Bioorganic Chemistry, Toxinology, and Pharmaceutical Uses of Datura Metabolites and Derivatives
Abstract
1. Introduction
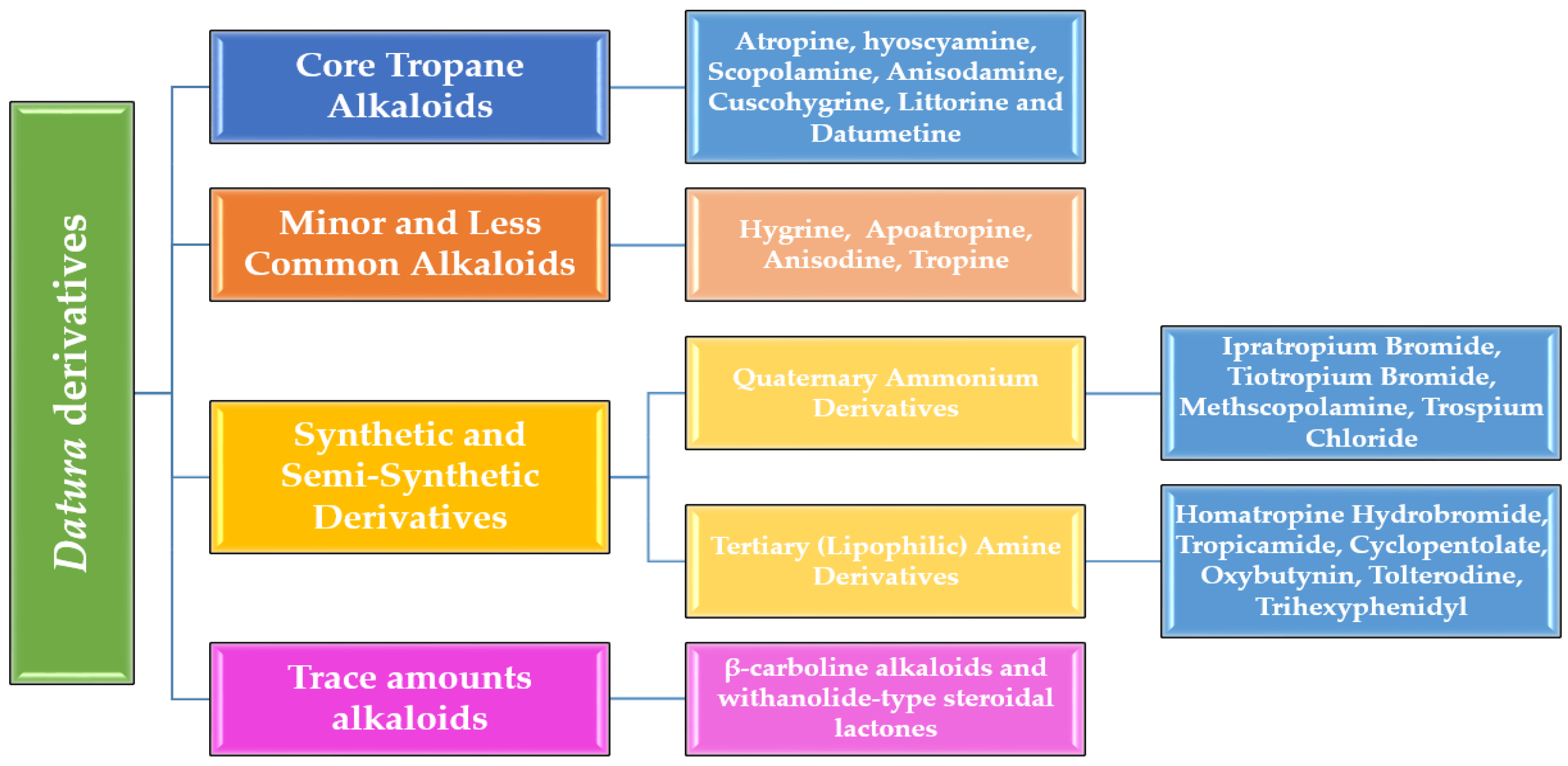
2. Classification of Alkaloids and Derivatives from Datura Species
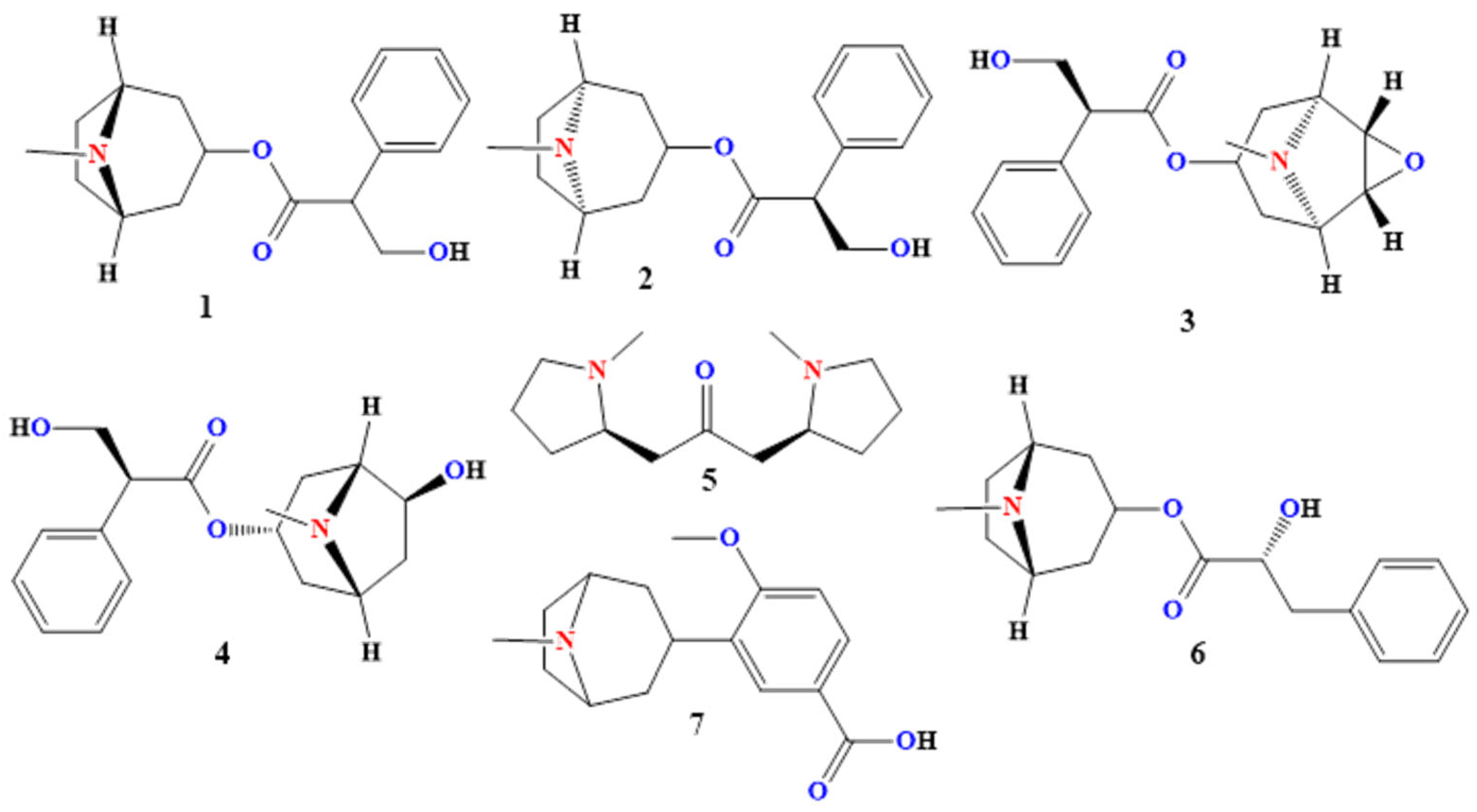
2.1. Core Tropane Alkaloids in Datura
2.1.1. Atropine
- Chemical Structure and Stereochemistry
- Mechanism of Action at the Molecular Level
- Toxinology of Atropine
- Pharmaceutical Uses of Atropine
2.1.2. Hyoscyamine (Also Called “Daturine” or “Levo-Atropine”)
- Bioorganic Chemistry of Hyoscyamine
- Toxinology of Hyoscyamine
- Pharmaceutical Uses of Hyoscyamine
2.1.3. Scopolamine (Hyoscine)
- Toxinology of Scopolamine
- Pharmaceutical Uses of Scopolamine
2.1.4. Anisodamine (7β-Hydroxyhyoscyamine)
- Toxinology of Anisodamine
- Pharmaceutical Uses of Anisodamine
2.1.5. Cuscohygrine
- Toxinology of Cuscohygrine
- Pharmaceutical and Analytical Applications
2.1.6. Littorine
- Toxinology of Littorine
- Pharmaceutical and Analytical Applications
2.1.7. Datumetine
- Toxinology of Datumetine
- Pharmaceutical Uses of Datumetine
2.2. Minor and Less Common Alkaloids
2.2.1. Hygrine
- Toxinology of Hygrine
- Pharmaceutical and Research Applications of Hygrine
2.2.2. Apoatropine
- Toxinology of Apoatropine
- Pharmaceutical and Research Applications
2.2.3. Anisodine
- Toxinology of Anisodine
- Pharmaceutical Uses of Anisodine
2.2.4. Tropine
- Toxinology of Tropine
- Pharmaceutical and Synthetic Applications of Tropine
- The classic synthesis of atropine begins with tropine, which is esterified at C-3 with tropic acid (3-hydroxy-2-phenylpropanoic acid). In practice, tropic acid is first converted to its acid chloride (SOCl2, catalytic DMF, 0 °C), then coupled to tropine in dry dichloromethane with pyridine as the base to give racemic atropine in 70–85% yield. Resolution of the racemate into (–)-hyoscyamine and its enantiomer is accomplished by formation of diastereomeric salts—typically with (–)-tartaric acid in ethanol—followed by fractional crystallization. Filtration and basification liberate enantiopure (S)-hyoscyamine, which exhibits ≈approximately 50–100 times greater affinity for muscarinic receptors than its (R) counterpart [41,117,118].
- Scopolamine can be generated from hyoscyamine via selective epoxidation of the C-6, C-7 bond. Chemically, this is achieved by treating the hyoscyamine free base with a peracid (e.g., m-CPBA) in chloroform at 0 °C, yielding the 6β,7β-epoxide in 50–70% yield after silica gel chromatography. Alternatively, biosynthetic conversion employs hyoscyamine 6β-hydroxylase (H6H) in recombinant microbial or plant-cell systems, where NADPH and O2 drive sequential hydroxylation and intramolecular epoxide closure, routinely achieving over 90% conversion under optimized fermentation conditions. Scopolamine’s rigid epoxide ring enhances central nervous system penetration and receptor affinity, underpinning its superior antiemetic potency [38,119].
