Aflatoxin Exposure in Immunocompromised Patients: Current State and Future Perspectives
Abstract
1. Introduction
2. Biosynthetic Pathway of AFs in Aspergillus Species
3. AF Exposure Pathways in Humans
3.1. Exposure to AF Through Ingestion
3.1.1. Exposure Through Carryover from Animal Sources
3.1.2. In Utero and Maternal Exposure
3.2. Exposure to AF Through Inhalation
3.3. Exposure to AF Through Dermal Contact
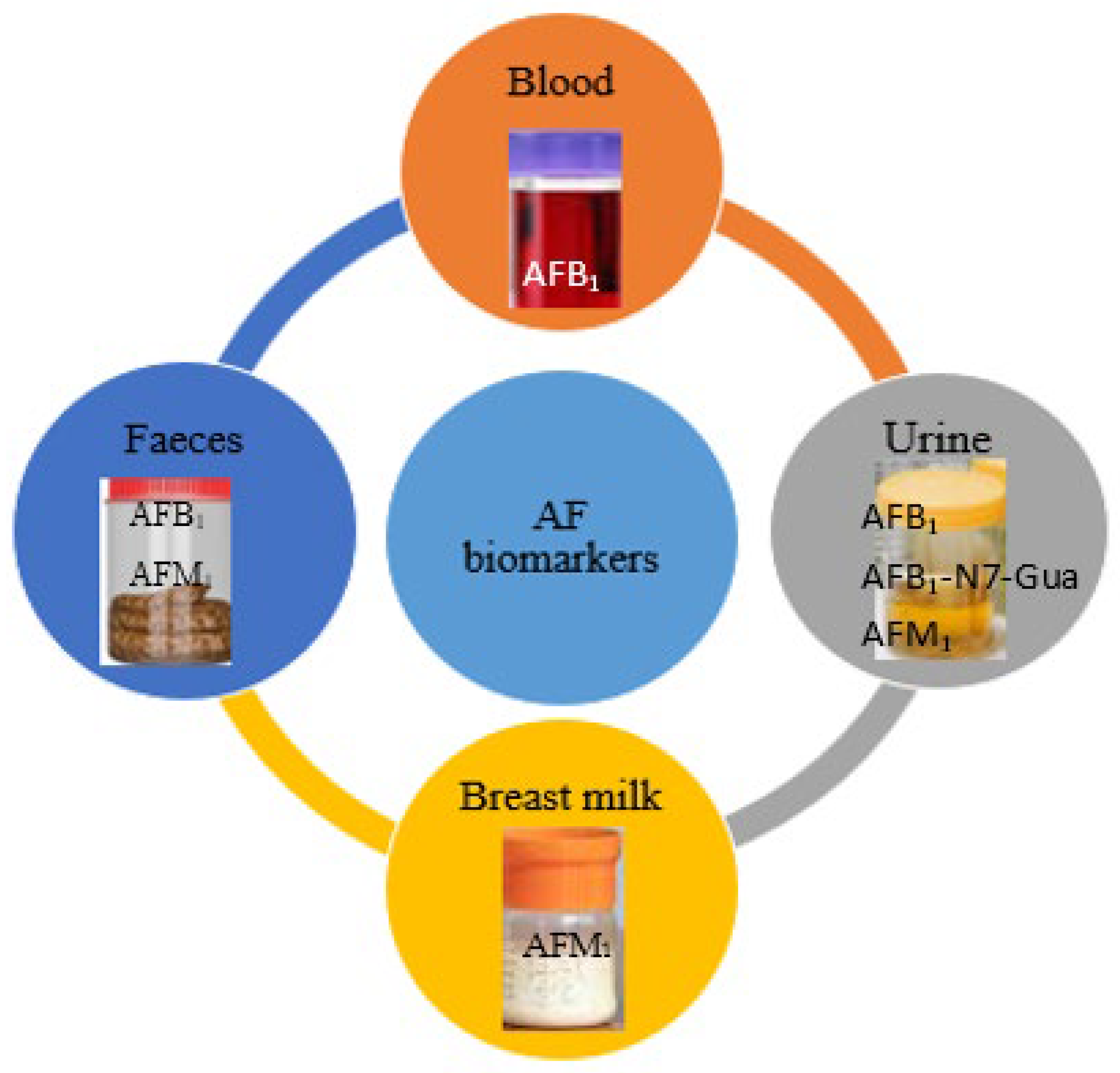
4. AF Biomarkers
4.1. Urine
4.2. Blood
4.3. Breast Milk
4.4. Feces
| Matrix | Biomarker | Technique | Sample Size | % Contamination | Concentration Range (ppb) | References |
|---|---|---|---|---|---|---|
| Urine | AFM1 | HPLC | 72 | 29.17 | 0.00067–0.00787 | [157] |
| Urine | AFM1 | HPLC | 69 | 78.26 | 0.0006–0.0399 | [158] |
| Urine | AFM1 | LC-MS/MS | 220 | 14 | 0.06–4.7 | [159] |
| Feces | AFB1 | PLE and HPLC-MS/MS | 3 | 1 | 0.02 | [160] |
| Urine | AFM1 | HPLC | 300 | 27.67 | 0.01–0.33 | [161] |
| Urine | AFM1 | LC-ESI-MS/MS | 145 | 10.34 | 0.17–1.38 | [162] |
| Urine | AFB1-N7-Gua | HPLC-MS/MS | 20 | 80 | 0.9–7.2 | [163] |
| Urine | AFB1-N7-Gua | HPLC | 27 | 40.74 | 6.6–494.9 | [164] |
| Urine | AFM1 | ELISA | 205 | 57.56 | 0.00020–0.0193 creatinine | [165] |
| Urine | AFs | 2-D TLC | 60 | 100 | 0.07–0.2 | [166] |
| Urine | AFM1 | HPLC | 50 | 64 | 0.008–0.801 | [167] |
| Urine | AFM1 | ELISA | 93 | 47.31 | 500–59,900 creatinine | [168] |
| Urine | AFM1 | ELISA | 160 | 61.25 | LOD-0.0747 | [169] |
| Urine | AFM1 | HPLC-ESI-MS/MS | 175 | - | 0.005-0.5 | [170] |
| Urine | AFM1 | HPLC-MS/MS | 28 | 10.74 | LOD-0.33 | [171] |
| Urine | AFM1 | LC-MS/MS | 120 | 14.2 | 0.3–1.5 | [172] |
| Plasma | AFB1-lys | LC-MS/MS | 260 | 19.6 | 10.5–74.5 | [115] |
| Plasma | AFB1 AFB2 | LC-MS/MS | 60 | 39.4 74.91 | 1.23–4.56 1.16–3.75 | [173] |
| Plasma | AFB1-lys | LC-Orbitrap | 58 | 23.45 | 0.2–59.2 | [174] |
| Serum | AFB1-lys | LC-FLDLC-MS/MS | 34 | 83 | 1.08–102.6 | [31] |
| Plasma | AFB1-lys | LC-MS | 32 | 46.88 | - | [175] |
| Serum | AFB1-lys | LC-MS | 160 | 61 | 0.80–20.24 | [176] |
| Serum | AFB1-lys | LC-FLD | 220 | 100 | 0.71–95.6 | [177] |
| Serum | AFB1-lys | LC-FLD | 347 | 99.4 | - | [178] |
| Serum | AFB1-lys | LC-FLD | 884 | 100 | 6.04–8.90 | [179] |
| Serum | AFB1-lys | LC-MS | 461 | 100 | 0.2–814.8 | [180] |
| Plasma | AFB1-lys | LC-MS/MS | 60 | 72 | 3.5–6.6 | [181] |
| Plasma | AFB1-lys | LC-MS/MS | 85 | - | - | [181] |
| Plasma | AFB1-lys | LC-MS/MS | 167 | 62 | 0.04–123.5 | [182] |
| Plasma | AFB1-lys | ELISA | 115 | 100 | 3.9–458.4 | [183] |
| Serum | AFB1-lys | ELISA | 230 | 67 | - | [184] |
| Serum | AFB1-lys | ELISA | 305 | 88.2 | - | [185] |
| Plasma | AFB1-lys | ELISA | 374 | 95 | - | [186] |
| Serum | AFB1 AFM1 | LC-MS/MS | 213 | 23 50 | 0.0–0.73 0.0–1.91 | [187] |
| Serum | AFB1-lys | LC-FLD | 713 | 90 | 0.4–168 | [188] |
| Plasma | AFB1-lys | ELISA | 166 | 67–98 | 4.7–23.50 | [189] |
| Serum | AFB1-alb | ELISA | 34 | 98 | 3.0–35.1 | [168] |
| Blood/serum | AFB1-alb | ELISA | 24 | 100 | LOD-32.8 | [190] |
| Blood/serum | AFs | 2-D TLC | 60 | 100 | 0.15–0.38 | [166] |
| Blood/serum | AFB1-alb | HPLC | 507 | 100 | 0.44–268.73 | [191] |
| Blood/serum | AFB1-alb | ELISA | 250 | 98 | LOD-66 | [192] |
| Blood/serum | AFB1-alb | LC-MS/MS | 597 | 78 | 0.02–211 | [193] |
| Blood/serum | AFB1-alb | HPLC | 170 | 97 | 0.2–23.16 | [194] |
| Blood/serum | AFM1 | HPLC | 131 | 39 | 0.3–56 | [195] |
| Blood/serum | AFB1-alb | ELISA | 119 | 100 | 4.8–260.8 | [196] |
| Blood/serum | AFB1-alb | HPLC | 170 | 20.6 | 1.01–16.57 | [197] |
| Breastmilk | AFM1 | HPLC | 388 | 36 | 5.5–5131 | [198] |
| Breastmilk | AFM1 | HPLC | 75 | 100 | 60.9–299.9 | [199] |
| Breastmilk | AFB1 | HPLC | 75 | 100 | 94.5–4123.8 | [199] |
| Breastmilk | AFM1 | HPLC | 140 | 92 | 5–3400 | [200] |
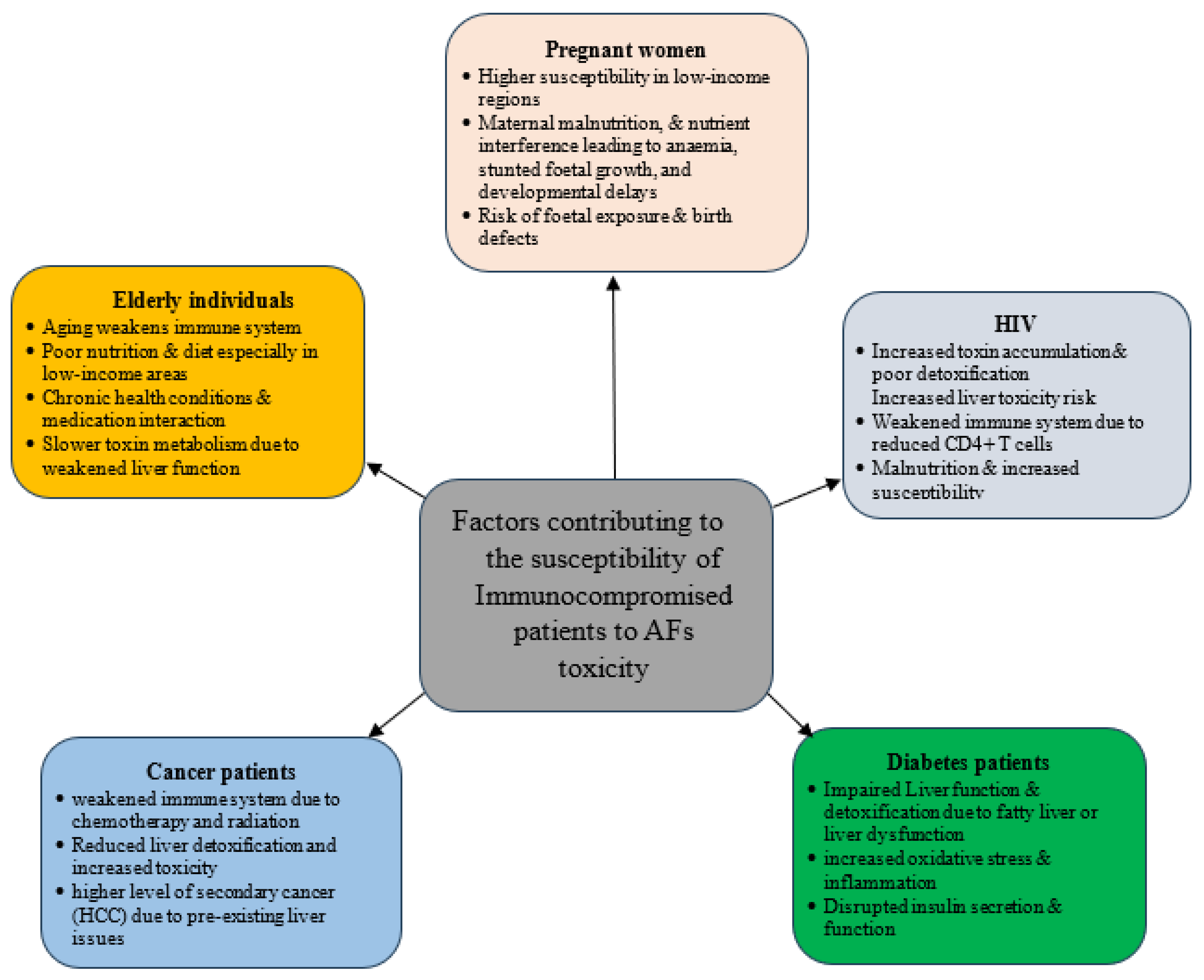
5. AF Biomonitoring in Immunocompromised Individuals
5.1. Elderly Individuals
5.2. Pregnant Women
5.3. Malnourished Individuals
5.4. AF Biomonitoring in HIV Individuals
5.5. AF Biomonitoring in Diabetes Patients
5.6. AF Biomonitoring in Cancer Patients
5.7. AF Biomonitoring in Hepatitis Patients
6. Conclusions
