Chlorogenic Acid and VX765 Alleviate Deoxynivalenol-Induced Enterohepatic Injury and Lipid Metabolism Disorders by Improving Intestinal Microecology
Abstract
1. Introduction
2. Results
2.1. CGA and VX765 Alleviate DON-Induced Intestinal Barrier Breakdown
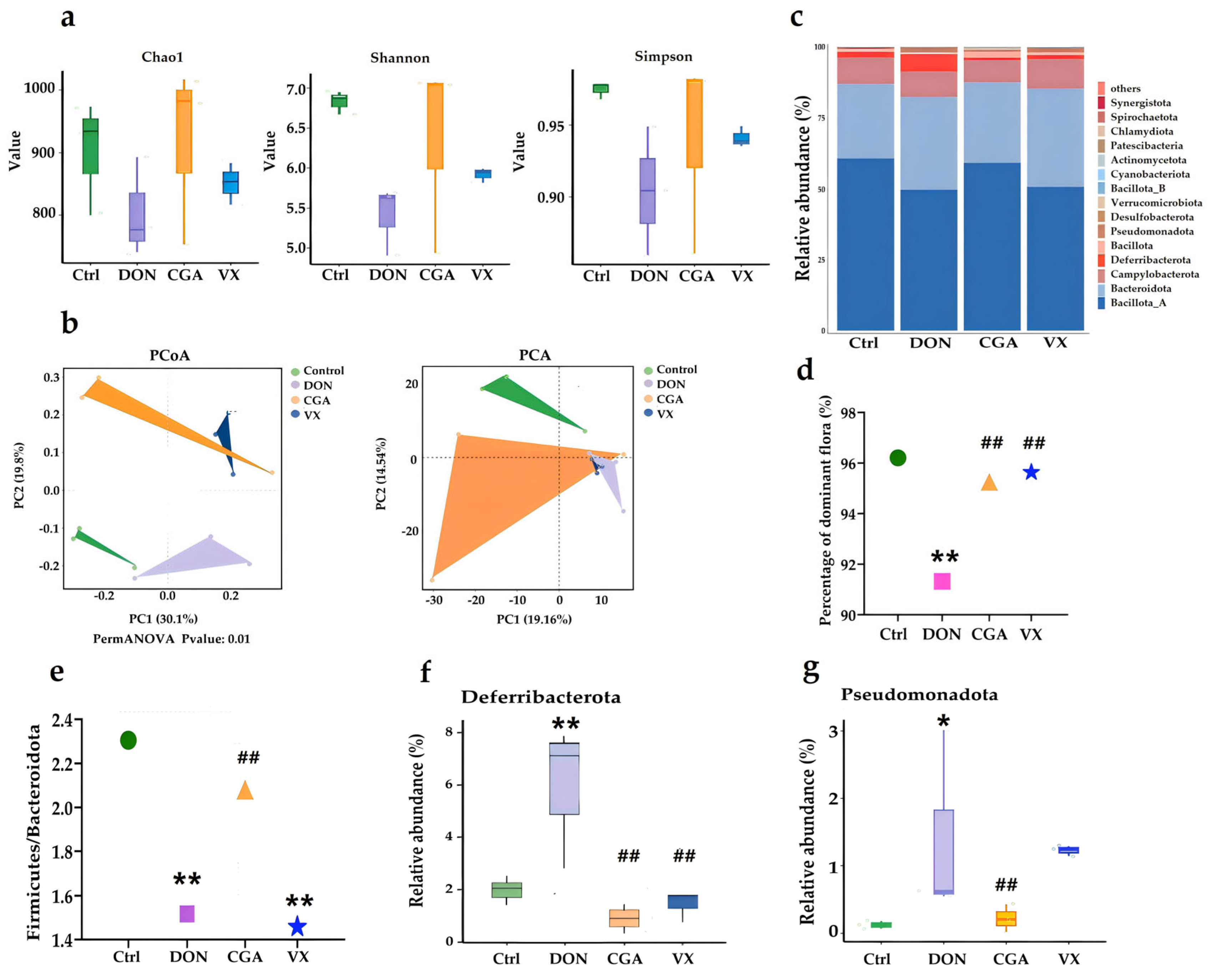
2.2. CGA and VX765 Intervene in the Intestinal Flora Disturbance Induced by DON
2.3. CGA and VX765 Intervene in the Cecal Fungal Flora Induced by DON
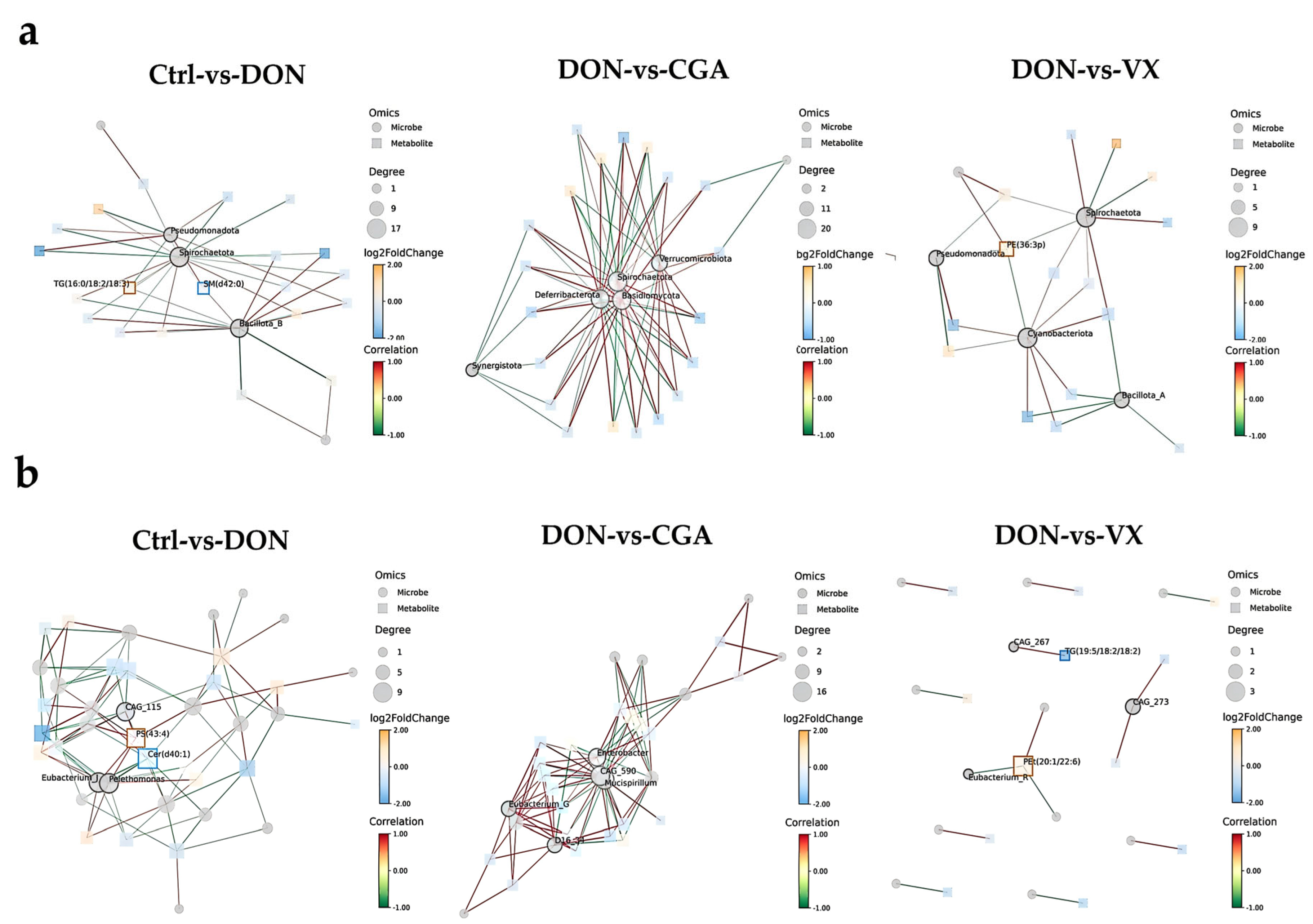
2.4. Correlation Analysis of Liver Lipid Metabolism and Cecal Microbiota
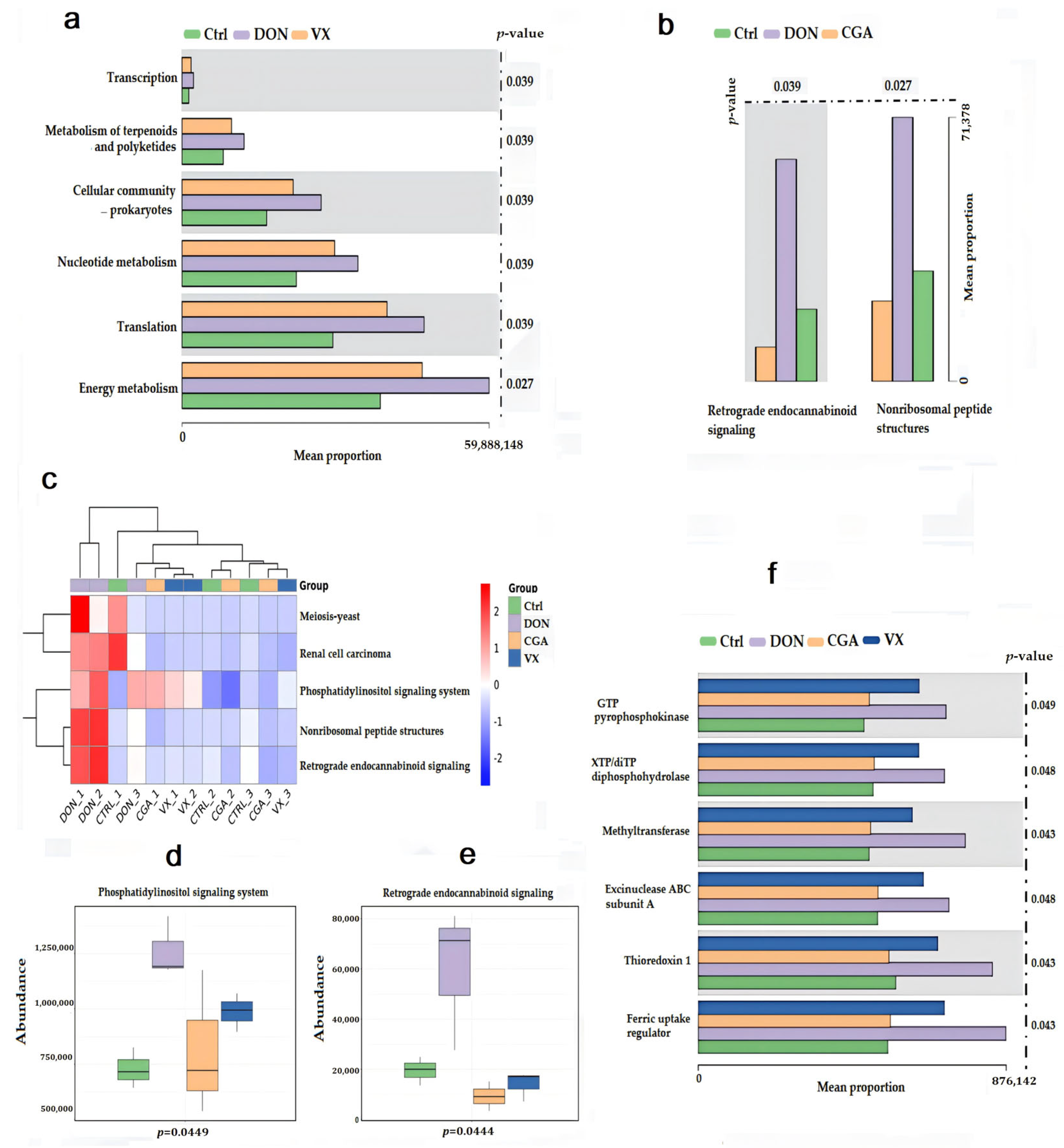
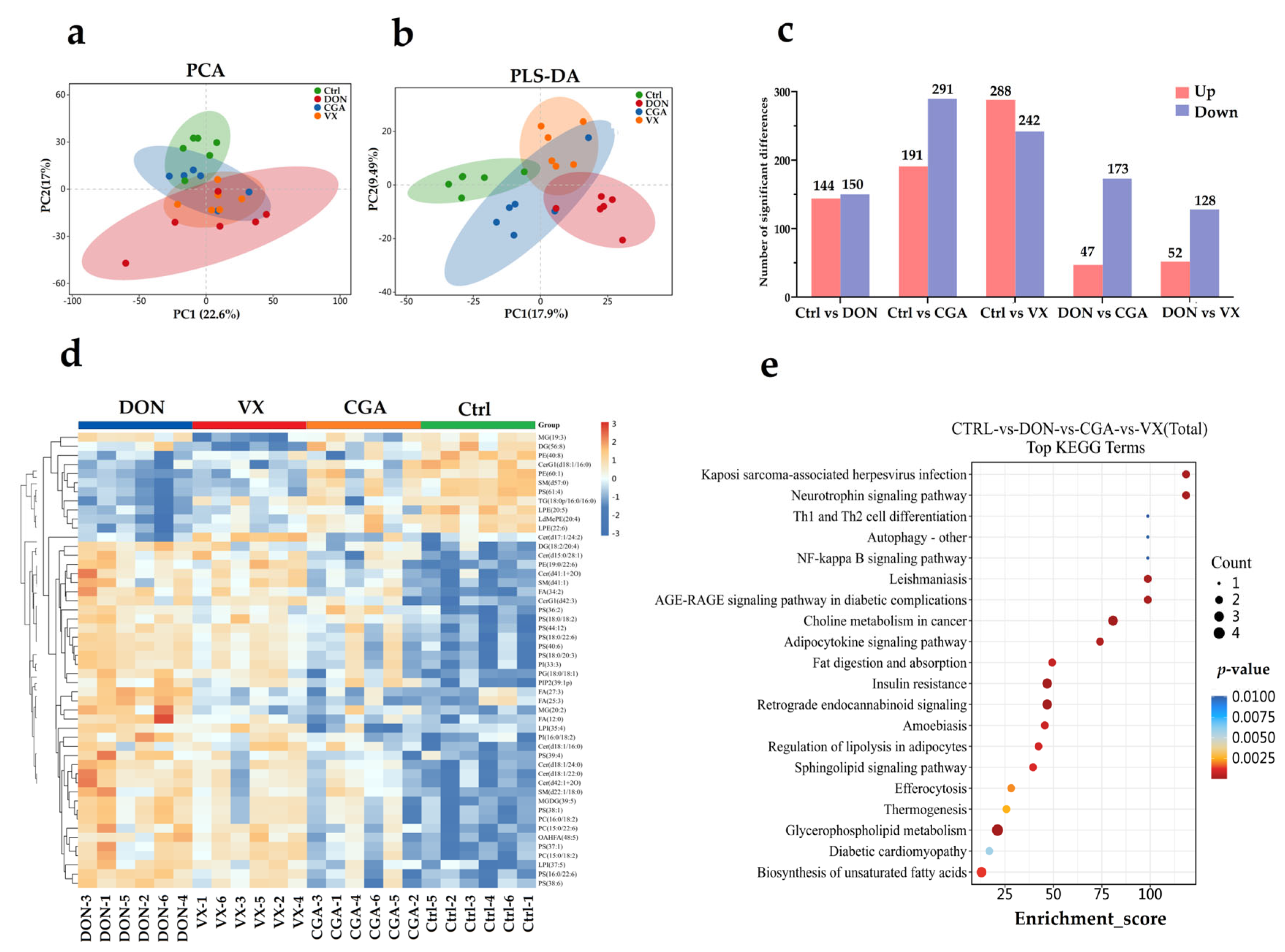
2.5. CGA and VX765 Intervene in Gene Expression Related to Lipid Metabolism in the Mice Liver
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animal, Experimental Design and Sampling
4.3. Hematoxylin-Eosin (HE) Staining
4.4. qRT-PCR
4.5. Western Blotting (WB)
4.6. Immunofluorescence (IF)
4.7. UPLC-MS/MS
4.8. 2bRAD-M
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| CDS2 | CDP—diacylglycerol synthase 2 |
| Cer | Ceramide |
| CGA | Chlorogenic acid |
| DON | Deoxynivalenol |
| DGKA | Diacylglycerol Kinase Alpha |
| F/B ratio | Firmicutes to Bacteroidetes |
| GPX4 | Glutathione peroxidase 4 |
| IMPA | Inositol Monophosphatase |
| MAPK1 | Mitogen-Activated Protein Kinase-1 |
| MUC2 | Mucin2 |
| NDUFS8 | NADH:Ubiquinone Oxidoreductase Core Subunit S8 |
| PCA | Principal component analysis |
| PCoA | Principal coordinate analysis |
| PS | Phosphatidylserine |
| VIP | Variable importance in projection |
| ZO-1 | Zonula occludens protein 1 |
References
