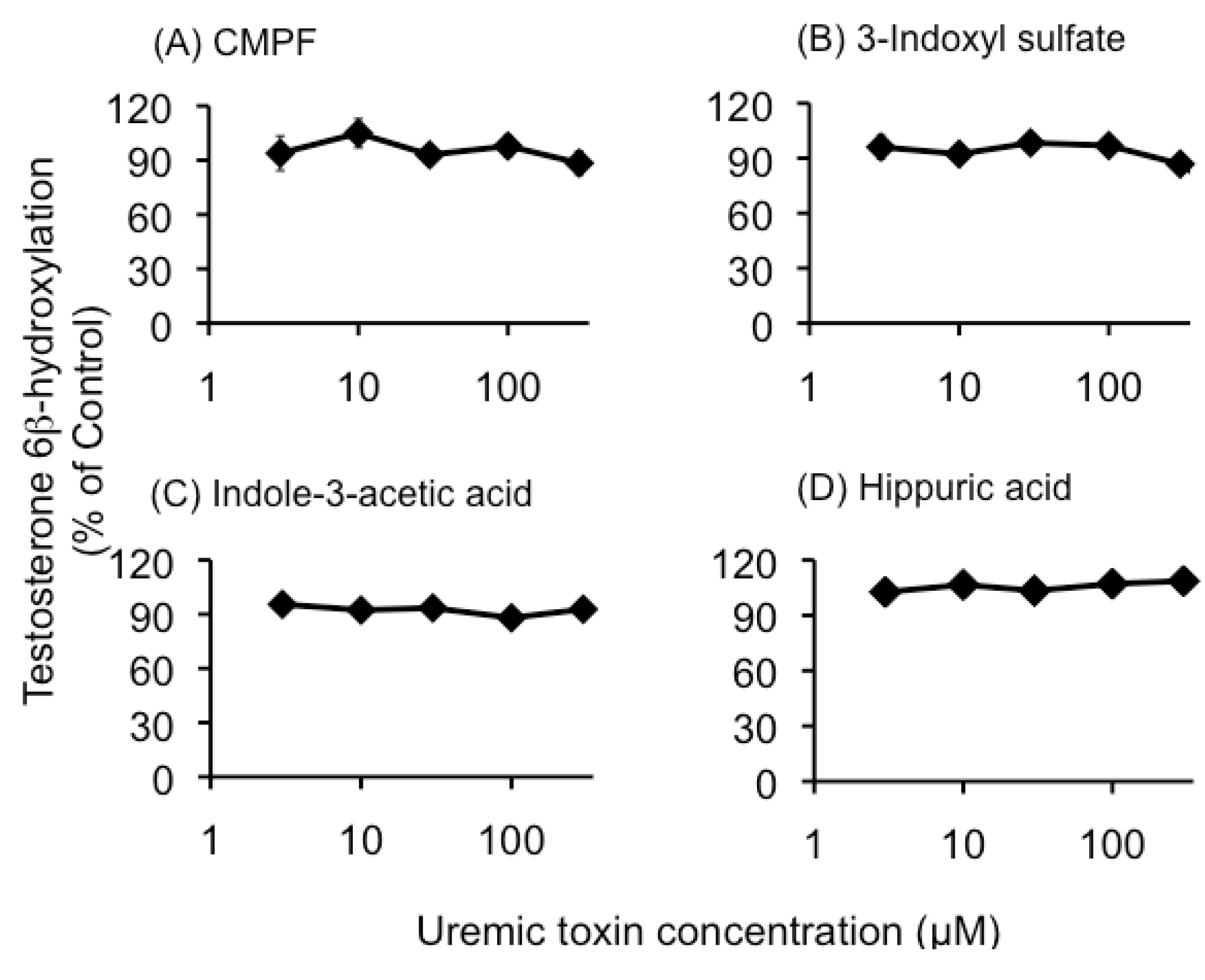
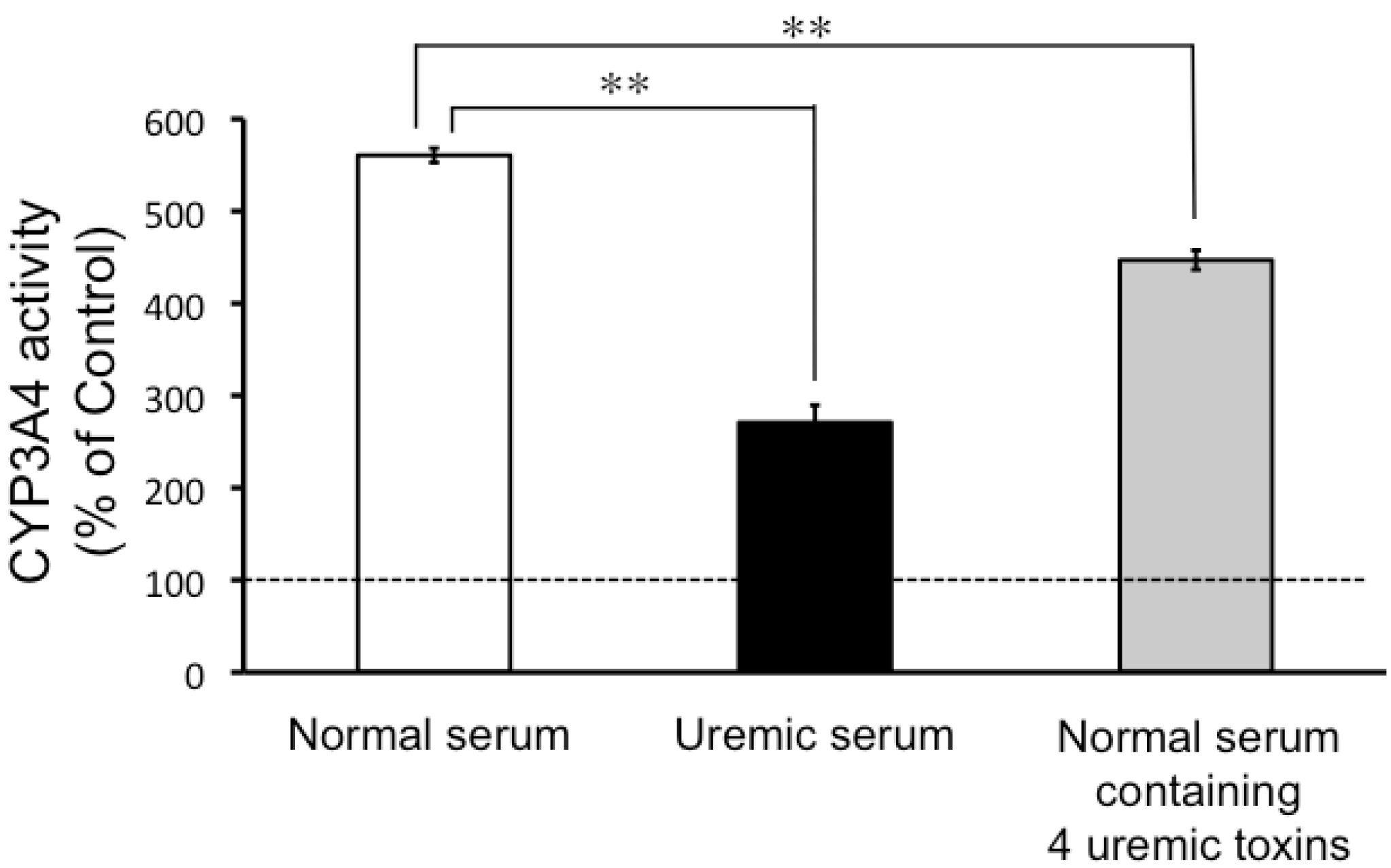
2.2. CYP3A4 Activity in LS180 Cells Exposed to Uremic Serum or Uremic Toxins
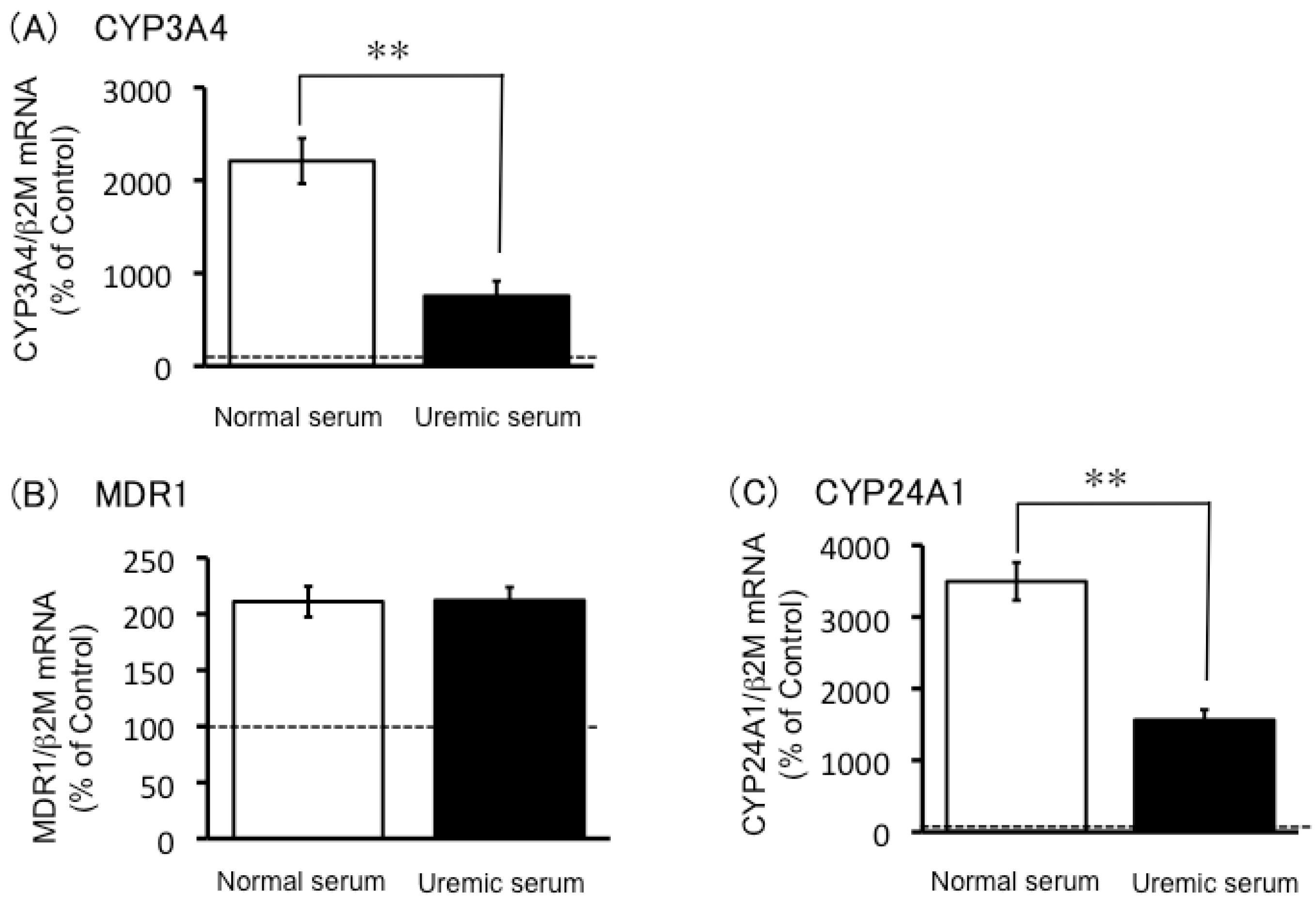
2.3. Expression of CYP3A4, MDR1 and CYP24A1 mRNA in Uremic Serum-Treated LS180 Cells
2.4. Effects of 1,25-Dihydroxyvitamin D and Uremic Toxins on Induction of CYP3A4 mRNA Expression

3. Discussion
| Uremic toxin | Serum concentration (μM) | Unbound fraction (%) | |
|---|---|---|---|
| Total | Unbound | ||
| CMPF | 179.1 ± 6.9 | Not detected | Not calculation |
| 3-Indoxyl sulfate | 134.6 ± 0.7 | 20.2 ± 0.2 | 14 |
| Indole-3-acetic acid | 9.2 ± 0.5 | 1.7 ± 0.04 | 18.5 |
| Hippuric acid | 238.5 ± 2.1 | 131.4 ± 0.2 | 55.5 |
4. Materials and Methods
4.1. Materials
4.2. Sera
4.3. Analysis of Uremic Toxins in Uremic Serum
4.4. Detection of CYP3A4 Activity Using Testosterone 6β-Hydroxylation
4.5. Detection of CYP3A4 Activity in LS180 Cells by Using the P450-GloTM Assays Kit
4.6. Quantification of mRNA by Real-Time Reverse Transcription-Polymerase Chain Reaction Analysis
4.7. Quantification of 1,25-Dihydroxyvitamin D
4.8. Data Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Fenster, P.E.; Hager, W.D.; Perrier, D.; Powell, J.R.; Graves, P.E.; Michael, U.F. Digoxin-quinidine interaction in patients with chronic renal failure. Circulation 1982, 66, 1277–1280. [Google Scholar] [CrossRef]