7. Future Perspectives and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adelusi, O.A.; Gbashi, S.; Adebo, J.A.; Aasa, A.O.; Oladeji, O.M.; Kah, G.; Adebo, O.A.; Changwa, R.; Njobeh, P.B. Seasonal and geographical impact on the mycotoxigenicity of Aspergillus and Fusarium species isolated from smallholder dairy cattle feeds and feedstuffs in Free State and Limpopo provinces of South Africa. Toxins 2023, 15, 128. [Google Scholar] [CrossRef]
- Okechukwu, V.O.; Kappo, A.P.; Njobeh, P.B.; Mamo, M.A. Morphed aflaxotin concentration produced by Aspergillus flavus strain VKMN22 on maize grains inoculated on agar culture. Food Chem. Mol. Sci. 2024, 8, 100197. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Chronic and acute toxicities of Aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, N.A.; Al-Ameri, H.A. Aflatoxins, in Aflatoxins-Occurrence, Detoxification, Determination and Health Risks; IntechOpen: London, UK, 2022. [Google Scholar]
- Danso, J.K.; Mbata, G.N.; Holton, R.L. Preharvest insect pests of peanuts and associated aflatoxin contaminants in Georgia, USA. J. Econ. Entomol. 2024, 117, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Aminou, M.M.; Falalou, H.; Abdou, H.; Mendu, V. Aflatoxin B1 Contamination Association with the Seed Coat Biochemical Marker Polyphenol in Peanuts Under Intermittent Drought. J. Fungi 2024, 10, 850. [Google Scholar] [CrossRef]
- Muaz, K.; Manzoor, S.; Akhtar, S.; Riaz, M.; Amir, M.; Akram, K.; Ismail, A. Aflatoxin Biosynthesis. In Aflatoxins in Food: A Recent Perspective; Springer: Berlin/Heidelberg, Germany, 2022; pp. 19–40. [Google Scholar]
- Okechukwu, V.O.; Adelusi, O.A.; Kappo, A.P.; Njobeh, P.B.; Mamo, M.A. Aflatoxins: Occurrence, biosynthesis, mechanism of action and effects, conventional/emerging detection techniques. Food Chem. 2024, 436, 137775. [Google Scholar] [CrossRef]
- Boadu, R.O.; Dankyi, E.; Apalangya, V.A.; Osei-Safo, D. Aflatoxins in maize and groundnuts on markets in Accra and consumers risk. Food Addit. Contam. Part B 2024, 17, 213–222. [Google Scholar] [CrossRef]
- Kos, J.; Radić, B.; Radović, R.; Šarić, B.; Jovanov, P.; Šarić, L. Aflatoxins in maize, milk and dairy products from Serbia. Food Addit. Contam. Part B 2024, 17, 296–307. [Google Scholar] [CrossRef]
- Falade, T.D.; Neya, A.; Bonkoungou, S.; Dagno, K.; Basso, A.; Senghor, A.L.; Atehnkeng, J.; Ortega-Beltran, A.; Bandyopadhyay, R. Aflatoxin contamination of maize, groundnut, and sorghum grown in Burkina Faso, Mali, and Niger and aflatoxin exposure assessment. Toxins 2022, 14, 700. [Google Scholar] [CrossRef]
- Wenndt, A.; Sudini, H.K.; Pingali, P.; Nelson, R. Exploring aflatoxin contamination and household-level exposure risk in diverse Indian food systems. PLoS ONE 2020, 15, e0240565. [Google Scholar] [CrossRef]
- Xia, L.; Routledge, M.N.; Rasheed, H.; Ismail, A.; Dong, Y.; Jiang, T.; Gong, Y.Y. Biomonitoring of aflatoxin B1 and deoxynivalenol in a rural pakistan population using ultra-sensitive LC-MS/MS method. Toxins 2020, 12, 591. [Google Scholar] [CrossRef]
- Heshmati, A.; Mozaffari Nejad, A.S.; Mehri, F. Occurrence, dietary exposure, and risk assessment of Aflatoxins in wheat flour from Iran. Int. J. Environ. Anal. Chem. 2023, 103, 9395–9408. [Google Scholar] [CrossRef]
- Houissa, H.; Lasram, S.; Sulyok, M.; Šarkanj, B.; Fontana, A.; Strub, C.; Krska, R.; Schorr-Galindo, S.; Ghorbel, A. Multimycotoxin LC-MS/MS analysis in pearl millet (Pennisetum glaucum) from Tunisia. Food Control 2019, 106, 106738. [Google Scholar] [CrossRef]
- Tadesse, S.; Berhanu, T.; Woldegiorgis, A.Z. Aflatoxin M1 in milk and milk products marketed by local and industrial producers in Bishoftu town of Ethiopia. Food Control 2020, 118, 107386. [Google Scholar] [CrossRef]
- Mathew, G.; Pingle, S. Assessing Aflatoxin Exposure: Exploring Types of Exposure and Diverse Biomarkers—A Minireview. Curr. Fungal Infect. Rep. 2024, 18, 163–171. [Google Scholar] [CrossRef]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef]
- Nabwire Wangia-Dixon, R.; Xue, K.S.; Alcala, J.; Quach, T.H.T.; Song, X.; Tang, L.; Ombaka, J.; Githanga, D.P.; Anzala, O.A.; Wang, J.-S. Nutrition and growth outcomes are affected by aflatoxin exposures in Kenyan children. Food Addit. Contam. Part A 2020, 37, 2123–2134. [Google Scholar] [CrossRef]
- Costa, J.; Lima, N.; Santos, C. An overview on possible links between aflatoxin B1 exposure and gallbladder cancer. Mycotoxin Res. 2021, 37, 205–214. [Google Scholar] [CrossRef]