- Abraham, N.; Schroeter, K.L.; Zhu, Y.; Chan, J.; Evans, N.; Kimber, M.S.; Carere, J.; Zhou, T.; Seah, S.Y.K. Structure-function characterization of an aldo-keto reductase involved in detoxification of the mycotoxin, deoxynivalenol. Sci. Rep. 2022, 12, 14737. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ji, J.; Wang, J.S.; Sun, X. Deoxynivalenol: Masked forms, fate during food processing, and potential biological remedies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 895–926. [Google Scholar] [CrossRef]
- Li, Y.; Gao, H.; Wangm, R.; Xu, Q. Deoxynivalenol in food and feed: Recent advances in decontamination strategies. Front. Microbiol. 2023, 14, 1141378. [Google Scholar] [CrossRef]
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef]
- Faeste, C.K.; Pierre, F.; Ivanova, L.; Sayyari, A.; Massotte, D. Behavioural and metabolomic changes from chronic dietary exposure to low-level deoxynivalenol reveal impact on mouse well-being. Arch. Toxicol. 2019, 93, 2087–2102. [Google Scholar] [CrossRef]
- Li, F.; Huang, L.; Liu, Q.; Wang, P.; Chen, H.; Wang, C. Different metabolites induced by deoxynivalenol in the serum and urine of weaned rabbits detected using LC-MS-based metabolomics. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 250, 109184. [Google Scholar] [CrossRef]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef]
- Gonya, S.; Kallmerten, P.; Dinapoli, P. Are Infants and Children at Risk of Adverse Health Effects from Dietary Deoxynivalenol Exposure? An Integrative Review. Int. J. Environ. Res. Public Health 2024, 21, 808. [Google Scholar] [CrossRef] [PubMed]
- Serkan, Y.; Beyazit, U.; Ayhan, A.B. Mycotoxin Exposure and Autism: A Systematic Review of the Molecular Mechanism. Curr. Mol. Pharmacol. 2021, 14, 853–859. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha Sharma, K.K. Gut-organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.H.; Jin, Z.; Yang, X.X.; Lou, J.; Shan, W.X.; Hu, Y.X.; Du, Q.; Liao, Q.S.; Xie, R.; Xu, J.Y. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J. Gastroenterol. 2020, 26, 6141–6162. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Mande, S.S. Host-microbiome interactions: Gut-Liver axis and its connection with other organs. NPJ Biofilms Microbiomes 2022, 8, 89. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef]
