Towards Clinical Applications of Anti-endotoxin Antibodies; A Re-appraisal of the Disconnect
Abstract
:1. Introduction and Overview
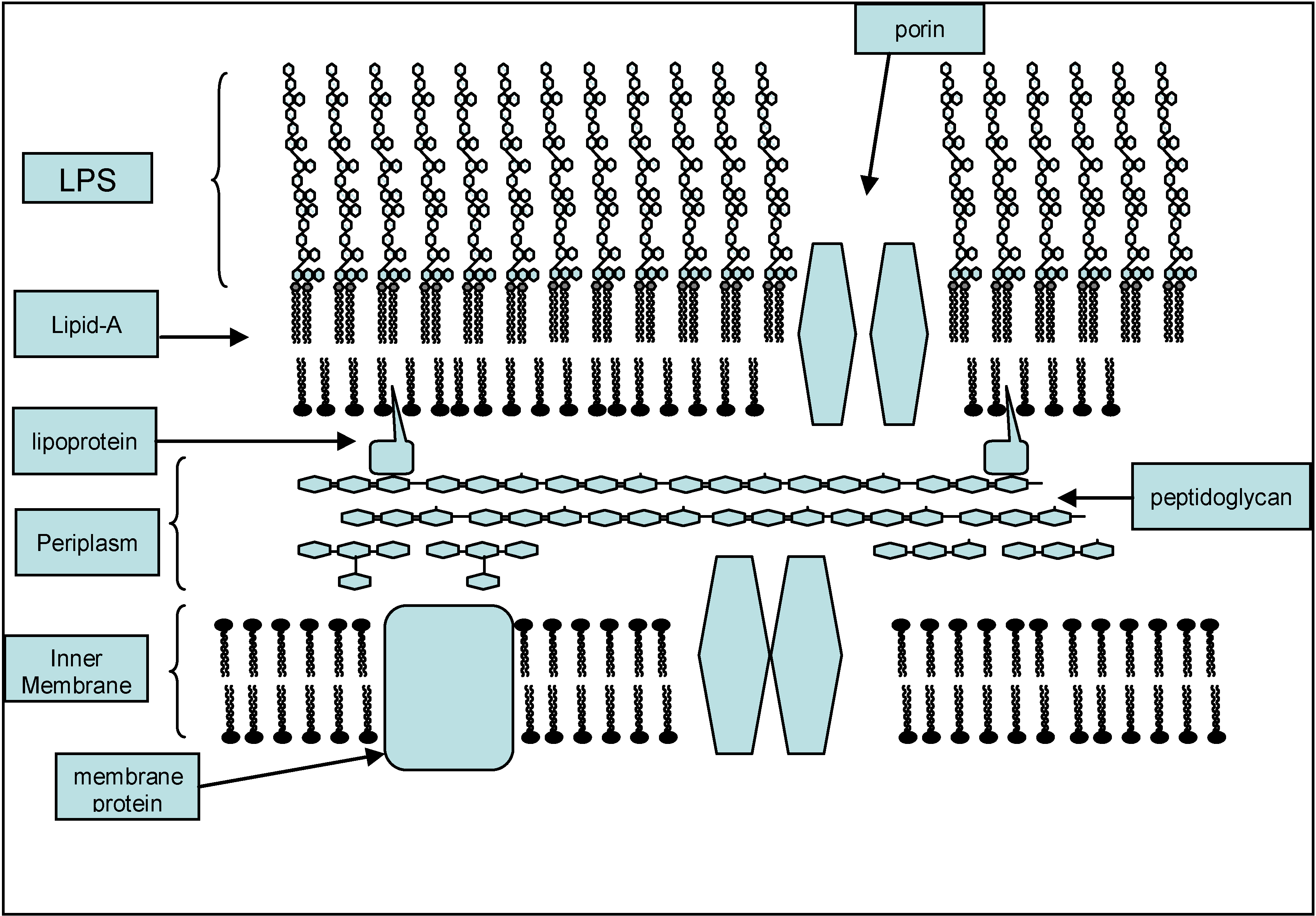
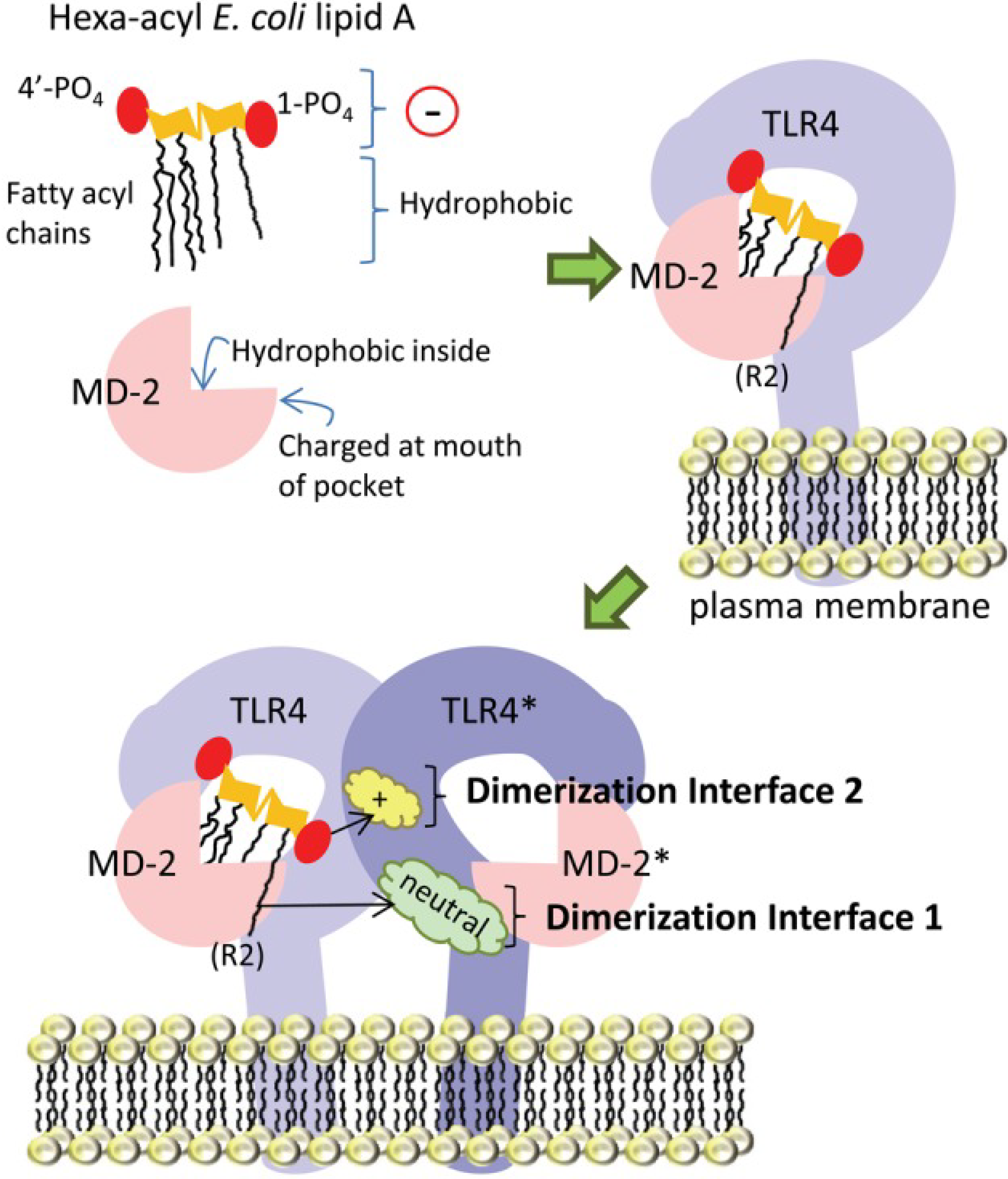
2. Structure Activity

3. Identifying the Target Population
- Body temperature >38 °C or <36 °C;
- Heart rate > 90 beats per minute;
- Respiratory rate > 20 breaths per minute of hyperventilation evident with a PaCO2 < 32 mmHg;
- White blood cell count (WCC) >12,000/mm, <4000/mm3 or with >10% immature neutrophils.