- Otedo, A.; Simbiri, K.O.; Were, V.; Ongati, O.; Estambale, B.A. Risk factors for liver Cancer in HIV endemic areas of Western Kenya. Infect. Agents Cancer 2018, 13, 41. [Google Scholar] [CrossRef]
- Kabak, B. Aflatoxins in foodstuffs: Occurrence and risk assessment in Turkey. J. Food Compos. Anal. 2021, 96, 103734. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Haq, M.E.U.; Qader, A.; Rehman, K. Biochemical investigation of human exposure to aflatoxin M1 and its association with risk factors of diabetes mellitus. Environ. Sci. Pollut. Res. 2021, 28, 62907–62918. [Google Scholar] [CrossRef]
- Saha Turna, N.; Comstock, S.S.; Gangur, V.; Wu, F. Effects of aflatoxin on the immune system: Evidence from human and mammalian animal research. Crit. Rev. Food Sci. Nutr. 2024, 64, 9955–9973. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.J.; Yang, H.I.; Wu, H.C.; Liu, J.; Wang, L.Y.; Lu, S.N.; Lee, M.H.; Jen, C.L.; You, S.L.; Santella, R.M.; et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int. J. Cancer 2017, 141, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, S.K.; Crothers, K.; Morris, A. Pathogenesis of HIV-related lung disease: Immunity, infection, and inflammation. Physiol. Rev. 2020, 100, 603–632. [Google Scholar] [CrossRef] [PubMed]
- Boni, S.; Beed, F.; Kimanya, M.; Koyano, E.; Mponda, O.; Mamiro, D.; Kaoneka, B.; Bandyopadhyay, R.; Korie, S.; Mahuku, G. Aflatoxin contamination in Tanzania: Quantifying the problem in maize and groundnuts from rural households. World Mycotoxin J. 2021, 14, 553–564. [Google Scholar] [CrossRef]
- Rasheed, H.; Xu, Y.; Kimanya, M.E.; Pan, X.; Li, Z.; Zou, X.; Shirima, C.P.; Holmes, M.; Routledge, M.N.; Gong, Y.Y. Estimating the health burden of aflatoxin attributable stunting among children in low income countries of Africa. Sci. Rep. 2021, 11, 1619. [Google Scholar] [CrossRef]
- Phan, J.M.; Kim, S.; Linh, Đ.T.T.; Cosimi, L.A.; Pollack, T.M. Telehealth interventions for HIV in low-and middle-income countries. Curr. HIV/AIDS Rep. 2022, 19, 600–609. [Google Scholar] [CrossRef]
- Saltzmann, J.; Xu, Y.; Gong, Y.Y.; Lindahl, J.; Kersten, S.; Dänicke, S.; Routledge, M.N. Preliminary study on the relationship between aflatoxin-bovine serum albumin adducts in blood and aflatoxin M1 levels in milk of dairy cows. Mycotoxin Res. 2020, 36, 207–211. [Google Scholar] [CrossRef]
- Diaz de Leon-Martinez, L.; Rodríguez-Aguilar, M.; Wong-Arce, A.; Díaz-Barriga, F.; Bañuelos-Hernández, B.; Rosales-Mendoza, S.; Flores-Ramírez, R. Evaluation of acute and chronic exposure to aflatoxin B1 in indigenous women of the Huasteca Potosina, Mexico. Environ. Sci. Pollut. Res. 2020, 27, 30583–30591. [Google Scholar] [CrossRef]
- Autrup, H.; Autrup, J.L. Human exposure to aflatoxins-biological monitoring. In Handbook of Applied Mycology; CRC Press: Boca Raton, FL, USA, 2024; pp. 213–230. [Google Scholar]
- Gichohi-Wainaina, W.N.; Kumwenda, N.; Zulu, R.; Munthali, J.; Okori, P. Aflatoxin contamination: Knowledge disparities among agriculture extension officers, frontline health workers and small holder farming households in Malawi. Food Control 2021, 121, 107672. [Google Scholar] [CrossRef]
- Marshall, H.; Meneely, J.P.; Quinn, B.; Zhao, Y.; Bourke, P.; Gilmore, B.F.; Zhang, G.; Elliott, C.T. Novel decontamination approaches and their potential application for post-harvest aflatoxin control. Trends Food Sci. Technol. 2020, 106, 489–496. [Google Scholar] [CrossRef]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin contamination, its impact and management strategies: An updated review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef]
- Yusuf Aliyu, A.; Adeleke, O.A. Latest Progress on Tuberculosis and HIV Co-Infection: A Closer Look at People of Different Ages. Adv. Ther. 2025, 8, 2400033. [Google Scholar] [CrossRef]
- Liao, J.; He, Z.; Xia, Y.; Lei, Y.; Liao, B. A review on biosynthesis and genetic regulation of aflatoxin production by major Aspergillus fungi. Oil Crop Sci. 2020, 5, 166–173. [Google Scholar] [CrossRef]
- Ma, L.; Li, X.; Xing, F.; Ma, J.; Ma, X.; Jiang, Y. Fus3, as a critical kinase in MAPK cascade, regulates aflatoxin biosynthesis by controlling the substrate supply in Aspergillus flavus, rather than the cluster genes modulation. Microbiol. Spectr. 2022, 10, e01269-21. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Tian, J.; Keller, N.P. Post-translational modifications drive secondary metabolite biosynthesis in Aspergillus: A review. Environ. Microbiol. 2022, 24, 2857–2881. [Google Scholar] [CrossRef] [PubMed]
