Integrative Analysis of Metabolome and Transcriptome Identifies the Role of γ-Glutamylcysteine in Mitigating Deoxynivalenol-Induced Toxicity
Abstract
1. Introduction
2. Results
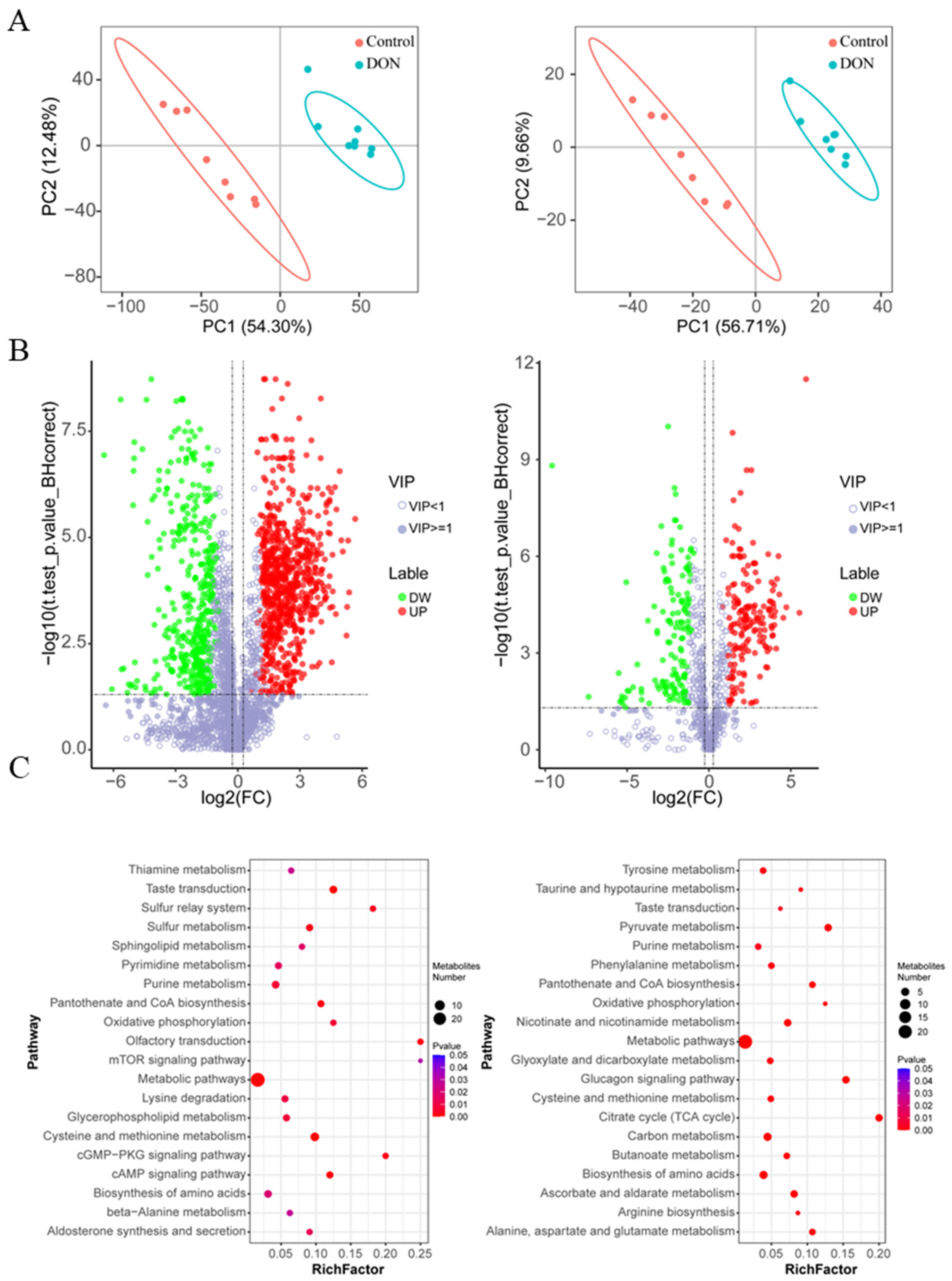
2.1. Metabolomic Analysis in IPEC-J2 Cells Exposed to DON
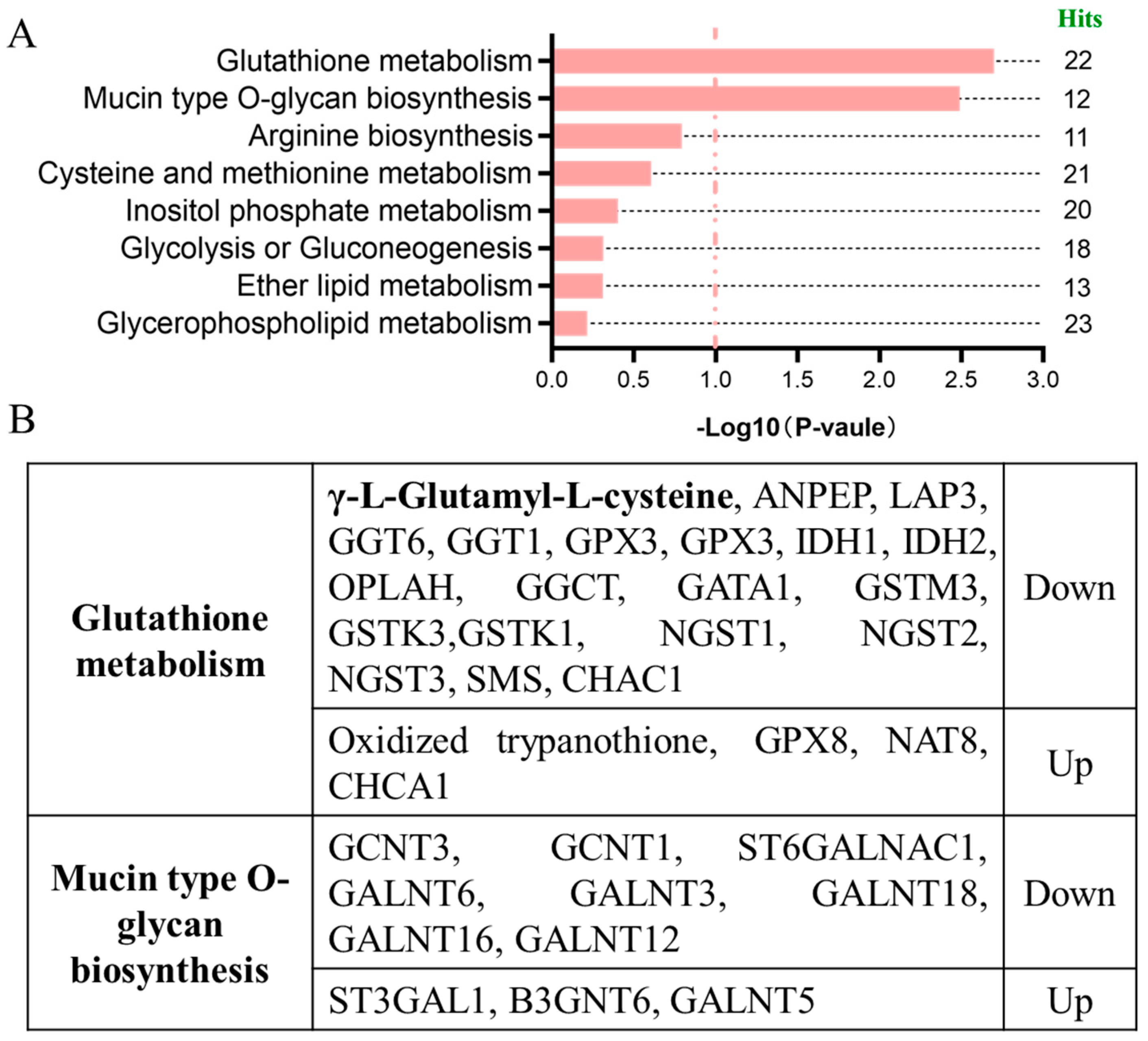
2.2. Integrated Analysis of Metabolome and Transcriptome of DON-Exposed Cells
2.3. γGC Attenuated DON-Induced Cytotoxicity in IPEC-J2 Cells
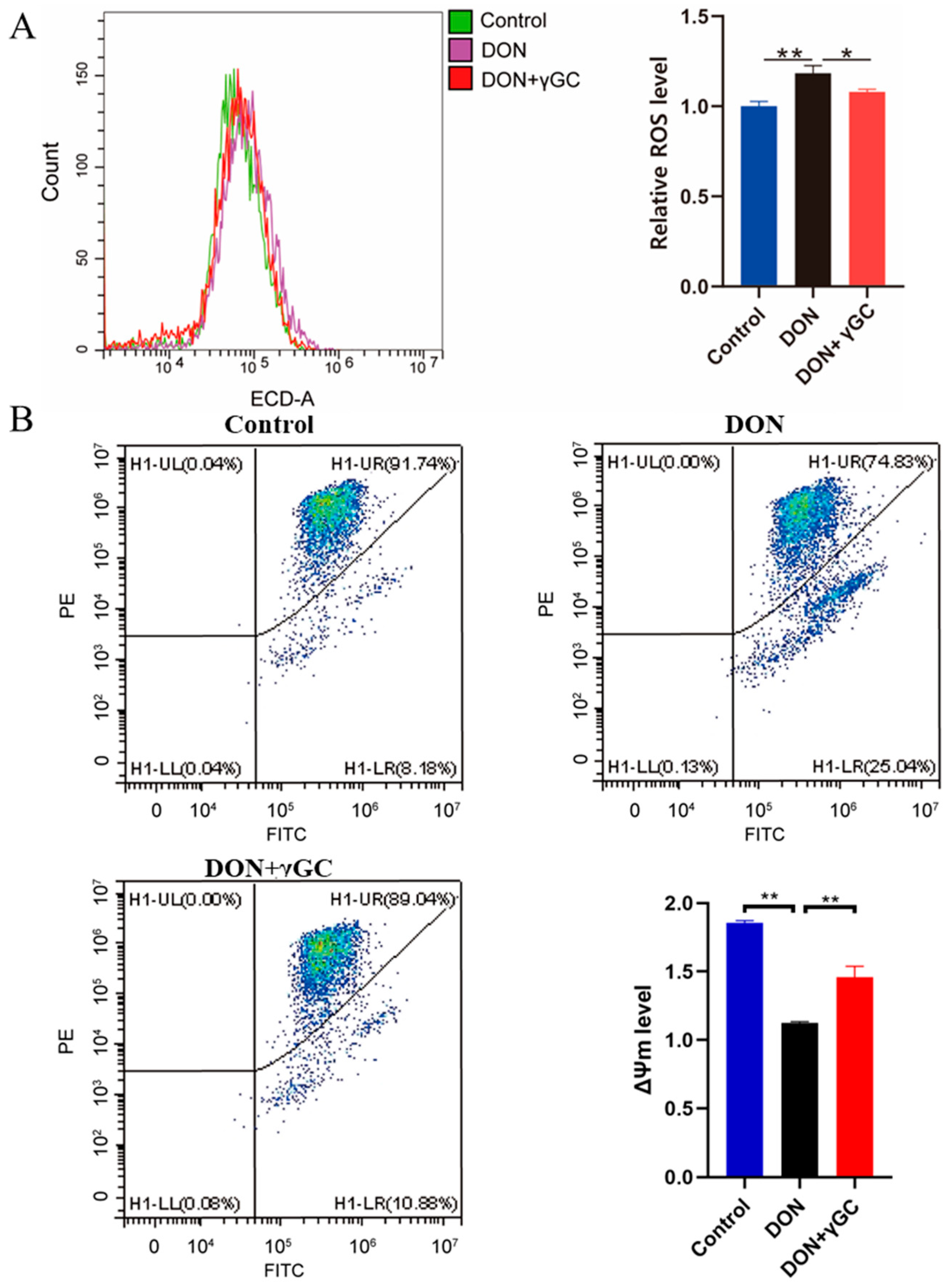
2.4. γGC Attenuated DON-Induced Oxidative Stress in IPEC-J2 Cells
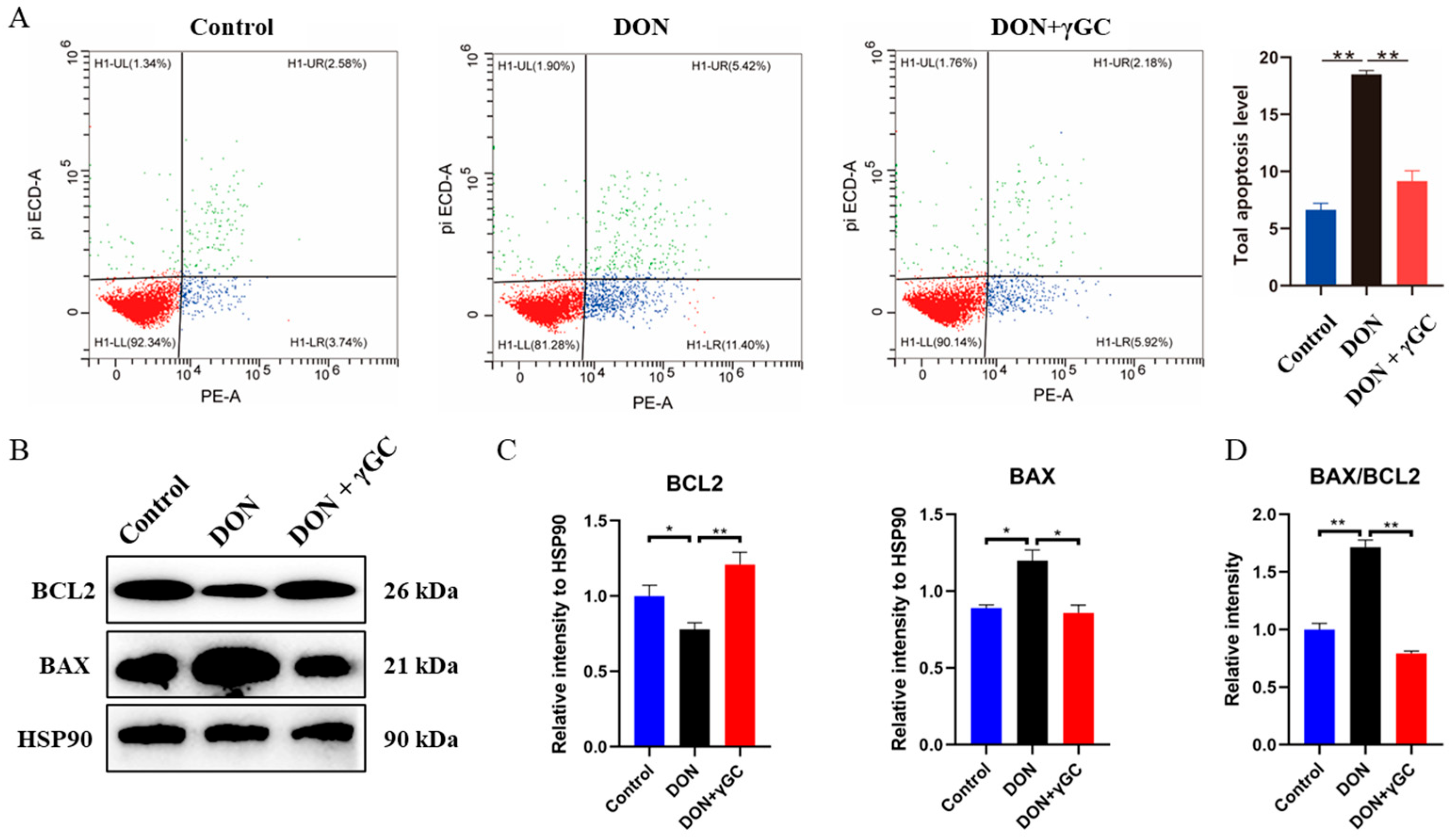
2.5. γGC Attenuated DON-Induced Apoptosis in IPEC-J2 Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture and DON Treatment
5.2. Metabolites Extraction and LC-MS/MS Analysis
5.3. Metabolomics Analysis
5.4. Integrated Analysis of Metabolome and Transcriptome
5.5. Detection of Cell Viability
5.6. Flow Cytometry
5.7. Western Blotting
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DON | Deoxynivalenol |
| γGC | γ-Glutamylcysteine, γ-Glu-Cys |
| ROS | Reactive oxygen species |
References
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Knutsen, H.K.; Sandvik, M.; Brantsæter, A.-L. Urinary deoxynivalenol as a biomarker of exposure in different age, life stage and dietary practice population groups. Environ. Int. 2021, 157, 106804. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; Demeyere, K.; Berthiller, F.; Michlmayr, H.; Varga, E.; Adam, G.; Meyer, E.; Croubels, S. Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem. Toxicol. 2016, 95, 103–109. [Google Scholar] [CrossRef]