4. Endotoxemia as a Therapeutic Target in Sepsis
- What do bacteremia and endotoxemia separately contribute towards the attributable mortality of Gram negative sepsis?
- Is GN bacteremia a single entity?
- Can endotoxemia be considered as a single entity?
- Is the attributable mortality dependent on the underlying mortality risk?
- Is the onset of severe sepsis an indication that the detrimental pathophysiological process has passed the point of no return and hence anti-endotoxemia therapy at this point is futile?
- Is the disconnect bridgeable or is a more fundamental explanation required?

5. Anti-endotoxin Therapies and the Disconnect
5.1. Anti-endotoxin Therapies: Pre-clinical Disconnect
| Author | Year | Reference | Agent | Setting | N | Mort | GNI |
|---|---|---|---|---|---|---|---|
| McCabe; Zinner | 1972, 1976 | [101,102] | core Ab § | Hospital | 182 | ↓ | ND |
| Pollack | 1983 | [103] | core Ab | Hospital | 43 | ↓ | ND |
| Baumgartner | 1985 | [104] | J5 IVIG | ICU | 262 | ↓* | ND |
| McCutchan | 1983 | [105] | J5 IVIG | Oncology | 100 | ↔ | ↔ |
| Cometta | 1992 | [106] | J5 IVIG | Surgical | 329 | ↔ | ↓ |
| Bennett-Guerrero | 1997 | [107] | core Ab § | Surgical | 301 | ND | ↓ |
| Author | Year | Reference | Agent | Setting | N | Mort |
|---|---|---|---|---|---|---|
| Meningococcal disease | ||||||
| J5 study group | 1992 | [108] | J5 PC | ICU | 73 | ↔ |
| Derkx | 1999 | [109] | HA-1A | ICU | 267 | ↔ |
| Levin | 2000 | [110] | rBPI21 | ICU | 892 | ↓* |
| Author | Year | Reference | Agent | Setting | N | Mort | GNI |
|---|---|---|---|---|---|---|---|
| Polyclonal anti-sera | |||||||
| Ziegler | 1982 | [111] | JS PC | Hospital | 212 | ↓ | ND |
| Calandra | 1988 | [112] | J5 PC | ICU | 71 | ↔ | ↔ |
| Grundmann | 1988 | [113] | IVIG | ICU | 46 | ↔ | ND |
| Schedel | 1991 | [114] | IVIG | Hospital | 55 § | ↓ | ND |
| Behre | 1992 | [115] | IVIG | Oncology | 21 | ↓ | ND |
| Behre | 1995 | [116] | IVIG | Oncology | 52 | ↓ | ND |
| Monoclonal antibodies | |||||||
| Ziegler | 1991 | [117] | HA-1A | Hospital | 200 § | ↓* | ND |
| Greenman | 1991 | [118] | E5 | Hospital | 212 | ↓* | ND |
| Fisher | 1990 | [119] | HA-1A | 34 | ND | ND | |
| French Reg | 1994 | [120] | HA-1A | Hospital | 600 | ↑ | ND |
| McCloskey | 1994 | [121] | HA-1A | Hospital | 2199 | ↔ | ND |
| Angus | 2000 | [122] | E5 | ICU | 1090 | ↔ | ND |
| Daifuku | 1992 | [123] | MAB-T88 | 9 | ND | ND | |
| Greenberg | 1991 | [124] | E5 | ||||
| Greenberg | 1992 | [125] | E5 | 39 | ND | ND | |
| Albertson | 2003 | [126] | ECA-Ab | ICU | 411 | ↔ | ND |
| Other endotoxin agents | |||||||
| Willats | 1995 | [127] | Taurolidine | ICU | 100 | ↔ | ↔ |
| Reinhart | 2004 | [128] | iHSA | ICU | 143 | ↔ | ND |
| Bennett-Guerrero | 2007 | [129] | E5564 | Surgical | 152 | ↔ | ND |
| Tidswell | 1910 | [130] | E5564 | ICU | 235 | ↔ | ND |
| Opal | 2013 | [131] | E5564 | ICU | 1961 | ↔ | ND |
| Dellinger | 2009 | [132] | PLE | ICU | 1379 | ↔ | ND |
| Heemskerk | 2009 | [133] | ALP | ICU | 36 | ↔ | ND |
| Pickkers | 2012 | [134] | ALP | ICU | 36 | ↔ | ND |
5.2. Anti-endotoxin Therapies: Clinical Disconnect
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Braude, A.I. Bacterial endotoxins. Sci. Am. 1964, 210, 36–45. [Google Scholar] [CrossRef]
- Rietschel, E.T.; Brade, H. Bacterial endotoxins. Sci. Am. 1992, 267, 54–61. [Google Scholar] [CrossRef]
- Raetz, C.R. Bacterial endotoxins extraordinary lipids that activate eucaryotic signal transduction. J. Bacteriol. 1993, 175, 5745–5753. [Google Scholar]
- Hurley, J.C. Endotoxemia: Concordance with Gram Negative Bacteremia and Association with Outcome. D. Med. Sci. Thesis; University of Melbourne: Melbourne, Australia, September 2013. Available online: http://repository.unimelb.edu.au/10187/17991 (accessed on 22 October 2013).