- Hasuda, A.L.; Person, E.; Khoshal, A.K.; Bruel, S.; Puel, S.; Oswald, I.P.; Bracarense, A.P.F.R.L.; Pinton, P. Deoxynivalenol induces apoptosis and inflammation in the liver: Analysis using precision-cut liver slices. Food Chem. Toxicol. 2022, 163, 112930. [Google Scholar] [CrossRef]
- Mao, X.; Li, J.; Xie, X.; Chen, S.; Huang, Q.; Mu, P.; Jiang, J.; Deng, Y. Deoxynivalenol induces caspase-3/GSDME-dependent pyroptosis and inflammation in mouse liver and HepaRG cells. Arch. Toxicol. 2022, 96, 3091–3112. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, Z.; Wang, H.; Sun, J.H.; Wang, G.; Jia, A.; Yan, Y.X. Protective Mechanisms of Various Active Substances on Cell DNA Damage and Apoptosis Induced by Deoxynivalenol. J. Agric. Food Chem. 2024, 72, 6651–6659. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Vázquez, S.; Codina Moreno, R.; Della Badia, A.; Castro, O.; Riahi, I. Promising Phytogenic Feed Additives Used as Anti-Mycotoxin Solutions in Animal Nutrition. Toxins 2024, 16, 434. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, F.; Li, R.; Zhang, X.; Guo, H.; Wang, C. Chlorogenic acid alleviates IPEC-J2 pyroptosis induced by deoxynivalenol by inhibiting activation of the NF-κB/NLRP3/caspase-1 pathway. J. Anim. Sci. Biotechnol. 2024, 15, 159. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Z.; Wu, N.; Li, J.; Xi, N.; Xu, M.; Wu, F.; Fu, Q.; Yan, G.; Liu, Y.; et al. Chlorogenic acid attenuates deoxynivalenol-induced apoptosis and pyroptosis in human keratinocytes via activating Nrf2/HO-1 and inhibiting MAPK/NF-κB/NLRP3 pathways. Biomed. Pharmacother. 2024, 170, 116003. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Wang, J.; Liu, W.; Zhao, X.; Qin, Y.; Zhang, C.; Yin, H.; Zhao, C. VX-765 inhibits pyroptosis and reduces inflammation to prevent acute liver failure by upregulating PPARα expression. Ann. Hepatol. 2023, 28, 101082. [Google Scholar] [CrossRef]