- Yue, J.; Guo, D.; Gao, X.; Wang, J.; Nepovimova, E.; Wu, W.; Kuca, K. Deoxynivalenol (Vomitoxin)-Induced Anorexia Is Induced by the Release of Intestinal Hormones in Mice. Toxins 2021, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018-2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef]
- Goossens, J.; Vandenbroucke, V.; Pasmans, F.; De Baere, S.; Devreese, M.; Osselaere, A.; Verbrugghe, E.; Haesebrouck, F.; De Saeger, S.; Eeckhout, M.; et al. Influence of mycotoxins and a mycotoxin adsorbing agent on the oral bioavailability of commonly used antibiotics in pigs. Toxins 2012, 4, 281–295. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Du, Y.; Yao, F.; Zhao, M.; Cai, C.; Zhu, R.; Shao, S. Metabolomics study reveals DON-induced intestinal toxicity in adult zebrafish through disruption of amino acid metabolism and sphingolipid signaling pathway. Aquat. Toxicol. 2025, 282, 107324. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zong, Q.; Wang, S.; Zhao, C.; Wu, S.; Bao, W. Genome-Wide DNA Methylome and Transcriptome Analysis of Porcine Intestinal Epithelial Cells upon Deoxynivalenol Exposure. J. Agric. Food Chem. 2019, 67, 6423–6431. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, S.; Wang, H.; Sun, M.; Wu, S.; Bao, W. Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects. Antioxidants 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Yuan, D.; Liao, P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ. Pollut. 