- Quaternization of tropine’s bridgehead nitrogen provides a route to inhaled anticholinergics with minimal systemic exposure. For ipratropium bromide, tropine is stirred with excess isopropyl bromide in dry acetone at reflux for 12–24 h, yielding the isopropyl quaternary ammonium salt in an isolated yield of 60–75%. Recrystallization from ethanol affords the pharmaceutically standardized monohydrate. Tiotropium bromide is prepared analogously by SN2 alkylation with 2-thienylmethyl chloride, followed by the introduction of two thienyl rings via subsequent alkylation steps, and finally, counterion exchange to the bromide salt—overall yields for the multi-step sequence range from 45% to 55%. The permanent positive charge of these agents prevents blood–brain barrier crossing, thereby focusing their M3-selective antagonism on bronchial smooth muscle for the treatment of COPD and asthma [120,121].
- Tropine metabolites serve as lead scaffolds in medicinal chemistry campaigns targeting central and peripheral nervous system disorders. By modifying the C-3 hydroxyl group (e.g., carbamate linkages, ether conjugates), researchers have generated novel compounds with tailored muscarinic subtype selectivity and pharmacokinetic profiles, underscoring tropine’s versatility as a synthetic and pharmacophoric template [43,122,123].
2.3. Synthetic and Semi-Synthetic Derivatives
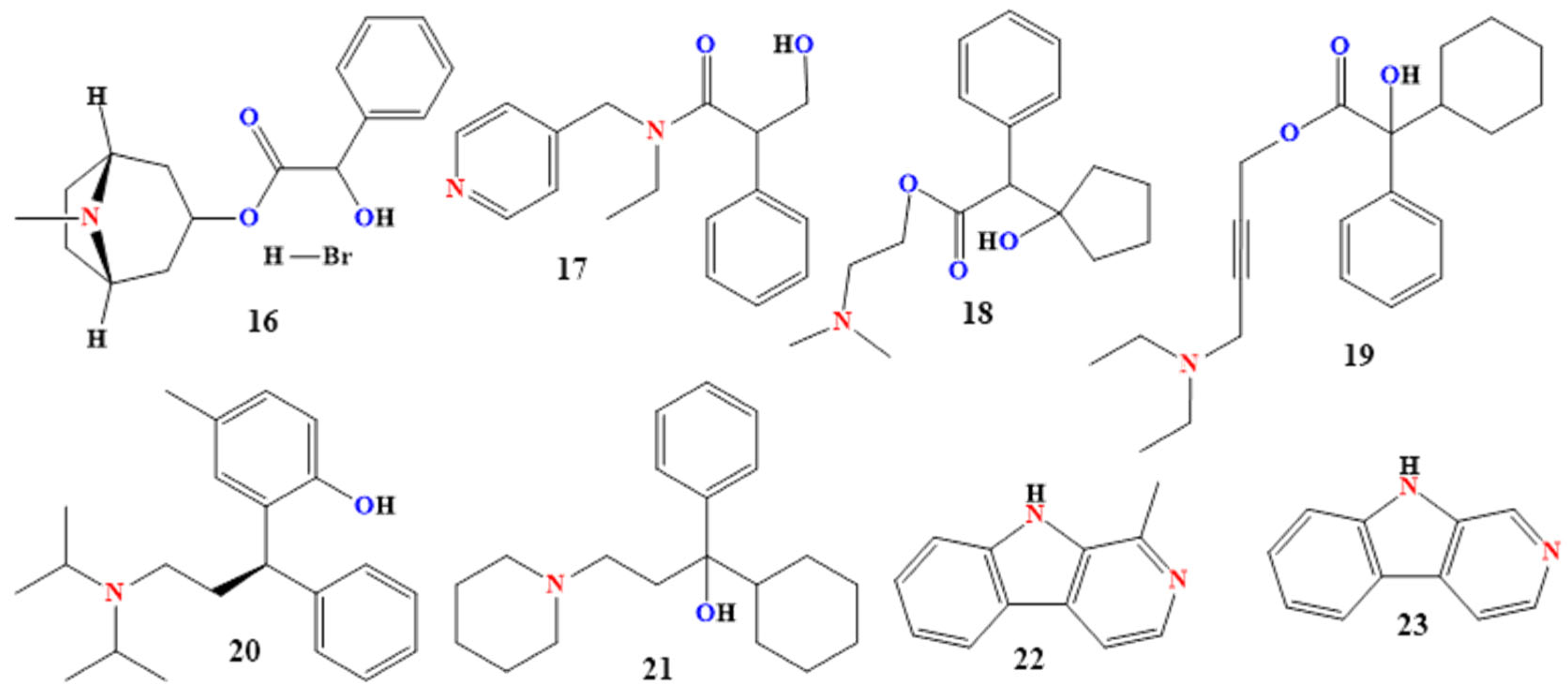
2.3.1. Quaternary Ammonium Derivatives
2.3.2. Tertiary (Lipophilic) Amine Derivatives
2.4. Other Alkaloid Classes and Their Datura Metabolites
2.4.1. Harmane and Norharmane
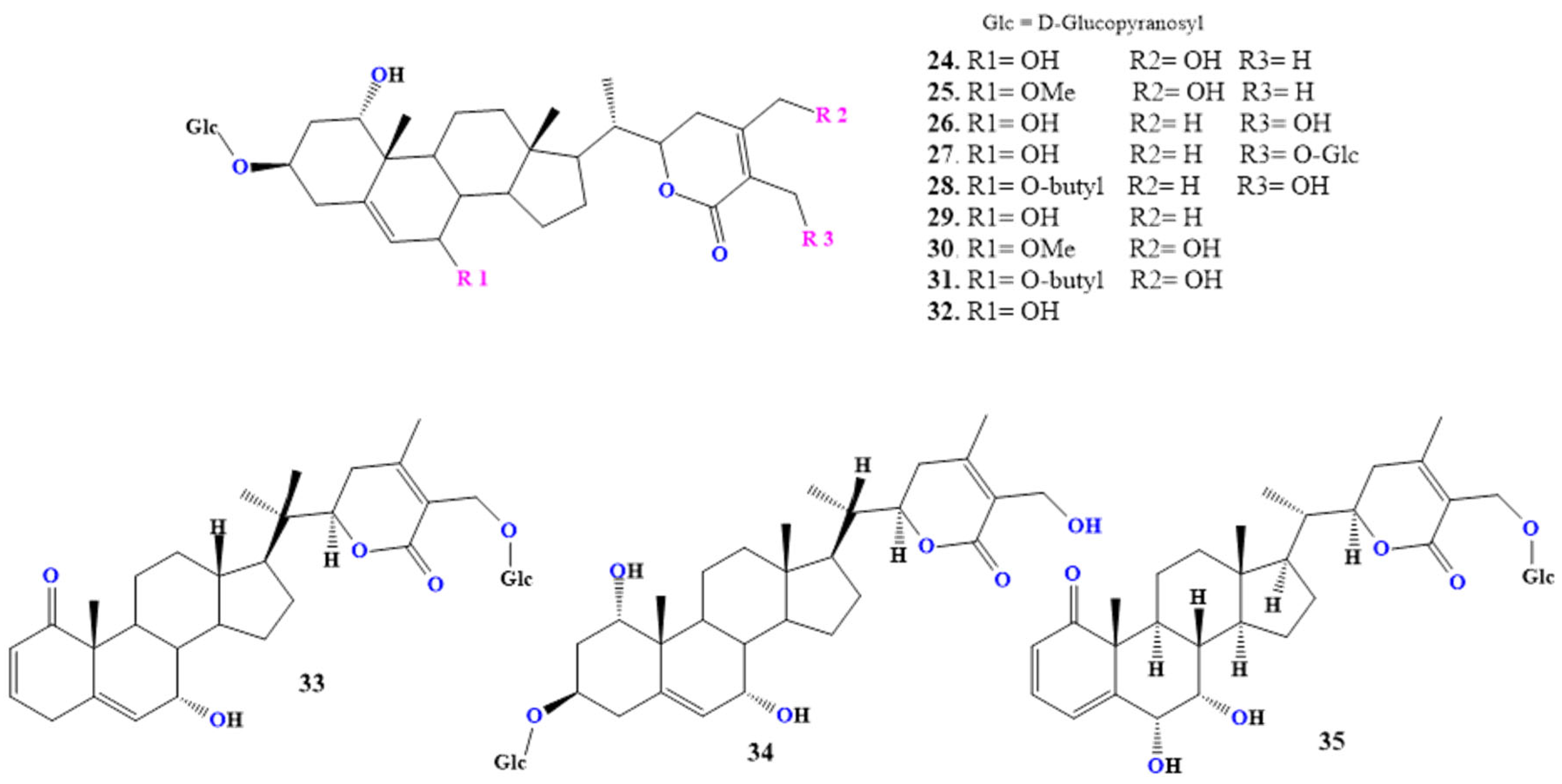
2.4.2. Daturafolisides
2.4.3. Daturataturin
2.4.4. Withametelins
2.4.5. Daturametelin J
3. Future Directions and Challenges
3.1. Metabolic Pathway Elucidation
3.2. Pharmacological Profiling and Toxicology
3.3. Pharmacogenomics and Personalized Medicine
3.4. Synthetic Derivatives and Analog Development
3.5. Ethnobotanical and Traditional Use Validation
3.6. Regulatory and Ethical Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lian, W.; Wang, Y.; Zhang, J.; Yan, Y.; Xia, C.; Gan, H.; Wang, X.; Yang, T.; Xu, J.; He, J.; et al. The genus Datura L. (Solanaceae): A systematic review of botany, traditional use, phytochemistry, pharmacology, and toxicology. Phytochemistry 2022, 204, 113446. [Google Scholar] [CrossRef]
- Chen, L.-L.; Shen, Y.-C.; Ke, C.-C.; Imtiyaz, Z.; Chen, H.-I.; Chang, C.-H.; Lee, M.-H. Efficacy of cinnamon patch treatment for alleviating symptoms of overactive bladder: A double-blind, randomized, placebo-controlled trial. Phytomedicine 2020, 80, 153380. [Google Scholar] [CrossRef]
- Jmii, G.; Zorrilla, J.G.; Keffala, C.; Jupsin, H.; Haouala, R. Effect of Datura metel L. and Inula viscosa L. applied separately or in combination on coexisting plants, Solanum elaeagnifolium Cav. and Capsicum annuum L. Sci. Hortic. 2024, 328, 112963. [Google Scholar] [CrossRef]
- Masum, S.M.; Nowroz, F.; Talha, M.A.; Islam, M.; Jalal, M.J.; Uddin, M.A. Invasive weed (Parthenium hysterophorus) response to chemical and allelopathic extracts at different stages. SAARC J. Agric. 2023, 21, 239–252. [Google Scholar] [CrossRef]
- Al-Andal, A.; Ewas, M.; Donia, A.E.R.M.; Radwan, A.M.; Suliman, M.N.S.; Nishawy, E.; El-Shabasy, A.; Khames, E. A three-sided story: A biosystematic revision of genus Datura reveals novel tropane alkaloids for the first time in certain species. Front. Plant Sci. 2025, 16, 1555237. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.I.; Rabellino, L.C.; Rotter, M.C. Tropane Alkaloid Variation in the Genus Datura and its Consequences for Cultural Practices. Econ. Bot. 2025, 79, 100–107. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Dang, T.-T.T. Cytochrome P450 enzymes as key drivers of alkaloid chemical diversification in plants. Front. Plant Sci. 2021, 12, 682181. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.V.; Jugulam, M. Role of cytochrome P450 enzymes in plant stress response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Babiker, F.; Jamal, P.; Mirghani, M.E.S.; Ansari, A.H. Characterization, purification, and identification of some Alkaloids in Datura stramonium. EBSCOhost 2017, 24, 540. [Google Scholar]
- Shi, Z.; Zou, W.; Zhu, Z.; Xiong, Z.; Li, S.; Dong, P.; Zhu, Z. Tropane alkaloids (hyoscyamine, scopolamine and atropine) from genus Datura: Extractions, contents, syntheses and effects. Ind. Crops Prod. 2022, 186, 115283. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Ogunsuyi, O.B.; Oboh, G. Alkaloid extracts from Jimson weed (Datura stramonium L.) modulate purinergic enzymes in rat brain. NeuroToxicology 2016, 56, 107–117. [Google Scholar] [CrossRef]
- Céspedes-Méndez, C.; Iturriaga-Vásquez, P.; Hormazábal, E. Secondary Metabolites and Biological Profiles of Datura Genus. J. Chil. Chem. Soc. 2021, 66, 5183–5189. [Google Scholar] [CrossRef]
- Benítez, G.; March-Salas, M.; Villa-Kamel, A.; Cháves-Jiménez, U.; Hernández, J.; Montes-Osuna, N.; Moreno-Chocano, J.; Cariñanos, P. The genus Datura L. (Solanaceae) in Mexico and Spain – Ethnobotanical perspective at the interface of medical and illicit uses. J. Ethnopharmacol. 2018, 219, 133–151. [Google Scholar] [CrossRef]
- Yin, S.; Hu, J.; Mohamed, K.H.; Hu, X.; Gao, W.; Li, Y.; Hu, W.; Ding, C. Ethnobotany, phytochemistry, pharmacology, and toxicology of the genus Datura (Solanaceae). Nat. Prod. Res. 2025, 39, 1–16. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Thawabteh, A.; Lelario, F.; Bufo, S.A.; Scrano, L. Classification, toxicity, and bioactivity of natural diterpenoid alkaloids. Molecules 2021, 26, 4103. [Google Scholar] [CrossRef]
- Melese, G.M.; Aychiluhim, T.B.; Yessuf, A.M.; Eshete, M. Identification and characterization of phytochemicals in methanolic extract of roots of Datura fastuosa using various techniques. Future J. Pharm. Sci. 2024, 10, 108. [Google Scholar] [CrossRef]
- Okpashi, V.E.; Oyo-Ita, E.E.A.; Jones, B.B.; Obeten, U.N.; Ucho, K.M.; Ofoelo, L.I. Identification and Characterization of Bioactive Components in Datura stramonium Leaves: An insight into Drug Discovery. J. Appl. Sci. Environ. Manag. 2022, 25, 1789–1799. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, K.; Purohit, D.; Verma, R.; Pandey, P.; Bhatia, S.; Malik, V.; Mittal, V.; Rahman, M.H.; Albadrani, G.M.; et al. Exploration of therapeutic applicability and different signaling mechanisms of various phytopharmacological agents for treatment of breast cancer. Biomed. Pharmacother. 2021, 139, 111584. [Google Scholar] [CrossRef] [PubMed]
