Sorption of Ochratoxin A from Aqueous Solutions Using β-Cyclodextrin-Polyurethane Polymer
Abstract
:1. Introduction

2. Materials and Methods
2.1. Chemicals
2.2. Polymer Synthesis
2.3. Surface Analysis and Scanning Electron Microscopy
2.4. Batch Rebinding Assays
2.5. LC-Analysis
2.6. Ochratoxin A Determination in Red Wine
3. Results and Discussion


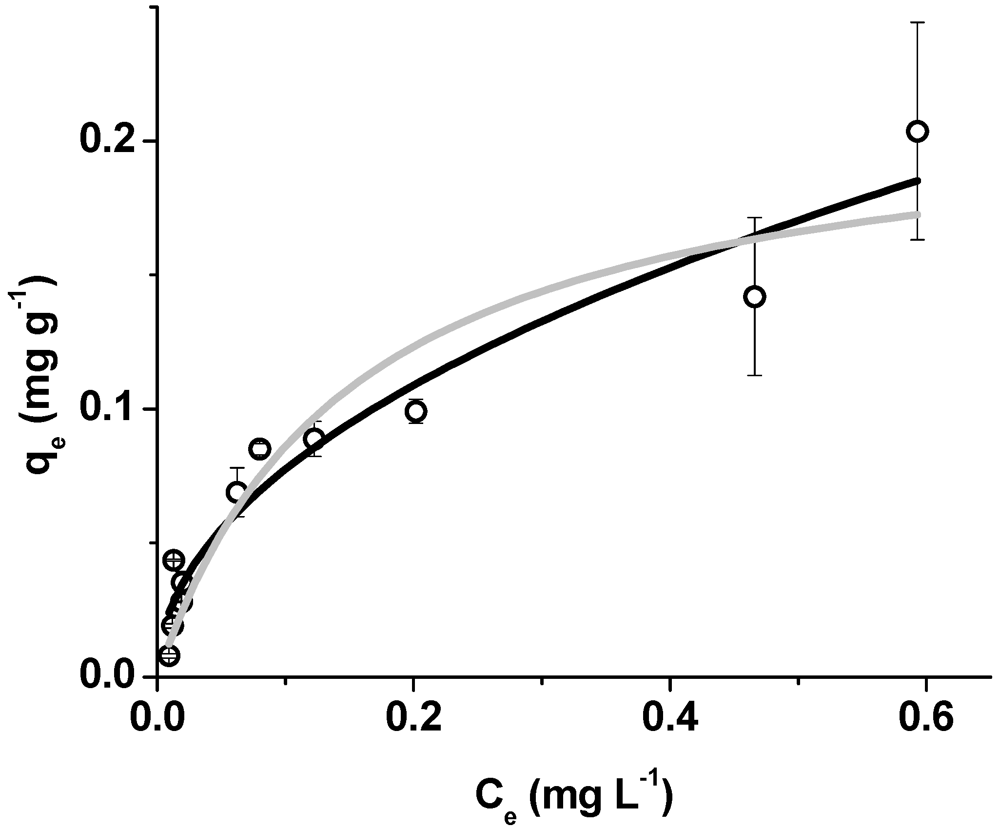
| Model | R2 | ||
|---|---|---|---|
| Langmuir | KL (L·mg−1) | Q0 (mg·g−1) | |
| 6.60 ± 2.24 | 0.22 ± 0.03 | 0.909 | |
| Freundlich | KF (mg·g−1)(L·mg−1)1/n | 1/n | |
| 0.24 ± 0.02 | 0.49 ± 0.05 | 0.946 | |
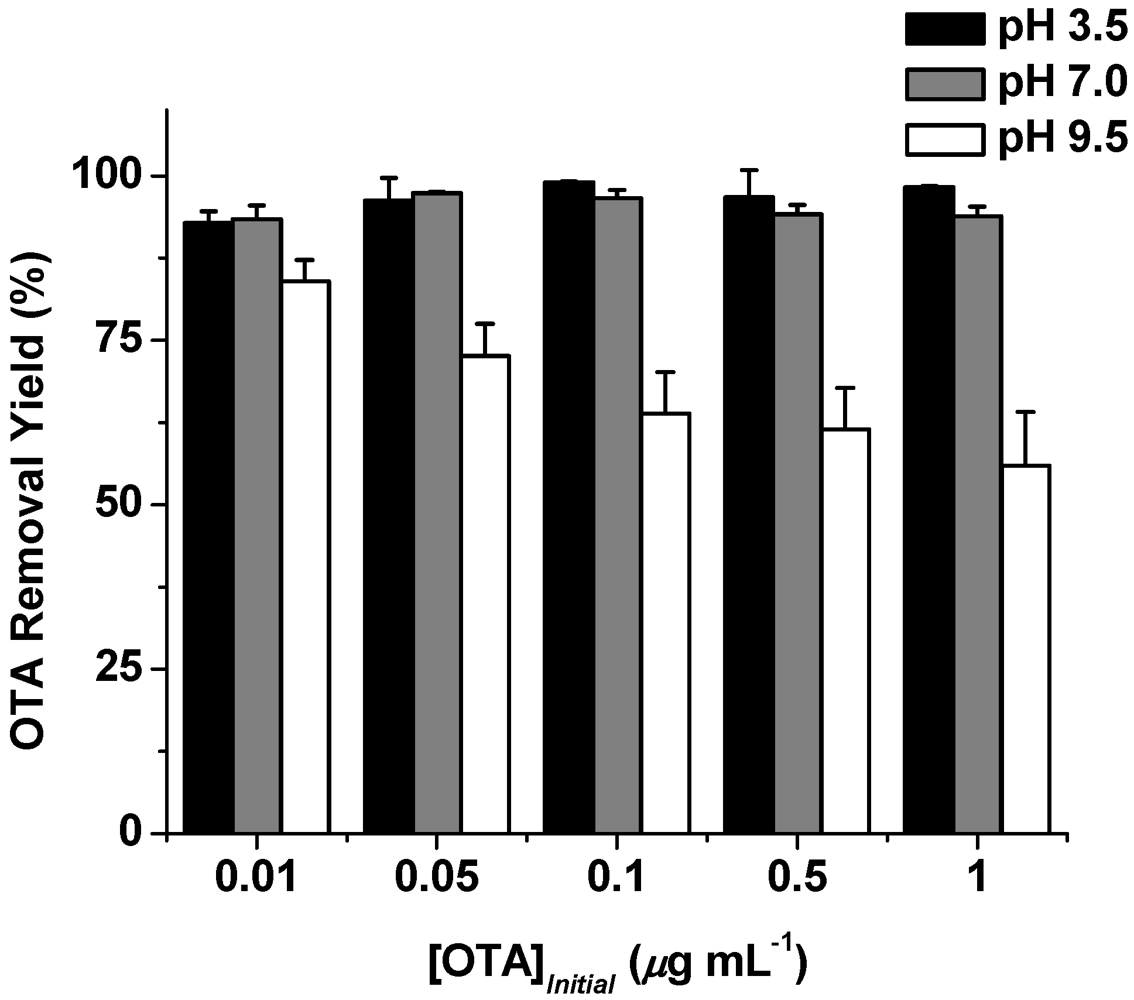
| [OTA] Initial (μg L−1) | OTABound b (μg) | % OTABound |
|---|---|---|
| 10 | 8.82 ± 0.23 | 88 |
| 7.5 | 7.11 ± 0.11 | 95 |
| 5 | 4.62 ± 0.20 | 92 |
| 2.5 | 2.33 ± 0.12 | 93 |
| 1 | ± 0.14 | 61 |
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Duarte, S.C.; Lino, C.M.; Pena, A. Mycotoxin food and feed regulation and the specific case of ochratoxin A: A review of the worldwide status. Food Addit. Contam. 2010, 27, 1440–1450. [Google Scholar]
- El Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar]
- Tozlovanu, M.; Pfohl-Leszkowicz, A. Ochratoxin A in roasted coffee from french supermarkets and transfer in coffee beverages: Comparison of analysis methods. Toxins 2010, 2, 1928–1942. [Google Scholar]
- Varga, J.; Kocsubé, S.; Péteri, Z.; Vágvölgyi, C.; Tóth, B. Chemical, physical and biological approaches to prevent ochratoxin induced toxicoses in humans and animals. Toxins 2010, 2, 1718–1750. [Google Scholar]
- Abrunhosa, L.; Paterson, R.; Venâncio, A. Biodegradation of ochratoxin A for food and feed decontamination. Toxins 2010, 2, 1078–1099. [Google Scholar]
- Amézqueta, S.; González-Peñas, E.; Murillo-Arbizu, M.; López de Cerain, A. Ochratoxin A decontamination: A review. Food Control 2009, 20, 326–333. [Google Scholar]