- Ye, X.; Lin, Z.J.; Hong, G.H.; Wang, Z.M.; Dou, R.T.; Lin, J.Y.; Xie, J.H.; Shen, Y.W. Pyroptosis inhibitors MCC950 and VX-765 mitigate myocardial injury by alleviating oxidative stress, inflammation, and apoptosis in acute myocardial hypoxia. Exp. Cell Res. 2024, 438, 114061. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Fang, H.; Yu, H.; Zhao, Y.; Shen, J.; Zhou, C.; Jin, Y. Pterostilbene inhibits deoxynivalenol-induced oxidative stress and inflammatory response in bovine mammary epithelial cells. Toxicon 2020, 189, 10–18. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Y.; Wu, S.; Yu, H.; Zhao, Y.; Fang, H.; Shen, J.; Zhou, C.; Fu, Y.; Li, R.; et al. Deoxynivalenol induces oxidative stress, inflammatory response and apoptosis in bovine mammary epithelial cells. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lu, X.; Guo, E.; Mo, J.; Xv, Y. Preclinical evidence for luteolin in ulcerative colitis: A meta-analysis and systematic review. Front. Pharmacol. 2025, 16, 1639644. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Gremmels, H.; Koelink, P.J.; Verheijden, K.A.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol: A trigger for intestinal integrity breakdown. FASEB J. 2014, 28, 2414–2429. [Google Scholar] [CrossRef]
- Pinto, A.C.S.M.; De Pierri, C.R.; Evangelista, A.G.; Gomes, A.S.L.P.B.; Luciano, F.B. Deoxynivalenol: Toxicology, Degradation by Bacteria, and Phylogenetic Analysis. Toxins 2022, 14, 90. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, S.; Zhu, P.; Tzehau, L.; Zhao, H.; Lv, J.; Zhang, R.; Zhou, L.; Niu, Q.; Wang, X.; et al. Species-resolved sequencing of low-biomass or degraded microbiomes using 2bRAD-M. Genome Biol. 2022, 23, 36. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, J.; Li, S.; Chen, J.; Wang, H.; Li, C.; Zhang, S. 16S rRNA, metagenomics and 2bRAD-M sequencing to decode human thanatomicrobiome. Sci. Data 2024, 11, 736. [Google Scholar] [CrossRef]
- Duca, F.A.; Lam, T.K. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes. Metab. 2014, 1, 68–76. [Google Scholar] [CrossRef]
- Baek, G.H.; Yoo, K.M.; Kim, S.Y.; Lee, D.H.; Chung, H.; Jung, S.C.; Park, S.K.; Kim, J.S. Collagen Peptide Exerts an Anti-Obesity Effect by Influencing the Firmicutes/Bacteroidetes Ratio in the Gut. Nutrients 2023, 15, 2610. [Google Scholar] [CrossRef] [PubMed]
- Paziewska, M.; Szelest, M.; Kiełbus, M.; Masternak, M.; Zaleska, J.; Wawrzyniak, E.; Kotkowska, A.; Siemieniuk-Ryś, M.; Morawska, M.; Kalicińska, E.; et al. Increased abundance of Firmicutes and depletion of Bacteroidota predicts poor outcome in chronic lymphocytic leukemia. Oncol. Lett. 2024, 28, 552. [Google Scholar] [CrossRef]
- Hao, Y.; Feng, Y.; Yan, X.; Chen, L.; Ma, X.; Tang, X.; Zhong, R.; Sun, Z.; Agarwal, M.; Zhang, H.; et al. Gut Microbiota-Testis Axis: FMT Mitigates High-Fat Diet-Diminished Male Fertility via Improving Systemic and Testicular Metabolome. Microbiol. Spectr. 2022, 10, e0002822. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, T.; Moens de Hase, E.; Van Hul, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Depommier, C.; Druart, C.; Everard, A.; Maiter, D.; et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut 2022, 71, 534–543. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, J.; Shan, Y.; Hong, J.; Ye, Q.; Dai, Y.; Hu, J.; Zhang, J.; Li, C.; Wen, H. Comprehensive assessment of HF-rTMS treatment mechanism for post-stroke dysphagia in rats by integration of fecal metabolomics and 16S rRNA sequencing. Front. Cell. Infect. Microbiolpgy 2024, 14, 1373737. [Google Scholar] [CrossRef] [PubMed]