- Turnaturi, R.; Piana, S.; Spoto, S.; Costanzo, G.; Reina, L.; Pasquinucci, L.; Parenti, C. From Plant to Chemistry: Sources of Antinociceptive Non-Opioid Active principles for medicinal chemistry and drug design. Molecules 2024, 29, 815. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.M.; Ghanem, A.W.; AbuMadi, S.; Thaher, D.; Jaghama, W.; Karaman, R.; Scrano, L.; Bufo, S.A. Antibacterial activity and antifungal activity of monomeric alkaloids. Toxins 2024, 16, 489. [Google Scholar] [CrossRef]
- Nithya, M.; Fathima, S.S.; Sankar, R.; Ajay, A.; Kondiba, K.V.; Sedhupathi, S. In Vitro Production of Atropine from Datura metel. BIO Web Conf. 2025, 172, 04006. [Google Scholar] [CrossRef]
- Jaber, A.; Al-Sayegh, H.; Zein, M.; Ibrahim, G.; Cheble, E. Quantification of atropine and scopolamine in different plant organs of Datura metel: Development and validation of High-Performance Liquid Chromatography Method. J. Clin. Lab. Res. 2021, 3, 01–09. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Talapatra, B. Atropine [(±)-Hyoscyamine] and cocaine (Ornithine-Derived alkaloids). In Chemistry of Plant Natural Products: Stereochemistry, Conformation, Synthesis, Biology, and Medicine; Springer: Berlin/Heidelberg, Germany, 2014; pp. 767–780. [Google Scholar] [CrossRef]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.; Karaman, R. The Biological Activity of Natural Alkaloids Against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, E.F.; Juárez, Z.N.; Hernández, L.R.; Bach, H. Natural antispasmodics: Source, stereochemical configuration, and biological activity. BioMed Res. Int. 2018, 2018, 3819714. [Google Scholar] [CrossRef]
- Turgunov, K.K.; Kadirova, D.; Okmanov, R.; Aripova, S.F.; Tashkhodjaev, B. Stereochemistry of tropane alkaloids of convolvine and their derivatives. Eur. J. Chem. 2019, 10, 376–380. [Google Scholar] [CrossRef]
- Upadhyay, A.; Beuerman, R.W. Biological mechanisms of atropine control of myopia. Eye Contact Lens Sci. Clin. Pract. 2020, 46, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.; Salzano, A.D.; Jenewein, E.C.; Weise, K.K.; Schaeffel, F.; Mathis, U.; Khanal, S. Topical review: Potential mechanisms of atropine for myopia control. Optom. Vis. Sci. 2025, 102, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.; Kelly, T.; Karouta, C.; Morgan, I.; Ashby, R. Insights into the mechanism by which atropine inhibits myopia: Evidence against cholinergic hyperactivity and modulation of dopamine release. Br. J. Pharmacol. 2021, 178, 4501–4517. [Google Scholar] [CrossRef]
- Lochner, M.; Thompson, A.J. The muscarinic antagonists scopolamine and atropine are competitive antagonists at 5-HT 3 receptors. Neuropharmacology 2016, 108, 220–228. [Google Scholar] [CrossRef]
- Diaz, J.H. Poisoning by herbs and Plants: Rapid toxidromic classification and diagnosis. Wilderness Environ. Med. 2016, 27, 136–152. [Google Scholar] [CrossRef]
- Pawar, S.D.; Bhutekar, S.S.; Gaikwad, R.R.; Gaikwad, M.S. Comprehensive review on medicinal value of poisonous plants. J. Drug Deliv. Ther. 2025, 15, 143–152. [Google Scholar] [CrossRef]
- Kapoor, V.K.; Kaur, N.; Rana, S. Safety concern of drugs of herbal origin. Phytochem. Rev. 2025, 24, 1–11. [Google Scholar] [CrossRef]
- Boroughf, W.J. Anticholinergic syndrome. In Critical Care Toxicology; Springer: Cham, Switzerland, 2017; pp. 519–537. [Google Scholar] [CrossRef]
- Boddupalli, R.S. A review on the most important poisonous plants and their medicinal properties. J. Med. Bot. 2021, 1–13. [Google Scholar] [CrossRef]
- Rates, S.M.K.; Betti, A.H.; Müller, L.G.; De Matos Nunes, J. Plant toxins as sources of drugs. In Plant Toxins; Springer: Dordrecht, The Netherlands, 2015; pp. 1–21. [Google Scholar] [CrossRef]
- Basher, A.; Islam, Q.T. Plants and herbal poisoning in Bangladesh. In Clinical Toxinology; Springer: Dordrecht, The Netherlands, 2015; pp. 609–631. [Google Scholar] [CrossRef]
- Amer, A.M.; Amer, M.M. Pharmacology of Tropane Alkaloids. In Tropane Alkaloids; Springer Nature: Singapore, 2025; pp. 103–132. [Google Scholar] [CrossRef]
- Doncheva, T.; Kostova, N.; Philipov, S. Alkaloids Derived from Ornithine: Tropane Alkaloids. In Natural Products: Phytochemistry, Botany, Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–20. [Google Scholar] [CrossRef]
- Mulder, P.P.J.; De Nijs, M.; Castellari, M.; Hortos, M.; MacDonald, S.; Crews, C.; Hajslova, J.; Stranska, M. Occurrence of tropane alkaloids in food. EFSA Support. Publ. 2016, 13, 1140E. [Google Scholar] [CrossRef]
- Amer, A.M.; Amer, M.M. Tropane Alkaloids: Biosimilar and Biopharmaceutics. In Tropane Alkaloids Sources, Chemistry, Pharmacology and Biotechnology; Springer: Singapore, 2025; pp. 163–181. [Google Scholar] [CrossRef]
- González-Gómez, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Occurrence and Chemistry of Tropane Alkaloids in Foods, with a Focus on Sample Analysis Methods: A Review on Recent Trends and Technological Advances. Foods 2022, 11, 407. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Masaret, G.S.; Abdulwahab, H.G. The patent review of the biological activity of tropane-containing compounds. Expert Opin. Ther. Pat. 2023, 33, 875–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, S.; Zheng, P.; Ren, Z.; Zhang, H.; Zhang, J.; Shao, B.; Wu, C.; Jiang, H. Emerging tropane alkaloids: Global development and potential health threats. Food Qual. Saf. 2023, 8, fyad043. [Google Scholar] [CrossRef]
- Kim, N.; Estrada, O.; Chavez, B.; Stewart, C.; D’Auria, J. Tropane and granatane alkaloid biosynthesis: A Systematic analysis. Molecules 2016, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, D.; Rikanati, R.D.; Volis, S.; Faigenboim, A.; Vendramin, V.; Cattonaro, F.; Hooper, M.; Oren, E.; Taylor, M.; Sitrit, Y.; et al. Alkaloid chemodiversity in Mandragora spp. is associated with loss-of-functionality of MoH6H, a hyoscyamine 6β-hydroxylase gene. Plant Sci. 2019, 283, 301–310. [Google Scholar] [CrossRef]
- Devaki, R.; Kumar, R.S. Anticholinergic herbs featured in siddha system of medicine—A review. World J. Pharm. Res. 2022, 11, 460–467. Available online: https://www.wjpr.net/abstract_show/19206 (accessed on 15 August 2025).