- Smit, S.J.; Lichman, B.R. Plant biosynthetic gene clusters in the context of metabolic evolution. Nat. Prod. Rep. 2022, 39, 1465–1482. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, S.; Cannon, M.; O’Neill, B. Climate change risks to human development in sub-Saharan Africa: A review of the literature. Clim. Dev. 2022, 14, 571–589. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—A revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Ashby, E.; Nicholson, A.; Haag, T. The Role of Plant Agricultural Practices on Development of Antimicrobial Resistant Fungi Affecting Human Health; Proceedings of a Workshop Series; National Academies Press: Washington, DC, USA, 2023; pp. 43–56. [Google Scholar]
- Marchante, H.; Palhas, J.; Núñez, F.A.L.; Marchante, E. Invasive species impacts and management. In Life on Land; Springer: Cham, Switzerland, 2021; pp. 560–571. [Google Scholar]
- Nji, Q.N.; Babalola, O.O.; Ekwomadu, T.I.; Nleya, N.; Mwanza, M. Six Main Contributing Factors to High Levels of Mycotoxin Contamination in African Foods. Toxins 2022, 14, 318. [Google Scholar] [CrossRef]
- Williams, P.R.; Paustenbach, D.J. Risk characterization. In Human and Ecological Risk Assessment: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2024; Volume 1, pp. 263–331. [Google Scholar]
- Kibugu, J.; Munga, L.; Mburu, D.; Maloba, F.; Auma, J.E.; Grace, D.; Lindahl, J.F. Dietary mycotoxins: An overview on toxicokinetics, toxicodynamics, toxicity, epidemiology, detection, and their mitigation with special emphasis on aflatoxicosis in humans and animals. Toxins 2024, 16, 483. [Google Scholar] [CrossRef]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. General considerations of dose-effect and dose-response relationships. In Handbook on the Toxicology of Metals; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- FAO/WHO. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods; WHO, Geneva 9th Session; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Marrez, D.A.; Ayesh, A.M. Mycotoxins: The threat to food safety. Egypt. J. Chem. 2022, 65, 353–372. [Google Scholar] [CrossRef]
- Waseem, A.; Ahmad Shah, S.; Sajjad, A.; Rauf Siddiqi, A.; Nafees, M. Human Exposure to Mycotoxins: A Retrospective Review of Leading Toxins and Metabolites in Human Biological Matrices. J. Chem. Soc. Pak. 2014, 36, 1196. [Google Scholar]
- Partnership for Aflatoxin Control in Africa (PACA) Secretariat, PACA Strategy: Overview of Intervention and Results. African Union Commission. 2020. Available online: https://archives.au.int/handle/123456789/5549 (accessed on 25 March 2025).
- Alberts, J.; Lilly, M.; Rheeder, J.; Burger, H.-M.; Shephard, G.S.; Gelderblom, W. Technological and community-based methods to reduce mycotoxin exposure. Food Control 2017, 73, 101–109. [Google Scholar] [CrossRef]
- Misihairabgwi, J.; Cheikhyoussef, A. Traditional fermented foods and beverages of Namibia. J. Ethn. Foods 2017, 4, 145–153. [Google Scholar] [CrossRef]
- Zebib, H.; Abate, D.; Woldegiorgis, A.Z. Aflatoxin M1 in raw milk, pasteurized milk and cottage cheese collected along value chain actors from three regions of Ethiopia. Toxins 2022, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Maggiore, A.; Afonso, A.; Barrucci, F.; Sanctis, G.D. Climate change as a driver of emerging risks for food and feed safety, plant, animal health and nutritional quality. EFSA Support. Publ. 2020, 17, 1881E. [Google Scholar]
- Martins, C.; Assunção, R.; Cunha, S.C.; Fernandes, J.O.; Jager, A.; Petta, T.; Oliveira, C.A.; Alvito, P. Assessment of multiple mycotoxins in breakfast cereals available in the Portuguese market. Food Chem. 2018, 239, 132–140. [Google Scholar] [CrossRef]