- Marion, S.; Desharnais, L.; Studer, N.; Dong, Y.; Notter, M.D.; Poudel, S.; Menin, L.; Janowczyk, A.; Hettich, R.L.; Hapfelmeier, S.; et al. Biogeography of microbial bile acid transformations along the murine gut. J. Lipid Res. 2020, 61, 1450–1463. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Beyoğlu, D.; Schwalm, S.; Semmo, N.; Huwiler, A.; Idle, J.R.; Hepatitis, C. Virus Infection Upregulates Plasma Phosphosphingolipids and End ocannabinoids and Downregulates Lysophosphoinositols. Int. J. Mol. Sci. 2023, 24, 1407. [Google Scholar] [CrossRef]
- Biernacki, M.; Skrzydlewska, E. Metabolism of endocannabinoids. Postep. Hig. Med. Dosw. 2016, 70, 830–843. [Google Scholar] [CrossRef]
- Hu, P.; Liu, Y.; Li, S.; Zhao, Y.; Gu, H.; Zong, Q.; Ahmed, A.A.; Bao, W.; Liu, H.Y.; Cai, D. Lactoferrin Relieves Deoxynivalenol-Induced Oxidative Stress and Inflammatory Response by Modulating the Nrf2/MAPK Pathways in the Liver. J. Agric. Food Chem. 2023, 71, 8182–8191. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Ho, H.C. Roles of gut microbes in metabolic-associated fatty liver disease. Tzu Chi Med. J. 2023, 35, 279–289. [Google Scholar] [CrossRef]
- Frazier, K.; Manzoor, S.; Carroll, K.; DeLeon, O.; Miyoshi, S.; Miyoshi, J.; St George, M.; Tan, A.; Chrisler, E.A.; Izumo, M.; et al. Gut microbes and the liver circadian clock partition glucose and lipid metabolism. J. Clin. Investig. 2023, 133, e162515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shen, L.; Li, S. Emerging role of inositol monophosphatase in cancer. Biomed. Pharmacother. 2023, 161, 114442. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; Janardan, V.; Barneda, D.; Anderson, K.E.; Niewczas, I.; Taylor, D.; Qiu, D.; Jessen, H.J.; Lopez-Clavijo, A.F.; Walker, S.; et al. CDS2 expression regulates de novo phosphatidic acid synthesis. Biochem. J. 2024, 481, 1449–1473. [Google Scholar] [CrossRef]
