Cost-Effective and Rapid Detection of Tetrodotoxin Using Indium Tin Oxide Electrodes via In Vitro Electrophysiology and Electrochemistry
Abstract
1. Introduction
2. Results
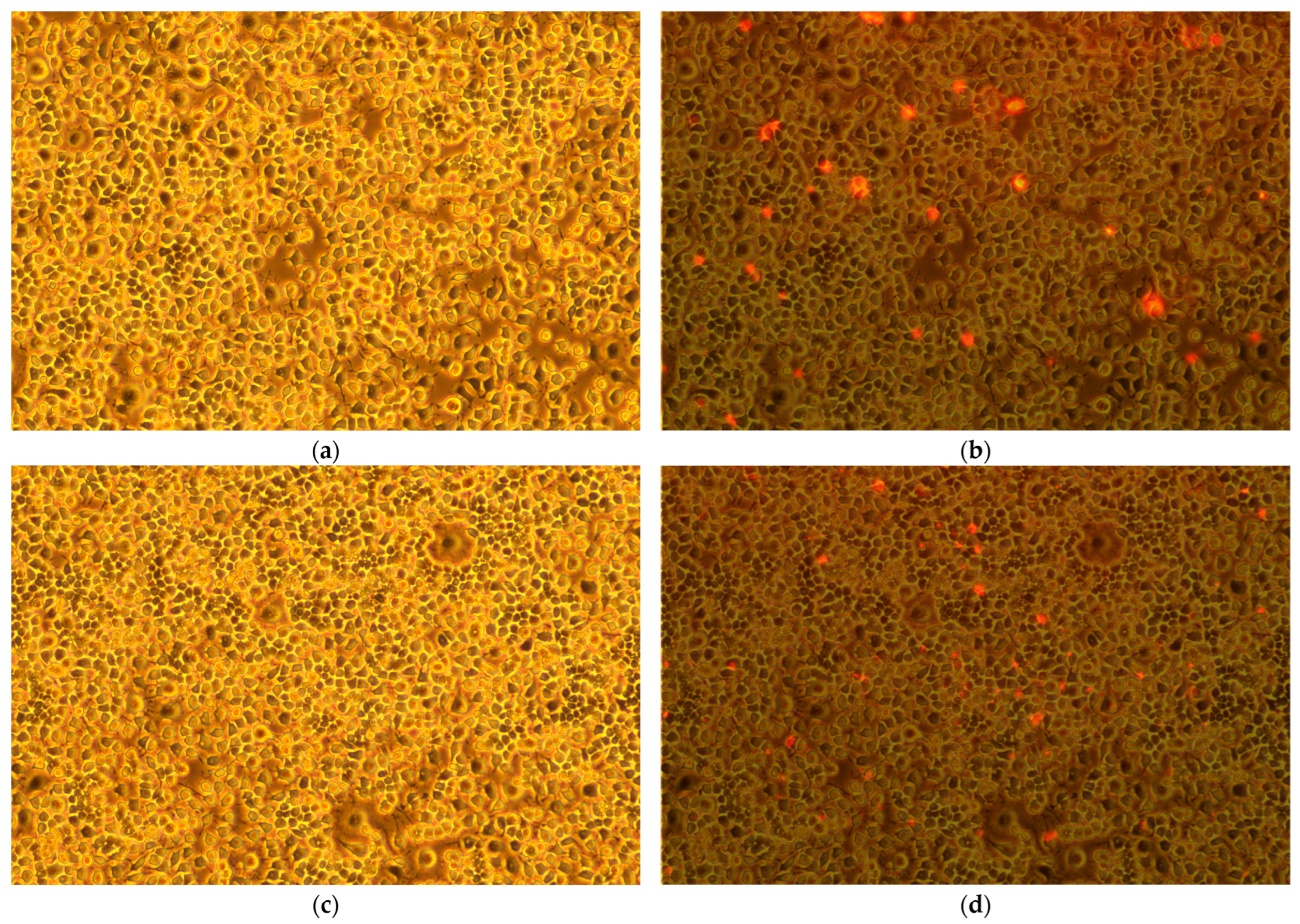
2.1. Confluency and Viability Studies
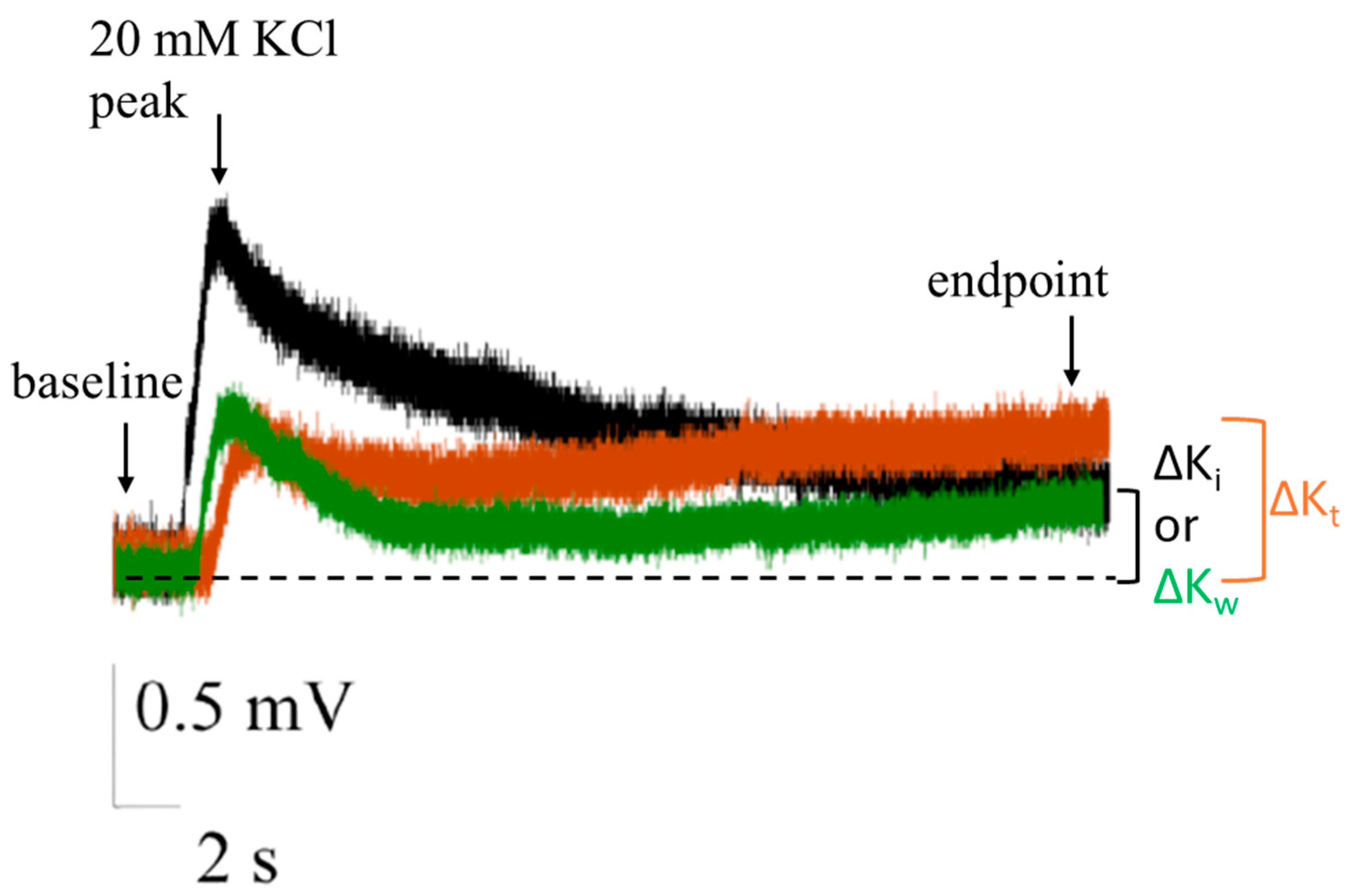
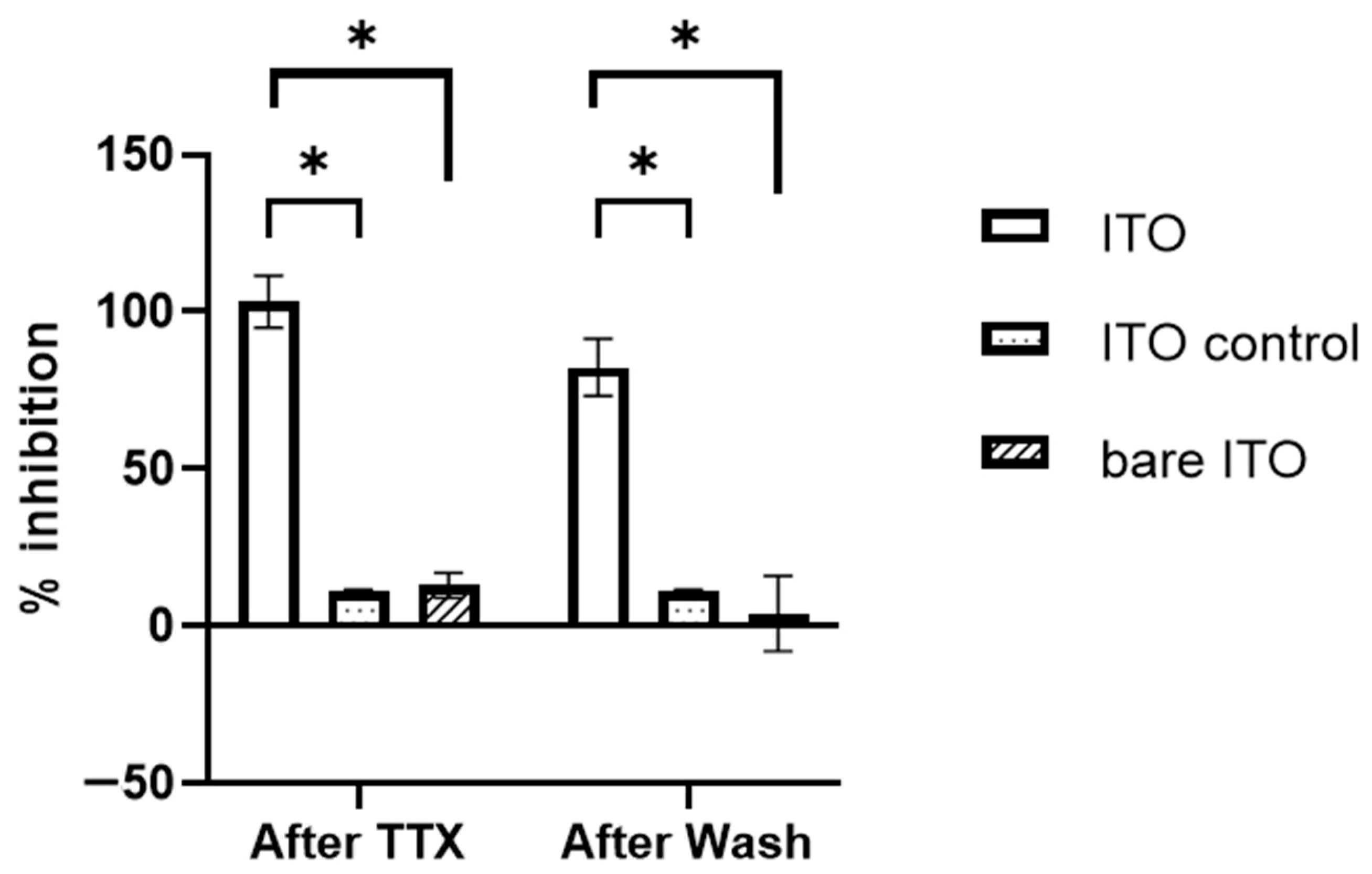
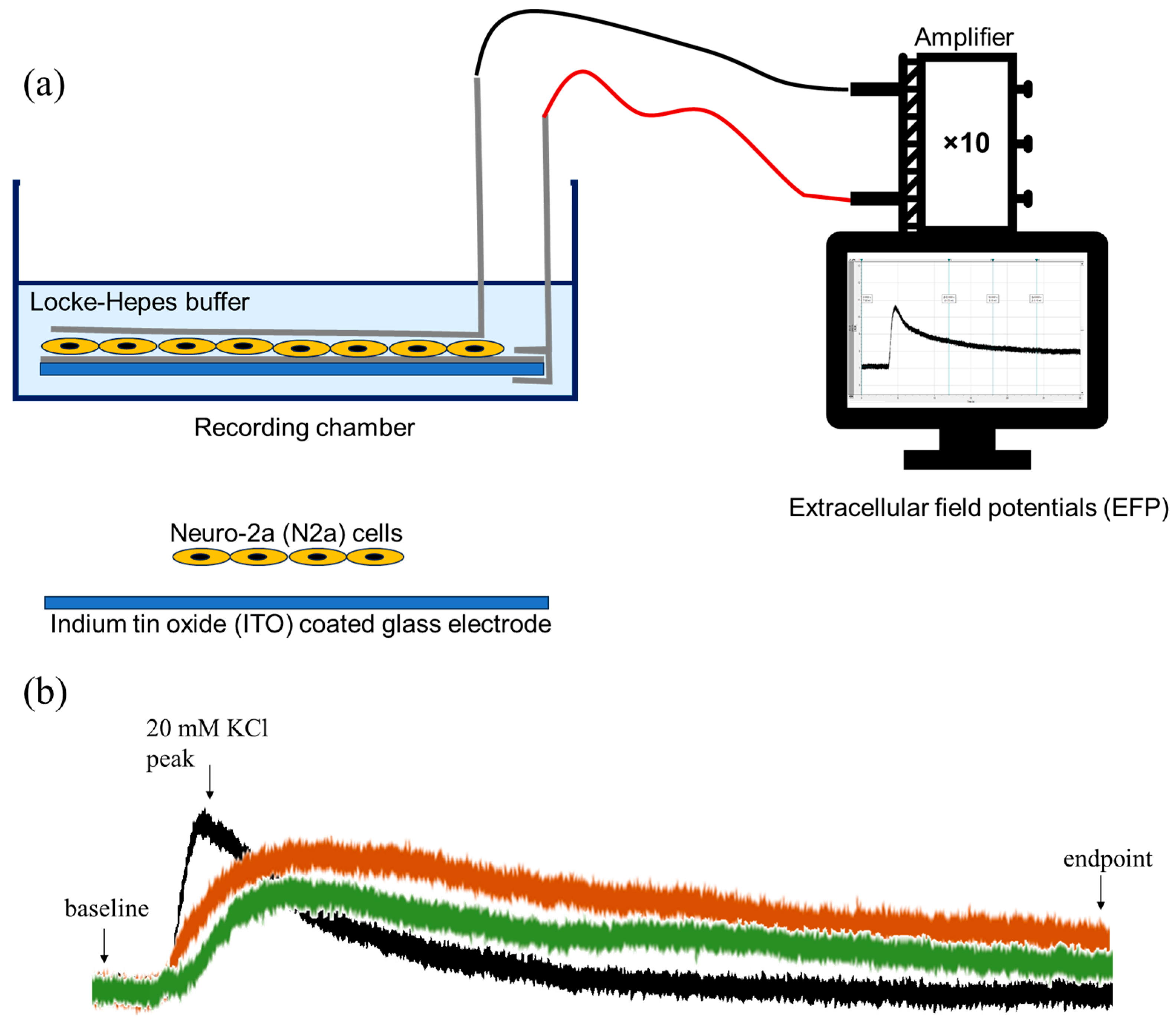
2.2. Extracellular Field Potential (EFP) Recordings on ITO
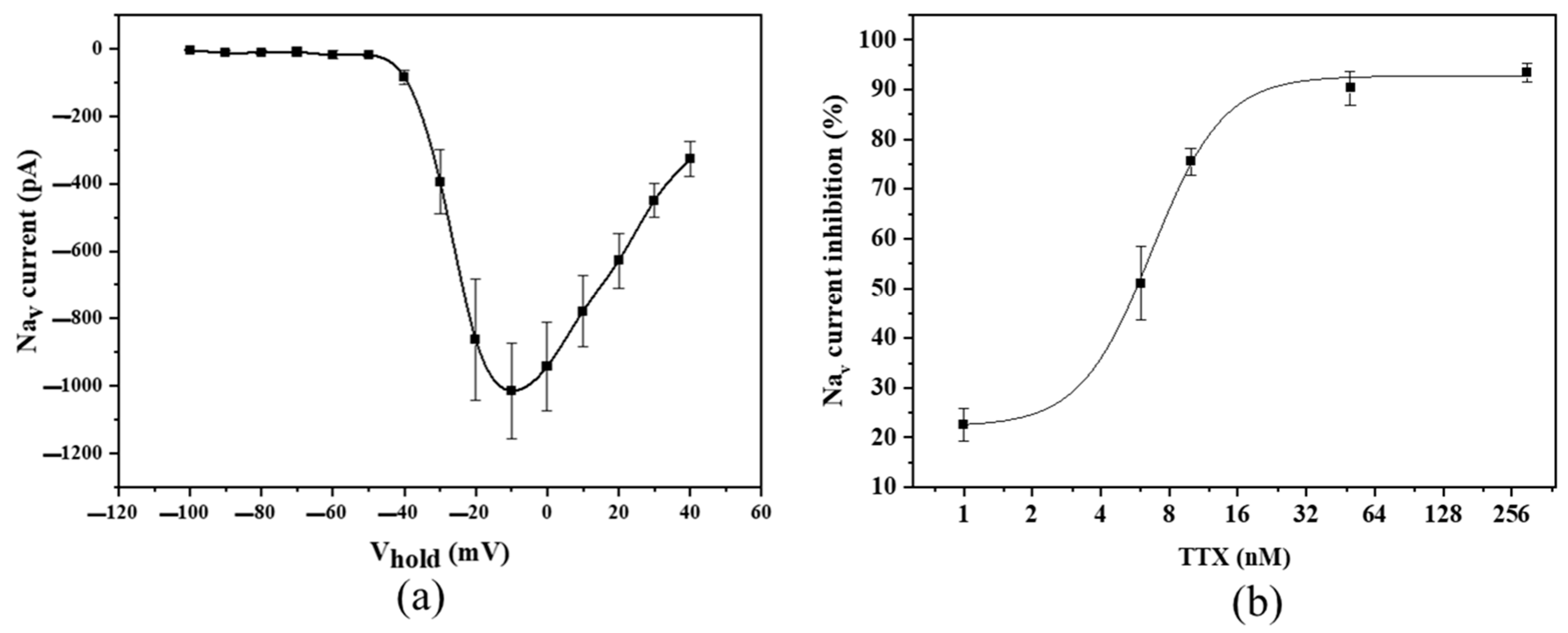
2.3. Patch Clamp Recordings on ITO
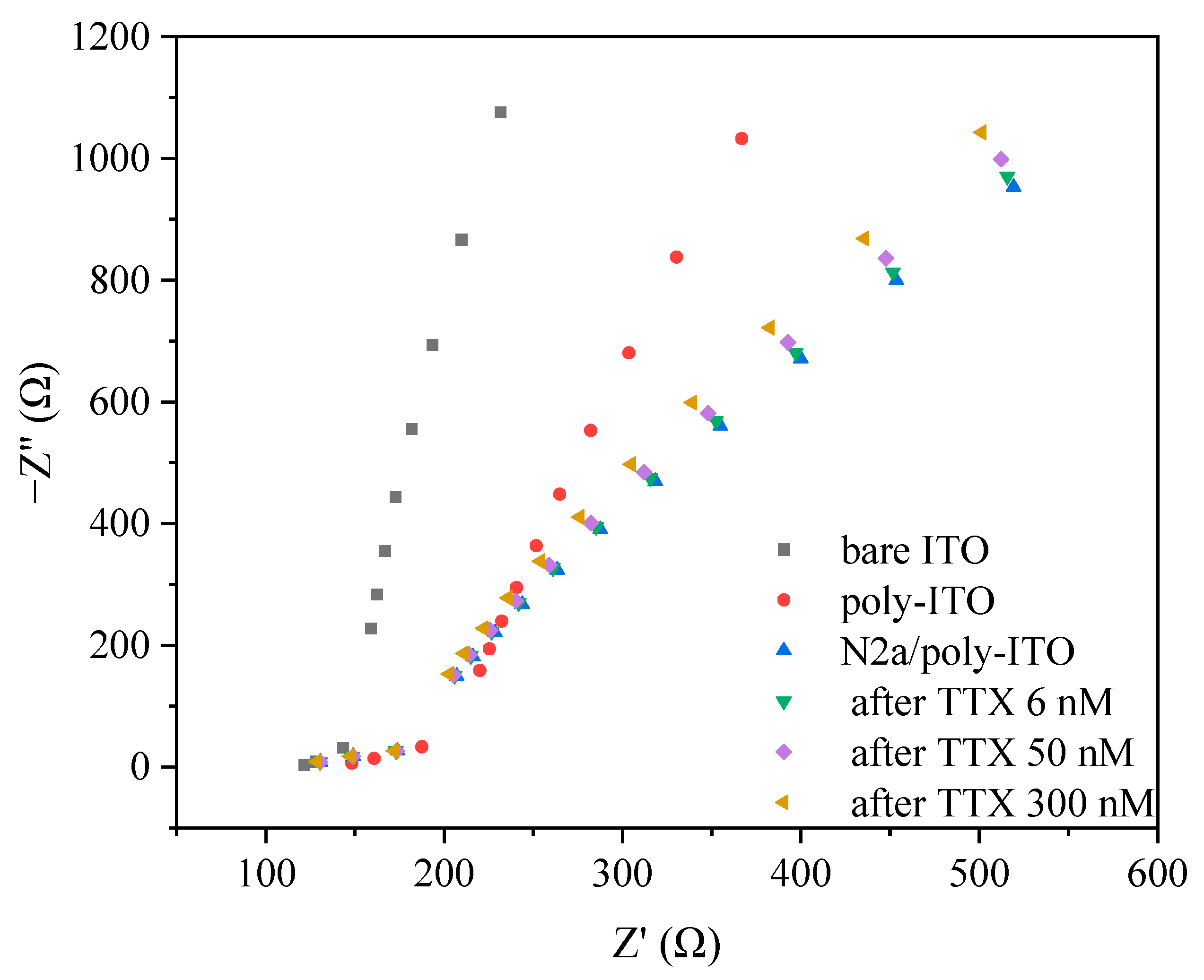
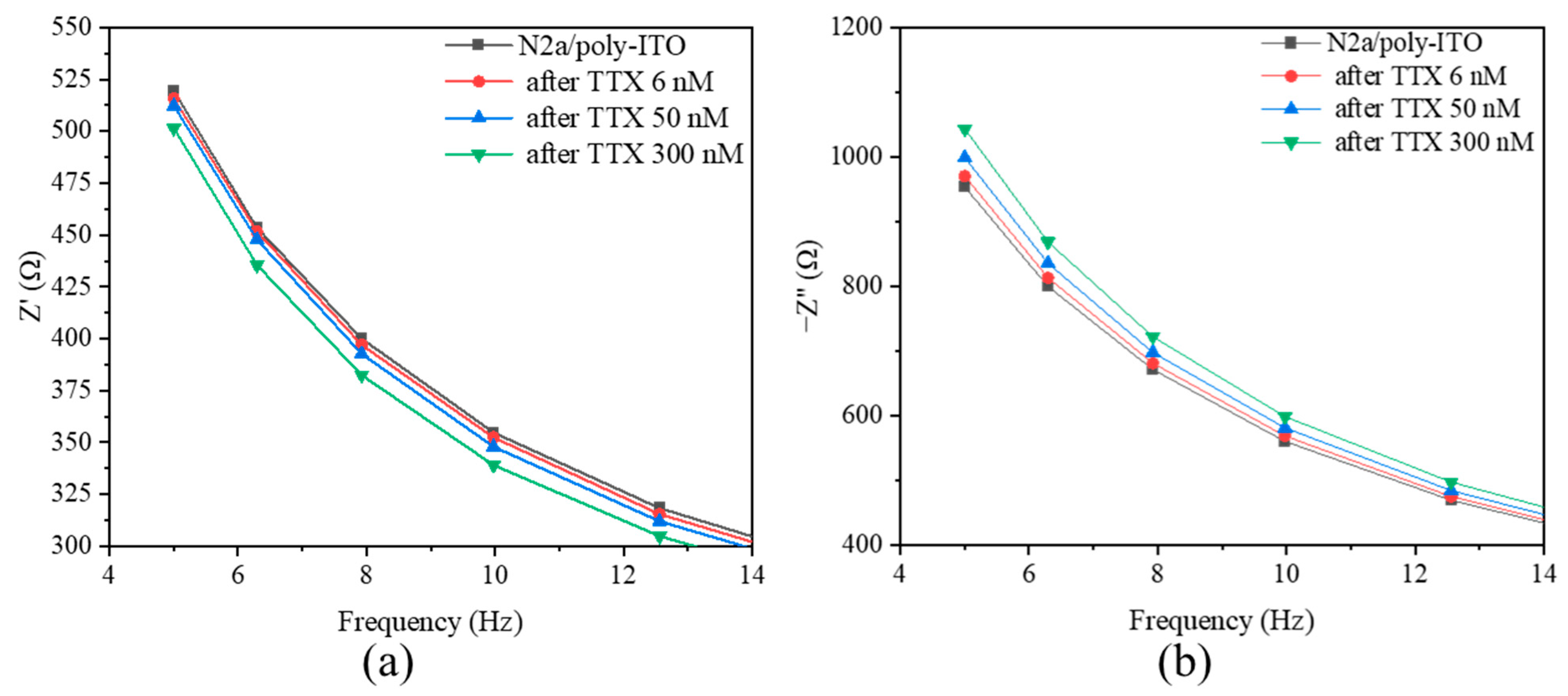
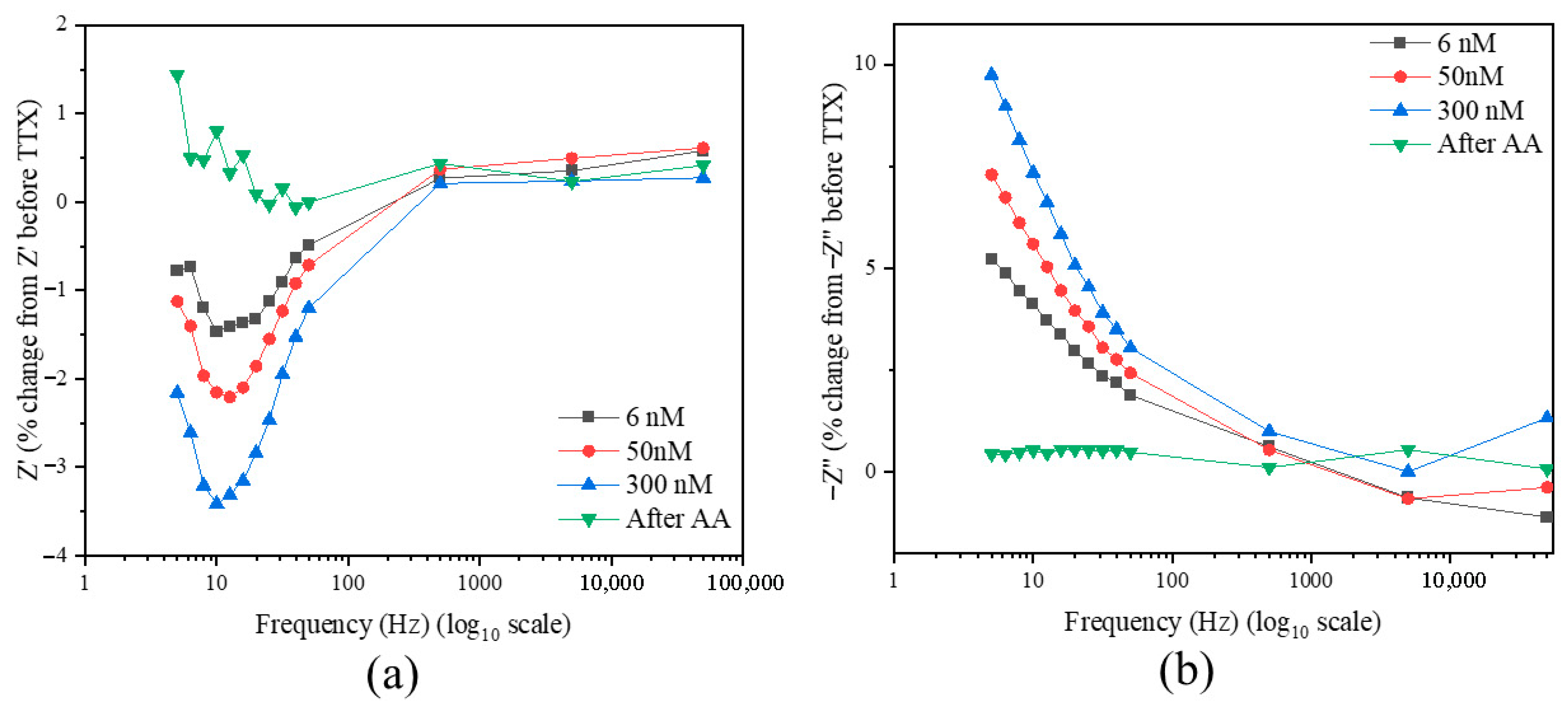
2.4. Electrochemical Experiments on ITO
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cells
5.2. Reagents
5.3. Cell Plating and Viability
5.4. Extracellular Field Potential (EFP) Recordings
5.5. Patch Clamp Experiments on ITO
5.6. Electrochemical Experiments
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Automated patch clamp |
| CBA | Cell-based assay |
| EDTA | Ethylenediaminetetraacetic acid |
| EFP | Extracellular field potential |
| EFSA | European Food Safety Authority |
| EIS | Electrochemical Impedance Spectroscopy |
| ELISA | Enzyme-linked immunosorbent assay |
