Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals
Abstract
:Abbreviations
| AF | Aflatoxins |
| APC | Antigen-Presenting Cell |
| DON | Deoxynivalenol |
| FB | Fumonisins |
| FUS | Fusarium toxins |
| GALT | Gut-Associated Lymphoid Tissue |
| GIT | Gastrointestinal Tract |
| GLUT2 | facilitated glucose transporter |
| GLUT5 | fructose transporter |
| IEC | Intestinal Epithelial Cell |
| OTA | Ochratoxin A |
| PP | Peyer’s Patches |
| SGLT1 | sodium-dependent glucose cotransporter 1 |
| TCT | Trichothecenes |
| TEER | Transepithelial Electrical Resistance |
| TJ | Tight Junction |
| T-2 | T-2 toxin |
| UC | Ussing Chamber |
| ZEA | Zearalenone |
1. Introduction
2. Intestinal Absorption and Fate of Mycotoxins with the Gut
| Nutrient digestibility | Enzyme activities | Nutrient uptake 1 | Digestive microflora | Barrier integrity | Mucosal immunity 2 | Pathogen clearance | Total 3 | ||
|---|---|---|---|---|---|---|---|---|---|
| Experiments | 13 | 5 | 17 | 5 | 16 | 13 | 14 | 83 | |
| in vitro / ex vivo / in vivo 4 | 0/0/13 | 0/0/5 | 1/10/12 | 1/2/4 | 13/2/5 | 7/1/10 | 1/1/13 | 23/16/62 | |
| Aflatoxin (AF) | 5 | 4 | 1 | 0 | 2 | 1 | 1 | 14 | |
| Ochratoxin A (OTA) | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 6 | |
| Deoxynivalenol (DON) | 1 | 0 | 11 | 3 | 8 | 7 | 2 | 32 | |
| T-2 toxin (T-2) | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 5 | |
| Zearalenone (ZEA) 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Fumonisin (FB) | 2 | 1 | 2 | 1 | 2 | 4 | 2 | 14 | |
| Multi-contamination | 5 | 0 | 2 | 0 | 1 | 1 | 3 | 12 | |
| References | |||||||||
| Aflatoxin (AF) | [17,18,19,20,21] | [17,19,22,23] | [24] | [25,26] | [27] | [28] | |||
| Ochratoxin A (OTA) | [29,30,31] | [32,33,34] | |||||||
| Deoxynivalenol (DON) | [35] | [36,38,39,40,41,42,43,44,45,46] | [47,48,49] | [50,51,52, 53,54,55,56,57] | [51,53,58,59,60,61,62] | [58,63] | |||
| T-2 toxin (T-2) | [64] | [65] | [28,66,67] | ||||||
| Fumonisin (FB) | [68,69] | [70] | [70,71] | [72] | [51,73] | [51,74,75,76] | [75,77] | ||
| Multi-contamination | [21,78,79,81] | [82,83] | [51] | [51] | [84,85,86] | ||||
| Deoxynivalenol | T-2 Toxin | Zearalenone | Fumonisins | Aflatoxin | Ochratoxin A | ||
|---|---|---|---|---|---|---|---|
| (DON; mg/kg) | (T-2; mg/kg) | (ZEA; mg/kg) | (FB; mg/kg) | (AF; mg/kg) | (OTA; mg/kg) | ||
| Realistic doses (RD) 1 | <5 | <0.5 | <1 | <10 | <0.3 | <0.3 | |
| Representative of field conditions | |||||||
| Occasional doses (OD) 1 | >5 | >0.5 | >1 | >10 | >0.3 | >0.3 | |
| Unfavorable weather conditions | <25 | <2 | <5 | <40 | <2 | <2 | |
| Unrealistic doses (UD) 1 | >25 | >2 | >5 | >40 | >2 | >2 | |
| Unlikely to occur in nature | |||||||
| EU Limits (EC guidance) 2 | |||||||