- De Martin, S.; Orlando, R.; Bertoli, M.; Pegoraro, P.; Palatini, P. Differential effect of chronic renal failure on the pharmacokinetics of lidocaine in patients receiving and not receiving hemodialysis. Clin. Pharmacol. Ther. 2006, 80, 597–606. [Google Scholar] [CrossRef]
- Sun, H.; Frassetto, L.A.; Huang, Y.; Benet, L.Z. Hepatic clearance, but not gut availability, of erythromycin is altered in patients with end-stage renal disease. Clin. Pharmacol. Ther. 2010, 87, 465–472. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Y.; Frassetto, L.; Benet, L.Z. Effects of uremic toxins on hepatic uptake and metabolism of erythromycin. Drug Metab. Dispos. 2004, 32, 1239–1246. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Higuchi, K.; Shima, D.; Yokota, H.; Furukubo, T.; Izumi, S.; Yamakawa, T.; Otagiri, M.; Hirata, S.; Takara, K.; et al. Inhibitory effects of uraemic toxins 3-indoxyl sulfate and p-cresol on losartan metabolism in vitro. J. Pharm. Pharmacol. 2010, 62, 133–138. [Google Scholar] [CrossRef]
- Istrate, M.A.; Nussler, A.K.; Eichelbaum, M.; Burk, O. Regulation of CYP3A4 by pregnane X receptor: The role of nuclear receptors competing for response element binding. Biochem. Biophys. Res. Commun. 2010, 393, 688–693. [Google Scholar] [CrossRef]
- Drocourt, L.; Ourlin, J.C.; Pascussi, J.M.; Maurel, P.; Vilarem, M.J. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J. Biol. Chem. 2002, 277, 25125–25132. [Google Scholar]