| HABs | Harmful algal blooms |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| HPLC | High-performance liquid chromatography |
| IC50 | Half-maximal inhibitory concentration |
| ITO | Indium tin oxide |
| LFA | Lateral flow assay |
| LOD | Limit of detection |
| MEA | Multi-electrode array |
| N2a | Neuro-2a cell lines |
| PET | Polyethylene terephthalate |
| P-Os redox polymer | Poly(1-vinylimidazole-co-allylamine)-[Os(2,2′-bipyridine)2Cl]Cl |
| RPMI | Rosewell Park Memorial Institute |
| RTCA | Real-time cell analysis |
| SD | Standard deviation |
| SEM | Standard error of means |
| STX | Saxitoxin |
| TTX | Tetrodotoxin |
| TCO | Transparent conductive oxide |
| VGSC | Voltage-gated sodium channels |
References
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, e04752. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortega, J.F.; Santos, J.M.M.L.; Herrera-Gutiérrez, M.E.; Fernández-Sánchez, V.; Loureo, P.R.; Rancaño, A.A.; Téllez-Andrade, A. Seafood Intoxication by Tetrodotoxin: First Case in Europe. J. Emerg. Med. 2010, 39, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Alkassar, M.; Sanchez-Henao, A.; Reverté, J.; Barreiro, L.; Rambla-Alegre, M.; Leonardo, S.; Mandalakis, M.; Peristeraki, P.; Diogène, J.; Campàs, M. Evaluation of Toxicity Equivalency Factors of Tetrodotoxin Analogues with a Neuro-2a Cell-Based Assay and Application to Puffer Fish from Greece. Mar. Drugs 2023, 21, 432. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chou, H.-N. Detection of Tetrodotoxin by High Performance Liquid Chromatography in Lined-Moon Shell and Puffer Fish. Acta Zool. Taiwanica 1998, 9, 41–48. [Google Scholar]
- Campàs, M.; Reverté, J.; Tudó, À.; Alkassar, M.; Diogène, J.; Sureda, F.X. Automated Patch Clamp for the Detection of Tetrodotoxin in Pufferfish Samples. Mar. Drugs 2024, 22, 176. [Google Scholar] [CrossRef]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef]
- Reverté, L.; de la Iglesia, P.; del Río, V.; Campbell, K.; Elliott, C.T.; Kawatsu, K.; Katikou, P.; Diogène, J.; Campàs, M. Detection of Tetrodotoxins in Puffer Fish by a Self-Assembled Monolayer-Based Immunoassay and Comparison with Surface Plasmon Resonance, LC-MS/MS, and Mouse Bioassay. Anal. Chem. 2015, 87, 10839–10847. [Google Scholar] [CrossRef]
- Reverté, L.; Rambla-Alegre, M.; Leonardo, S.; Bellés, C.; Campbell, K.; Elliott, C.T.; Gerssen, A.; Klijnstra, M.D.; Diogène, J.; Campàs, M. Development and validation of a maleimide-based enzyme-linked immunosorbent assay for the detection of tetrodotoxin in oysters and mussels. Talanta 2018, 176, 659–666. [Google Scholar] [CrossRef]