| Pig (young) | 0.9 (0.9) | no advisory or guidance levels established | 0.25 (0.1) | 5 (5) | 0.02 | 0.05 (0.05) | |
| Poultry | 5 | - | 20 | 0.02 | 0.1 | ||
| Ruminant (young) | 5 (2) | 0.5 (0.5) | 50 (20) | 0.02 (0.01) | - | ||
| USA Limits (FDA guidance) 3 | |||||||
| Pig (young) | 1 | no advisory or guidance levels established | no advisory or guidance levels established | 10 | 0.1 (0.02) | no advisory or guidance levels established | |
| Poultry (young) | 5 | 50 4 | 0.1 (0.02) | ||||
| Ruminant (young) | 5 | 30 4 | 0.3 (0.02) | ||||
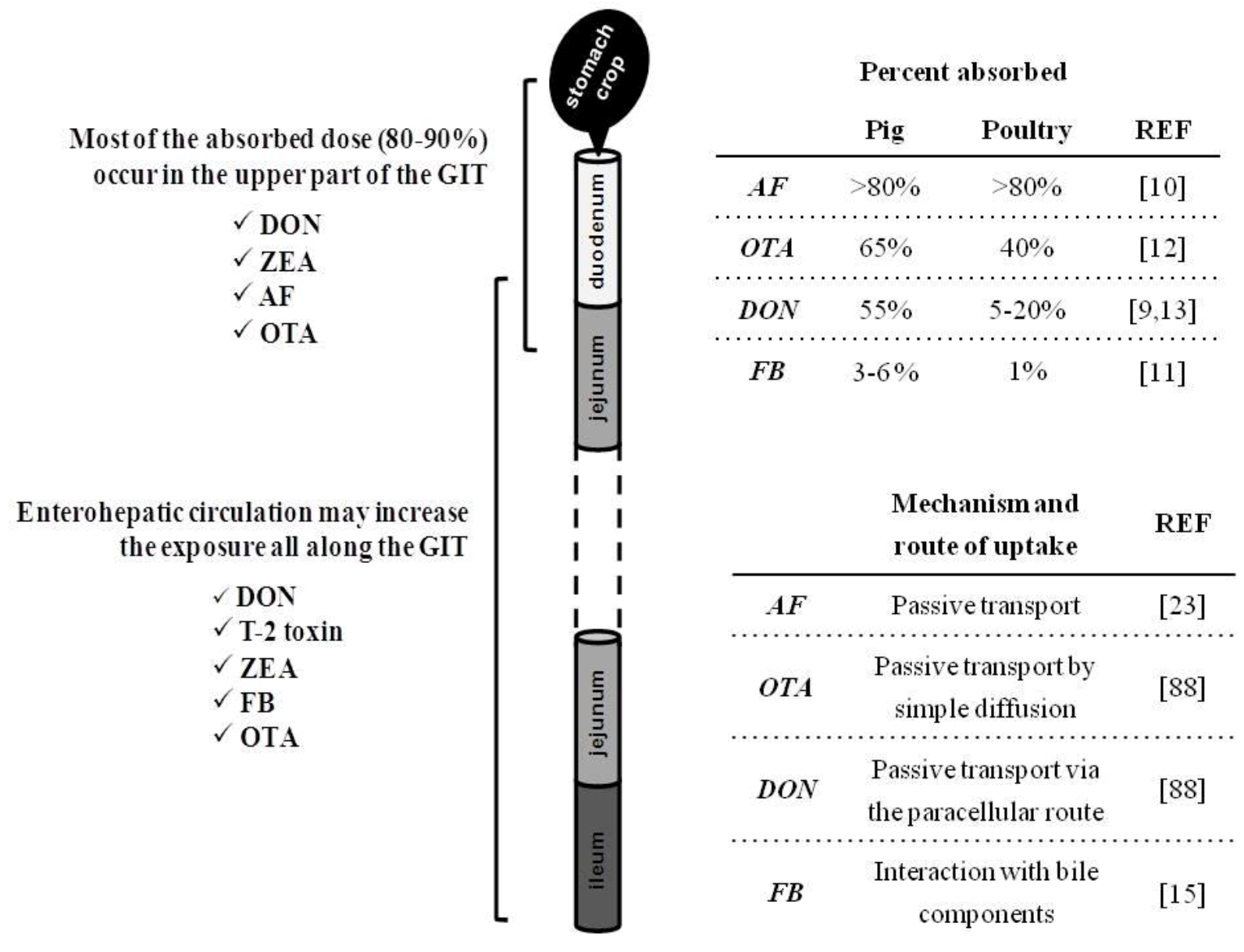
3. Consequence of Mycotoxins for Nutrient Metabolism
3.1. Nutrient Digestibility and Metabolizable Energy
| MYCOTOXIN CONCENTRATION IN STUDIES | |||||||
|---|---|---|---|---|---|---|---|
| Realistic doses | Occasional doses | Unrealistic doses | |||||
| Digestive processes | |||||||
| Enzyme activities | AF (hen): amlysase activity ↗ in pancreas and ↘ in duodenum, lipase activity ↘ in pancreas and duodenum, trypsin and chymotrypsin activity ↗ in pancreas [22]. | FB1 (pig): aminopeptidase activity ↘ in jejunum [70]. | |||||
| AF (duck): protease, amlysase, trypsin and chymotrypsin activity ↗ in duodenum [17]. | AF (hen): disaccharidase, maltase activity ↗ in jejunum [19]. | ||||||
| AF (mouse): alkaline phosphatase activity ↘ in isolated duodenal enterocytes [23]. | |||||||
| Nutrient digestibility | AF (duck): reduced apparent digestibility of crude protein [17,18]. | FUS (hen): slightly depressed nutrient digestibility & metabolizable energy [78]. | |||||
| FUS (dog): improved nutrient digestibility [81]. | FUS (chicken): increased protein digestibility & net protein utilization [80]. | ||||||
| FB1 (pig): reduced digestibility of ether extract [68]. | FB1 (rat, pig): reduced nutrient digestibility [68,69]. | ||||||
| DON (chicken): reduced intestinal viscosity [35]. | AF (chicken/hen): reduced apparent digestibility, digestible & metabolizable energy [19,20,21]. | ||||||
| Absorptive processes | |||||||
| Sugar transport | DON (HT-29 cells): strong inhibition of SGLT1 & | DON (chicken-hen/UCj 1): reduced Isc after glucose addition [42,43], inhibition of intestinal SGLT1 [45]. | T-2 toxin (rat/explant): reduced glucose absorption in jejunum and its rate of appearance in venous plasma [64]. | ||||
| GLUT5 [44]. | FB1 (pig/UCj 1): enhanced Isc after glucose | AF (UCj 2): reduced Isc after glucose addition [24]. | |||||
| DON (chicken): reduced intestinal expression of SGLT1, GLUT2 [40] & GLUT5 [46]. | addition [70]. | OTA (HT-29 cells): strong inhibition of SGLT1 [29]. | |||||
| Amino-acid transport | DON (HT-29 cells): inhibition of active and passive | DON (UCj 2): reduced Isc after proline addition [41]. | |||||