- Thawabteh, A.M.; Ghanem, A.W.; AbuMadi, S.; Thaher, D.; Jaghama, W.; Karaman, D.; Karaman, R. Recent advances in therapeutics for the treatment of Alzheimer’s disease. Molecules 2024, 29, 5131. [Google Scholar] [CrossRef]
- Sahu, P.K.; Pradhan, S.P.; Kumar, P.S. Isolation, elucidation, and structure–activity relationships of phytoalkaloids from Solanaceae. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–389. [Google Scholar] [CrossRef]
- Kramer, L.; Jayanty, S.; Reckhow, D.A.; Sathuvalli, V. Identification of Tropane Alkaloid Chemotypes and Genotypes in Hyoscyamus niger L. J. Am. Soc. Hortic. Sci. 2023, 148, 304–314. [Google Scholar] [CrossRef]
- Bhakshu, L.M.; Pullaiah, T. Chemistry of Tropane Alkaloids: A Comprehensive Study of Biologically Important Group of Nitrogen-Containing Heterocyclic Phyto-secondary Metabolites. In Tropane Alkaloids; Springer: Singapore, 2025; pp. 49–88. [Google Scholar] [CrossRef]
- Harfi, B.; Khelifi, L.; Khelifi-Slaoui, M.; Assaf-Ducrocq, C.; Gontier, E. Tropane alkaloids GC/MS analysis and low dose elicitors’ effects on hyoscyamine biosynthetic pathway in hairy roots of Algerian Datura species. Sci. Rep. 2018, 8, 17951. [Google Scholar] [CrossRef]
- Rasi, A.; Sabokdast, M.; Naghavi, M.R.; Jariani, P.; Dedičová, B. Modulation of Tropane Alkaloids’ Biosynthesis and Gene Expression by Methyl Jasmonate in Datura stramonium L.: A Comparative Analysis of Scopolamine, Atropine, and Hyoscyamine Accumulation. Life 2024, 14, 618. [Google Scholar] [CrossRef] [PubMed]
- Harfi, B.; Khelifi-Slaoui, M.; Bekhouche, M.; Benyammi, R.; Hefferon, K.; Makhzoum, A.; Khelifi, L. Hyoscyamine production in hairy roots of three Datura species exposed to high-salt medium. Vitr. Cell. Dev. Biol.-Plant 2015, 52, 92–98. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Jones, A.D. Alkaloids of the Genus Datura: Review of a Rich Resource for Natural Product Discovery. Molecules 2021, 26, 2629. [Google Scholar] [CrossRef]
- Karimian, R.; Nafari, M.; Khodabakhshi, M.R.; Davarpanah, S.J. Expression Profile of Hyoscyamine Biosynthesis-related Genes in Response to UV-C Radiation in Datura metel Plant. J. Appl. Biotechnol. Rep. 2023, 10, 1176–1181. [Google Scholar] [CrossRef]
- Mihálik, D.; Hančinský, R.; Kaňuková, Š.; Mrkvová, M.; Kraic, J. Elicitation of Hyoscyamine Production in Datura stramonium L. Plants Using Tobamoviruses. Plants 2022, 11, 3319. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Yan, Y.; Zeng, J.; Huang, J.-P.; Zeng, L.; Zhong, W.; Lan, X.; Chen, M.; Huang, S.-X.; Liao, Z. Biochemical and Metabolic Insights into Hyoscyamine Dehydrogenase. ACS Catal. 2021, 11, 2912–2924. [Google Scholar] [CrossRef]
- Rosecrans, J.A.; Young, R. Discriminative stimulus properties of S(−)-Nicotine: “A drug for all seasons”. Curr. Top. Behav. Neurosci. 2017, 39, 51–94. [Google Scholar] [CrossRef]
- Van Der Bijl, P.; Van Der Bijl, P. Cardiovascular toxicities of herbal products: An overview of selected compounds. In Toxicology of Herbal Products; Springer: Cham, Switzerland, 2017; pp. 363–383. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Kwiatek, K. Simultaneous determination of pyrrolizidine and tropane alkaloids in honey by liquid chromatography–mass spectrometry. J. Vet. Res. 2022, 66, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zahran, T.E.; Halabi, Z.; Barakat, A.; Hachem, R.I.E.; Nicolas, C.B.; Hassan, S.A.; Khalil, A. An overview of the poisonous plants of Lebanon and their effects. Toxicon 2024, 252, 108177. [Google Scholar] [CrossRef]
- Hung, D.-Z.; Hung, Y.-H. Anticholinergic syndrome related to plants and herbs. In Clinical Toxinology; Springer: Dordrecht, The Netherlands, 2015; pp. 569–586. [Google Scholar] [CrossRef]
- Demire, B.; Avcı, S. Physostigmine in Hyoscyamus Niger Intoxication: Is it an Antidote Myth? Indian J. Public Health Res. Dev. 2019, 10, 1359. [Google Scholar] [CrossRef]
- Bonde, A.R.; Pinjari, R.M. Therapeutic Importance of Hyoscyamus Species—A Review. Int. J. For. Anim. Fish. Res. 2023, 7, 18. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Therapeutic Importance of Hyoscyamus Species Grown in Iraq (Hyoscyamus Albus, Hyoscyamus Niger and Hyoscyamus Reticulatus)—A Review. IOSR J. Pharm. 2018, 8, 18–32. Available online: https://med.utq.edu.iq/wp-content/uploads/sites/7/2021/07/therapeutic-importance-of-hyoscyamus-species-grown-in-iraq-hyoscyamus-albus-hyoscyamus-niger-and-hyoscyamus-reticulates-a-review.pdf (accessed on 17 August 2025).
- Li, J.; Liu, S.-J.; Huang, Z.-L.; Yu, J. Physochlainae Radix, a review of its phytochemistry, pharmacology, toxicity and medicinal processing. Phytochem. Rev. 2024, 24, 1503–1533. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Naga, R.; Saha, M.; Roy, S. Viral Inhibitory potential of Hyoscyamine in Japanese Encephalitis virus infected Embryonated Chick involving multiple signaling pathways. Res. Sq. (Res. Sq.) 2022. [Google Scholar] [CrossRef]
- Mishra, P.; Sharma, P.; Tripathi, P.N.; Gupta, S.K.; Srivastava, P.; Seth, A.; Tripathi, A.; Krishnamurthy, S.; Shrivastava, S.K. Design and development of 1,3,4-oxadiazole derivatives as potential inhibitors of acetylcholinesterase to ameliorate scopolamine-induced cognitive dysfunctions. Bioorganic Chem. 2019, 89, 103025. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.; Wang, B.; Wang, Y.; Wang, M.; Yang, S.; Su, C.; Chang, J.; Zhu, B. Design, Synthesis, and Activity Evaluation of Fluorine-Containing Scopolamine Analogues as Potential Antidepressants. J. Med. Chem. 2024, 67, 5391–5420. [Google Scholar] [CrossRef]
- Ullrich, S.F.; Hagels, H.; Kayser, O. Scopolamine: A journey from the field to clinics. Phytochem. Rev. 2016, 16, 333–353. [Google Scholar] [CrossRef]
- Anacker, C. New insight into the mechanisms of Fast-Acting Antidepressants: What we learn from Scopolamine. Biol. Psychiatry 2017, 83, e5–e7. [Google Scholar] [CrossRef]
- Chen, W.N.; Yeong, K.Y. Scopolamine, a Toxin-Induced experimental model, used for research in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2020, 19, 85–93. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Ghanem, A.W.; AbuMadi, S.; Thaher, D.; Jaghama, W.; Karaman, D.; Karaman, R. Promising natural remedies for Alzheimer’s disease therapy. Molecules 2025, 30, 922. [Google Scholar] [CrossRef]
- Martin, A.E.; Schober, D.A.; Nikolayev, A.; Tolstikov, V.V.; Anderson, W.H.; Higgs, R.E.; Kuo, M.-S.; Laksmanan, A.; Catlow, J.T.; Li, X.; et al. Further Evaluation of Mechanisms Associated with the Antidepressantlike Signature of Scopolamine in Mice. CNS Neurol. Disord. Drug Targets 2017, 16, 492–500. [Google Scholar] [CrossRef]
- Wang, S.-B.; Yang, X.-Y.; Du, G.-H. Anisodine. In Natural Small Molecule Drugs from Plants; Springer: Singapore, 2018; pp. 175–180. [Google Scholar] [CrossRef]
- Xia, Q.; Pingcuo, R.; Yang, C.; Xiong, W.; Peng, X.; Xia, J.; Wang, W.; Hai, M. A review on the chemical properties, plant sources, anti-shock effects, pharmacokinetics, toxicity, and clinical applications of anisodamine. Chem. Biodivers. 2024, 21, e202301477. [Google Scholar] [CrossRef]
- Minoia, J.M.; Villanueva, M.E.; Copello, G.J.; Talou, J.R.; Cardillo, A.B. Recycling of hyoscyamine 6β-hydroxylase for the in vitro production of anisodamine and scopolamine. Appl. Microbiol. Biotechnol. 2023, 107, 3459–3478. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Wan, F.; Peng, F.; Peng, C. Update on the sources, pharmacokinetics, pharmacological action, and clinical application of anisodamine. Biomed. Pharmacother. 2023, 161, 114522. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, C.; Hong, Y.; Chen, L.; Huang, Z.; Zhou, J.; Tian, X.; Liu, D.; Ren, B.; Zhang, C.; et al. Effectiveness of anisodamine for the treatment of critically ill patients with septic shock: A multicentre randomized controlled trial. Crit. Care 2021, 25, 349. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Talapatra, B. Hygrine, hygroline, and cuscohygrine (Ornithine-Derived alkaloids). In Chemistry of Plant Natural Products: Stereochemistry, Conformation, Synthesis, Biology, and Medicine; Springer: Berlin/Heidelberg, Germany, 2014; pp. 725–732. [Google Scholar] [CrossRef]
- Petricevich, V.L.; Salinas-Sánchez, D.O.; Avilés-Montes, D.; Sotelo-Leyva, C.; Abarca-Vargas, R. Chemical Compounds, Pharmacological and Toxicological Activity of Brugmansia suaveolens: A Review. Plants 2020, 9, 1161. [Google Scholar] [CrossRef]
- Rubio, N.C.; Thurmann, D.; Krumbiegel, F.; Pragst, F. Behaviour of hygrine and cuscohygrine in illicit cocaine production establishes their use as markers for chewing coca leaves in contrast with cocaine abuse. Drug Test. Anal. 2016, 9, 323–326. [Google Scholar] [CrossRef]