- Akello, J.; Ortega-Beltran, A.; Katati, B.; Atehnkeng, J.; Augusto, J.; Mwila, C.M.; Mahuku, G.; Chikoye, D.; Bandyopadhyay, R. Prevalence of aflatoxin-and fumonisin-producing fungi associated with cereal crops grown in Zimbabwe and their associated risks in a climate change scenario. Foods 2021, 10, 287. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in analysis and detection of major mycotoxins in foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Mngqawa, P.; Shephard, G.; Green, I.; Ngobeni, S.; De Rijk, T.; Katerere, D. Mycotoxin contamination of home-grown maize in rural northern South Africa (Limpopo and Mpumalanga Provinces). Food Addit. Contam. Part B 2016, 9, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, O.P.; Omotayo, A.O.; Babalola, O.O.; Mwanza, M. Comparative study of aflatoxin contamination of winter and summer ginger from the North West Province of South Africa. Toxicol. Rep. 2019, 6, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Adekoya, I.; Obadina, A.; Adaku, C.C.; De Boevre, M.; Okoth, S.; De Saeger, S.; Njobeh, P. Mycobiota and co-occurrence of mycotoxins in South African maize-based opaque beer. Int. J. Food Microbiol. 2018, 270, 22–30. [Google Scholar] [CrossRef]
- Odhav, B.; Naicker, V. Mycotoxins in South African traditionally brewed beers. Food Addit. Contam. 2002, 19, 55–61. [Google Scholar] [CrossRef]
- Ndisio, B.; Wachira, P.; Kagot, V.; Okoth, S. Susceptibility of locally cultivated groundnut (Arachis hypogaea) varieties to aflatoxin accumulation in Homa Bay County, Kenya. Afr. J. Microbiol. Res. 2017, 11, 1329–1337. [Google Scholar] [CrossRef]
- Dada, T.A.; Ekwomadu, T.I.; Mwanza, M. Multi-mycotoxin determination in dried beef using liquid chromatography coupled with triple quadrupole mass spectrometry (LC-MS/MS). Toxins 2020, 12, 357. [Google Scholar] [CrossRef]
- Daou, R.; Afif, C.; Joubrane, K.; Khabbaz, L.R.; Maroun, R.; Ismail, A.; El Khoury, A. Occurrence of aflatoxin M1 in raw, pasteurized, UHT cows’ milk, and dairy products in Lebanon. Food Control 2020, 111, 107055. [Google Scholar] [CrossRef]
- Ekpakpale, D.O.; Kraak, B.; Meijer, M.; Ayeni, K.I.; Houbraken, J.; Ezekiel, C.N. Fungal diversity and Aflatoxins in maize and rice grains and cassava-based flour (Pupuru) from Ondo State, Nigeria. J. Fungi 2021, 7, 635. [Google Scholar] [CrossRef]
- Afolabi, C.G.; Ezekiel, C.N.; Ogunbiyi, A.E.; Oluwadairo, O.J.; Sulyok, M.; Krska, R. Fungi and mycotoxins in cowpea (Vigna unguiculata L.) on Nigerian markets. Food Addit. Contam. Part B 2020, 13, 52–58. [Google Scholar] [CrossRef]
- Onyedum, S.C.; Adefolalu, F.S.; Muhammad, H.L.; Apeh, D.O.; Agada, M.; Imienwanrin, M.; Makun, H.A. Occurrence of major mycotoxins and their dietary exposure in North-Central Nigeria staples. Sci. Afr. 2020, 7, e00188. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Malik, N. The seasonal variation of aflatoxin M1 in milk and dairy products and assessment of dietary intake in Punjab, Pakistan. Food Control 2017, 79, 292–296. [Google Scholar] [CrossRef]
- Hajnal, J.; Kos, J.; Krulj, J.; Krstović, S.; Jajić, I.; Pezo, L.; Šarić, B.; Nedeljković, N. Aflatoxins contamination of maize in Serbia: The impact of weather conditions in 2015. Food Addit. Contam. Part A 2017, 34, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Janić Hajnal, E.; Šarić, B.; Jovanov, P.; Mandić, A.; Đuragić, O.; Kokić, B. Aflatoxins in maize harvested in the Republic of Serbia over the period 2012–2016. Food Addit. Contam. Part B 2018, 11, 246–255. [Google Scholar] [CrossRef]
- Matumba, L.; Monjerezi, M.; Kankwamba, H.; Njoroge, S.M.; Ndilowe, P.; Kabuli, H.; Kambewa, D.; Njapau, H. Knowledge, attitude, and practices concerning presence of molds in foods among members of the general public in Malawi. Mycotoxin Res. 2016, 32, 27–36. [Google Scholar] [CrossRef]
- Phokane, S.; Flett, B.C.; Ncube, E.; Rheeder, J.P.; Rose, L.J. Agricultural practices and their potential role in mycotoxin contamination of maize and groundnut subsistence farming. S. Afr. J. Sci. 2019, 115, 1–6. [Google Scholar] [CrossRef]
- Nji, Q.N.; Babalola, O.O.; Nleya, N.; Mwanza, M. Underreported human exposure to mycotoxins: The case of South Africa. Foods 2022, 11, 2714. [Google Scholar] [CrossRef]
- Jiang, Y.; Ogunade, I.M.; Vyas, D.; Adesogan, A.T. Aflatoxin in dairy cows: Toxicity, occurrence in feedstuffs and milk and dietary mitigation strategies. Toxins 2021, 13, 283. [Google Scholar] [CrossRef]
- Smaoui, S.; D’Amore, T.; Tarapoulouzi, M.; Agriopoulou, S.; Varzakas, T. Aflatoxins contamination in feed commodities: From occurrence and toxicity to recent advances in analytical methods and detoxification. Microorganisms 2023, 11, 2614. [Google Scholar] [CrossRef]