| L-serine transporters [44]. | |||||||
| Lipid transport | DON (HT-29 cells): increase of palmitate transport [44]. | ||||||
| DON (chicken): reduced expression of palmitate transporter in jejunum [46]. | |||||||
| Other essential nutrients | DON (mouse/explant): reduced uptake and transfer of folate [36]. | ||||||
3.2. Digestive and Absorptive Processes
3.2.1. Activity of Digestive Enzymes
3.2.2. Morphology of Intestinal Villi
3.2.3. Nutrient Uptake
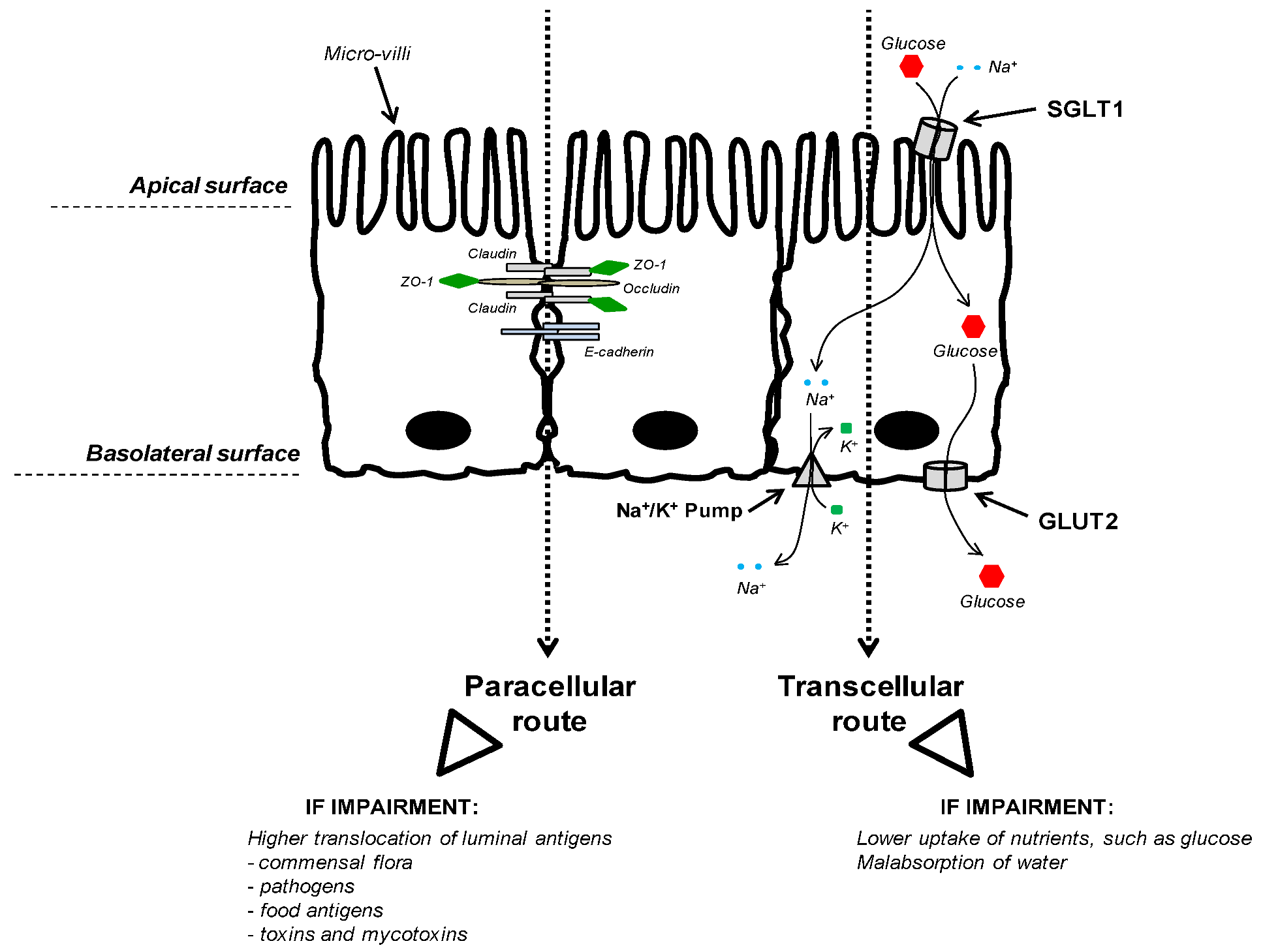
3.3. Connection between Intestinal Nutrient Metabolism and Animal Growth
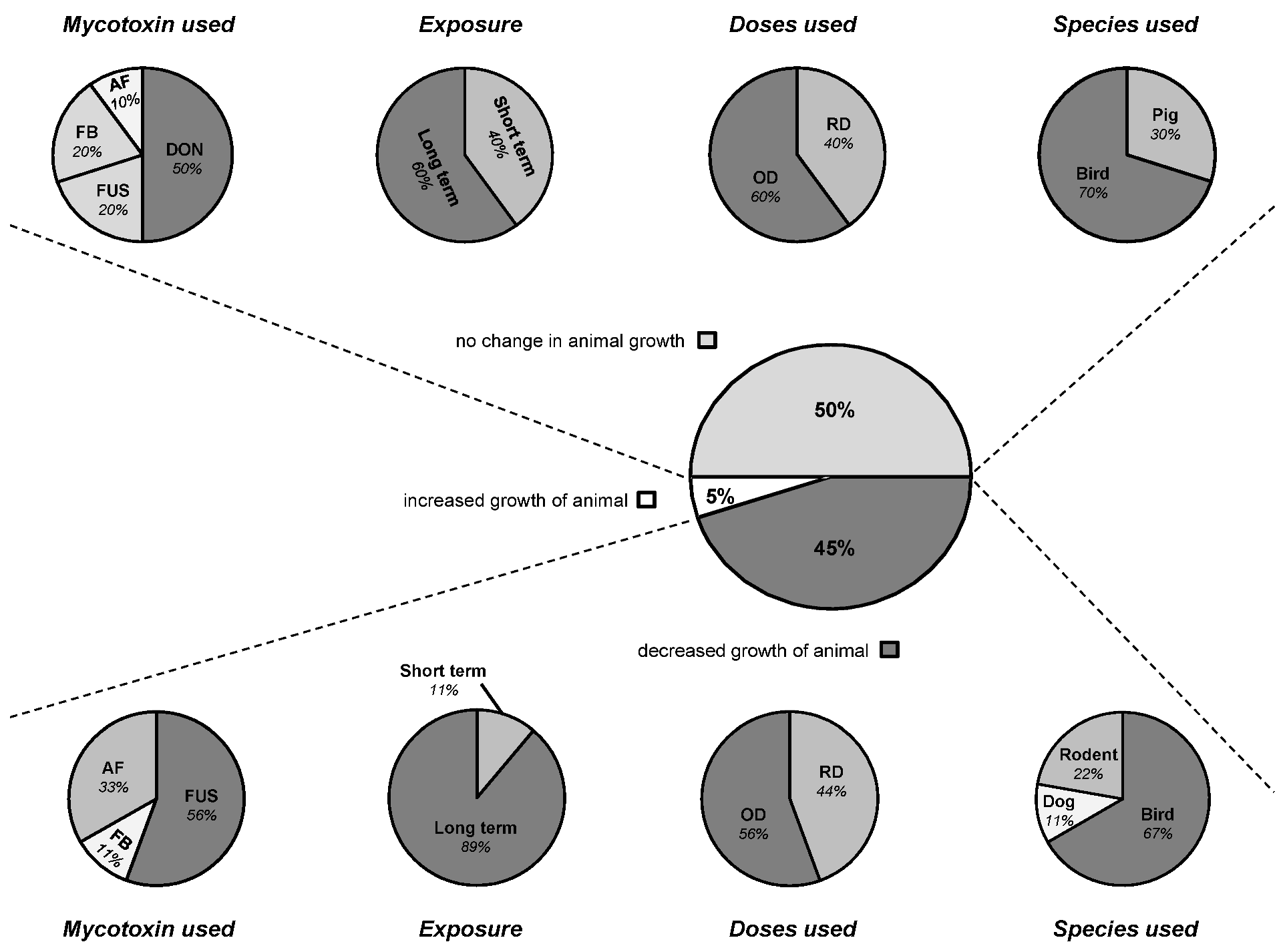
4. Consequence of Mycotoxins on Intestinal Defense
4.1. Pathogen Clearance
4.1.1. Parasitic Infections
| Microorganism in Contact with the Intestinal Epithelium | |||||
|---|---|---|---|---|---|
| Parasite | Bacteria | Virus | |||
| Realistic doses 1 | |||||
| FUS (chicken): impaired recovery of duodenal villi from coccidial lesions [84], upregulation of IFN-γ expression in CT [85]. | FB1 (pig): increased intestinal colonization by | ||||
| E. coli [77]. | |||||
| DON (porcine cells & ileal loop): enhanced | |||||
| S. typhimurium invasion and translocation, potentiation of pro-inflammatory cytokines [58]. | |||||
| Occasional doses 1 | |||||
| FUS (chicken): delayed recruitment of CD4+ and CD8+ cells in jejunum [86]. | FB1 (pig): longer shedding of E. coli, reduction of in vivo APC maturation (MHC-II, IL-12p40), T cell stimulatory capacity, specific Ig in PP [75]. | ||||
| Unrealistic doses 1 | |||||
| OTA (turkey, chicken): bloody diarrhea, higher lesions and oocyst in intestine [32,33], duodenal hemorrhages [32]. | OTA (chicken): higher number of S. typhimurium in duodenum & cecum, acute enteritis [34]. | T-2 (mouse): inability to clear reovirus from intestine, increased fecal shedding of the virus, suppression of IFN-γ expression in PP [67]. | |||
| DON (mouse): increased fecal shedding of reovirus, elevated intestinal virus-specific IgA, suppressed Th1 & enhanced Th2 cytokine expression [63]. | |||||
4.1.2. Digestive Bacterial Infections
4.1.3. Enteric Viral Infections
4.2. Mucosal Immunity—Cytokine Balance
4.2.1. Deoxynivalenol (DON) Interaction with the Gut Epithelium
4.2.2. Mycotoxin Interaction with the Gut Epithelium
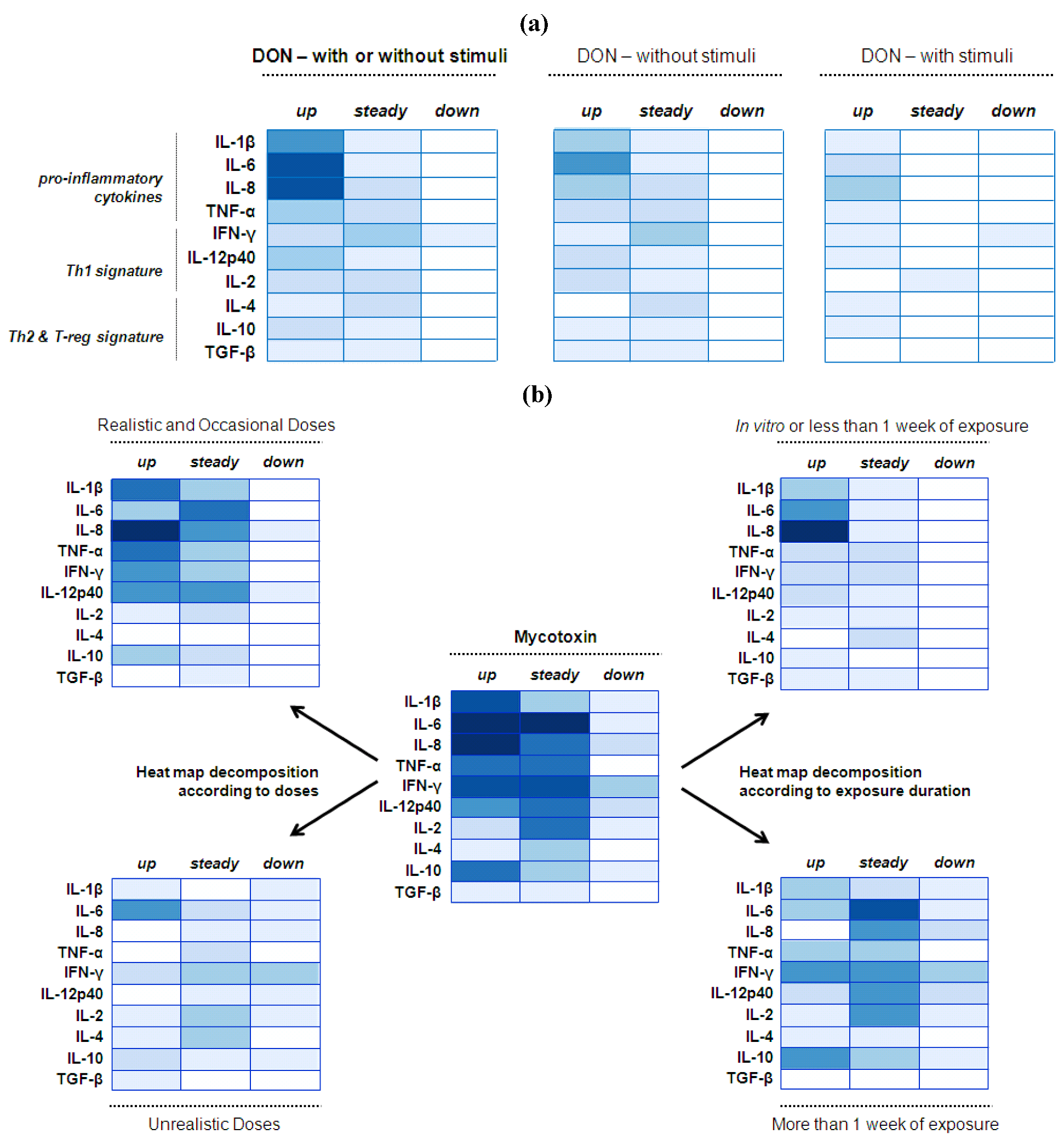
4.2.3. Implications
5. Consequence of Mycotoxins on Barrier Integrity
| Teer | Paracellular Flux | Junction Proteins | ||||
|---|---|---|---|---|---|---|
| DON | (RD) | IPEC-1: reduced TEER [54]. | Caco-2: increased paracellular flux of mannitol [55]. | IPEC-J2: reduced expression of ZO-1 [50,57] and claudin 3 [50]. | ||
| IPEC-J2: reduced TEER [50]. | IPEC-1: disappearance of ZO-1 [57]. | |||||