- Rubio, N.C.; Moreda-Piñeiro, A.; Álvarez-Freire, I.; Bermejo-Barrera, P.; Tabernero-Duque, M.J.; Bermejo, A.M. The probability to detect cocaine, methylecgonine, cinnamoylcocaine, hygrine, and cuscohygrine in urine samples of coca leaves chewers after six years. Microchem. J. 2019, 151, 104215. [Google Scholar] [CrossRef]
- Qiu, F.; Zeng, J.; Wang, J.; Huang, J.; Zhou, W.; Yang, C.; Lan, X.; Chen, M.; Huang, S.; Kai, G.; et al. Functional genomics analysis reveals two novel genes required for littorine biosynthesis. New Phytol. 2019, 225, 1906–1914. [Google Scholar] [CrossRef]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane alkaloids: Chemistry, pharmacology, biosynthesis and production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef]
- Huang, J.-P.; Wang, Y.-J.; Tian, T.; Wang, L.; Yan, Y.; Huang, S.-X. Tropane alkaloid biosynthesis: A centennial review. Nat. Prod. Rep. 2021, 38, 1634–1658. [Google Scholar] [CrossRef]
- Bhakshu, L.M.; Pullaiah, T. Chapter 4, Biosynthesis of Tropane Alkaloids. In Tropane Alkaloids; Springer: Singapore, 2025; pp. 89–101. [Google Scholar] [CrossRef]
- Parks, H.M.; Cinelli, M.A.; Bedewitz, M.A.; Grabar, J.M.; Hurney, S.M.; Walker, K.D.; Jones, A.D.; Barry, C.S. Redirecting tropane alkaloid metabolism reveals pyrrolidine alkaloid diversity in Atropa belladonna. New Phytol. 2022, 237, 1810–1825. [Google Scholar] [CrossRef]
- Aryal, B.; Raut, B.K.; Bhattarai, S.; Bhandari, S.; Tandan, P.; Gyawali, K.; Sharma, K.; Ranabhat, D.; Thapa, R.; Aryal, D.; et al. Potential Therapeutic Applications of Plant-Derived Alkaloids against Inflammatory and Neurodegenerative Diseases. Evid.-Based Complement. Altern. Med. 2022, 2022, 7299778. [Google Scholar] [CrossRef]
- Ishola, A.O.; Adetunji, A.E.; Abanum, I.C.; Adeyemi, A.A.; Faleye, C.K.; Martins, J.B.; Ogbe, N.C.; Ogundipe, T.C.; Okewulonu, K.E.; Okon, U.E.; et al. Datumetine preferentially upregulates NMDAR signalling pathways in different brain regions of mice. Basic Clin. Neurosci. J. 2021, 14, 103–116. [Google Scholar] [CrossRef]
- Ishola, A.O.; Imam, A.; Ajao, M.S. Datumetine exposure alters the hippocampal neurotransmitter system in C57BL/6 mice. Drug Chem. Toxicol. 2020, 45, 785–798. [Google Scholar] [CrossRef]
- Ishola, A.O.; Imam, A.; Ajao, M.S. Effects of datumetine on hippocampal NMDAR activity. Toxicol. Rep. 2021, 8, 1131–1142. [Google Scholar] [CrossRef]
- Jones, N.S.; Comparin, J.H. Interpol review of controlled substances 2016–2019. Forensic Sci. Int. Synerg. 2020, 2, 608–669. [Google Scholar] [CrossRef]
- Teuscher, E.; Lindequist, U. Chapter 1 Natural Poisons: Natural Poisons and Venoms, Plant Toxins: Terpenes and Steroids, 1st ed.; De Gruyter: Berlin, Germany, 2023. [Google Scholar] [CrossRef]
- Rubio, N.C.; Herbello-Hermelo, P.; Álvarez-Freire, I.; Cabarcos-Fernández, P.; Tabernero-Duque, M.J.; Sánchez-Sellero, I.; Bermejo-Barrera, P.; Bermejo-Barrera, A.M.; Moreda-Piñeiro, A. Impact of Coca Leaf Flour Candy Consumption on Cocaine and Benzoylecgonine Levels: The Role of Hygrine and Cuscohygrine in Distinguishing Licit from Illicit Cocaine Use. Forensic Sci. Int. 2025, 371, 112494. [Google Scholar] [CrossRef]
- Hong, J.-R.; Choi, K.-M. Analytical Characteristics of GC/MS and HPLC according to the Concentration Distribution of PAHs. Han-Guk Saneop Bogeon Hakoeji 2015, 25, 312–321. [Google Scholar] [CrossRef][Green Version]
- Burnier, C.; Massonnet, G.; Coulson, S.; DeTata, D.; Pitts, K. Characterization and classification of water-based compounds in condoms and personal hygiene products using GC-MS. Forensic Sci. Int. 2020, 317, 110513. [Google Scholar] [CrossRef]
- Bekeschus, S.; Favia, P.; Robert, E.; Von Woedtke, T. White paper on plasma for medicine and hygiene: Future in plasma health sciences. Plasma Process. Polym. 2018, 16, 1800033. [Google Scholar] [CrossRef]
- Willis, L.D.; Chandler, C. Quick fix for care, productivity, hygiene, and inequality: Reframing the entrenched problem of antibiotic overuse. BMJ Glob. Health 2019, 4, e001590. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Ravi, L.; Girish, S.; Harshini, M.; Sreenivas, B.K.A. Β-Sitosterol: An antibacterial agent in aquaculture management of Vibrio infections. J. Pure Appl. Microbiol. 2020, 14, 2699–2714. [Google Scholar] [CrossRef]
- Interactive Properties of Alkaloids from Datura stramonium, Moringa oleifera, and Carica papaya with Human Receptor Proteins of Psychoactive Compounds from Cannabis sativa and Nicotiana tabacum. Trop. J. Nat. Prod. Res. 2024, 8. [CrossRef]
- Da Costa, S.P.; Schuenck-Rodrigues, R.A.; Da Silva Cardoso, V.; Valverde, S.S.; Vermelho, A.B.; Ricci-Júnior, E. Therapeutic Potential of Bioactive Compounds from Brugmansia suaveolens Bercht. & J. Presl. Nutrients 2023, 15, 2912. [Google Scholar] [CrossRef]
- Yen, C.H.; Mohammad, A.; Schneider, M.; Poole, S.K.; Lowry, B.; Curdy, B.W.M.; Faustino, P.J.; Khan, S.R. Development and application of a validated UHPLC method for the determination of atropine and its major impurities in antidote treatment Nerve Agent Auto-Injectors (ATNAA) stored in the Strategic National Stockpiles. Pharmacol. Pharm. 2017, 8, 15–31. [Google Scholar] [CrossRef]
- Dai, C.; Snead, D.R.; Zhang, P.; Jamison, T.F. Continuous-Flow Synthesis and Purification of Atropine with Sequential In-Line Separations of Structurally Similar Impurities. J. Flow Chem. 2015, 5, 133–138. [Google Scholar] [CrossRef]
- Attar, U.A.; Zimare, S.B.; Mohite, A.V.; Zimare, P.S. Chapter 8, Biotechnological, Genetic, Molecular and Breeding Attempts and Strategies for the analysis and production of pharmaceutically important tropane alkaloids in Atropa belladonna L.: A review. In Biodiversity and Genetic Improvement of Medicinal and Aromatic Plants I; Springer: Cham, Switzerland, 2025; Volume 9, pp. 207–234. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, P. Epoxides: Developability as active pharmaceutical ingredients and biochemical probes. Bioorganic Chem. 2022, 125, 105862. [Google Scholar] [CrossRef]
- Tian, F.; Li, C.; Wang, X.; Ren, S.; Li, N.; Liu, Q.; Zhou, S.; Lu, Y.; Zhao, D.; Chen, X. Comparative study on pharmacokinetics of a series of anticholinergics, atropine, anisodamine, anisodine, scopolamine, and tiotropium in rats. Eur. J. Drug Metab. Pharmacokinet. 2014, 40, 245–253. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wan, F.; Yang, N. Effects of anisodine hydrobromide on the cardiovascular and respiratory functions in conscious dogs. Drug Des. Dev. Ther. 2020, 14, 4263–4276. [Google Scholar] [CrossRef]
- Chen, D.; Peng, C.; Xie, X.; Chen, Q.; Liu, H.; Zhang, S.; Wan, F.; Ao, H. Low dose of anisodine hydrobromide induced neuroprotective effects in chronic cerebral hypoperfusion rats. CNS Neurol. Disord. Drug Targets 2018, 16, 1111–1119. [Google Scholar] [CrossRef]
- Liu, W.-D.; Chen, L.-L.; Shen, C.-Y.; Jiang, L.-B. Neuroprotective Effect of Compound Anisodine in a Mouse Model with Chronic Ocular Hypertension. Chin. Med. J. 2015, 128, 2652–2657. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, F.; Hu, P.; He, B.; Hu, Y.; Liu, Y. Efficacy and safety of anisodine hydrobromide injection for acute ischemic stroke: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1290755. [Google Scholar] [CrossRef]
- Wang, Z. Investigation of effects of tropine-based surfactant structure on its thermal stability, foaming properties, and solubilization. J. Mol. Liq. 2023, 389, 122869. [Google Scholar] [CrossRef]
- Asztemborska, M.; Ceborska, M.; Pietrzak, M. Complexation of tropane alkaloids by cyclodextrins. Carbohydr. Polym. 2019, 209, 74–81. [Google Scholar] [CrossRef]
- French, R.N.E.; Walter, F.G. Atropine. In Critical Care Toxicology; Springer: Cham, Switzerland, 2017; pp. 2725–2731. [Google Scholar] [CrossRef]
- Alsamarrai, S.H. Chapter 3, Synthesis of tropane derivatives. In Alkaloids: Their Importance in Nature and Human Life; IntechOpen: London, UK, 2019; pp. 27–47. [Google Scholar] [CrossRef]
- Mehrotra, S.; Mishra, S.; Srivastava, V. Plant tropane alkaloids: Commercial stature and production developments. In Tropane Alkaloids; Springer eBooks: Singapore, 2021; pp. 51–65. [Google Scholar] [CrossRef]
- Najmi, A.A.; Xiao, Z.; Bischoff, R.; Dekker, F.J.; Permentier, H.P. Electrochemical N-demethylation of tropane alkaloids. Green Chem. 2020, 22, 6455–6463. [Google Scholar] [CrossRef]
- Dweib, K.; Jumaa, S.; Thawabteh, A.; Scrano, L.; Bufo, S.A.; Mecca, G.; Karaman, R. Diclofenac Codrugs and prodrugs-Three decades of design. World J. Pharm. Pharm. Sci. 2015, 4, 1960–1962. Available online: https://www.wjpps.com/Wjpps_controller/abstract_id/3486 (accessed on 16 September 2025).