- Espejo, F.; Armada, S. Effect of activated carbon on ochratoxin A reduction in “Pedro Ximenez” sweet wine made from off-vine dried grapes. Eur. Food Res. Technol. 2009, 229, 255–262. [Google Scholar]
- Solfrizzo, M.; Avantaggiato, G.; Panzarini, G.; Visconti, A. Removal of ochratoxin A from contaminated red wines by repassage over grape pomaces. J. Agric. Food Chem. 2009, 58, 317–323. [Google Scholar]
- Yiannikouris, A.; André, G.; Poughon, L.; François, J.; Dussap, C.-G.; Jeminet, G.; Bertin, G.; Jouany, J.-P. Chemical and conformational study of the interactions involved in mycotoxin complexation with β-D-glucans. Biomacromolecules 2006, 7, 1147–1155. [Google Scholar]
- Bazin, I.; Nabais, E.; Lopez-Ferber, M. Rapid visual tests: Fast and reliable detection of ochratoxin A. Toxins 2010, 2, 2230–2241. [Google Scholar]
- De Girolamo, A.; McKeague, M.; Miller, J.D.; DeRosa, M.C.; Visconti, A. Determination of ochratoxin A in wheat after clean-up through a DNA aptamer-based solid phase extraction column. Food Chem. 2011, 127, 1378–1384. [Google Scholar]
- Yu, J.C.C.; Lai, E.P.C. Molecularly imprinted polymers for ochratoxin A extraction and analysis. Toxins 2010, 2, 1536–1553. [Google Scholar]
- Appell, M.; Jackson, M. Synthesis and evaluation of cyclodextrin-based polymers for patulin extraction from aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 117–122. [Google Scholar]
- Mele, A.; Castiglione, F.; Malpezzi, L.; Ganazzoli, F.; Raffaini, G.; Trotta, F.; Rossi, B.; Fontana, A.; Giunchi, G. HR MAS NMR, powder XRD and Raman spectroscopy study of inclusion phenomena in βCD nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 403–409. [Google Scholar]
- Mhlanga, S.D.; Mamba, B.B.; Krause, R.W.; Malefetse, T.J. Removal of organic contaminants from water using nanosponge cyclodextrin polyurethanes. J. Chem. Technol. Biotechnol. 2007, 82, 382–388. [Google Scholar]
- Xiao, P.; Dudal, Y.; Corvini, P.F.X.; Pieles, U.; Shahgaldian, P. Cyclodextrin-based polyurethanes act as selective molecular recognition materials of active pharmaceutical ingredients (APIs). Polym. Chem. 2011, 2, 1264–1266. [Google Scholar]