| Caco-2: reduced TEER [54,55]. | Caco-2: reduced expression of claudin 4 but not occludin [55]. | |||||
| Pig: reduced expression of claudin 4 in jejunum [54], occludin & | ||||||
| E-cadherin in ileum [51]. | ||||||
| (OD) | IPEC-1: reduced TEER [52,54,56]. | IPEC-1: increased paracellular flux of 4-kDa dextran [54,56] and pathogenic E.coli [54]. | IPEC-1: reduced expression of claudins 4 [52,54,56] & 3 but not ZO-1 and occludin [54]. | |||
| Pig explant: increased paracellular flux of | ||||||
| 4-kDa dextran [54]. | ||||||
| OTA | (UD) | Caco-2: reduced | Caco-2: increased in the paracellular flux of | Caco-2: disappearance of claudins 3 & 4 but not claudin 1 [30,31], ZO-1 and occludin [30]. | ||
| TEER [29,30,31]. | 4- and 10-kDa dextrans, but not 20- and | |||||
| HT-29: reduced TEER [29]. | 40-kDa dextrans [30]. | |||||
| AF | (RD) | Caco-2: slightly reduced TEER [25]. | ||||
| (UD) | Caco-2: reduced TEER [26]. | |||||
| FB | (RD) | Pig: reduced expression of occludin & E-cadherin in ileum [51]. | ||||
| (OD) | IPEC-1: reduced TEER [73]. | |||||
6. Consequence of Mycotoxins on Intestinal Microflora
7. Conclusions
Acknowledgments
Conflict of Interest
References
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar]
- Desjardins, A.; Maragos, C.; Norred, W.; Pestka, J.; Phillips, T.; Vardon, P.; Whitaker, T.; Wood, G.; van Egmond, H. Mycotoxins:Risks in Plant,Animal,and Human System; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Tech. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Oswald, I.P.; Marin, D.E.; Bouhet, S.; Pinton, P.; Taranu, I.; Accensi, F. Immunotoxicological risk of mycotoxins for domestic animals. Food Addit. Contam. 2005, 22, 354–360. [Google Scholar] [CrossRef]
- Lorenzoni, G. Poultry Diseases Influenced by Gastrointestinal Health: Traditional Treatments and Innovative Solutions. In Poultry Diseases Influenced by Gastrointestinal Health: Traditional Treatments and Innovative Solutions; Nottingham University Press: Loughborough, UK, 2010; pp. 1–140. [Google Scholar]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Tech. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Swamy, H.; Breeding, L.; Jackson, L.; Yiannikouris, A. Surveillance program tracks mycotoxin levels. Feedstuffs 2012, 84, 1–3. [Google Scholar]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Cavret, S.; Lecoeur, S. Fusariotoxin transfer in animal. Food Chem.Toxicol. 2006, 44, 444–453. [Google Scholar] [CrossRef]
- Agence Française de Sécurité Sanitaire des Aliments, Évaluation des risques liés à la présence de mycotoxines dans les chaînes alimentaires humaine et animale; Agence Française de Sécurité Sanitaire des Aliments: Maisons-Alfort, France, 2009; pp. 1–308.