- Lowe, R.A.; Taylor, D.; Chibale, K.; Nelson, A.; Marsden, S.P. Synthesis and evaluation of the performance of a small molecule library based on diverse tropane-related scaffolds. Bioorganic Med. Chem. 2020, 28, 115442. [Google Scholar] [CrossRef]
- Szumilak, M.; Stanczak, A. Cinnoline Scaffold—A molecular heart of medicinal chemistry? Molecules 2019, 24, 2271. [Google Scholar] [CrossRef]
- Lakstygal, A.M.; Kolesnikova, T.O.; Khatsko, S.L.; Zabegalov, K.N.; Volgin, A.D.; Demin, K.A.; Shevyrin, V.A.; Wappler-Guzzetta, E.A.; Kalueff, A.V. DARK classics in chemical neuroscience: Atropine, scopolamine, and other anticholinergic deliriant hallucinogens. ACS Chem. Neurosci. 2018, 10, 2144–2159. [Google Scholar] [CrossRef]
- Bhaskar, S.; Golab, J.T.; Kaduk, J.A.; Gindhart, A.M.; Blanton, T.N. Crystal structure of ipratropium bromide monohydrate, C20H30NO3Br(H2O). Powder Diffr. 2020, 35, 61–66. [Google Scholar] [CrossRef]
- Cassambai, S.; Mee, C.J.; Renshaw, D.; Hussain, A. Tiotropium bromide, a long-acting muscarinic receptor antagonist, triggers intracellular calcium signalling in the heart. Toxicol. Appl. Pharmacol. 2019, 384, 114778. [Google Scholar] [CrossRef]
- Singh, M.P.; Kumar, M.; Shankar, R. Development and optimization of methscopolamine bromide gastroretentive floating tablets using a 32 factorial design. Drug Res. 2020, 70, 576–582. [Google Scholar] [CrossRef]
- Geller, E.J.; Dumond, J.B.; Bowling, J.M.; Khandelwal, C.M.; Wu, J.M.; Busby-Whitehead, J.; Kaufer, D.I. Effect of trospium chloride on cognitive function in women aged 50 and older: A randomized trial. Female Pelvic Med. Reconstr. Surg. 2017, 23, 118–123. [Google Scholar] [CrossRef]
- Joyce, M.D.; Jennings, M.C.; Santiago, C.N.; Fletcher, M.H.; Wuest, W.M.; Minbiole, K.P. Natural product-derived quaternary ammonium compounds with potent antimicrobial activity. J. Antibiot. 2015, 69, 344–347. [Google Scholar] [CrossRef]
- Khaled, E.; Hassan, H.N.A.; Ahmed, M.A.; El-Attar, R.O. Novel ipratropium bromide nanomaterial-based screen-printed sensors. Anal. Methods 2016, 9, 304–311. [Google Scholar] [CrossRef]
- Alvarado-Gonzalez, A.; Arce, I. Tiotropium bromide in chronic obstructive pulmonary disease and bronchial asthma. J. Clin. Med. Res. 2015, 7, 831–839. [Google Scholar] [CrossRef]
- Robert, A.; Nezamis, J.E. Effect of an anti-acetylcholine drug, methscopolamine bromide, on ulcer formation and gastric mucus. J. Pharm. Pharmacol. 1964, 16, 690–695. [Google Scholar] [CrossRef]
- Syed, Y.Y. Xanomeline/Trospium chloride: First approval. Drugs 2024, 85, 103–109. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Sharma, S.; Iftikhar, H. Unexplained bromide toxicity presenting as hyperchloremia and a negative anion gap. Cureus 2023, 15, e36218. [Google Scholar] [CrossRef]
- Kokulu, K.; Öner, H.; Özen, C.; Eroğlu, S.E.; Altunok, İ.; Akça, H.Ş. Pharmacologic anisocoria due to nebulized ipratropium bromide: A diagnostic challenge. Am. J. Emerg. Med. 2019, 37, e3–e1217. [Google Scholar] [CrossRef]
- Dusser, D.; Ducharme, F.M. Safety of tiotropium in patients with asthma. Ther. Adv. Respir. Dis. 2019, 13. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Janowsky, D.S. Cholinergic regulation of mood: From basic and clinical studies to emerging therapeutics. Mol. Psychiatry 2018, 24, 694–709. [Google Scholar] [CrossRef]
- Calzetta, L.; Ciaprini, C.; Puxeddu, E.; Cazzola, M. Olodaterol + tiotropium bromide for the treatment of COPD. Expert Rev. Respir. Med. 2016, 10, 379–386. [Google Scholar] [CrossRef]
- Ramadan, W.; Kabbara, W.; Khoury, G.E.; Al-Assir, S. Combined bronchodilators (tiotropium plus olodaterol) for patients with chronic obstructive pulmonary disease. Int. J. COPD 2015, 10, 2347–2356. [Google Scholar] [CrossRef][Green Version]
- Truzzi, J.C.; Sartori, M.G.F.; Kemp, V.L. Trospium chloride in the treatment of overactive bladder syndrome and detrusor overactivity. Adv. Ther. 2025. [Google Scholar] [CrossRef]
- Mostafaei, H.; Shariat, S.F.; Salehi-Pourmehr, H.; Janisch, F.; Mori, K.; Quhal, F.; Hajebrahimi, S. The clinical pharmacology of the medical treatment for overactive bladder in adults. Expert Rev. Clin. Pharmacol. 2020, 13, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Padró, J.M.; Osorio-Grisales, J.; Arancibia, J.A.; Olivieri, A.C.; Castells, C.B. Enantiomeric analysis of overlapped chromatographic profiles in the presence of interferences. Determination of ibuprofen in a pharmaceutical formulation containing homatropine. J. Chromatogr. A 2016, 1467, 255–260. [Google Scholar] [CrossRef]
- Yazdani, N.; Sadeghi, R.; Momeni-Moghaddam, H.; Zarifmahmoudi, L.; Ehsaei, A. Comparison of cyclopentolate versus tropicamide cycloplegia: A systematic review and meta-analysis. J. Optom. 2017, 11, 135–143. [Google Scholar] [CrossRef]
- Lin, J.; Bu, G.; Unge, J.; Gonen, T. An updated structure of oxybutynin hydrochloride. Adv. Sci. 2024, 11, 2406494. [Google Scholar] [CrossRef]
- Stock, V.; Hofer, R.; Lochmann, F.; Spanke, V.; Liedl, K.R.; Troppmair, J.; Langer, T.; Gstach, H.; Dank, C.; Mayhew, C.A.; et al. Tolterodine is a novel candidate for assessing CYP3A4 activity through metabolic volatiles to predict drug responses. Sci. Rep. 2025, 15, 2462. [Google Scholar] [CrossRef]
- Mahal, P.; Nishanth, K.N.; Mahapatra, A.; Sarkar, S.; Balhara, Y.P.S. Trihexyphenidyl misuse in delusional disorder. J. Neurosci. Rural Pract. 2018, 9, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Vouri, S.M.; Schootman, M.; Strope, S.A.; Birge, S.J.; Olsen, M.A. Differential Prescribing of Antimuscarinic Agents in Older Adults with Cognitive Impairment. Drugs Aging 2018, 35, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Drug-Induced Disorders of Memory and Dementia. In Drug-Induced Neurological Disorders; Springer eBooks; Springer International Publishing: Cham, Switzerland, 2021; pp. 209–231. [Google Scholar] [CrossRef]
- Curi, I.; Nakayama, S.A.; Pereira, É.M.; Hopker, L.M.; Ejzenbaum, F.; Barcellos, R.B.; Da Cruz Ferreira, R.; Cronemberger, M.F.; McKeown, C.A.; Rossetto, J.D. Brazilian guideline for pediatric cycloplegia and mydriasis. Arq. Bras. Oftalmol. 2022, 86, 388–396. [Google Scholar] [CrossRef]
- Ejzenbaum, F.; Schaefer, T.M.C.; Cunha, C.; Rossetto, J.D.; Godinho, I.F.; Nakanami, C.R.; Noma, R.K.; Hopker, L.M. Guidelines for preventing and slowing myopia progression in Brazilian children. Arq. Bras. Oftalmol. 2024, 87, 01–10. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, B.M. Crossing the Blood–Brain Barrier: Recent advances in drug delivery to the brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Müller-Arteaga, C.; Batista-Miranda, J.E.; Libano, C.Z.; Moreno, R.E.K.; Solchaga, G.M.; Nebra, J.C.; Vallejo, M.L.; Garcia, O.G.; Errando-Smet, C.; Arlandis-Guzman, S. Evaluación de la función cognitiva en pacientes de edad avanzada con vejiga hiperactiva tratados con oxibutinina transdémica. Actas Urológicas Españolas 2018, 43, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Al-Thawabieh, W.; Al-Omari, R.; Abu-Hassan, D.W.; Abuawwad, M.T.; Al-Awadhi, A.; Serhan, H.A. Tropicamide versus cyclopentolate for cycloplegic refraction in pediatric patients with brown irides: A randomized clinical trial. Am. J. Ophthalmol. 2023, 257, 218–226. [Google Scholar] [CrossRef]
- Major, E.; Dutson, T.; Moshirfar, M. Cycloplegia in Children: An Optometrist’s Perspective. Clin. Optom. 2020, 12, 129–133. [Google Scholar] [CrossRef]
- Vozmediano-Chicharro, R.; Hernández, P.B.; Madurga-Patuel, B. Insights into the Management of Overactive Bladder with Transdermal Oxybutynin: A Practical Review. Res. Rep. Urol. 2020, 12, 321–330. [Google Scholar] [CrossRef]
- Duong, V.; Iwamoto, A.; Pennycuff, J.; Kudish, B.; Iglesia, C. A systematic review of neurocognitive dysfunction with overactive bladder medications. Int. Urogynecol. J. 2021, 32, 2693–2702. [Google Scholar] [CrossRef]
- Esteban, M.; Méndez, S.; Salinas, J. AEU positioning statement on transdermal drug administration: Determinant evolution of functional urologic therapy. Actas Urológicas Españolas (Engl. Ed.) 2020, 44, 571–573. [Google Scholar] [CrossRef]
- Khan, H.; Patel, S.; Kamal, M.A. Pharmacological and toxicological profile of Harmane-Β-Carboline alkaloid: Friend or foe. Curr. Drug Metab. 2017, 18, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Sammi, S.R.; Agim, Z.S.; Cannon, J.R. From the Cover: Harmane-Induced Selective Dopaminergic Neurotoxicity in Caenorhabditis elegans. Toxicol. Sci. 2017, 161, 335–348. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Paidesetty, S.K.; Padhy, R.N. Norharmane as a potential chemical entity for the development of anticancer drugs. Eur. J. Med. Chem. 2018, 162, 752–764. [Google Scholar] [CrossRef]
- Lantz, S.M.; Cuevas, E.; Robinson, B.L.; Paule, M.G.; Ali, S.F.; Imam, S.Z. The role of Harmane and Norharmane in in vitro dopaminergic function. J. Drug Alcohol Res. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Li, S.; Teng, L.; Liu, W.; Cheng, X.; Jiang, B.; Wang, Z.; Wang, C.-H. Pharmacokinetic study of harmane and its 10 metabolites in rats after intravenous and oral administration by UPLC-ESI-MS/MS. Pharm. Biol. 2016, 54, 1768–1781. [Google Scholar] [CrossRef]