- Hurley, J.C.; Levin, J. Relevance of Endotoxin Detection in Sepsis. In Endotoxin in Health and Disease; Brade, H., Stefanie Vogel, S.O., Morrison, D., Eds.; Marcel Dekker Limited: New York, NY, USA, 1999; pp. 841–854. [Google Scholar]
- Opal, S.M. The clinical relevance of endotoxin in human sepsis: A critical analysis. J. Endotoxin. Res. 2002, 8, 473–476. [Google Scholar]
- Corriveau, C.C.; Danner, R.L. Antiendotoxin therapies for septic shock. Infect. Agents Dis. 1993, 2, 44–52. [Google Scholar]
- Opal, S.M.; Gluck, T. Endotoxin as a drug target. Crit. Care Med. 2003, 31 (Suppl. 1), S57–S64. [Google Scholar] [CrossRef]
- Corriveau, C.C.; Danner, R.L. Endotoxin as a therapeutic target in septic shock. Infect. Agents Dis. 1993, 2, 35–43. [Google Scholar]
- Hurley, J.C. Antibiotic induced release of endotoxin: A therapeutic paradox. Drug Saf. 1995, 12, 183–195. [Google Scholar] [CrossRef]
- Hitchcock, P.J.; Leive, L.; Makela, P.H.; Rietschel, E.T.; Strittmatter, W.; Morrison, D.C. Lipopolysaccharide nomenclature—past, present, and future. J. Bacteriol. 1986, 166, 699–705. [Google Scholar]
- Kotani, S.; Takada, H.; Tsujimoto, M.; Ogawa, T.; Takahashi, I.; Ikeda, T.; Otsuka, K.; Shimauchi, H.; Kasai, N.; Mashimo, J.; et al. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect. Immun. 1985, 49, 225–237. [Google Scholar]
- Maeshima, N.; Fernandez, R.C. Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Front. Cell. Infect. Microbiol. 2013, 3, 3. [Google Scholar]
- Trent, M.S.; Stead, C.M.; Tran, A.X.; Hankins, J.V. Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin Res. 2006, 12, 205–223. [Google Scholar] [CrossRef]
- Raetz, C.R.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef]
- Morrison, D.C.; Ryan, J.L. Endotoxins and disease mechanisms. Annu. Rev. Med. 1987, 38, 417–432. [Google Scholar] [CrossRef]
- Tesh, V.L.; Vukajlovich, S.W.; Morrison, D.C. Endotoxin interactions with serum proteins relationship to biological activity. Prog. Clin. Biol. Res. 1988, 272, 47–62. [Google Scholar]
- Hurley, J.C.; Tosolini, F.A.; Louis, W.J. Quantitative Limulus lysate assay for endotoxin and the effect of plasma. J. Clin. Pathol. 1991, 44, 849–854. [Google Scholar] [CrossRef]
- Beller, F.K.; Debrovner, C.H.; Douglas, G.W. Potentiation of the lethal effect of endotoxin by heterologous plasma. J. Exp. Med. 1963, 118, 245–256. [Google Scholar] [CrossRef]
- Andra, J.; Gutsmann, T.; Muller, M.; Schromm, A.B. Interactions between lipid A and serum proteins. Adv. Exp. Med. Biol. 2009, 667, 39–51. [Google Scholar] [CrossRef]
- Cross, A.S.; Sadoff, J.C.; Kelly, N.; Bemton, E.; Gemski, P. Pre-treatment with recombinant murine tumour necrosis factor and murine inter-leukin 1 protects mice from lethal infection. J. Exp. Med. 1989, 169, 2021–2027. [Google Scholar] [CrossRef]
- Eden, C.S.; Shahin, R.; Briles, D. Host resistance to mucosal gram-negative infection. Susceptibility of lipopolysaccharide nonresponder mice. J. Immunol. 1988, 140, 3180–3185. [Google Scholar]
- Hochstein, H.D.; Fitzgerald, E.A.; McMahon, F.G.; Vargas, R. Properties of US standard endotoxin (EC-5) in human male volunteers. J. Endotoxin Res. 1994, 1, 52–56. [Google Scholar]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zahringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar]
- Suffredini, A.F.; Fromm, R.E.; Parker, M.M.; Brenner, M.; Kovacs, J.A.; Wesley, R.A.; Parrillo, J.E. The cardiovascular response of normal humans to the administration of endotoxin. N. Engl. J. Med. 1989, 321, 280–287. [Google Scholar]
- Mileski, W.J.; Winn, R.K.; Harlan, J.M.; Rice, C.L. Sensitivity to endotoxin in rabbits is increased after hemorrhagic shock. J. Appl. Physiol. 1992, 73, 1146–1149. [Google Scholar]
- Noshima, S.; Noda, H.; Herndon, D.N.; Traber, L.D.; Traber, D.L. Left ventricular performance during continuous endotoxin-induced hyperdynamic endotoxemia in sheep. J. Appl. Physiol. 1993, 74, 1528–1533. [Google Scholar]
- Bahrami, S.; Redl, H.; Leichtfried, G.; Yu, Y.; Schlag, G. Similar cytokine but different coagulation responses to lipopolysaccharide injection in D-galactosamine-sensitized versus nonsensitized rats. Infect. Immun. 1994, 62, 99–105. [Google Scholar]
- Holzer, K.; Thiel, M.; Moritz, S.; Kreimeier, U.; Messmer, K. Expression of adhesion molecules on circulating PMN during hyperdynamic endotoxemia. J. Appl. Physiol. 1996, 81, 341–348. [Google Scholar]
- Levi, M.; ten Cate, H.; Bauer, K.A.; van der Poll, T.; Edgington, T.S.; Buller, H.R.; van Deventer, S.J.; Hack, C.E.; ten Cate, J.W.; Rosenberg, R.D. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J. Clin. Investig. 1994, 93, 114–120. [Google Scholar]
- Kneidinger, R.; Bahrami, S.; Redl, H.; Schlag, G. Comparison of endothelial acitavion during endotoxic and post-traumatic conditions by serum analysis of soluble E-selectin in non-human primates. J. Lab. Clin. Med. 1996, 128, 515–519. [Google Scholar] [CrossRef]
- Redl, H.; Schlag, G.; Bahrami, S. Endotoxemia in Primate Models. In Endotoxin in Health and Disease; Brade, H., Stefanie Vogel, S.O., Morrison, D., Eds.; Marcel Dekker Limited: New York, NY, USA, 1999; pp. 795–808. [Google Scholar]
- Van der Poll, T.; Levi, M.; van Deventer, S.J.; ten Cate, H.; Haagmans, B.L.; Biemond, B.J.; Buller, H.R.; Hack, C.E.; ten Cate, J.W. Differential effects of anti-tumor necrosis factor monoclonal antibodies on systemic inflammatory responses in experimental endotoxemia in chimpanzees. Blood 1994, 83, 446–451. [Google Scholar]
- West, M.A.; Heagy, W. Endotoxin tolerance: A review. Crit. Care Med. 2002, 30 (Suppl. 1), S64–S73. [Google Scholar] [CrossRef]
- Greisman, S.E.; Hornick, R.B. Mechanisms of endotoxin tolerance with special reference to man. J. Infect. Dis. 1973, 128 (Suppl.), 265–276. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Cross, A.S. Endotoxin tolerance-current concepts in historical perspective. J. Endotoxin Res. 2002, 8, 83–98. [Google Scholar]
- Greisman, S.E.; Hornick, R.B.; Wagner, H.N., Jr.; Woodward, W.E.; Woodward, T.E. The role of endotoxin during typhoid fever and tularemia in man. IV. The integrity of the endotoxin tolerance mechanisms during infection. J. Clin. Investig. 1969, 48, 613–629. [Google Scholar] [CrossRef]
- Greisman, S.E.; Wagner, H.N.; Iio, M.; Hornick, R.B. Mechanisms of endotoxin tolerance. II. Relationship between endotoxin tolerance and reticuloendothelial system phagocytic activity in man. J. Exp. Med. 1964, 119, 241–264. [Google Scholar]
- Greisman, S.E.; Young, E.J.; DuBuy, B. Mechanisms of endotoxin tolerance. VIII. Specificity of serum transfer. J. Immunol. 1973, 111, 1349–1360. [Google Scholar]
- Pechous, R.D.; McCarthy, T.R.; Zahrt, T.C. Working toward the future: Insights into Francisella tularensis pathogenesis and vaccine development. Microbiol. Mol. Biol. Rev. 2009, 73, 684–711. [Google Scholar] [CrossRef]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Caroff, M.; Karibian, D.; Cavaillon, J.M.; Haeffner-Cavaillon, N. Structural and functional analyses of bacterial lipopolysaccharides. Microbes Infect. 2002, 4, 915–926. [Google Scholar] [CrossRef]