- Schroeder, J.C.; Dinatale, B.C.; Murray, I.A.; Flaveny, C.A.; Liu, Q.; Laurenzana, E.M.; Lin, J.M.; Strom, S.C.; Omiecinski, C.J.; Amin, S.; et al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 2010, 49, 393–400. [Google Scholar] [CrossRef]
- Michaud, J.; Nolin, T.D.; Naud, J.; Dani, M.; Lafrance, J.P.; Leblond, F.A.; Himmelfarb, J.; Pichette, V. Effect of hemodialysis on hepatic cytochrome P450 functional expression. J. Pharmacol. Sci. 2008, 108, 157–163. [Google Scholar] [CrossRef]
- Leblond, F.; Guévin, C.; Demers, C.; Pellerin, I.; Gascon-Barré, M.; Pichette, V. Downregulation of hepatic cytochrome P450 in chronic renal failure. J. Am. Soc. Nephrol. 2001, 12, 326–332. [Google Scholar]
- Geick, A.; Eichelbaum, M.; Burk, O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J. Biol. Chem. 2001, 276, 14581–14587. [Google Scholar] [CrossRef]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha, 25-dihydroxyvitamin D3. J. Biol. Chem. 2010, 285, 15599–15610. [Google Scholar] [CrossRef]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef]
- Patel, S.R; Ke, H.Q.; Vanholder, R.; Koenig, R.J.; Hsu, C.H. Inhibition of calcitriol receptor binding to vitamin D response elements by uremic toxins. J. Clin. Invest. 1995, 96, 50–59. [Google Scholar] [CrossRef]