- Wilson, L.D.; Mohamed, M.H.; Headley, J.V. Surface area and pore structure properties of urethane-based copolymers containing [beta]-cyclodextrin. J. Colloid Interface Sci. 2011, 357, 215–222. [Google Scholar]
- Amadasi, A.; Dall’Asta, C.; Ingletto, G.; Pela, R.; Marchelli, R.; Cozzini, P. Explaining cyclodextrin-mycotoxin interactions using a “natural” force field. Bioorg. Med. Chem. 2007, 15, 4585–4594. [Google Scholar]
- Böhs, B.; Seidel, V.; Lindner, W. Analysis of selected mycotoxins by capillary electrophoresis. Chromatographia 1995, 41, 631–637. [Google Scholar]
- Cozzini, P.; Ingletto, G.; Singh, R.; Dall’Asta, C. Mycotoxin detection plays “cops and robbers”: Cyclodextrin chemosensors as specialized police? Int. J. Mol. Sci. 2008, 9, 2474–2494. [Google Scholar] [CrossRef]
- Hashemi, J.; Alizadeh, N. Investigation of solvent effect and cyclodextrins on fluorescence properties of ochratoxin A. Spectrochim. Acta Part A 2009, 73, 121–126. [Google Scholar]
- Seidel, V.; Poglits, E.; Schiller, K.; Lindner, W. Simultaneous determination of ochratoxin A and zearalenone in maize by reversed-phase high-performance liquid chromatography with fluorescence detectio. J. Chromatogr. A 1993, 635, 227–235. [Google Scholar]
- Verrone, R.; Catucci, L.; Cosma, P.; Fini, P.; Agostiano, A.; Lippolis, V.; Pascale, M. Effect of β-cyclodextrin on spectroscopic properties of ochratoxin A in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 475–479. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar]
- Tseng, R.-L.; Wu, F.-C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant. J. Hazard. Mater. 2008, 155, 277–287. [Google Scholar]
- Hernández, M.J.; García-Moreno, M.V.; Durán, E.; Guillén, D.; Barroso, C.G. Validation of two analytical methods for the determination of ochratoxin A by reversed-phased high-performance liquid chromatography coupled to fluorescence detection in musts and sweet wines from Andalusia. Anal. Chim. Acta 2006, 566, 117–121. [Google Scholar]