- Mueller, M.; Lindner, B.; Dedrick, R.; Schromm, A.B.; Seydel, U. Endotoxin: Physical requirements for cell activation. J. Endotoxin Res. 2005, 11, 299–303. [Google Scholar]
- Shnyra, A.; Hultenby, K.; Lindberg, A.A. Role of the physical state of Salmonella lipopolysaccharide in expression of biological and endotoxic properties. Infect. Immun. 1993, 61, 5351–5360. [Google Scholar]
- Kato, N. Crystallization and electron microscopy of bacterial lipopolysaccharide. Micron 1993, 24, 91–114. [Google Scholar] [CrossRef]
- Komuro, T.; Murai, T.; Kawasaki, H. Effect of sonication on the dispersion state of lipopolysaccharide and its pyrogenicity in rabbits. Chem. Pharm. Bull. (Tokyo) 1987, 35, 4946–4952. [Google Scholar]
- Opal, S.M.; Palardy, J.E.; Marra, M.N.; McKelligon, B.M.; Scott, R.W.; Fisher, C.J. Relative concentrations of endotoxin-binding proteins in body fluids during infection. Lancet 1994, 344, 429–431. [Google Scholar] [CrossRef]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef]
- Gutsmann, T.; Razquin-Olazarán, I.; Kowalski, I.; Kaconis, Y.; Howe, J.; Bartels, R.; Brandenburg, K. New antiseptic peptides to protect against endotoxin-mediated shock. Antimicrob. Agents Chemother. 2010, 54, 3817–3824. [Google Scholar] [CrossRef]
- Schuerholz, T.; Brandenburg, K.; Marx, G. Antimicrobial peptides and their potential application in inflammation and sepsis. Crit. Care 2012, 16, 207. [Google Scholar] [CrossRef]
- Brandenburg, K.; Andrä, J.; Garidel, P.; Gutsmann, T. Peptide-based treatment of sepsis. Appl. Microbiol. Biotechnol. 2011, 90, 799–808. [Google Scholar] [CrossRef]
- Munford, R.S.; Varley, A.W. Shield as signal: Lipopolysaccharides and the evolution of immunity to gram-negative bacteria. PLoS Pathog. 2006, 2, e67. [Google Scholar] [CrossRef]
- Munford, R.S. Sensing gram-negative bacterial lipopolysaccharides: A human disease determinant? Infect. Immun. 2008, 76, 454–465. [Google Scholar] [CrossRef]
- Dziarski, R. Deadly plague versus mild-mannered TLR4. Nat. Immunol. 2006, 7, 1017–1019. [Google Scholar] [CrossRef]
- Miller, S.I.; Ernst, R.K.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Hurley, J.C. Sepsis management and anti-endotoxin therapy after nebacumab; A reappraisal. (leading article). Drugs 1994, 47, 855–861. [Google Scholar] [CrossRef]
- Hurley, J.C. Endotoxemia and novel therapies for the treatment of sepsis. Exp. Opin. Investig. Drugs 1995, 4, 163–174. [Google Scholar] [CrossRef]
- Riedemann, N.C.; Guo, R.F.; Ward, P.A. The enigma of sepsis. J. Clin. Investig. 2003, 112, 460–467. [Google Scholar]
- Carlet, J.; Cohen, J.; Calandra, T.; Opal, S.M.; Masur, H. Sepsis: Time to reconsider the concept. Crit. Care Med. 2008, 36, 964–966. [Google Scholar] [CrossRef]
- Vincent, J.L.; Martinez, E.O.; Silva, E. Evolving concepts in sepsis definitions. Crit. Care Clin. 2009, 25, 665–675. [Google Scholar] [CrossRef]
- Thomas, L. Germs. N. Engl. J. Med. 1972, 287, 553–555. [Google Scholar] [CrossRef]
- Horn, D.L.; Morrison, D.C.; Opal, S.M.; Silverstein, R.; Visvanathan, K.; Zabriskie, J.B. What are the microbial components implicated in the pathogenesis of sepsis? Report on a symposium. Clin. Infect. Dis. 2000, 31, 851–858. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar]
- Annane, D.; Aegerter, P.; Jars-Guincestre, M.C.; Guidet, B. Current epidemiology of septic shock: The CUB-rea network. Am. J. Respir. Crit. Care Med. 2003, 168, 165–172. [Google Scholar] [CrossRef]
- Brun-Buisson, C.; Doyon, F.; Carlet, J.; Dellamonica, P.; Gouin, F.; Lepoutre, A.; Mercier, J.C.; Offenstadt, G.; Regnier, B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA 1995, 274, 968–974. [Google Scholar] [CrossRef]
- Rangel-Frausto, M.S.; Pittet, D.; Costigan, M.; Hwang, T.; Davis, C.S.; Wenzel, R.P. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 1995, 273, 117–123. [Google Scholar] [CrossRef]
- Sands, K.E.; Bates, D.W.; Lanken, P.N.; Graman, P.S.; Hibberd, P.L.; Kahn, K.L.; Parsonnet, J.; Panzer, R.; Orav, E.J.; Snydman, D.R.; et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 1997, 278, 234–240. [Google Scholar] [CrossRef]
- Bates, D.W.; Lee, T.H. Projected impact of monoclonal anti-endotoxin antibody therapy. Arch. Intern. Med. 1994, 154, 1241–1249. [Google Scholar] [CrossRef]
- James, S.K.; Armstrong, P.; Barnathan, E.; Califf, R.; Lindahl, B.; Siegbahn, A.; Simoons, M.L.; Topol, E.J.; Venge, P.; Wallentin, L. GUSTO-IV-ACS Investigators. Troponin and C-reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome. GUSTO-IV sub-study. J. Am. Coll. Cardiol. 2003, 41, 916. [Google Scholar] [CrossRef]
- Hurley, J.C. Endotoxemia of concordance with gram-negative bacteremia: A meta—analysis using ROC curves. Arch. Pathol. Lab. Med. 2000, 124, 1157–1164. [Google Scholar]
- Hurley, J.C. Does gram-negative bacteremia occur without endotoxemia? A meta-analysis using hierarchical summary ROC curves. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 29, 207–215. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Kierulf, P.; Gaustad, P.; Skulberg, A.; Bruun, J.N.; Halvorsen, S.; Sorensen, E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 1989, 159, 195–204. [Google Scholar] [CrossRef]
- Brock-Utne, J.G.; Gaffin, S.L.; Wells, M.T.; Gathiram, P.; Sohar, E.; James, M.F.; Morrell, D.F.; Norman, R.J. Endotoxaemia in exhausted runners after a long-distance race. S. Afr. Med. J. 1988, 73, 533–536. [Google Scholar]
- Hurley, J.C. The detection of endotoxemia with gram-negative bacteremia is bacterial species dependent. A meta-analysis of clinical studies. J. Clin. Microbiol. 2009, 47, 3826–3831. [Google Scholar] [CrossRef]
- Hurley, J.C.; Guidet, B.; Offenstadt, G.; Maury, E. Endotoxemia and mortality prediction in ICU and other settings. underlying risk and co-detection of gram negative bacteremia are confounders. Crit. Care 2012, 16, R418. [Google Scholar] [CrossRef]
- Hurley, J.C.; Opal, S. Prognostic value of endotoxemia in patients with gram-negative bacteremia is bacterial species dependent: An individual patient data meta-analysis. J. Innate Immun. 2013, 5, 555–564. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Cheang, M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Kumar, A.; Zarychanski, R.; Light, B.; Parrillo, J.; Maki, D.; Simon, D.; Doucette, S. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: A propensity-matched analysis. Crit. Care Med. 2010, 38, 1773–1785. [Google Scholar] [CrossRef]
- Hurley, J.C. Reappraisal of the role of endotoxin in the sepsis syndrome. Lancet 1993, 341, 1133–1135. [Google Scholar] [CrossRef]
- Errington, J. L-form bacteria, cell walls and the origins of life. Open Biol. 2013, 3. [Google Scholar] [CrossRef]