- Franke, R.M.; Baker, S.D.; Mathijssen, R.H.; Schuetz, E.G.; Sparreboom, A. Influence of solute carriers on the pharmacokinetics of CYP3A4 probes. Clin. Pharmacol. Ther. 2008, 84, 704–709. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Kinoshita, Y.; Hirata, S.; Otagiri, M.; Ohtani, H.; Sawada, Y. Effects of uremic serum and uremic toxins on hepatic uptake of digoxin. Ther. Drug Monit. 2008, 30, 576–582. [Google Scholar] [CrossRef]
- Reyes, M.; Benet, L.Z. Effects of uremic toxins on transport and metabolism of different biopharmaceutics drug disposition classification system xenobiotics. J. Pharm. Sci. 2011, 100, 3831–3842. [Google Scholar] [CrossRef]
- Lins, R.L.; Matthys, K.E.; Verpooten, G.A.; Peeters, P.C.; Dratwa, M.; Stolear, J.C.; Lameire, N.H. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrol. Dial. Transplant. 2003, 18, 967–976. [Google Scholar] [CrossRef]
- Nolin, T.D.; Frye, R.F.; Le, P.; Sadr, H.; Naud, J.; Leblond, F.A.; Pichette, V.; Himmelfarb, J. ESRD impaires nonrenal clearance of fexofenadine but not midazolam. J. Am. Soc. Nephrol. 2009, 20, 2269–2276. [Google Scholar] [CrossRef]
- Nishikawa, M.; Ariyoshi, N.; Kotani, A.; Ishii, I.; Nakamura, H.; Nakasa, H.; Ida, M.; Nakamura, H.; Kimura, N.; Kimura, M.; et al. Effects of continuous ingestion of green tea or grape seed extracts on the pharmacokinetics of midazolam. Drug Metab. Pharmacokinet. 2004, 19, 280–289. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsujimoto, M.; Nagano, Y.; Hosoda, S.; Shiraishi, A.; Miyoshi, A.; Hiraoka, S.; Furukubo, T.; Izumi, S.; Yamakawa, T.; Minegaki, T.; et al. Effects of Decreased Vitamin D and Accumulated Uremic Toxin on Human CYP3A4 Activity in Patients with End-Stage Renal Disease. Toxins 2013, 5, 1475-1485. https://doi.org/10.3390/toxins5081475
Tsujimoto M, Nagano Y, Hosoda S, Shiraishi A, Miyoshi A, Hiraoka S, Furukubo T, Izumi S, Yamakawa T, Minegaki T, et al. Effects of Decreased Vitamin D and Accumulated Uremic Toxin on Human CYP3A4 Activity in Patients with End-Stage Renal Disease. Toxins. 2013; 5(8):1475-1485. https://doi.org/10.3390/toxins5081475
Chicago/Turabian StyleTsujimoto, Masayuki, Yui Nagano, Satomi Hosoda, Asuka Shiraishi, Ayaka Miyoshi, Shima Hiraoka, Taku Furukubo, Satoshi Izumi, Tomoyuki Yamakawa, Tetsuya Minegaki, and et al. 2013. "Effects of Decreased Vitamin D and Accumulated Uremic Toxin on Human CYP3A4 Activity in Patients with End-Stage Renal Disease" Toxins 5, no. 8: 1475-1485. https://doi.org/10.3390/toxins5081475
APA StyleTsujimoto, M., Nagano, Y., Hosoda, S., Shiraishi, A., Miyoshi, A., Hiraoka, S., Furukubo, T., Izumi, S., Yamakawa, T., Minegaki, T., & Nishiguchi, K. (2013). Effects of Decreased Vitamin D and Accumulated Uremic Toxin on Human CYP3A4 Activity in Patients with End-Stage Renal Disease. Toxins, 5(8), 1475-1485. https://doi.org/10.3390/toxins5081475