- Bouhet, S.; Oswald, I.P. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2007, 51, 925–931. [Google Scholar] [CrossRef]
- Ringot, D.; Chango, A.; Schneider, Y.J.; Larondelle, Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef]
- Osselaere, A.; Devreese, M.; Goossens, J.; Vandenbroucke, V.; de Baere, S.; de Backer, P.; Croubels, S. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food Chem. Toxicol. 2012, in press. [Google Scholar]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Env. Health 1996, 48, 1–34. [Google Scholar]
- Mahfoud, R.; Maresca, M.; Santelli, M.; Pfohl-Leszkowicz, A.; Puigserver, A.; Fantini, J. pH-dependent interaction of fumonisin B-1 with cholesterol: Physicochemical and molecular modeling studies at the air-water interface. J. Agric. Food Chem. 2002, 50, 327–331. [Google Scholar] [CrossRef]
- Danicke, S.; Matthäus, K.; Lebzien, P.; Valenta, H.; Stemme, K.; Ueberschär, K.H.; Razzazi-Fazeli, E.; Böhm, J.; Flachowsky, G. Effects of Fusarium toxin-contaminated wheat grain on nutrient turnover, microbial protein synthesis and metabolism of deoxynivalenol and zearalenone in the rumen of dairy cows. J. Anim. Physiol. Anim. Nutr. 2005, 89, 303–315. [Google Scholar] [CrossRef]
- Han, X.-Y.; Huang, Q.-C.; Li, W.-F.; Jiang, J.-F.; Xu, Z.-R. Changes in growth performance, digestive enzyme activities and nutrient digestibility of cherry valley ducks in response to aflatoxin B1 levels. Livest. Sci. 2008, 119, 216–220. [Google Scholar] [CrossRef]
- Ostrowski-Meissner, H.T. Effect of contamination of foods by Aspergillus flavus on the nutritive value of protein. J. Sci. Food Agric. 1984, 35, 47–58. [Google Scholar] [CrossRef]
- Applegate, T.J.; Schatzmayr, G.; Pricket, K.; Troche, C.; Jiang, Z. Effect of aflatoxin culture on intestinal function and nutrient loss in laying hens. Poult. Sci. 2009, 88, 1235–1241. [Google Scholar] [CrossRef]
- Kermanshahi, H.; Akbari, M.R.; Maleki, M.; Behgar, M. Effect of prolonged low level inclusion of aflatoxin B1 into diet on performance, nutrient digestibility, histopathology and blood enzymes of broiler chicken. J. Anim. Vet. Adv. 2007, 6, 686–692. [Google Scholar]
- Verma, J.; Johri, T.S.; Swain, B.K. Effect of aflatoxin, ochratoxin and their combination on protein and energy utilisation in white leghorn laying hens. J. Sci. Food Agric. 2007, 87, 760–764. [Google Scholar] [CrossRef]
- Matur, E.; Ergul, E.; Akyazi, I.; Eraslan, E.; Cirakli, Z.T. The effects of Saccharomyces cerevisiae extract on the weight of some organs, liver, and pancreatic digestive enzyme activity in breeder hens fed diets contaminated with aflatoxins. Poult. Sci. 2010, 89, 2213–2220. [Google Scholar] [CrossRef]
- Tomková, I.; Ševčíková, Z.; Levkut, M.; Revajová, V.; Čonková, E.; Laciaková, A.; Lenhardt, L. Effect of aflatoxin B1 on CD3 T cells andalkaline phosphatase in the intestine of mice. Mycopathologia 2002, 154, 15–19. [Google Scholar] [CrossRef]
- Yunus, A.W.; Awad, W.A.; Kroger, S.; Zentek, J.; Bohm, J. In vitro aflatoxin B(1) exposure decreases response to carbamylcholine in the jejunal epithelium of broilers. Poult. Sci. 2010, 89, 1372–1378. [Google Scholar] [CrossRef]
- Caloni, F.; Cortinovis, C.; Pizzo, F.; de Angelis, I. Transport of aflatoxin M1 in human intestinal Caco-2/TC7 cells. Front. Pharmacol. 2012, 3, 111. [Google Scholar]
- Gratz, S.; Wu, Q.K.; El-Nezami, H.; Juvonen, R.O.; Mykkanen, H.; Turner, P.C. Lactobacillus rhamnosus strain GG reduces aflatoxin B1 transport, metabolism, and toxicity in Caco-2 cells. Appl. Environ. Microbiol. 2007, 73, 3958–3964. [Google Scholar] [CrossRef]
- Watzl, B.; Neudecker, C.; Hansch, G.M.; Rechkemmer, G.; Pool-Zobel, B.L. Short-term moderate aflatoxin B1 exposure has only minor effects on the gut-associated lymphoid tissue of Brown Norway rats. Toxicology 1999, 138, 93–102. [Google Scholar] [CrossRef]
- Kubena, L.F.; Bailey, R.H.; Byrd, J.A.; Young, C.R.; Corrier, D.E.; Stanker, L.H.; Rottinghaust, G.E. Cecal volatile fatty acids and broiler chick susceptibility to Salmonella typhimurium colonization as affected by aflatoxins and T-2 toxin. Poult. Sci. 2001, 80, 411–417. [Google Scholar]
- Maresca, M.; Mahfoud, R.; Pfohl-Leszkowicz, A.; Fantini, J. The mycotoxin ochratoxin A alters intestinal barrier and absorption functions but has no effect on chloride secretion. Toxicol. Appl. Pharm. 2001, 176, 54–63. [Google Scholar] [CrossRef]
- McLaughlin, J.; Padfield, P.J.; Burt, J.P.H.; O’Neill, C.A. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am. J. Physiol. Cell Physiol. 2004, 287, C1412–C1417. [Google Scholar] [CrossRef]
- Lambert, D.; Padfield, P.J.; McLaughlin, J.; Cannell, S.; O’Neill, C.A. Ochratoxin A displaces claudins from detergent resistant membrane microdomains. Biochem. Biophys. Res. Commun. 2007, 358, 632–636. [Google Scholar] [CrossRef]
- Koynarski, V.; Stoev, S.; Grozeva, N.; Mirtcheva, T.; Daskalov, H.; Mitev, J.; Mantle, P. Experimental coccidiosis provoked by Eimeria acervulina in chicks simultaneously fed on ochratoxin A contaminated diet. Res. Vet. Sci. 2007, 82, 225–231. [Google Scholar] [CrossRef]
- Koynarsky, V.; Stoev, S.D.; Grozeva, N.; Mirtcheva, T. Experimental coccidiosis provoked by Eimeria adenoeides in turkey poults given ochratoxin A. Veterinarski Arhiv 2007, 77, 113–128. [Google Scholar]
- Fukata, T.; Sasai, K.; Baba, E.; Arakawa, A. Effect of ochratoxin A on Salmonella typhimurium-challenged layer chickens. Avian Dis. 1996, 40, 924–926. [Google Scholar] [CrossRef]