- Briers, Y.; Staubli, T.; Schmid, M.C.; Wagner, M.; Schuppler, M.; Loessner, M.J. Intracellular vesicles as reproduction elements in cell wall-deficient L-form bacteria. PLoS One 2012, 7, e38514. [Google Scholar]
- Rittirsch, D.; Hoesel, L.M.; Ward, P.A. The disconnect between animal models of sepsis and human sepsis. J. Leukoc. Biol. 2007, 81, 137–143. [Google Scholar] [CrossRef]
- Deitch, E.A. Animal models of sepsis and shock: A review and lessons learned. Shock 1998, 9, 1–11. [Google Scholar] [CrossRef]
- Marshall, J.C. Sepsis: Rethinking the approach to clinical research. J. Leukoc. Biol. 2008, 83, 471–482. [Google Scholar] [CrossRef]
- Eichacker, P.Q.; Parent, C.; Kalil, A.; Esposito, C.; Cui, X.; Banks, S.M.; Gerstenberger, E.P.; Fitz, Y.; Danner, R.L.; Natanson, C. Risk and the efficacy of anti-inflammatory agents: Retrospective and confirmatory studies of sepsis. Am. J. Respir. Crit. Care Med. 2002, 166, 1197–1205. [Google Scholar] [CrossRef]
- Dyson, A.; Singer, M. Animal models of sepsis: Why does preclinical efficacy fail to translate to the clinical setting? Crit. Care Med. 2009, 37 (Suppl. 1), S30–S37. [Google Scholar]
- Riedemann, N.C.; Guo, R.F.; Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 2003, 9, 517–524. [Google Scholar] [CrossRef]
- Suffredini, A.F.; Munford, R.S. Novel therapies for septic shock over the past 4 decades. JAMA 2011, 306, 194–199. [Google Scholar]
- Warren, H.S.; Danner, R.L.; Munford, R.S. Anti-endotoxin monoclonal antibodies. N. Engl. J. Med. 1992, 326, 1153–1157. [Google Scholar] [CrossRef]
- Cross, A.S.; Opal, S.M.; Bhattacharjee, A.K.; Donta, S.T.; Peduzzi, P.N.; Furer, E.; Que, J.U.; Cryz, S.J. Immunotherapy of sepsis: Flawed concept or faulty implementation? Vaccine 1999, 17 (Suppl. 2), S13–S21. [Google Scholar] [CrossRef]
- Hurley, J.C. Meta analysis and investigation of anti—infective therapies. Exp. Opin. Investig. Drugs 1996, 6, 159–167. [Google Scholar] [CrossRef]
- Opal, S.M. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int. J. Med. Microbiol. 2007, 297, 365–377. [Google Scholar] [CrossRef]
- Greisman, S.E.; Johnston, C.A. Review: Evidence against the hypothesis that antibodies to the inner core of lipopolysaccharides in antisera raised by immunization with enterobacterial deep-rough mutants confer broad-spectrum protection during Gram-negative bacterial sepsis. J. Endotoxin Res. 1997, 4, 123–153. [Google Scholar]
- Chong, K.T.; Huston, M. Implications of endotoxin contamination in the evaluation of antibodies to lipopolysaccharides in a murine model of gram-negative sepsis. J. Infect. Dis. 1987, 156, 713–719. [Google Scholar]
- Wakelin, S.J.; Sabroe, I.; Gregory, C.D.; Poxton, I.R.; Forsythe, J.L.; Garden, O.J.; Howie, S.E. “Dirty little secrets”—endotoxin contamination of recombinant proteins. Immunol. Lett. 2006, 106, 1–7. [Google Scholar] [CrossRef]
- Warren, H.S.; Amato, S.F.; Fitting, C.; Black, K.M.; Loiselle, P.M.; Pasternack, M.S.; Cavaillon, J.M. Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J. Exp. Med. 1993, 177, 89–97. [Google Scholar] [CrossRef]
- Opal, S.M.; Patrozou, E. Translational research in the development of novel sepsis therapeutics. Logical deductive reasoning or mission impossible? Crit. Care Med. 2009, 37, S10–S15. [Google Scholar] [CrossRef]
- Lamontagne, F.; Briel, M.; Duffett, M.; Fox-Robichaud, A.; Cook, D.J.; Guyatt, G.; Lesur, O.; Meade, M.O. Systematic review of reviews including animal studies addressing therapeutic interventions for sepsis. Crit. Care Med. 2010, 38, 2401–2408. [Google Scholar] [CrossRef]
- McCabe, W.R.; Kreger, B.E.; Johns, M. Type-specific and cross-reactive antibodies in gram-negative bacteremia. N. Engl. J. Med. 1972, 287, 261–267. [Google Scholar] [CrossRef]
- Zinner, S.H.; McCabe, W.R. Effects of IgM and IgG antibody in patients with bacteremia due to gram-negative bacilli. J. Infect. Dis. 1976, 133, 37–45. [Google Scholar] [CrossRef]
- Pollack, M.; Huang, A.I.; Prescott, R.K.; Young, L.S.; Hunter, K.W.; Cruess, D.F.; Tsai, C.M. Enhanced survival in Pseudomonas aeruginosa septicemia associated with high levels of circulating antibody to Escherichia coli endotoxin core. J. Clin. Investig. 1983, 72, 1874–1881. [Google Scholar] [CrossRef]
- Baumgartner, J.D.; Glauser, M.P.; McCutchan, J.A.; Ziegler, E.J.; van Melle, G.; Klauber, M.R.; Vogt, M.; Muehlen, E.; Luethy, R.; Chiolero, R.; et al. Prevention of gram-negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet 1985, 2, 59–63. [Google Scholar]
- McCutchan, J.A.; Wolf, J.L.; Ziegler, E.J.; Braude, A.I. Ineffectiveness of single-dose human antiserum to core glycolipid (E. coli J5) for prophylaxis of bacteremic, gram-negative infections in patients with prolonged neutropenia. Schweiz. Med. Wochenschr. 1983, 14 (Suppl.), 40–45. [Google Scholar]
- The Intravenous Immunoglobulin Collaborative Study Group. Prophylactic intravenous administration of standard immune globulin as compared with core-lipopolysaccharide immune globulin in patients at high risk of postsurgical infection. N. Engl. J. Med. 1992, 327, 234–240. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Ayuso, L.; Hamilton-Davies, C.; White, W.D.; Barclay, G.R.; Smith, P.K.; King, S.A.; Muhlbaier, L.H.; Newman, M.F.; Mythen, M.G. Relationship of preoperative antiendotoxin core antibodies and adverse outcomes following cardiac surgery. JAMA 1997, 277, 646–650. [Google Scholar] [CrossRef]
- J5 Study Group. Treatment of severe infectious purpura in children with human plasma from donors immunized with Escherichia coli J5: A prospective double-blind study. J. Infect. Dis. 1992, 165, 695–701. [Google Scholar] [CrossRef]
- Derkx, B.; Wittes, J.; McCloskey, R. Randomized, placebo-controlled trial of HA-1A; a human monoclonal antibody to endotoxin, in children with meningococcal septic shock. European Pediatric Meningococcal Septic Shock Trial Study Group. Clin. Infect. Dis. 1999, 28, 770–777. [Google Scholar]
- Levin, M.; Quint, P.A.; Goldstein, B.; Barton, P.; Bradley, J.S.; Shemie, S.D.; Yeh, T.; Kim, S.S.; Cafaro, D.P.; Scannon, P.J.; et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: A randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet 2000, 356, 961–967. [Google Scholar] [CrossRef]
- Ziegler, E.J.; McCutchan, J.A.; Fierer, J.; Glauser, M.P.; Sadoff, J.C.; Douglas, H.; Braude, A.I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N. Engl. J. Med. 1982, 307, 1225–1230. [Google Scholar] [CrossRef]
- Calandra, T.; Glauser, M.P.; Schellekens, J.; Verhoef, J. Treatment of gram-negative septic shock with human IgG antibody to Escherichia coli J5: A prospective, double-blind, randomized trial. J. Infect. Dis. 1988, 158, 312–319. [Google Scholar] [CrossRef]
- Grundmann, R.; Hornung, M. Immunoglobulin therapy in patients with endotoxemia and postoperative sepsis—A prospective randomized study. Prog. Clin. Biol. Res. 1988, 272, 339–349. [Google Scholar]