- Zhao, W.; Abdelsattar, M.M.; Wang, X.; Zhang, N.; Chai, J. In vitro modulation of rumen fermentation by microbiota from the recombination of rumen fluid and solid phases. Microbiol. Spectr. 2023, 11, e03387-22. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; van der Merwe, D. Mycotoxins in the food chain: Contamination of foods of animal origin. In Chemical Hazards in Foods of Animal Origin; Wageningen Academic: Wageningen, The Netherlands, 2019; pp. 241–261. [Google Scholar]
- Anelli, P.; Haidukowski, M.; Epifani, F.; Cimmarusti, M.T.; Moretti, A.; Logrieco, A.; Susca, A. Fungal mycobiota and mycotoxin risk for traditional artisan Italian cave cheese. Food Microbiol. 2019, 78, 62–72. [Google Scholar] [CrossRef]
- Younis, G.; Ibrahim, D.; Awad, A.; El Bardisy, M. Determination of aflatoxin M1 and ochratoxin A in milk and dairy products in supermarkets located in Mansoura City, Egypt. Adv. Anim. Vet. Sci. 2016, 4, 114–121. [Google Scholar] [CrossRef]
- Mwihia, E.W.; Mbuthia, P.G.; Eriksen, G.S.; Gathumbi, J.K.; Maina, J.G.; Mutoloki, S.; Waruiru, R.M.; Mulei, I.R.; Lyche, J.L. Occurrence and Levels of Aflatoxins in Fish Feeds and Their Potential Effects on Fish in Nyeri, Kenya. Toxins 2018, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Namulawa, V.T.; Mutiga, S.; Musimbi, F.; Akello, S.; Ngángá, F.; Kago, L.; Kyallo, M.; Harvey, J.; Ghimire, S. Assessment of fungal contamination in fish feed from the Lake Victoria Basin, Uganda. Toxins 2020, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Chelimo, S.J.; Isaboke, J.; Osano, O.; Menya, D. Risk assessment of aflatoxin and fumonisin in fish feeds, Kenya a review. Risk 2021, 11, 31–42. [Google Scholar]
- Matejova, I.; Svobodova, Z.; Vakula, J.; Mares, J.; Modra, H. Impact of mycotoxins on aquaculture fish species: A review. J. World Aquac. Soc. 2017, 48, 186–200. [Google Scholar] [CrossRef]
- Triarico, S.; Rivetti, S.; Capozza, M.A.; Romano, A.; Maurizi, P.; Mastrangelo, S.; Attinà, G.; Ruggiero, A. Transplacental passage and fetal effects of antineoplastic treatment during pregnancy. Cancers 2022, 14, 3103. [Google Scholar] [CrossRef]
- Magoha, H.; Kimanya, M.; De Meulenaer, B.; Roberfroid, D.; Lachat, C.; Kolsteren, P. Risk of dietary exposure to Aflatoxins and fumonisins in infants less than 6 months of age in Rombo, Northern Tanzania. Matern. Child Nutr. 2016, 12, 516–527. [Google Scholar] [CrossRef]
- Mahdavi, R.; Nikniaz, L.; Arefhosseini, S.; Vahed Jabbari, M. Determination of Aflatoxin M1 in breast milk samples in Tabriz–Iran. Matern. Child Health J. 2010, 14, 141–145. [Google Scholar] [CrossRef]
- Benkerroum, N.; Ismail, A. Human breast milk contamination with Aflatoxins, impact on children’s health, and possible control means: A review. Int. J. Environ. Res. Public Health 2022, 19, 16792. [Google Scholar] [CrossRef]
- Kayanda, R.A.; Ngure, F.M.; Kassim, N. Dietary Exposure of Infants and Young Children to Aflatoxins and Fumonisins in the East African Region: A Review. Curr. Res. Nutr. Food Sci. J. 2024, 12, 471–489. [Google Scholar] [CrossRef]
- Viegas, S.; Assunção, R.; Martins, C.; Nunes, C.; Osteresch, B.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Ribeiro, E.; Viegas, C. Occupational exposure to mycotoxins in swine production: Environmental and biological monitoring approaches. Toxins 2019, 11, 78. [Google Scholar] [CrossRef]
- Viegas, S.; Assunção, R.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Viegas, C. Mycotoxins feed contamination in a dairy farm–potential implications for milk contamination and workers’ exposure in a One Health approach. J. Sci. Food Agric. 2020, 100, 1118–1123. [Google Scholar] [CrossRef]
- Viegas, S.; Assunção, R.; Nunes, C.; Osteresch, B.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Martins, C.; Alvito, P.; Almeida, A.; et al. Exposure assessment to mycotoxins in a Portuguese fresh bread dough company by using a multi-biomarker approach. Toxins 2018, 10, 342. [Google Scholar] [CrossRef]
- Viegas, S.; Viegas, C.; Oppliger, A. Occupational exposure to mycotoxins: Current knowledge and prospects. Ann. Work. Expo. Health 2018, 62, 923–941. [Google Scholar] [CrossRef]