- Danicke, S.; Valenta, H.; Matthes, S. On the interactions between Fusarium toxin-contaminated wheat and nonstarch polysaccharide hydrolyzing enzymes in diets of broilers on performance, intestinal viscosity, and carryover of deoxynivalenol. Poult. Sci. 2007, 86, 291–298. [Google Scholar]
- Hunder, G.; Schumann, K.; Strugala, G.; Gropp, J.; Fichtl, B.; Forth, W. Influence of subchronic exposure to low dietary deoxynivalenol, a trichothecene mycotoxin, on intestinal absorption of nutrients in mice. Food Chem. Toxicol. 1991, 29, 809–814. [Google Scholar] [CrossRef]
- Kolf-Clauw, M.; Castellote, J.; Joly, B.; Bourges-Abella, N.; Raymond-Letron, I.; Pinton, P.; Oswald, I.P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol. in Vitro 2009, 23, 1580–1584. [Google Scholar] [CrossRef]
- Awad, W.A.; Bohm, J.; Razzazi-Fazeli, E.; Ghareeb, K.; Zentek, J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult. Sci. 2006, 85, 974–979. [Google Scholar]
- Awad, W.A.; Bohm, J.; Razzazi-Fazeli, E.; Zentek, J. Effects of feeding deoxynivalenol contaminated wheat on growth performance, organ weights and histological parameters of the intestine of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2006, 90, 32–37. [Google Scholar] [CrossRef]
- Awad, W.A.; Vahjen, W.; Aschenbach, J.R.; Zentek, J. A diet naturally contaminated with the Fusarium mycotoxin deoxynivalenol (DON) downregulates gene expression of glucose transporters in the intestine of broiler chickens. Livest. Sci. 2011, 140, 72–79. [Google Scholar] [CrossRef]
- Awad, W.A.; Rehman, H.; Bohm, J.; Razzazi-Fazeli, E.; Zentek, J. Effects of luminal deoxynivalenol and L-proline on electrophysiological parameters in the Jejunums of laying hens. Poult. Sci. 2005, 84, 928–932. [Google Scholar]
- Awad, W.A.; Bohm, J.; Razzazi-Fazeli, E.; Hulan, H.W.; Zentek, J. Effects of deoxynivalenol on general performance and electrophysiological properties of intestinal mucosa of broiler chickens. Poult. Sci. 2004, 83, 1964–1972. [Google Scholar]
- Awad, W.A.; Bohm, J.; Razzazi-Fazeli, E.; Zentek, J. In vitro effects of deoxynivalenol on electrical properties of intestinal mucosa of laying hens. Poult. Sci. 2005, 84, 921–927. [Google Scholar]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002, 132, 2723–2731. [Google Scholar]
- Awad, W.A.; Aschenbach, J.R.; Setyabudi, F.; Razzazi-Fazeli, E.; Bohm, J.; Zentek, J. In vitro effects of deoxynivalenol on small intestinal D-glucose uptake and absorption of deoxynivalenol across the isolated jejunal epithelium of laying hens. Poult. Sci. 2007, 86, 15–20. [Google Scholar]
- Dietrich, B.; Neuenschwander, S.; Bucher, B.; Wenk, C. Fusarium mycotoxin-contaminated wheat containing deoxynivalenol alters the gene expression in the liver and the jejunum of broilers. Animal 2012, 6, 278–291. [Google Scholar] [CrossRef]
- Boguhn, J.; Neumann, D.; Helm, A.; Strobel, E.; Tebbe, C.C.; Danicke, S.; Rodehutscord, M. Effects of concentrate proportion in the diet with or without Fusarium toxin-contaminated triticale on ruminal fermentation and the structural diversity of rumen microbial communities in vitro. Arch. Anim. Nutr. 2010, 64, 467–483. [Google Scholar] [CrossRef]
- Hildebrand, B.; Boguhn, J.; Dänicke, S.; Rodehutscord, M. Effect of Fusarium toxin-contaminated triticale and forage-to-concentrate ratio on fermentation and microbial protein synthesis in the rumen. J. Anim. Physiol. Anim. Nutr. 2012, 96, 307–318. [Google Scholar] [CrossRef]
- Wache, Y.J.; Valat, C.; Postollec, G.; Bougeard, S.; Burel, C.; Oswald, I.P.; Fravalo, P. Impact of deoxynivalenol on the intestinal microflora of pigs. Int. J. Mol. Sci. 2009, 10, 1–17. [Google Scholar]
- Diesing, A.K.; Nossol, C.; Danicke, S.; Walk, N.; Post, A.; Kahlert, S.; Rothkotter, H.J.; Kluess, J. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS One 2011, 6, e17472. [Google Scholar]
- Bracarense, A.-P.F.L.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junctions proteins and MAPKinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef]
- Maresca, M.; Yahi, N.; Younes-Sakr, L.; Boyron, M.; Caporiccio, B.; Fantini, J. Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: Stimulation of interleukin-8 secretion, potentiation of interleukin-1 beta effect and increase in the transepithelial passage of commensal bacteria. Toxicol. Appl. Pharmacol. 2008, 228, 84–92. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrède, J.-P.; del Rio, J.-C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.-P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef]
- Van de Walle, J.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar] [CrossRef]
- Pinton, P.; Braicu, C.; Nougayrede, J.P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a Mitogen Activated Protein Kinase dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef]
- Diesing, A.K.; Nossol, C.; Panther, P.; Walk, N.; Post, A.; Kluess, J.; Kreutzmann, P.; Danicke, S.; Rothkotter, H.J.; Kahlert, S. Mycotoxin deoxynivalenol (DON) mediates biphasic cellular response in intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2. Toxicol. Lett. 2011, 200, 8–18. [Google Scholar] [CrossRef]
- Vandenbroucke, V.; Croubels, S.; Martel, A.; Verbrugghe, E.; Goossens, J.; van Deun, K.; Boyen, F.; Thompson, A.; Shearer, N.; de Backer, P.; et al. The mycotoxin deoxynivalenol potentiates intestinal inflammation by Salmonella typhimurium in porcine ileal loops. PLoS One 2011, 6, e23871. [Google Scholar]