- Schedel, I.; Dreikhausen, U.; Nentwig, B.; Hockenschnieder, M.; Rauthmann, D.; Balikcioglu, S.; Coldewey, R.; Deicher, H. Treatment of gram-negative septic shock with an immunoglobulin preparation: A prospective, randomized clinical trial. Crit. Care Med. 1991, 19, 1104–1113. [Google Scholar]
- Behre, G.; Schedel, I.; Nentwig, B.; Wormann, B.; Essink, M.; Hiddemann, W. Endotoxin concentration in neutropenic patients with suspected gram-negative sepsis: Correlation with clinical outcome and determination of anti-endotoxin core antibodies during therapy with polyclonal immunoglobulin M-enriched immunoglobulins. Antimicrob. Agents Chemother. 1992, 36, 2139–2146. [Google Scholar]
- Behre, G.; Ostermann, H.; Schedel, I. Endotoxin concentration and therapy with polyclonal immunoglobulin M-enriched immunoglobulins in neutropenic patients with sepsis syndrome. Pilot study and interim analysis of a randomized trial. Anti-infect. Drug Chemother. 1995, 13, 129–134. [Google Scholar]
- Ziegler, E.J.; Fisher, C.J., Jr.; Sprung, C.L.; Straube, R.C.; Sadoff, J.C.; Foulke, G.E.; Wortel, C.H.; Fink, M.P.; Dellinger, R.P.; Teng, N.N.; et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N. Engl. J. Med. 1991, 324, 429–436. [Google Scholar] [CrossRef]
- Greenman, R.L.; Schein, R.M.; Martin, M.A.; Wenzel, R.P.; MacIntyre, N.R.; Emmanuel, G.; Chmel, H.; Kohler, R.B.; McCarthy, M.; Plouffe, J.; et al. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. JAMA 1991, 266, 1097–1102. [Google Scholar] [CrossRef]
- Fisher, C.J., Jr.; Khazaeli, M.B.; Albertson, T.E.; Dellinger, R.P.; Panacek, E.A.; Foulke, G.E.; Dating, C.; Smith, C.R.; LoBuglio, A.F. Initial evaluation of human monoclonal anti-lipid A antibody (HA-1A) in patients with sepsis syndrome. Crit. Care Med. 1990, 18, 1311–1315. [Google Scholar] [CrossRef]
- The National Committee for the Evaluation of Centoxin. The French National Registry of HA-1A (Centoxin) in septic shock. A cohort study of 600 patients. Arch. Intern. Med. 1994, 154, 2484–2491. [Google Scholar] [CrossRef]
- McCloskey, R.V.; Straube, R.C.; Sanders, C.; Smith, S.M.; Smith, C.R. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. T Ann. Intern. Med. 1994, 121, 1–5. [Google Scholar] [CrossRef]
- Angus, D.C.; Birmingham, M.C.; Balk, R.A.; Scannon, P.J.; Collins, D.; Kruse, J.A.; Graham, D.R.; Dedhia, H.V.; Homann, S.; MacIntyre, N. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: A randomized controlled trial. E5 Study Investigators. JAMA 2000, 283, 1723–1730. [Google Scholar] [CrossRef]
- Daifuku, R.; Haenftling, K.; Young, J.; Groves, E.S.; Turrell, C.; Meyers, F.J. Phase I study of anti-lipopolysaccharide human monoclonal antibody MAB-T88. Antimicrob. Agents Chemother. 1992, 36, 2349–2351. [Google Scholar] [CrossRef]
- Greenberg, R.N.; Wilson, K.M.; Kunz, A.Y.; Wedel, N.I.; Gorelick, K.J. Randomized, double-blind phase II study of anti-endotoxin antibody (E5) as adjuvant therapy in humans with serious gram-negative infections. Prog. Clin. Biol. Res. 1991, 367, 179–186. [Google Scholar]
- Greenberg, R.N.; Wilson, K.M.; Kunz, A.Y.; Wedel, N.I.; Gorelick, K.J. Observations using antiendotoxin antibody (E5) as adjuvant therapy in humans with suspected, serious, gram-negative sepsis. Crit. Care Med. 1992, 20, 730–735. [Google Scholar] [CrossRef]
- Albertson, T.E.; Panacek, E.A.; MacArthur, R.D.; Johnson, S.B.; Benjamin, E.; Matuschak, G.M.; Zaloga, G.; Maki, D.; Silverstein, J.; Tobias, J.K.; et al. Multicenter evaluation of a human monoclonal antibody to Enterobacteriaceae common antigen in patients with Gram-negative sepsis. Crit. Care Med. 2003, 31, 419–427. [Google Scholar] [CrossRef]
- Willatts, S.M.; Radford, S.; Leitermann, M. Effect of the antiendotoxic agent, taurolidine, in the treatment of sepsis syndrome: A placebo-controlled, double-blind trial. Crit. Care Med. 1995, 23, 1033–1039. [Google Scholar] [CrossRef]
- Reinhart, K.; Meier-Hellmann, A.; Beale, R.; Forst, H.; Boehm, D.; Willatts, S.; Rothe, K.F.; Adolph, M.; Hoffmann, J.E.; Boehme, M.; et al. Open randomized phase II trial of an extracorporeal endotoxin adsorber in suspected Gram-negative sepsis. Crit. Care Med. 2004, 32, 1662–1668. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Grocott, H.P.; Levy, J.H.; Stierer, K.A.; Hogue, C.W.; Cheung, A.T.; Newman, M.F.; Carter, A.A.; Rossignol, D.P.; Collard, C.D. A phase II, double-blind, placebo-controlled, ascending-dose study of Eritoran (E5564), a lipid A antagonist, in patients undergoing cardiac surgery with cardiopulmonary bypass. Anesth. Analg. 2007, 104, 378–383. [Google Scholar] [CrossRef]
- Tidswell, M.; Tillis, W.; Larosa, S.P.; Lynn, M.; Wittek, A.E.; Kao, R.; Wheeler, J.; Gogate, J.; Opal, S.M. Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit. Care Med. 2010, 38, 72–83. [Google Scholar] [CrossRef]
- Opal, S.M.; Laterre, P.F.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.P.; Vincent, J.L. Effect of Eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis. The ACCESS Randomized Trial. JAMA 2013, 309, 1154–1162. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Tomayko, J.F.; Angus, D.C.; Opal, S.; Cupo, M.A.; McDermott, S.; Ducher, A.; Calandra, T.; Cohen, J. Efficacy and safety of a phospholipid emulsion (GR270773) in Gram-negative severe sepsis: Results of a phase II multicenter, randomized, placebo-controlled, dose-finding clinical trial. Crit. Care Med. 2009, 37, 2929–2938. [Google Scholar] [CrossRef]
- Heemskerk, S.; Masereeuw, R.; Moesker, O.; Bouw, M.P.; van der Hoeven, J.G.; Peters, W.H.; Russel, F.G.; Pickkers, P. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit. Care Med. 2009, 37, 417–423. [Google Scholar] [CrossRef]
- Pickkers, P.; Heemskerk, S.; Schouten, J.; Laterre, P.F.; Vincent, J.L.; Beishuizen, A.; Jorens, P.G.; Spapen, H.; Bulitta, M.; Peters, W.H.; et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: A prospective randomized double-blind placebo-controlled trial. Crit Care. 2012, 16, R14. [Google Scholar] [CrossRef]
- Berry, L.J. Cellular Biology of Endotoxin. In Handbook of Endotoxin; Berry, L.J., Ed.; Elsevier: New York, NY, USA, 1985; pp. xvii–xxi. [Google Scholar]
- Parker, S.J.; Watkins, P.E. Experimental models of gram-negative sepsis. Br. J. Surg. 2001, 88, 22–30. [Google Scholar] [CrossRef]
- Fink, M.P. Animal models of sepsis. Virulence 2014, 5, 1–11. [Google Scholar]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. PNAS 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Michie, H.R. The value of animal models in the development of new drugs for the treatment of the sepsis syndrome. J. Antimicrob. Chemother. 1998, 41 (Suppl. A), 47–49. [Google Scholar] [CrossRef]
- Cross, A.S.; Opal, S.M.; Sadoff, J.C.; Gemski, P. Choice of bacteria in animal models of sepsis. Infect. Immun. 1993, 61, 2741–2747. [Google Scholar]
- Hurley, J.C. Antibiotic action and Endotoxin. Ph.D. Thesis; University of Melbourne: Melbourne, Australia, March 1990. Available online: http://cat.lib.unimelb.edu.au/record=b1696516~S30 (accessed on 22 October 2013).