- Cramer, B.; Humpf, H.-U. Human biomonitoring of mycotoxins for the detection of nutritional, environmental and occupational exposure. In Exposure to Microbiological Agents in Indoor and Occupational Environments; Springer: Cham, Switzerland, 2017; pp. 191–212. [Google Scholar]
- Franco, L.T.; Oliveira, C.A. Assessment of occupational and dietary exposures of feed handling workers to mycotoxins in rural areas from São Paulo, Brazil. Sci. Total Environ. 2022, 837, 155763. [Google Scholar] [CrossRef]
- Huttunen, K.; Korkalainen, M. Microbial secondary metabolites and knowledge on inhalation effects. In Exposure to Microbiological Agents in Indoor and Occupational Environments; Springer: Cham, Switzerland, 2017; pp. 213–234. [Google Scholar]
- Guindon-Kezis, K.A.; Mulder, J.E.; Massey, T.E. In vivo treatment with aflatoxin B1 increases DNA oxidation, base excision repair activity and 8-oxoguanine DNA glycosylase 1 levels in mouse lung. Toxicology 2014, 321, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Veiga, L.; Almeida, A.; dos Santos, M.; Carolino, E.; Viegas, C. Occupational exposure to Aflatoxin B1 in a Portuguese poultry slaughterhouse. Ann. Occup. Hyg. 2016, 60, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Lai, H.; Yang, Y.; Xiao, J.; He, K.; Liu, C.; Chen, J.; Lin, Y. How does airway exposure of aflatoxin B1 affect serum albumin adduct concentrations? Evidence based on epidemiological study and animal experimentation. J. Toxicol. Sci. 2014, 39, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Mo, X.; Yang, Y.; He, K.; Xiao, J.; Liu, C.; Chen, J.; Lin, Y. Association between aflatoxin B1 occupational airway exposure and risk of hepatocellular carcinoma: A case-control study. Tumor Biol. 2014, 35, 9577–9584. [Google Scholar] [CrossRef]
- Brera, C.; Caputi, R.; Miraglia, M.; Iavicoli, I.; Salerno, A.; Carelli, G. Exposure assessment to mycotoxins in workplaces: Aflatoxins and Ochratoxin A occurrence in airborne dusts and human sera. Microchem. J. 2002, 73, 167–173. [Google Scholar] [CrossRef]
- Selim, M.I.; Juchems, A.M.; Popendorf, W. Assessing airborne aflatoxin B1 during on-farm grain handling activities. Am. Ind. Hyg. Assoc. J. 1998, 59, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Autrup, J.L.; Schmidt, J.; Seremet, T.; Autrup, H. Determination of exposure to Aflatoxins among Danish workers in animal-feed production through the analysis of aflatoxin B1 adducts to serum albumin. Scand. J. Work Environ. Health 1991, 17, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Veiga, L.; Figueiredo, P.; Almeida, A.; Carolino, E.; Viegas, C. Assessment of workers’ exposure to aflatoxin B1 in a Portuguese waste industry. Ann. Occup. Hyg. 2015, 59, 173–181. [Google Scholar]
- Viegas, S.; Veiga, L.; Malta-Vacas, J.; Sabino, R.; Figueredo, P.; Almeida, A.; Viegas, C.; Carolino, E. Occupational exposure to aflatoxin (AFB1) in poultry production. J. Toxicol. Environ. Health Part A 2012, 75, 1330–1340. [Google Scholar] [CrossRef]
- Saad-Hussein, A.; Beshir, S.; Moubarz, G.; Elserougy, S.; Ibrahim, M.I. Effect of occupational exposure to Aflatoxins on some liver tumor markers in textile workers. Am. J. Ind. Med. 2013, 56, 818–824. [Google Scholar] [CrossRef]
- Viegas, S.; Osteresch, B.; Almeida, A.; Cramer, B.; Humpf, H.-U.; Viegas, C. Enniatin B and Ochratoxin A in the blood serum of workers from the waste management setting. Mycotoxin Res. 2018, 34, 85–90. [Google Scholar] [CrossRef]
- Oluwafemi, F.; Odebiyi, T.; Kolapo, A. Occupational aflatoxin exposure among feed mill workers in Nigeria. World Mycotoxin J. 2012, 5, 385–389. [Google Scholar] [CrossRef]
- Wangia, R.N.; Tang, L.; Wang, J.-S. Occupational exposure to Aflatoxins and health outcomes: A review. J. Environ. Sci. Health Part C 2019, 37, 215–234. [Google Scholar] [CrossRef]
- Taevernier, L.; Veryser, L.; Roche, N.; Peremans, K.; Burvenich, C.; Delesalle, C.; De Spiegeleer, B. Human skin permeation of emerging mycotoxins (Beauvericin and Enniatins). J. Expo. Sci. Environ. Epidemiol. 2016, 26, 277–287. [Google Scholar] [CrossRef]
- Pallares, N.; Tolosa, J.; Ferrer, E.; Berrada, H. Mycotoxins in raw materials, beverages and supplements of botanicals: A review of occurrence, risk assessment and analytical methodologies. Food Chem. Toxicol. 2022, 165, 113013. [Google Scholar] [CrossRef] [PubMed]