- Xu, L.; Eicher, S.D.; Applegate, T.J. Effects of increasing dietary concentrations of corn naturally contaminated with deoxynivalenol on broiler and turkey poult performance and response to lipopolysaccharide. Poult. Sci. 2011, 90, 2766–2774. [Google Scholar] [CrossRef]
- De Walle, J.V.; Romier, B.; Larondelle, Y.; Schneider, Y.J. Influence of deoxynivalenol on NF-κ B activation and IL-8 secretion in human intestinal Caco-2 cells. Toxicol. Lett. 2008, 177, 205–214. [Google Scholar] [CrossRef]
- Azcona-Olivera, J.I.; Ouyang, Y.; Murtha, J.; Chu, F.S.; Pestka, J.J. Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): Relationship to toxin distribution and protein synthesis inhibition. Toxicol. Appl. Pharmacol. 1995, 133, 109–120. [Google Scholar] [CrossRef]
- Yan, D.; Zhou, H.R.; Brooks, K.H.; Pestka, J.J. Potential role for IL-5 and IL-6 in enhanced IgA secretion by Peyer’s patch cells isolated from mice acutely exposed to vomitoxin. Toxicology 1997, 122, 145–158. [Google Scholar] [CrossRef]
- Li, M.X.; Cuff, C.F.; Pestka, J. Modulation of murine host response to enteric reovirus infection by the trichothecene deoxynivalenol. Toxicol. Sci. 2005, 87, 134–145. [Google Scholar] [CrossRef]
- Kumagai, S.; Shimizu, T. Effects of Fusarenon-X and T-2 toxin on intestinal absorption of monosaccharide in rats. Arch. Toxicol. 1988, 61, 489–495. [Google Scholar] [CrossRef]
- Tenk, I.; Fodor, E.; Szathmary, C. The effect of pure Fusarium toxins (T-2, F-2, DAS) on the microflora of the gut and on plasma glucocorticoid levels in rat and swine. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1982, 252, 384–393. [Google Scholar]
- Varga, I.; Vanyi, A. Interaction of T-2 fusariotoxin with anticoccidial efficacy of lasalocid in chickens. Int. J. Parasitol. 1992, 22, 523–525. [Google Scholar] [CrossRef]
- Li, M.; Cuff, C.F.; Pestka, J.J. T-2 toxin impairment of enteric reovirus clearance in the mouse associated with suppressed immunoglobulin and IFN-γ responses. Toxicol. Appl. Pharmacol. 2006, 214, 318–325. [Google Scholar] [CrossRef]
- Gbore, F.A.; Egbunike, G.N. Influence of dietary fumonisin B1 on nutrient utilization by growing pigs. Livest. Res. Rural Dev. 2007, 19. #93. [Google Scholar]
- Gbore, F.A.; Yinusa, R.I.; Salleh, B. Evaluation of subchronic dietary fumonisin B1 on nutrient digestibility and growth performance of rats. Afr. J. Biotechnol. 2010, 9, 6442–6447. [Google Scholar]
- Lessard, M.; Boudry, G.; Sève, B.; Oswald, I.P.; Lallès, J.-P. Intestinal physiology and peptidase activity in male pigs are modulated by consumption of corn culture extracts containing fumonisins. J. Nutr. 2009, 139, 1303–1307. [Google Scholar] [CrossRef]
- Piva, A.; Casadei, G.; Pagliuca, G.; Cabassi, E.; Galvano, F.; Solfrizzo, M.; Riley, R.T.; Diaz, D.E. Activated carbon does not prevent the toxicity of culture material containing fumonisin B1 when fed to weanling piglets. J. Anim. Sci. 2005, 83, 1939–1947. [Google Scholar]
- Becker, B.; Bresch, H.; Schillinger, U.; Thiel, P.G. The effect of fumonisin B1 on the growth of bacteria. World J. Microbiol. Biotech. 1997, 13, 539–543. [Google Scholar] [CrossRef]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Martinez, S.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P. The mycotoxin fumonisin B-1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar]
- Grenier, B.; Bracarense, A.-P.F.L.; Schwartz, H.E.; Trumel, C.; Cossalter, A.-M.; Schatzmayr, G.; Kolf-Clauw, M.; Moll, W.-D.; Oswald, I.P. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochem. Pharmacol. 2012, 83, 1465–1473. [Google Scholar]
- Devriendt, B.; Gallois, M.; Verdonck, F.; Wache, Y.; Bimczok, D.; Oswald, I.P.; Goddeeris, B.M.; Cox, E. The food contaminant fumonisin B-1 reduces the maturation of porcine CD11R1(+) intestinal antigen presenting cells and antigen-specific immune responses, leading to a prolonged intestinal ETEC infection. Vet. Res. 2009. [Google Scholar] [CrossRef]
- Bouhet, S.; le Dorze, E.; Peres, S.; Fairbrother, J.M.; Oswald, I.P. Mycotoxin fumonisin B-1 selectively down-regulates the basal IL-8 expression in pig intestine: In vivo and in vitro studies. Food Chem. Toxicol. 2006, 44, 1768–1773. [Google Scholar] [CrossRef]
- Oswald, I.P.; Desautels, C.; Laffitte, J.; Fournout, S.; Peres, S.Y.; Odin, M.; le Bars, P.; le Bars, J.; Fairbrother, J.M. Mycotoxin fumonisin B-1 increases intestinal colonization by pathogenic Escherichia coli in pigs. Appl. Environ. Microbiol. 2003, 69, 5870–5874. [Google Scholar]
- Danicke, S.; Ueberschar, K.H.; Halle, I.; Matthes, S.; Valenta, H.; Flachowsky, G. Effect of addition of a detoxifying agent to laying hen diets containing uncontaminated or Fusarium toxin-contaminated maize on performance of hens and on carryover of zearalenone. Poult. Sci. 2002, 81, 1671–1680. [Google Scholar]
- Danicke, S.; Valenta, H.; Klobasa, F.; Doll, S.; Ganter, M.; Flachowsky, G. Effects of graded levels of Fusarium toxin contaminated wheat in diets for fattening pigs on growth performance, nutrient digestibility, deoxynivalenol balance and clinical serum characteristics. Arch. Anim. Nutr. 2004, 58, 1–17. [Google Scholar] [CrossRef]
- Danicke, S.; Matthes, S.; Halle, I.; Ueberschar, K.H.; Doll, S.; Valenta, H. Effects of graded levels of Fusarium toxin-contaminated wheat and of a detoxifying agent in broiler diets on performance, nutrient digestibility and blood chemical parameters. Br. Poult. Sci. 2003, 44, 113–126. [Google Scholar] [CrossRef]