2021, 289, 117865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol Induces Inflammation in IPEC-J2 Cells by Activating P38 Mapk and Erk1/2. Toxins 2020, 12, 180. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, E.-Q.; Fang, Y.-X.; Liu, G.-W.; Cheng, Y.-J.; Huang, K.; Xu, E.; Zhang, Y.-Y.; Wang, X.-J. Piceatannol Alleviates Deoxynivalenol-Induced Damage in Intestinal Epithelial Cells via Inhibition of the NF-κB Pathway. Molecules 2024, 29, 855. [Google Scholar] [CrossRef]
- Zeinvand-Lorestani, H.; Sabzevari, O.; Setayesh, N.; Amini, M.; Nili-Ahmadabadi, A.; Faramarzi, M.A. Comparative study of in vitro prooxidative properties and genotoxicity induced by aflatoxin B1 and its laccase-mediated detoxification products. Chemosphere 2015, 135, 1–6. [Google Scholar] [CrossRef]
- Wang, J.; Lu, F.; Gu, S.; Cao, C.; Xiao, Y.; Bao, W.; Wang, H. Lycopene alleviates Deoxynivalenol-induced toxicity in Porcine intestinal epithelial cells by mediating mitochondrial function. Toxicology 2024, 506, 153880. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Chen, W.; Zhang, W.; Zhang, H.; Yu, H.; Yi, Y. Integrated metabolomics and transcriptomics reveal metabolites difference between wild and cultivated Ophiocordyceps sinensis. Food Res. Int. 2023, 163, 112275. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.; Gong, X.; Xiang, J.; Zhang, Y.; Xiang, Q.; Li, J. Integrated transcriptome and metabolome analysis to investigate the mechanism of intranasal insulin treatment in a rat model of vascular dementia. Front. Pharmacol. 2023, 14, 1182803. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Y.; Xu, C.; Cao, Y.; Jing, P.; Wu, S.; Liu, J.; Bao, W. Integrated Metabolomics and Transcriptomics Analyses Reveal Metabolic Mechanisms in Porcine Intestinal Epithelial Cells under Zearalenone Stress. J. Agric. Food Chem. 2022, 70, 6561–6572. [Google Scholar] [CrossRef]
- Li, M.; Yang, J.; Ye, C.; Bian, P.; Yang, X.; Zhang, H.; Luo, C.; Xue, Z.; Lei, Y.; Lian, J. Integrated Metabolomics and Transcriptomics Analyses Reveal Metabolic Landscape in Neuronal Cells during JEV Infection. Virol. Sin. 2021, 36, 1554–1565. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, W.; Wang, J.; Bai, X. Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci. 2020, 259, 118356. [Google Scholar] [CrossRef] [PubMed]
- Moine, L.; Rivoira, M.; Díaz de Barboza, G.; Pérez, A.; Tolosa de Talamoni, N. Glutathione depleting drugs, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 2018, 24, 4979–4988. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Hang, Q.; Fang, Y.; Dong, X.; Cao, P.; Yin, Z.; Luo, L. γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019, 20, 157–166. [Google Scholar] [CrossRef]
- Zarka, M.H.; Bridge, W.J. Oral administration of γ-glutamylcysteine increases intracellular glutathione levels above homeostasis in a randomised human trial pilot study. Redox Biol. 2017, 11, 631–636. [Google Scholar] [CrossRef]