- Campàs, M.; Reverté, J.; Rambla-Alegre, M.; Campbell, K.; Gerssen, A.; Diogène, J. A fast magnetic bead-based colorimetric immunoassay for the detection of tetrodotoxins in shellfish. Food Chem. Toxicol. 2020, 140, 111315. [Google Scholar] [CrossRef]
- Turner, A.D.; Dean, K.J.; Dhanji-Rapkova, M.; Dall’Ara, S.; Pino, F.; McVey, C.; Haughey, S.; Logan, N.; Elliott, C.; Gago-Martinez, A.; et al. Interlaboratory Evaluation of Multiple LC–MS/MS Methods and a Commercial ELISA Method for Determination of Tetrodotoxin in Oysters and Mussels. J. AOAC Int. 2023, 106, 356–369. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Lu, S.; Ren, H.; Li, Z.; Zhang, Y.; Pan, F.; Liu, W.; Zhang, J.; Liu, Z. Gold nanoparticle probe-based immunoassay as a new tool for tetrodotoxin detection in puffer fish tissues. Sens. Actuators B Chem. 2010, 146, 368–372. [Google Scholar] [CrossRef]
- Thattiyaphong, A.; Unahalekhaka, J.; Mekha, N.; Nispa, W.; Kluengklangdon, P.; Rojanapantip, L. EFFICIENCY OF A RAPID TEST FOR DETECTION OF TETRODOTOXIN IN PUFFER FISH. J. Immunoass. Immunochem. 2014, 35, 111–119. [Google Scholar] [CrossRef]