- Millings, E.J.; De Rosa, M.C.; Fleet, S.; Watanabe, K.; Rausch, R.; Egli, D.; Li, G.; Leduc, C.A.; Zhang, Y.; Fischer, S.G.; et al. ILDR2 has a negligible role in hepatic steatosis. PLoS ONE 2018, 13, e0197548. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Zhao, Q.; Tao, C.; Qu, W.; Hou, Y.; Huang, R.; Sun, Z.; Zhu, G.; Jiang, X.; et al. Multiomics analysis reveals metabolic subtypes and identifies diacylglycerol kinase α (DGKA) as a potential therapeutic target for intrahepatic cholangiocarcinoma. Cancer Commun. 2024, 44, 226–250. [Google Scholar] [CrossRef]
- Wang, S.; Kang, Y.; Wang, R.; Deng, J.; Yu, Y.; Yu, J.; Wang, J. Emerging Roles of NDUFS8 Located in Mitochondrial Complex I in Different Diseases. Molecules 2022, 27, 8754. [Google Scholar] [CrossRef] [PubMed]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Clevers, H. Organoids and organs-on-chips: Insights into human gut-microbe interactions. Cell Host Microbe 2021, 29, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porc. Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sequence | Length (bp) | Gene ID | GenBank No. | |
|---|---|---|---|---|---|
| Mapk1 | F 1 | CAGGTGTTCGACGTAGGGC | 139 | 26413 | NM_011949.3 |
| R 2 | TCTGGTGCTCAAAAGGACTGA | ||||
| GPX4 | F | GCAGGAGCCAGGAAGTAATCAAG | 112 | 625249 | NM_001037741.4 |
| R | ACAGTGGGTGGGCATCGTC | ||||
| CTA | F | CGTCCGTCCCTGCTGTCTC | 129 | 12359 | NM_009804.2 |
| R | GCTCCTTCCACTGCTTCATCTG | ||||
| NDUFS8 | F | TGCAAACTCTGTGAGGCCAT | 94 | 225887 | NM_144870.5 |
| R | TGTCATAGCGTGTCGTTCGG | ||||
| IMPA | F | GCTGCTGTTAATATGTGCCTTGTG | 110 | 55980 | NM_018864.7 |
| R | CCTGCCTCGGTGACAATGATG | ||||