- Jiang, D.; Guo, Y.; Wang, T.; Wang, L.; Yan, Y.; Xia, L.; Bam, R.; Yang, Z.; Lee, H.; Iwawaki, T.; et al. IRE1α determines ferroptosis sensitivity through regulation of glutathione synthesis. Nat. Commun. 2024, 15, 4114. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.; Wu, Z.; Wang, H.; Chen, Z.; Wang, J.; Bao, W. Protective effects of melatonin on deoxynivalenol-induced oxidative stress and autophagy in IPEC-J2 cells. Food Chem. Toxicol. 2023, 177, 113803. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Han, L.; Xu, S.; Zhou, D.; Chen, R.; Ding, Y.; Zhang, M.; Bao, M.; He, B.; Li, S. Unveiling the causal link between metabolic factors and ovarian cancer risk using Mendelian randomization analysis. Front. Endocrinol. 2024, 15, 1401648. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, J.; Zhou, J.; Li, J.; Wang, M.; Wu, Y.; Zhao, D.; Chen, X.; Chen, X.; Wang, Y.; et al. Bioinspired Claw-Engaged Adhesive Microparticles Armed with γGC Alleviate Ulcerative Colitis via Targeted Suppression of Macrophage Ferroptosis. Adv. Sci. 2025, 12, e2503903. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.; Meister, A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc. Natl. Acad. Sci. USA 1983, 80, 707–711. [Google Scholar] [CrossRef]
- Salama, S.A.; Al-Harbi, M.S.; Abdel-Bakky, M.S.; Omar, H.A. Glutamyl cysteine dipeptide suppresses ferritin expression and alleviates liver injury in iron-overload rat model. Biochimie 2015, 115, 203–211. [Google Scholar] [CrossRef]