- Shen, H.; Xu, F.; Xiao, M.; Fu, Q.; Cheng, Z.; Zhang, S.; Huang, C.; Tang, Y. A new lateral-flow immunochromatographic strip combined with quantum dot nanobeads and gold nanoflowers for rapid detection of tetrodotoxin. Analyst 2017, 142, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, S.; Fu, Q.; Xiao, W.; Wang, S.; Yu, S.; Xiao, M.; Bian, H.; Tang, Y. A membrane-based fluorescence-quenching immunochromatographic sensor for the rapid detection of tetrodotoxin. Food Control 2017, 81, 101–106. [Google Scholar] [CrossRef]
- Reverté, L.; Campbell, K.; Rambla-Alegre, M.; Elliott, C.T.; Diogène, J.; Campàs, M. Immunosensor array platforms based on self-assembled dithiols for the electrochemical detection of tetrodotoxins in puffer fish. Anal. Chim. Acta 2017, 989, 95–103. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Liu, L.; Kuang, H.; Xu, L.; Xu, C. A gold nanoparticle-based lateral flow immunosensor for ultrasensitive detection of tetrodotoxin. Analyst 2020, 145, 2143–2151. [Google Scholar] [CrossRef]
- Reverté, J.; Alkassar, M.; Diogène, J.; Campàs, M. Detection of Ciguatoxins and Tetrodotoxins in Seafood with Biosensors and Other Smart Bioanalytical Systems. Foods 2023, 12, 2043. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shi, Z.; Zhang, T.; Wang, L.; Dong, R.; Zhang, Y.; Sun, X. Highly sensitive and quantitative fluorescent strip immunosensor based on an independent control system for rapid detection of tetrodotoxin in shellfish. Food Control 2023, 145, 109403. [Google Scholar] [CrossRef]