| DGKA | F | GCCACATCTGAGTCCATCTTCT | 120 | 13139 | NM_016811.4 |
| R | GTTCAATACCGCAATGCCTTCT | ||||
| CDS2 | F | ACCAACCTCCGTAGATGACAC | 87 | 110911 | NM_138651.7 |
| R | CCTCTCACCCACCAGTTCTTC | ||||
| MUC2 | F | ACCTCACAAGCAGTATCAGGC | 136 | 17831 | NM_023566.4 |
| R | GTCATAGCCAGGGGCAAACT | ||||
| Claudin | F | TAAGGCACGGGTAGCACTCAC | 88 | 12741 | NM_009386.3 |
| R | GATGTTGGCGAACCAGCAGAG | ||||
| ZO-1 | F | TCCCACAAGGAGCCATTCCTG | 115 | 21872 | NM_009386.3 |
| R | GGGCTCAGCAGAGTTTCACCT | ||||
| Occludin | F | AGGAGGACTGGGTCAGGGAATA | 123 | 18260 | NM_008756.2 |
| R | TGACGTCGTCTAGTTCTGCCTG | ||||
| GAPDH | F | TGTGTCCGTCGTGGATCTGA | 150 | 14433 | NM_001289726.2 |
| R | TTGCTGTTGAAGTCGCAGGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, T.; Li, S.; Guo, H.; Wang, J.; Zhang, X.; Wang, C. Chlorogenic Acid and VX765 Alleviate Deoxynivalenol-Induced Enterohepatic Injury and Lipid Metabolism Disorders by Improving Intestinal Microecology. Toxins 2025, 17, 467. https://doi.org/10.3390/toxins17090467
Wen T, Li S, Guo H, Wang J, Zhang X, Wang C. Chlorogenic Acid and VX765 Alleviate Deoxynivalenol-Induced Enterohepatic Injury and Lipid Metabolism Disorders by Improving Intestinal Microecology. Toxins. 2025; 17(9):467. https://doi.org/10.3390/toxins17090467
Chicago/Turabian StyleWen, Tao, Sirui Li, Huijun Guo, Jinbo Wang, Xinru Zhang, and Chunyang Wang. 2025. "Chlorogenic Acid and VX765 Alleviate Deoxynivalenol-Induced Enterohepatic Injury and Lipid Metabolism Disorders by Improving Intestinal Microecology" Toxins 17, no. 9: 467. https://doi.org/10.3390/toxins17090467
APA StyleWen, T., Li, S., Guo, H., Wang, J., Zhang, X., & Wang, C. (2025). Chlorogenic Acid and VX765 Alleviate Deoxynivalenol-Induced Enterohepatic Injury and Lipid Metabolism Disorders by Improving Intestinal Microecology. Toxins, 17(9), 467. https://doi.org/10.3390/toxins17090467