- Danner, R.L.; Natanson, C.; Elin, R.J.; Hosseini, J.M.; Banks, S.; MacVittie, T.J.; Parrillo, J.E. Pseudomonas aeruginosa compared with Escherichia coli produces less endotoxemia but more cardiovascular dysfunction and mortality in a canine model of septic shock. Chest 1990, 98, 1480–1487. [Google Scholar] [CrossRef]
- Hoffman, W.D.; Pollack, M.; Banks, S.M.; Koev, L.A.; Solomon, M.A.; Danner, R.L.; Koles, N.; Guelde, G.; Yatsiv, I.; Mouginis, T.; et al. Distinct functional activities in canine septic shock of monoclonal antibodies specific for the O polysaccharide and core regions of Escherichia coli lipopolysaccharide. J. Infect. Dis. 1994, 169, 553–561. [Google Scholar] [CrossRef]
- Natanson, C.; Danner, R.L.; Reilly, J.M.; Doerfler, M.L.; Hoffman, W.D.; Akin, G.L.; Hosseini, J.M.; Banks, S.M.; Elin, R.J.; MacVittie, T.J.; et al. Antibiotics versus cardiovascular support in a canine model of human septic shock. Am. J. Physiol. 1990, 259, H1440–H1447. [Google Scholar]
- Solomon, S.B.; Cui, X.; Gerstenberger, E.; Danner, R.L.; Fitz, Y.; Banks, S.M.; Natanson, C.; Eichacker, P.Q. Effective dosing of lipid A analogue E5564 in rats depends on the timing of treatment and the route of Escherichia coli infection. J. Infect. Dis. 2006, 193, 634–644. [Google Scholar] [CrossRef]
- Quezado, Z.M.; Natanson, C.; Alling, D.W.; Banks, S.M.; Koev, C.A.; Elin, R.J.; Hosseini, J.M.; Bacher, J.D.; Danner, R.L.; Hoffman, W.D. A controlled trial of HA-1A in a canine model of gram-negative septic shock. JAMA 1993, 269, 2221–2227. [Google Scholar] [CrossRef]
- Eichacker, P.Q.; Hoffman, W.D.; Farese, A.; Danner, R.L.; Suffredini, A.F.; Waisman, Y.; Banks, S.M.; Mouginis, T.; Wilson, L.; Rothlein, R.; et al. Leukocyte CD18 monoclonal antibody worsens endotoxemia and cardiovascular injury in canines with septic shock. J. Appl. Physiol. 1993, 74, 1885–1892. [Google Scholar] [CrossRef]
- Hoffman, W.D.; Danner, R.L.; Quezado, Z.M.; Banks, S.M.; Elin, R.J.; Hosseini, J.M.; Natanson, C. Role of endotoxemia in cardiovascular dysfunction and lethality. Virulent and nonvirulent Escherichia coli challenges in a canine model of septic shock. Infect. Immun. 1996, 64, 406–412. [Google Scholar]
- Natanson, C.; Danner, R.L.; Elin, R.J.; Hosseini, J.M.; Peart, K.W.; Banks, S.M.; MacVittie, T.J.; Walker, R.I.; Parrillo, J.E. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J. Clin. Investig. 1989, 83, 243–251. [Google Scholar] [CrossRef]
- Natanson, C.; Fink, M.P.; Ballantyne, H.K.; MacVittie, T.J.; Conklin, J.J.; Parrillo, J.E. Gram-negative bacteremia produces both severe systolic and diastolic cardiac dysfunction in a canine model that simulates human septic shock. J. Clin. Investig. 1986, 78, 259–270. [Google Scholar] [CrossRef]
- Natanson, C.; Hoffman, W.D.; Koev, L.A.; Dolan, D.P.; Banks, S.M.; Bacher, J.; Danner, R.L.; Klein, H.G.; Parrillo, J.E. Plasma exchange does not improve survival in a canine model of human septic shock. Transfusion 1993, 33, 243–248. [Google Scholar]
- Quezado, Z.M.; Natanson, C.; Banks, S.M.; Alling, D.W.; Koev, C.A.; Danner, R.L.; Elin, R.J.; Hosseini, J.M.; Parker, T.S.; Levine, D.M.; et al. Therapeutic trial of reconstituted human high-density lipoprotein in a canine model of gram-negative septic shock. J. Pharmacol. Exp. Ther. 1995, 272, 604–611. [Google Scholar]
- Quezado, Z.M.; Hoffman, W.D.; Winkelstein, J.A.; Yatsiv, I.; Koev, C.A.; Cork, L.C.; Elin, R.J.; Eichacker, P.Q.; Natanson, C. The third component of complement protects against Escherichia coli endotoxin-induced shock and multiple organ failure. J. Exp. Med. 1994, 179, 569–578. [Google Scholar] [CrossRef]
- Freeman, B.D.; Quezado, Z.; Zeni, F.; Natanson, C.; Danner, R.L.; Banks, S.; Quezado, M.; Fitz, Y.; Bacher, J.; Eichacker, P.Q. rG-CSF reduces endotoxemia and improves survival during E. coli pneumonia. J. Appl. Physiol. 1997, 83, 1467–1475. [Google Scholar]
- Hurley, J.C. Endotoxin: Methods of detection and clinical correlates. Clin. Microbiol. Rev. 1995, 8, 268–292. [Google Scholar]
- Rogy, M.A.; Moldawer, L.L.; Oldenburg, H.S.; Thompson, W.A.; Montegut, W.J.; Stackpole, S.A.; Kumar, A.; Palladino, M.A.; Marra, M.N.; Lowry, S.F. Anti-endotoxin therapy in primate bacteremia with HA-1A and BPI. Ann. Surg. 1994, 220, 77–85. [Google Scholar] [CrossRef]
- Barclay, G.R. Endotoxin-core antibodies: Time for a reappraisal? Intensiv. Care Med. 1999, 25, 427–429. [Google Scholar] [CrossRef]
- Cometta, A.; Baumgartner, J.D.; Glauser, M.P. Polyclonal intravenous immune globulin for prevention and treatment of infections in critically ill patients. Clin. Exp. Immunol. 1994, 97 (Suppl. 1), 69–72. [Google Scholar]
- Turgeon, A.F.; Hutton, B.; Fergusson, D.A.; McIntyre, L.; Tinmouth, A.A.; Cameron, D.W.; Hebert, P.C. Meta-analysis: Intravenous immunoglobulin in critically ill adult patients with sepsis. Ann. Intern. Med. 2007, 146, 193–203. [Google Scholar] [CrossRef]
- Werdan, K.; Pilz, G.; Bujdoso, O.; Fraunberger, P.; Neeser, G.; Schmieder, R.E.; Viell, B.; Marget, W.; Seewald, M.; Walger, P.; et al. Score-based immunoglobulin G therapy of patients with sepsis: The SBITS study. Crit. Care Med. 2007, 35, 2693–2701. [Google Scholar] [CrossRef]