- Sui, M.; Zhang, J.; Li, J.; Wang, L.; Gao, Z.; Dan, W.; Dai, J. Antibacterial activity and multi-target mechanism of harmane against Escherichia coli O157:H7 and its application on ready-to-eat leafy greens. Int. J. Food Microbiol. 2025, 431, 111084. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Shim, S.H.; Lee, J. Antibiofilm activities of norharmane and its derivatives against Escherichia coli O157:H7 and other bacteria. Phytomedicine 2017, 36, 254–261. [Google Scholar] [CrossRef]
- Senhaji, S.; Lamchouri, F.; Akabli, T.; Toufik, H. In vitro antioxidant activities of five β-carboline alkaloids, molecular docking, and dynamic simulations. Struct. Chem. 2022, 33, 883–895. [Google Scholar] [CrossRef]
- Nirmala, F.S.; Lee, H.; Cho, Y.; Um, M.Y.; Seo, H.D.; Jung, C.H.; Hahm, J.-H.; Ahn, J. Norharmane prevents muscle aging via activation of SKN-1/NRF2 stress response pathways. Redox Biol. 2025, 80, 103512. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-Y.; Guo, R.; Li, T.; Wu, J.-J.; Zhang, J.; Liu, Y.; Wang, Q.-H.; Kuang, H.-X. New anti-inflammatory withanolides from the leaves of Datura metel L. Steroids 2014, 87, 26–34. Steroids 2014, 87, 26–34. [Google Scholar] [CrossRef]
- Guo, R.; Liu, Y.; Xu, Z.-P.; Xia, Y.-G.; Yang, B.-Y.; Kuang, H.-X. Withanolides from the leaves of Datura metel L. Phytochemistry 2018, 155, 136–146. Phytochemistry 2018, 155, 136–146. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Tan, J.; Sun, Y.; Guan, W.; Jiang, P.; Yang, B.; Kuang, H. Integrated serum metabolomics and network pharmacology approach to reveal the potential mechanisms of withanolides from the leaves of Datura metel L. on psoriasis. J. Pharm. Biomed. Anal. 2020, 186, 113277. [Google Scholar] [CrossRef]
- Damergi, B.; Essid, R.; Fares, N.; Khadraoui, N.; Ageitos, L.; Alaya, A.B.; Gharbi, D.; Abid, I.; Alothman, M.R.; Limam, F.; et al. Datura stramonium Flowers as a Potential Natural Resource of Bioactive Molecules: Identification of Anti-Inflammatory Agents and Molecular Docking Analysis. Molecules 2023, 28, 5195. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-Y.; Zhou, Y.-Q.; Liu, Y.; Lu, Z.-K.; Kuang, H.-X. Withanolides as Potential Immunosuppressive Agents against RAW264.7 Cells from the Pericarps of Datura metel. Nat. Prod. Commun. 2017, 12. [Google Scholar] [CrossRef]
- Wei, Z.; Zhong, H.; Yuan, S.; Chen, C. Daturataturin A ameliorates psoriasis by regulating PPAR pathway. Biochem. Genet. 2024, 62, 4952–4966. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Xiang, L.; Zhou, F.; Li, H.; Su, Y.; Xu, X.; Wang, Q. Metabolites Identification of Bioactive Compounds Daturataturin A, Daturametelin I, N-Trans-Feruloyltyramine, and Cannabisin F from the Seeds of Datura metel in Rats. Front. Pharmacol. 2018, 9, 731. [Google Scholar] [CrossRef]
- Wei, Z.; Li, T.; Sun, Y.; Su, H.; Zeng, Y.; Wang, Q.; Kuang, H. Daturataturin A, a withanolide in Datura metel L., induces HaCaT autophagy through the PI3K-Akt-mTOR signaling pathway. Phytother. Res. 2021, 35, 1546–1558. [Google Scholar] [CrossRef]
- Marahatha, R.; Gyawali, K.; Sharma, K.; Gyawali, N.; Tandan, P.; Adhikari, A.; Timilsina, G.; Bhattarai, S.; Lamichhane, G.; Acharya, A.; et al. Pharmacologic activities of phytosteroids in inflammatory diseases: Mechanism of action and therapeutic potentials. Phytother. Res. 2021, 35, 5103–5124. [Google Scholar] [CrossRef]
- Pan, J.; Liu, Y.; Li, X.; Wang, S.; Wu, J.; Li, M.; Guan, W.; Algradi, A.M.; Kuang, H.; Yang, B. Cytotoxic withanolides from the leaves of Datura stramonium L. Chem. Biodivers. 2023, 21, e202301655. [Google Scholar] [CrossRef]
- Baig, M.W.; Nasir, B.; Waseem, D.; Majid, M.; Khan, M.Z.I.; Haq, I.-U. Withametelin: A biologically active withanolide in cancer, inflammation, pain, and depression. Saudi Pharm. J. 2020, 28, 1526–1537. [Google Scholar] [CrossRef]
- Baig, M.W.; Majid, M.; Nasir, B.; Hassan, S.S.U.; Bungau, S.; Haq, I.-U. Toxicity evaluation induced by single and 28-days repeated exposure of withametelin and Daturaolone in Sprague Dawley rats. Front. Pharmacol. 2022, 13, 999078. [Google Scholar] [CrossRef]
- Rao, P.C.; Begum, S.; Jahromi, M.A.F.; Jahromi, Z.H.; Sriram, S.; Sahai, M. Cytotoxicity of withasteroids: Withametelin induces cell cycle arrest at G2/M phase and mitochondria-mediated apoptosis in non-small cell lung cancer A549 cells. Tumor Biol. 2016, 37, 12579–12587. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Ahmed, M.; Baig, M.W.; Tahir, M.N.; Kondratyuk, T.P.; Pezzuto, J.M.; Ul-Haq, I. Cancer Chemopreventive and Cytotoxic Activities of Isowithametelin from Datura innoxia. Rev. Bras. Farmacogn. 2020, 30, 723–728. [Google Scholar] [CrossRef]
- Fathima, A.; Jahromi, M.A.F.; Begum, S.A.; Jamma, T. Withametelin inhibits TGF-β induced Epithelial-to-Mesenchymal Transition and Programmed-Death Ligand-1 expression in vitro. Front. Oncol. 2024, 14, 1435516. [Google Scholar] [CrossRef]
- Maldonado, E.; Ramírez-Apan, T.; Martínez, M. Cytotoxic withanolides from Datura innoxia. Z. Für Naturforschung C 2020, 76, 251–255. [Google Scholar] [CrossRef]
- Akhtar, N.; Baig, M.W.; Haq, I.-U.; Rajeeve, V.; Cutillas, P.R. Withanolide metabolites inhibit PI3K/AKT and MAPK Pro-Survival pathways and induce apoptosis in acute myeloid leukemia cells. Biomedicines 2020, 8, 333. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Cao, S.; Kang, N.; Qiu, F. Withanolides: Promising candidates for cancer therapy. Phytother. Res. 2024, 38, 1104–1158. [Google Scholar] [CrossRef]
- Rocha, K.A.D.; De Freitas Paulo, T.; Ayala, A.P.; Da Silva Sampaio, V.; Nunes, P.I.G.; Santos, F.A.; Canuto, K.M.; Silveira, E.R.; Pessoa, O.D.L. Anti-inflammatory withajardins from the leaves of Athenaea velutina. Phytochemistry 2022, 203, 113338. [Google Scholar] [CrossRef]
- Khan, A.; Shal, B.; Khan, A.U.; Ullah, R.; Baig, M.W.; Haq, I.U.; Seo, E.K.; Khan, S. Suppression of TRPV1/TRPM8/P2Y nociceptors by withametelin via downregulating MAPK signaling in mouse model of Vincristine-Induced neuropathic pain. Int. J. Mol. Sci. 2021, 22, 6084. [Google Scholar] [CrossRef]
- Xia, G.-Y.; Cao, S.-J.; Chen, L.-X.; Qiu, F. Natural withanolides, an update. Nat. Prod. Rep. 2021, 39, 784–813. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Shal, B.; Khan, A.U.; Baig, M.W.; Haq, I.U.; Khan, S. Withametelin, a steroidal lactone isolated from Datura innoxia, attenuates STZ-induced diabetic neuropathic pain in rats through inhibition of NF-kB/MAPK signaling. Food Chem. Toxicol. 2023, 175, 113742. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Liu, S.; Liu, Y.; Li, C.; Wang, L. Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis. Antioxidants 2022, 11, 1495. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.U.; Ahmed, S.; Choudhury, A.; Hussain, A.; Alajmi, M.F.; Mohammad, T.; Hassan, M.I. Discovering novel inhibitors of RfaH from Klebsiella pneumoniae to combat antimicrobial resistance. Arch. Microbiol. 2024, 206, 472. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Cheng, Y.-B.; Chuang, Y.-T.; Yang, K.-H.; Chang, F.-R.; Liu, W.; Chang, H.-W. Oxidative stress and AKT-associated angiogenesis in a zebrafish model and its potential application for withanolides. Cells 2022, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, W.; Yang, C.-L.; Luo, Y.-M.; Liu, Y.; Zhou, Y.-Y.; Liu, L.-N.; Yang, B.-Y.; Kuang, H.-X. Steroids with potential anti-inflammatory activity from the roots of Datura metel L. Can. J. Chem. 2019, 98, 74–78. [Google Scholar] [CrossRef]
- Das, S.; Mazumder, A.; Pentela, B.; Das, S.; Ghai, R. Phytochemical components in Datura metel plant and their therapeutic properties. Allelopath. J. 2023, 60, 209–220. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Medical importance of Datura fastuosa (syn: Datura metel) and Datura stramonium—A review. IOSR J. Pharm. 2017, 7, 43–58. [Google Scholar] [CrossRef]
- Sharma, M.; Dhaliwal, I.; Rana, K.; Delta, A.K.; Kaushik, P. Phytochemistry, Pharmacology, and Toxicology of Datura Species—A review. Antioxidants 2021, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, A.S.; Bharadwaj, R.; Baxi, M.; Zhylkybekova, A. Evaluating the Therapeutic Potential of Datura stramonium and Datura inoxia: A Mini Review. West Kazakhstan Med. J. 2024, 66, 119–125. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Tain, T.; Yu, J.-Y.; Li, J.; Xu, B.; Chen, J.; D’Auria, J.C.; Huang, J.-P.; Huang, S.-X. Genomic and Structural Basis for the Evolution of Tropane Alkaloid Biosynthesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2302448120. [Google Scholar] [CrossRef]
- Srinivasan, P.; Smolke, C.D. Engineering a microbial biosynthesis platform for de novo production of tropane alkaloids. Nat. Commun. 2019, 10, 3634. [Google Scholar] [CrossRef]
- Shim, K.H.; Kang, M.J.; Sharma, N.; An, S.S.A. Beauty of the beast: Anticholinergic tropane alkaloids in therapeutics. Nat. Prod. Bioprospecting 2022, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Aka, I.; Bernal, C.J.; Carroll, R.; Maxwell-Horn, A.; Oshikoya, K.A.; Van Driest, S.L. Clinical Pharmacogenetics of Cytochrome P450-Associated Drugs in Children. J. Pers. Med. 2017, 7, 14. [Google Scholar] [CrossRef]