- Chin, Y.P.; Mohamad, S.; Abas, M.R.B. Removal of parabens from aqueous solution using β-cyclodextrin cross-linked polymer. Int. J. Mol. Sci. 2010, 11, 3459–3471. [Google Scholar]
- Yamasaki, H.; Makihata, Y.; Fukunaga, K. Efficient phenol removal of wastewater from phenolic resin plants using crosslinked cyclodextrin particles. J. Chem. Technol. Biotechnol. 2006, 81, 1271–1276. [Google Scholar]
- Salipira, K.L.; Krause, R.W.; Mamba, B.B.; Malefetse, T.J.; Cele, L.M.; Durbach, S.H. Cyclodextrin polyurethanes polymerized with multi-walled carbon nanotubes: Synthesis and characterization. Mater. Chem. Phys. 2008, 111, 218–224. [Google Scholar]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar]
- Fan, Y.; Feng, Y.-Q.; Da, S.-L.; Feng, P.-Y. Evaluation of beta-cyclodextrin bonded silica as a selective sorbent for the solid-phase extraction of 4-nitrophenol and 2,4-dinitrophenol. Anal. Sci. 2003, 19, 709–714. [Google Scholar]
- Putra, E.K.; Pranowo, R.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009, 43, 2419–2430. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Appell, M.; Jackson, M.A. Sorption of Ochratoxin A from Aqueous Solutions Using β-Cyclodextrin-Polyurethane Polymer. Toxins 2012, 4, 98-109. https://doi.org/10.3390/toxins4020098
Appell M, Jackson MA. Sorption of Ochratoxin A from Aqueous Solutions Using β-Cyclodextrin-Polyurethane Polymer. Toxins. 2012; 4(2):98-109. https://doi.org/10.3390/toxins4020098
Chicago/Turabian StyleAppell, Michael, and Michael A. Jackson. 2012. "Sorption of Ochratoxin A from Aqueous Solutions Using β-Cyclodextrin-Polyurethane Polymer" Toxins 4, no. 2: 98-109. https://doi.org/10.3390/toxins4020098
APA StyleAppell, M., & Jackson, M. A. (2012). Sorption of Ochratoxin A from Aqueous Solutions Using β-Cyclodextrin-Polyurethane Polymer. Toxins, 4(2), 98-109. https://doi.org/10.3390/toxins4020098