- Díaz-Avello, U.G.; Skouridou, V.; Shkembi, X.; Reverté, J.; Mandalakis, M.; Peristeraki, P.; Campàs, M.; O’Sullivan, C.K. Aptamer-antibody sandwich lateral flow test for rapid visual detection of tetrodotoxin in pufferfish. Sci. Total Environ. 2025, 978, 179419. [Google Scholar] [CrossRef]
- Creative Diagnostics Tetrodotoxin ELISA Kit. Available online: https://www.creative-diagnostics.com/tetrodotoxin-elisa-kit-item-deianj48ns-119427.html (accessed on 21 August 2025).
- REAGEN LLC Tetrodotoxin ELISA Test Kit. Available online: https://www.reagen.us/ (accessed on 21 August 2025).
- R-Biopharm AG EuroProxima Tetrodotoxin Sensitive. Available online: https://food.r-biopharm.com/products/europroxima-tetrodotoxin-sensitive/ (accessed on 21 August 2025).
- Logothetis, N.K.; Wandell, B.A. Interpreting the BOLD Signal. Annu. Rev. Physiol. 2004, 66, 735–769. [Google Scholar] [CrossRef]
- Memarian, H.; Patel, H. Transparent Conductive Stratiform Coating of Indium Tin Oxide. US Patent 6743488B2; U.S. Patent and Trademark Office, 1 June 2004. Available online: https://patents.google.com/patent/US6743488B2/en (accessed on 21 August 2025).