- Le, T.M.; Jiang, H.; Cunningham, G.R.; Magarik, J.A.; Barge, W.S.; Cato, M.C.; Farina, M.; Rocha, J.B.; Milatovic, D.; Lee, E.; et al. γ-Glutamylcysteine ameliorates oxidative injury in neurons and astrocytes in vitro and increases brain glutathione in vivo. Neurotoxicology 2011, 32, 518–525. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, J.; Chen, X.; Shi, D.; Hui, P.; Wang, H.; Wu, Z.; Wu, S.; Bao, W.; Fan, H.; et al. DNA ligase III mediates deoxynivalenol exposure-induced DNA damage in intestinal epithelial cells by regulating oxidative stress and interaction with PCNA. Int. J. Biol. Macromol. 2024, 282, 137137. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Chen, X.; Chen, S.; Sun, M.-A.; Fan, H. Integrative Analysis of Metabolome and Transcriptome Identifies the Role of γ-Glutamylcysteine in Mitigating Deoxynivalenol-Induced Toxicity. Toxins 2025, 17, 457. https://doi.org/10.3390/toxins17090457
Bao X, Chen X, Chen S, Sun M-A, Fan H. Integrative Analysis of Metabolome and Transcriptome Identifies the Role of γ-Glutamylcysteine in Mitigating Deoxynivalenol-Induced Toxicity. Toxins. 2025; 17(9):457. https://doi.org/10.3390/toxins17090457
Chicago/Turabian StyleBao, Xiaocheng, Xiaolei Chen, Shuai Chen, Ming-An Sun, and Hairui Fan. 2025. "Integrative Analysis of Metabolome and Transcriptome Identifies the Role of γ-Glutamylcysteine in Mitigating Deoxynivalenol-Induced Toxicity" Toxins 17, no. 9: 457. https://doi.org/10.3390/toxins17090457
APA StyleBao, X., Chen, X., Chen, S., Sun, M.-A., & Fan, H. (2025). Integrative Analysis of Metabolome and Transcriptome Identifies the Role of γ-Glutamylcysteine in Mitigating Deoxynivalenol-Induced Toxicity. Toxins, 17(9), 457. https://doi.org/10.3390/toxins17090457