- Leung, M.C.K.; Smith, T.K.; Karrow, N.A.; Boermans, H.J. Effects of foodborne Fusarium mycotoxins with and without a polymeric glucomannan mycotoxin adsorbent on food intake and nutrient digestibility, body weight, and physical and clinicopathologic variables of mature dogs. Am. J. Vet. Res. 2007, 68, 1122–1129. [Google Scholar] [CrossRef]
- Yunus, A.W.; Blajet-Kosicka, A.; Kosicki, R.; Khan, M.Z.; Rehman, H.; Böhm, J. Deoxynivalenol as a contaminant of broiler feed: Intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poult. Sci. 2012, 91, 852–861. [Google Scholar] [CrossRef]
- Girish, C.K.; Smith, T.K. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on small intestinal morphology of turkeys. Poult. Sci. 2008, 87, 1075–1082. [Google Scholar] [CrossRef]
- Girgis, G.N.; Barta, J.R.; Brash, M.; Smith, T.K. Morphologic changes in the intestine of broiler breeder pullets fed diets naturally contaminated with Fusarium mycotoxins with or without coccidial challenge. Avian Dis. 2010, 54, 67–73. [Google Scholar]
- Girgis, G.N.; Sharif, S.; Barta, J.R.; Boermans, H.J.; Smith, T.K. Immunomodulatory effects of feed-borne Fusarium mycotoxins in chickens infected with coccidia. Exp. Biol. Med. 2008, 233, 1411–1420. [Google Scholar] [CrossRef]
- Girgis, G.N.; Barta, J.R.; Girish, C.K.; Karrow, N.A.; Boermans, H.J.; Smith, T.K. Effects of feed-borne Fusarium mycotoxins and an organic mycotoxin adsorbent on immune cell dynamics in the jejunum of chickens infected with Eimeria maxima. Vet. Immunol. Immunopathol. 2010, 138, 218–223. [Google Scholar] [CrossRef]
- Sergent, T.; Parys, M.; Garsou, S.; Pussemier, L.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol. Lett. 2006, 164, 167–176. [Google Scholar] [CrossRef]
- Sergent, T.; Ribonnet, L.; Kolosova, A.; Garsou, S.; Schaut, A.; de Saeger, S.; van Peteghem, C.; Larondelle, Y.; Pussemier, L.; Schneider, Y.-J. Molecular and cellular effects of food contaminants and secondary plant components and their plausible interactions at the intestinal level. Food Chem. Toxicol. 2008, 46, 813–841. [Google Scholar] [CrossRef]
- De Angelis, I.; Frigge, G.; Raimondi, F.; Stammati, A.; Zucco, F.; Caloni, F. Absorption of fumonisin B-1 and aminopentol on an in vitro model of intestinal epithelium; the role of P-glycoprotein. Toxicon 2005, 45, 285–291. [Google Scholar] [CrossRef]
- Abdelhamid, A.M.; Dorra, T.M.; Mansy, S.E.; Sallam, A.E. Effect of raising dietary protein, amino acids and/or energy levels as an attempt to alleviate severity of the chronic aflatoxicosis by broiler chicks. Arch. Tierernahr. 1994, 46, 339–345. [Google Scholar] [CrossRef]
- Grenier, B.; Oswald, I. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Flannery, B.M.; Clark, E.S.; Pestka, J.J. Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY (PYY). Toxicol. Sci. 2012, 30, 289–297. [Google Scholar] [CrossRef]
- Pastorelli, H.; van Milgen, J.; Lovatto, P.; Montagne, L. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 2012, 6, 952–961. [Google Scholar] [CrossRef]
- R Development Core Team, R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010.
- Pestka, J.J. Deoxynivalenol-induced IgA production and IgA nephropathy-aberrant mucosal immune response with systemic repercussions. Toxicol. Lett. 2003, 140, 287–295. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta 2009, 1788, 864–871. [Google Scholar] [CrossRef]
- Maresca, M.; Fantini, J. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon 2010, 56, 282–294. [Google Scholar] [CrossRef]
- Loiseau, N.; Debrauwer, L.; Sambou, T.; Bouhet, S.; Miller, J.D.; Martin, P.G.; Viadere, J.L.; Pinton, P.; Puel, O.; Pineau, T.; et al. Fumonisin B-1 exposure and its selective effect on porcine jejunal segment: Sphingolipids, glycolipids and trans-epithelial passage disturbance. Biochem. Pharmacol. 2007, 74, 144–152. [Google Scholar]
- Pestka, J.J.; Zhou, H.-R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef]
- Grenier, B.; Loureiro-Bracarense, A.-P.; Lucioli, J.; Pacheco, G.D.; Cossalter, A.-M.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. Mol. Nutr. Food Res. 2011, 55, 761–771. [Google Scholar] [CrossRef]
- Schatzmayr, G.; Zehner, F.; Täubel, M.; Schatzmayr, D.; Klimitsch, A.; Loibner, A.P.; Binder, E.M. Microbiologicals for deactivating mycotoxins. Mol. Nutr. Food Res. 2006, 50, 543–551. [Google Scholar] [CrossRef]
- Diaz, G.J.; Calabrese, E.; Blain, R. Aflatoxicosis in chickens (Gallus gallus): An example of hormesis? Poult. Sci. 2008, 87, 727–732. [Google Scholar]
- Dall’Asta, C.; Falavigna, C.; Galaverna, G.; Dossena, A.; Marchelli, R. In vitro digestion assay for determination of hidden fumonisins in maize. J. Agric. Food Chem. 2010, 58, 12042–12047. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grenier, B.; Applegate, T.J. Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals . Toxins 2013, 5, 396-430. https://doi.org/10.3390/toxins5020396
Grenier B, Applegate TJ. Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals . Toxins. 2013; 5(2):396-430. https://doi.org/10.3390/toxins5020396
Chicago/Turabian StyleGrenier, Bertrand, and Todd J. Applegate. 2013. "Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals " Toxins 5, no. 2: 396-430. https://doi.org/10.3390/toxins5020396
APA StyleGrenier, B., & Applegate, T. J. (2013). Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals . Toxins, 5(2), 396-430. https://doi.org/10.3390/toxins5020396