- Aoki, T.; Tanino, M.; Sanui, K.; Ogata, N.; Kumakura, K.; Okano, T.; Sakurai, Y.; Watanabe, M. Culture of mammalian cells on polypyrrole-coated ITO as a biocompatible electrode. Synth. Met. 1995, 71, 2229–2230. [Google Scholar] [CrossRef]
- Aoki, T.; Tanino, M.; Sanui, K.; Ogata, N.; Kumakura, K. Secretory function of adrenal chromaffin cells cultured on polypyrrole films. Biomaterials 1996, 17, 1971–1974. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, Q.; Wang, H.; Wang, Y.; Nakayama, M.; Ren, D. Extracellular Calcium Controls Background Current and Neuronal Excitability via an UNC79-UNC80-NALCN Cation Channel Complex. Neuron 2010, 68, 488–499. [Google Scholar] [CrossRef]
- Martiszus, B.J.; Tsintsadze, T.; Chang, W.; Smith, S.M. Enhanced excitability of cortical neurons in low-divalent solutions is primarily mediated by altered voltage-dependence of voltage-gated sodium channels. Elife 2021, 10, e67914. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.L.; Smith, S.M. Calcium-Sensing Receptor: A Key Target for Extracellular Calcium Signaling in Neurons. Front. Physiol. 2016, 7, 116. [Google Scholar] [CrossRef]
- Weaver, I.A.; Li, A.W.; Shields, B.C.; Tadross, M.R. An open-source transparent microelectrode array. J. Neural Eng. 2022, 19, 024001. [Google Scholar] [CrossRef]
- Hammack, A.; Rihani, R.T.; Black, B.J.; Pancrazio, J.J.; Gnade, B.E. A patterned polystyrene-based microelectrode array for in vitro neuronal recordings. Biomed. Microdevices 2018, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Abend, A.; Steele, C.; Schmidt, S.; Frank, R.; Jahnke, H.G.; Zink, M. Neuronal and glial cell co-culture organization and impedance spectroscopy on nanocolumnar TiN films for lab-on-a-chip devices. Biomater. Sci. 2022, 10, 5719–5730. [Google Scholar] [CrossRef]
- Kappalakandy Valapil, K.; Filipiak, M.S.; Rekiel, W.; Jarosińska, E.; Nogala, W.; Jönsson-Niedziółka, M.; Witkowska Nery, E. Fabrication of ITO microelectrodes and electrode arrays using a low-cost CO2 laser plotter. Lab Chip 2023, 23, 3802–3810. [Google Scholar] [CrossRef]
- Wang, Q.; Su, K.; Hu, L.; Zou, L.; Wang, T.; Zhuang, L.; Hu, N.; Wang, P. A novel and functional assay for pharmacological effects of marine toxins, saxitoxin and tetrodotoxin by cardiomyocyte-based impedance biosensor. Sens. Actuators B Chem. 2015, 209, 828–837. [Google Scholar] [CrossRef]
- FREW, J.E.; HILL, H.A.O. Direct and indirect electron transfer between electrodes and redox proteins. Eur. J. Biochem. 1988, 172, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Medina, Z.; Wang, P.; Lielpetere, A.; Bashammakh, A.S.; Alyoubi, A.O.; Katakis, I.; Conzuelo, F.; Schuhmann, W. A biophotoelectrode based on boronic acid-modified Chlorella vulgaris cells integrated within a redox polymer. Bioelectrochemistry 2022, 146, 108128. [Google Scholar] [CrossRef]
- Kogure, K.; Tamplin, M.L.; Simidu, U.; Colwell, R.R. A tissue culture assay for tetrodotoxin, saxitoxin and related toxins. Toxicon 1988, 26, 191–197. [Google Scholar] [CrossRef]
- Ayala-Charca, G.; Forouhi, S.; Zoidl, G.; Magierowski, S.; Ghafar-Zadeh, E. Resonance-Like Impedance Measurement Technique for Life Science Applications. IEEE Trans. Instrum. Meas. 2022, 71, 9510811. [Google Scholar] [CrossRef]
- Gold, C.; Henze, D.A.; Koch, C.; Buzsáki, G. On the Origin of the Extracellular Action Potential Waveform: A Modeling Study. J. Neurophysiol. 2006, 95, 3113–3128. [Google Scholar] [CrossRef]
- Lothman, E.W.; Somjen, G.G. Extracellular potassium activity, intracellular and extracellular potential responses in the spinal cord. J. Physiol. 1975, 252, 115–136. [Google Scholar] [CrossRef]
- Müller, M.; Somjen, G.G. Na+ and K+ Concentrations, Extra- and Intracellular Voltages, and the Effect of TTX in Hypoxic Rat Hippocampal Slices. J. Neurophysiol. 2000, 83, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Chiba, Y.; Wakamori, M.; Yamada, T.; Tsunogae, S.; Cho, Y.; Sakakibara, R.; Imazu, T.; Tokoro, S.; Satake, Y.; et al. Differential binding of tetrodotoxin and its derivatives to voltage-sensitive sodium channel subtypes (Nav1.1 to Nav1.7). Br. J. Pharmacol. 2017, 174, 3881–3892. [Google Scholar] [CrossRef] [PubMed]
- Frelin, C.; Cognard, C.; Vigne, P.; Lazdunski, M. Tetrodotoxin-sensitive and tetrodotoxin-resistant Na+ channels differ in their sensitivity to Cd2+ and Zn2+. Eur. J. Pharmacol. 1986, 122, 245–250. [Google Scholar] [CrossRef]
- Katikou, P. Public Health Risks Associated with Tetrodotoxin and Its Analogues in European Waters: Recent Advances after The EFSA Scientific Opinion. Toxins 2019, 11, 240. [Google Scholar] [CrossRef]
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels in pufferfish (Lagocephalus sceleratus) caught in the Northeastern Mediterranean Sea. Food Chem. 2016, 210, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, Q.; Liu, D.; Feng, Q.; Zhou, H.; Shi, B.; Zhang, X.; Hu, Y.; Jiang, X.; Sun, X.; et al. Recent research progress in tetrodotoxin detection and quantitative analysis methods. Front. Chem. 2024, 12, 1447312. [Google Scholar] [CrossRef]
- Ling, S.; Chen, Q.-A.; Zhang, Y.; Wang, R.; Jin, N.; Pang, J.; Wang, S. Development of ELISA and colloidal gold immunoassay for tetrodotoxin detetcion based on monoclonal antibody. Biosens. Bioelectron. 2015, 71, 256–260. [Google Scholar] [CrossRef]