- Sadre, R.; Anthony, T.M.; Grabar, J.M.; Bedewitz, M.A.; Jones, A.D.; Barry, C.S. Metabolomics-guided discovery of cytochrome P450s involved in pseudotropine-dependent biosynthesis of modified tropane alkaloids. Nat. Commun. 2022, 13, 3832. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Marx, J. Datura stramonium in asthma treatment and possible effects on prenatal development. Environ. Toxicol. Pharmacol. 2006, 21, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Rifa’i, M.; Satwika, D.; Am, A. Datura metel Linn ameliorates asthma symptoms in BALB/c mice. J. Bio-Sci. 2016, 22, 1–8. [Google Scholar] [CrossRef][Green Version]
- Mutebi, R.R.; Ario, A.R.; Nabatanzi, M.; Kyamwine, I.B.; Wibabara, Y.; Muwereza, P.; Eurien, D.; Kwesiga, B.; Bulage, L.; Kabwama, S.N.; et al. Large outbreak of Jimsonweed (Datura stramonium) poisoning due to consumption of contaminated humanitarian relief food: Uganda, March–April 2019. BMC Public Health 2022, 22, 623. [Google Scholar] [CrossRef] [PubMed]

| Compound | Core Structure | Occurrence | Structural Modification | Primary Use/Utility | Distribution |
|---|---|---|---|---|---|
| Atropine | Tropane (7-azabicyclo[3.2.1]octane) esterified with tropic acid | Major alkaloid in seeds and leaves of D. stramonium, D. metel | Racemic mixture of (S)- and (R)-hyoscyamine enantiomers | Organophosphate antidote; preanesthetic antisialagogue; mydriatic/cycloplegic | Widespread—reported in multiple Solanaceae genera (Atropa, Hyoscyamus, Scopolia, Duboisia, Datura) |
| Hyoscyamine | Tropane core + tropic acid ester | Predominates in young leaves, seeds and roots of D. stramonium/D. metel | Pure (S)-enantiomer of hyoscyamine (levo-form) | GI antispasmodic (IBS, colic); adjunct in Parkinson’s; visceral pain relief | Widespread—multiple Solanaceae (Atropa, Hyoscyamus, Scopolia, Duboisia, Datura) |
| Scopolamine | Tropane core + tropic acid ester + 6β-epoxide bridge | High in flowers and young leaves of D. stramonium, D. innoxia | 6β-Epoxide on the hyoscyamine backbone | Motion sickness prophylaxis (TTS patch); PONV antiemetic; preanesthetic; ophthalmic mydriatic | Widespread—multiple Solanaceae genera |
| Anisodamine | Tropane core + tropic acid ester + 6β-hydroxyl | Trace in stems, leaves and seeds of D. stramonium | Addition of C-6β hydroxyl to hyoscyamine | Septic shock therapy (China); slowing of myopia progression ophthalmically | Widespread—reported in Anisodus, Hyoscyamus, Datura |
| Cuscohygrine | Bis-N-methylpyrrolidine rings linked by a propanone bridge | Trace alkaloid in seeds, roots and leaves of Datura spp. | Lacks bicyclic tropane core; two N-methylpyrrolidine moieties | Analytical/chemotaxonomic marker in alkaloid profiling; biosynthetic intermediate | Widespread—Solanaceae and Erythroxylaceae (Erythroxylum coca) |
| Littorine | Tropane core + phenyllactic acid ester | Concentrated in the roots of D. stramonium; lower in the aerial parts | Tropine esterified with phenyllactic (instead of tropic) acid | Biosynthetic precursor to hyoscyamine; metabolic tracer; species authentication | Widespread—multiple tropane-producing Solanaceae |
| Datumetine | Tropane core + 4-methoxybenzoic acid ester | Trace in leaves of D. metel | Ester linkage to 4-methoxybenzoate rather than tropic acid | Research tool as NMDAR modulator; neuroprotective/excitotoxicity studies | Reported primarily from Datura (esp. D. metel); no records in other genera to date |
| Compound | Core Structure | Occurrence | Structural Modification | Primary Use/Utility |
|---|---|---|---|---|
| Hygrine | N-Methylpyrrolidin-2-one (monopyrrolidine ring + ketone side chain) | Trace in seeds, roots and young leaves (0.02–0.1 mg/g DW *) | Lacks bicyclic tropane; simple pyrrolidine with an acetone moiety | Early biosynthetic intermediate; stable isotope tracer; analytical internal standard for HPLC–MS profiling |
| Apoatropine | Tropine core esterified to 2-phenylprop-2-enoic (atropic) acid | Minor in stems and leaves of Datura spp. | An unsaturated atropic acid ester replaces tropic acid | Structure–activity probe in tropane SAR studies; analytical marker differentiating tropane esters |
| Anisodine | 7-Azatricyclo[3.2.1.02,4]-nonane core with 3-oxa epoxide + benzoate ester | Trace in flowers, leaves and seeds (≤ 0.05 mg/g DW *) | Epoxide bridge (C-6→C-7) and esterified to α-hydroxybenzoic acid | Research tool for M1–M5/α1 receptor pharmacology; template for anticholinergic–vasodilator hybrids |
| Tropine | 7-Azabicyclo[3.2.1]octan-3-ol (tropane ring + C-3 hydroxyl) | Abundant in roots (0.3–0.5 mg/g DW *) & aerial parts (0.1–0.2 mg/g DW *) | De-esterified tropane (no acyl side chain) | Universal precursor for tropane esters (atropine, scopolamine); synthetic scaffold for inhaled/quaternary anticholinergics; analytical standard |
| Group | Compound | Key Structural Features | CNS Penetration | Main Use | Mechanism of Action |
|---|---|---|---|---|---|
| Quaternary Ammonium Derivatives | Ipratropium Bromide | Tropane derivative; isopropyl on ester | Minimal | COPD | Non-selective muscarinic antagonist; peripheral bronchodilation |
| Tiotropium Bromide | thiophene rings; tropane core | Minimal | Long-acting COPD | Selective M3 antagonist; prolonged bronchodilation | |
| Methscopolamine | 6,7-epoxide tropane; scopolamine-derived | Minimal | Peptic ulcers; GI spasms | Peripheral muscarinic antagonist; antisecretory and antispasmodic | |
| Trospium Chloride | benzilic acid ester with a tropane backbone | Minimal | Overactive bladder; urinary frequency | M3-preferring antagonist; inhibits detrusor overactivity | |
| Tertiary Amine Derivatives | Homatropine hydrobromide | Tropine and mandelic acid ester | Moderate | Ophthalmic mydriasis; cough suppressant adjunct | Non-selective muscarinic antagonist; central and peripheral action |
| Tropicamide | Tropic acid and pyridyl amide | Low to moderate | Diagnostic mydriasis/cycloplegia | Non-selective muscarinic antagonist; rapid receptor dissociation | |
| Cyclopentolate | Cyclopentyl tropate ester and dimethylaminoethanol | Moderate | Pediatric eye exams; diagnostic cycloplegia | Muscarinic antagonist; short-acting cycloplegic | |
| Oxybutynin (Oxytrol) | Cyclohexyl phenyl ester and butynyl diethylamino chain | High (oral); Low (patch) | Overactive bladder; neurogenic bladder | Primarily, it is an M3 antagonist, inhibiting detrusor muscle contraction. | |
| Tolterodine | Phenylpropanolamine scaffold; hydroxylated aryl and diisopropylamine | Low | Overactive bladder; urinary urgency | M2/M3 antagonist; reduces bladder contractions | |
| Trihexyphenidyl (Benzhexol) | Cyclohexyl phenyl tertiary alcohol and piperidine | High | Parkinsonism; antipsychotic-induced EPS | Central M1 antagonist; modulates basal ganglia cholinergic tone |
| Compound | Structural Class | Key Activities | Target Pathways/Mechanisms | Therapeutic Applications |
|---|---|---|---|---|
| Harmane | β-Carboline alkaloid | Antioxidant, neuroprotective, MAO inhibitor, tremor-inducing | MAO-A/B inhibition, serotonin modulation | Parkinson’s, Alzheimer’s, depression, HIV2 |
| Norharmane | β-Carboline alkaloid | Antidepressant, anticancer, MAO inhibitor, photosensitizer | MAO-A/B inhibition, PIN transport inhibition | Depression, cancer, neurodegeneration4 |
| Daturafolisides A–I | Steroidal glycosides (withanolides) | Anti-inflammatory, immunosuppressive, cytotoxic | NF-κB, MAPK, COX-2, and iNOS inhibition | Psoriasis, cancer, and autoimmune disorders |
| Daturataturin A and B | Withanolide glycosides | Anti-inflammatory, autophagy-inducing, immunosuppressive, cytotoxic | PI3K-Akt-mTOR, ERK/JNK/p38, NF-κB | Psoriasis, cancer, chronic inflammation5 |
| Withametelins I–P | Withanolide-type lactones | Cytotoxic, anti-inflammatory, antioxidant, and kinase inhibition | NF-κB, MAPK, CDKs, Nrf2/Keap1/HO-1 | Cancer, neuroinflammation, and autoimmune diseases |
| Daturametelin J | Withanolide lactone glycoside | Anti-inflammatory, cytotoxic, antioxidant, neuroprotective | NF-κB, COX-2, iNOS, Nrf2/HO-1 | Psoriasis, cancer, Alzheimer’s, Parkinson’s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thawabteh, A.M.; Sulaiman, S.; Alabed, I.O.; Scrano, L.; Karaman, D.; Karaman, R.; Bufo, S.A. Bioorganic Chemistry, Toxinology, and Pharmaceutical Uses of Datura Metabolites and Derivatives. Toxins 2025, 17, 469. https://doi.org/10.3390/toxins17090469
Thawabteh AM, Sulaiman S, Alabed IO, Scrano L, Karaman D, Karaman R, Bufo SA. Bioorganic Chemistry, Toxinology, and Pharmaceutical Uses of Datura Metabolites and Derivatives. Toxins. 2025; 17(9):469. https://doi.org/10.3390/toxins17090469
Chicago/Turabian StyleThawabteh, Amin Mahmood, Saleh Sulaiman, Ilaf Omar Alabed, Laura Scrano, Donia Karaman, Rafik Karaman, and Sabino A. Bufo. 2025. "Bioorganic Chemistry, Toxinology, and Pharmaceutical Uses of Datura Metabolites and Derivatives" Toxins 17, no. 9: 469. https://doi.org/10.3390/toxins17090469
APA StyleThawabteh, A. M., Sulaiman, S., Alabed, I. O., Scrano, L., Karaman, D., Karaman, R., & Bufo, S. A. (2025). Bioorganic Chemistry, Toxinology, and Pharmaceutical Uses of Datura Metabolites and Derivatives. Toxins, 17(9), 469. https://doi.org/10.3390/toxins17090469