- Jackson, S.K.; Parton, J.; Barnes, R.A.; Poynton, C.H.; Fegan, C. Effect of IgM-enriched intravenous immunoglobulin (Pentaglobin) on endotoxaemia and anti-endotoxin antibodies in bone marrow transplantation. Eur. J. Clin. Investig. 1993, 23, 540–545. [Google Scholar] [CrossRef]
- Munster, A.M.; Moran, K.T.; Thupari, J.; Allo, M.; Winchurch, R.A. Prophylactic intravenous immunoglobulin replacement in high-risk burn patients. J. Burn Care Rehabil. 1987, 8, 376–380. [Google Scholar] [CrossRef]
- Poynton, C.H.; Jackson, S.; Fegan, C.; Barnes, R.A.; Whittaker, J.A. Use of IgM enriched intravenous immunoglobulin (Pentaglobin) in bone marrow transplantation. Bone Marrow Transplant. 1992, 9, 451–457. [Google Scholar]
- Flynn, P.M.; Shenep, J.L.; Stokes, D.C.; Fairclough, D.; Hildner, W.K. Polymyxin B moderates acidosis and hypotension in established, experimental gram-negative septicemia. J. Infect. Dis. 1987, 156, 706–712. [Google Scholar] [CrossRef]
- Ovstebo, R.; Brandtzaeg, P.; Brusletto, B.; Haug, K.B.; Lande, K.; Hoiby, E.A.; Kierulf, P. Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J. Clin. Microbiol. 2004, 42, 2980–2987. [Google Scholar] [CrossRef]
- Wortel, C.H.; von der Mohlen, M.A.; van Deventer, S.J.; Sprung, C.L.; Jastremski, M.; Lubbers, M.J.; Smith, C.R.; Allen, I.E.; ten Cate, J.W. Effectiveness of a human monoclonal anti-endotoxin antibody (HA-1A) in gram-negative sepsis: Relationship to endotoxin and cytokine levels. J. Infect. Dis. 1992, 166, 1367–1374. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Haeffner-Cavaillon, N. Polymyxin-B inhibition of LPS-induced interleukin-1 secretion by human monocytes is dependent upon the LPS origin. Mol. Immunol. 1986, 23, 965–969. [Google Scholar] [CrossRef]
- Davies, B.; Cohen, J. Endotoxin removal devices for the treatment of sepsis and septic shock. Lancet Infect. Dis. 2011, 11, 65–71. [Google Scholar] [CrossRef]
- Opal, S.M. Hemofiltration-absorption systems for the treatment of experimental sepsis: Is it possible to remove the “evil humors” responsible for septic shock? Crit. Care Med. 2000, 28, 1681–1682. [Google Scholar] [CrossRef]
- Cruz, D.N.; Bellomo, R.; Ronco, C. Clinical effects of polymyxin B-immobilized fiber column in septic patients. Contrib. Nephrol. 2007, 156, 444–451. [Google Scholar] [CrossRef]
- Cruz, D.N.; Perazella, M.A.; Bellomo, R.; de Cal, M.; Polanco, N.; Corradi, V.; Lentini, P.; Nalesso, F.; Ueno, T.; Ranieri, V.M.; et al. Effectiveness of polymyxin B-immobilized fiber column in sepsis: A systematic review. Crit. Care 2007, 11, R47. [Google Scholar] [CrossRef]
- Aoki, H.; Kodama, M.; Tani, T.; Hanasawa, K. Treatment of sepsis by extracorporeal elimination of endotoxin using polymyxin B-immobilized fiber. Am. J. Surg. 1994, 167, 412–417. [Google Scholar] [CrossRef]
- Buttenschoen, K.; Radermacher, P.; Bracht, H. Endotoxin elimination in sepsis: Physiology and therapeutic application. Langenbecks Arch. Surg. 2010, 395, 597–605. [Google Scholar] [CrossRef]
- Vincent, J.L.; Laterre, P.F.; Cohen, J.; Burchardi, H.; Bruining, H.; Lerma, F.A.; Wittebole, X.; de Backer, D.; Brett, S.; Marzo, D.; et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock 2005, 23, 400–405. [Google Scholar] [CrossRef]
- Cruz, D.N.; Antonelli, M.; Fumagalli, R.; Foltran, F.; Brienza, N.; Donati, A.; Malcangi, V.; Petrini, F.; Volta, G.; Bobbio Pallavicini, F.M.; et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 2009, 301, 2445–2452. [Google Scholar] [CrossRef]
- Novelli, G.; Ferretti, G.; Poli, L.; Pretagostini, R.; Ruberto, F.; Perrella, S.M.; Levi Sandri, G.B.; Morabito, V.; Berloco, P.B. Clinical results of treatment of postsurgical endotoxin-mediated sepsis with polymyxin-B direct hemoperfusion. Transplant. Proc. 2010, 42, 1021–1024. [Google Scholar] [CrossRef]
- Novelli, G.; Ferretti, G.; Ruberto, F.; Morabito, V.; Pugliese, F. Early management of endotoxemia using the endotoxin activity assay and polymyxin B-based hemoperfusion. Contrib. Nephrol. 2010, 167, 91–101. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Polymyxin B for endotoxin removal in sepsis. Lancet Infect. Dis. 2011, 11, 426–427. [Google Scholar] [CrossRef]
- Amaral, A.C. Polymyxin B hemoperfusion and mortality in abdominal septic shock. JAMA 2009, 302, 1968–1969. [Google Scholar] [CrossRef]
- Vincent, J.-L. Polymyxin B hemoperfusion and mortality in abdominal septic shock. JAMA 2009, 302, 1969–1970. [Google Scholar]
- Ullrich, H.; Jakob, W.; Frohlich, D.; Rothe, G.; Prasser, C.; Drobnik, W.; Taeger, K.; Meier-Hellmann, A.; Reinhart, K.; Zimmermann, M.; et al. A new endotoxin adsorber: First clinical application. Ther. Apher. 2001, 5, 326–334. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hurley, J.C. Towards Clinical Applications of Anti-endotoxin Antibodies; A Re-appraisal of the Disconnect. Toxins 2013, 5, 2589-2620. https://doi.org/10.3390/toxins5122589
Hurley JC. Towards Clinical Applications of Anti-endotoxin Antibodies; A Re-appraisal of the Disconnect. Toxins. 2013; 5(12):2589-2620. https://doi.org/10.3390/toxins5122589
Chicago/Turabian StyleHurley, James C. 2013. "Towards Clinical Applications of Anti-endotoxin Antibodies; A Re-appraisal of the Disconnect" Toxins 5, no. 12: 2589-2620. https://doi.org/10.3390/toxins5122589
APA StyleHurley, J. C. (2013). Towards Clinical Applications of Anti-endotoxin Antibodies; A Re-appraisal of the Disconnect. Toxins, 5(12), 2589-2620. https://doi.org/10.3390/toxins5122589