- Yin, L.; Fan, M.; She, Q.; You, R.; Lu, Y.; Lu, D.; Li, M. Facilely self-assembled and dual-molecule calibration aptasensor based on SERS for ultra-sensitive detection of tetrodotoxin in pufferfish. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121275. [Google Scholar] [CrossRef]
- Zheng, C.; Ge, R.; Wei, J.; Jiao, T.; Chen, Q.; Chen, Q.; Chen, X. NIR-responsive photoelectrochemical sensing platform for the simultaneous determination of tetrodotoxin and okadaic acid in Nassariidae. Food Chem. 2024, 430, 136999. [Google Scholar] [CrossRef]
- Arman, S.; Gonçales, V.R.; Yang, Y.; Tilley, R.D.; Gaus, K.; Gooding, J.J. Toward development of dual optical and electrical cell-based biosensor: An investigation on electrode geometry and transparent conductive material function. Electroanalysis 2023, 35, e202300124. [Google Scholar] [CrossRef]
- Láng, G.; Inzelt, G. An advanced model of the impedance of polymer film electrodes. Electrochim. Acta 1999, 44, 2037–2051. [Google Scholar] [CrossRef]
- Deiss, E.; Sullivan, M.; Haas, O. A numerical ac-impedance model used for the analysis of a polyvinylferrocene coated electrode. J. Electroanal. Chem. 1994, 378, 93–102. [Google Scholar] [CrossRef]
- NARAHASHI, T. Tetrodotoxin -A brief history-. Proc. Japan Acad. Ser. B 2008, 84, 147–154. [Google Scholar] [CrossRef]
- Viallon, J.; Chinain, M.; Darius, H.T. Revisiting the Neuroblastoma Cell-Based Assay (CBA-N2a) for the Improved Detection of Marine Toxins Active on Voltage Gated Sodium Channels (VGSCs). Toxins 2020, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Kenausis, G.; Katakis, I.; Heller, A. “Wiring” of glucose oxidase within a hydrogel made with polyvinyl imidazole complexed with [(Os-4,4′-dimethoxy-2,2′-bipyridine)Cl]+/2+1. J. Electroanal. Chem. 1995, 396, 511–515. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
| Method | Lowest LOD (ng/mL 1) | Linear Detection Range (ng/mL) | Advantages | Disadvantages and Requirements | Reference |
|---|---|---|---|---|---|
| Mouse Bioassay (MBA) | 1.1–2.2 mg/g of mouse | NS 2 | Assesses overall toxicity, no equipment needed | Ethical concerns; low specificity, time-consuming (24–48 h) | [45] |
| Neuro-2a (N2a) cell-based assay (CBA) | 0.32–1.6 | 0.96–95.7 | Measures functional toxicity and TEFs, sensitive, cost-effective | Requires cell culture and pretreatment (e.g., ouabain/veratridine), 24–72 h duration, labor-intensive | [3] |
| High-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) | 0.1–1 | 0.3–100 | High accuracy, quantifies analogs, gold standard | Expensive equipment, sample preparation required, lab-based | [46] |
| Automated patch clamp (N2a cell-based) | 0.32 | 15.95–95.7 | Real-time, high throughput | Costly, requires skilled operators | [5] |
| Enzyme-linked immunosorbent assay (ELISA) | 4.44 | 5–500 | fast (1–2 h), portable, low cost | Development cost | [47] |
| Lateral flow assay (LFA) (immuno/aptamer-based) | 0.2 (competitive), 0.3 (sandwich) | 1.56–100 | On-site, visual readout; quick (<15 min) | Semi-quantitative; lower sensitivity | [13,19] |
| Aptasensors | 6 pg/mL | 0.01–300 | High specificity, stable aptamers | Development cost, limited commercial availability | [48] |
| Immunosensors | 7 pg/mL | 0.001–100 | Ultra-high specificity | Development cost, limited commercial availability | [49] |
| Special cell-based EIS sensor, real-time cell analysis (RTCA) | 89 | 0.001–100 | Real-time, sensitive, portable | Potential fouling, requires real sample validation | [34] |
| N2a/ITO-based extracellular field potential (EFP) | 95.8 3 | NA 4 | Cost-effective; rapid (<10 min) | Non-specific; requires dose–response curve and real sample validation | This study |
| N2a/ITO-based patch clamp | 0.32 | 0.63–10.21 | Real-time | Expensive and requires skilled operators and real-sample validation | “ |
| N2a/ITO-based, electrochemical impedance spectroscopy (EIS) | 1.92 | 1.92–95.8 | Cost-effective; rapid (<10 min) | Non-specific and requires real sample validation | “ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandurangi, N.A.C.; Santafe, M.M.; Tudo, A.; Ozsoy, N.; Sureda, F.X.; Dallas, M.L.; Katakis, I. Cost-Effective and Rapid Detection of Tetrodotoxin Using Indium Tin Oxide Electrodes via In Vitro Electrophysiology and Electrochemistry. Toxins 2025, 17, 462. https://doi.org/10.3390/toxins17090462
Pandurangi NAC, Santafe MM, Tudo A, Ozsoy N, Sureda FX, Dallas ML, Katakis I. Cost-Effective and Rapid Detection of Tetrodotoxin Using Indium Tin Oxide Electrodes via In Vitro Electrophysiology and Electrochemistry. Toxins. 2025; 17(9):462. https://doi.org/10.3390/toxins17090462
Chicago/Turabian StylePandurangi, Naga Adithya Chandra, Manel M. Santafe, Angels Tudo, Nagihan Ozsoy, Fransesc X. Sureda, Mark L. Dallas, and Ioanis Katakis. 2025. "Cost-Effective and Rapid Detection of Tetrodotoxin Using Indium Tin Oxide Electrodes via In Vitro Electrophysiology and Electrochemistry" Toxins 17, no. 9: 462. https://doi.org/10.3390/toxins17090462
APA StylePandurangi, N. A. C., Santafe, M. M., Tudo, A., Ozsoy, N., Sureda, F. X., Dallas, M. L., & Katakis, I. (2025). Cost-Effective and Rapid Detection of Tetrodotoxin Using Indium Tin Oxide Electrodes via In Vitro Electrophysiology and Electrochemistry. Toxins, 17(9), 462. https://doi.org/10.3390/toxins17090462