- Carballo, D.; Fernández-Franzón, M.; Ferrer, E.; Pallarés, N.; Berrada, H. Dietary exposure to mycotoxins through alcoholic and non-alcoholic beverages in Valencia, Spain. Toxins 2021, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Xu, J.; Jiang, K.; Liu, X.; Meng, J.; Di Mavungu, J.D.; Guo, W.; Zhang, Z.; Jing, J.; Li, H.; et al. Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ. Pollut. 2019, 248, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guo, X. Emerging strategies in fluorescent aptasensor toward food hazard Aflatoxins detection. Trends Food Sci. Technol. 2022, 129, 621–633. [Google Scholar] [CrossRef]
- Zarba, A.; Wild, C.P.; Hall, A.J.; Montesano, R.; Hudson, G.J.; Groopman, J.D. Aflatoxrn M1 in human breast milk from The Gambia, West Africa, quantified by combined monoclonal antibody immunoaffinity chromatography and HPLC. Carcinogenesis 1992, 13, 891–894. [Google Scholar] [CrossRef]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2010, 31, 71–82. [Google Scholar] [CrossRef]
- Mamo, F.T.; Abate, B.A.; Zheng, Y.; Nie, C.; He, M.; Liu, Y. Distribution of Aspergillus fungi and recent aflatoxin reports, health risks, and advances in developments of biological mitigation strategies in China. Toxins 2021, 13, 678. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the presence of mycotoxins in biological samples: An overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef]
- Vallero, D.A. Translating Diverse Environmental Data into Reliable Information: How to Coordinate Evidence from Different Sources; Academic Press: Cambridge, MA, USA, 2017; pp. 251–296. [Google Scholar]
- Belbasis, L.; Mavrogiannis, M.C.; Emfietzoglou, M.; Evangelou, E. Environmental factors, serum biomarkers and risk of atrial fibrillation: An exposure-wide umbrella review of meta-analyses. Eur. J. Epidemiol. 2020, 35, 223–239. [Google Scholar] [CrossRef]
- Filipoiu, D.C.; Bungau, S.G.; Endres, L.; Negru, P.A.; Bungau, A.F.; Pasca, B.; Radu, A.-F.; Tarce, A.G.; Bogdan, M.A.; Behl, T.; et al. Characterization of the toxicological impact of heavy metals on human health in conjunction with modern analytical methods. Toxics 2022, 10, 716. [Google Scholar] [CrossRef]
- Frusciante, L.; Visibelli, A.; Geminiani, M.; Santucci, A.; Spiga, O. Artificial Intelligence Approaches in Drug Discovery: Towards the Laboratory of the Future. Curr. Top. Med. Chem. 2022, 22, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Quig, D. Metal toxicity: Assessment of exposure and retention. In Textbook of Natural Medicine; Elsevier: Amsterdam, The Netherlands, 2020; 187p. [Google Scholar]
- Habschied, K.; Kanižai Šarić, G.; Krstanović, V.; Mastanjević, K. Mycotoxins—Biomonitoring and human exposure. Toxins 2021, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Battistini, B.; Petrucci, F. Silver and gold nanoparticles characterization by SP-ICP-MS and AF4-FFF-MALS-UV-ICP-MS in human samples used for biomonitoring. Talanta 2020, 220, 121404. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, A.M.; Zamora-Sanabria, R.; Granados-Chinchilla, F. A focus on Aflatoxins in feedstuffs: Levels of contamination, prevalence, control strategies, and impacts on animal health. Aflatoxin-Control. Anal. Detect. Health Risks 2017, 2017, 116–152. [Google Scholar]
- Thompson, L.E.; Joy, M.S. Endogenous markers of kidney function and renal drug clearance processes of filtration, secretion, and reabsorption. Curr. Opin. Toxicol. 2022, 31, 100344. [Google Scholar] [CrossRef]
- Owolabi, I.O.; Siwarak, K.; Greer, B.; Rajkovic, A.; Dall’asta, C.; Karoonuthaisiri, N.; Uawisetwathana, U.; Elliott, C.T.; Petchkongkaew, A. Applications of mycotoxin biomarkers in human biomonitoring for exposome-health studies: Past, present, and future. Expo. Health 2024, 16, 837–859. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human biomonitoring of mycotoxins in blood, plasma and serum in recent years: A review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef]
- Bore, M.G. Nutritional Status Among Adolescents Living with Human Immuno-Deficiency Virus on Anti-Retroviral Therapy Follow-Up Living in Selected Regions of Ethiopia: A Mixed Methods Study. 2024. Available online: http://hdl.handle.net/10453/187481 (accessed on 29 April 2025).
- Wobudeya, E.; Bonnet, M.; Walters, E.G.; Nabeta, P.; Song, R.; Murithi, W.; Mchembere, W.; Dim, B.; Taguebue, J.-V.; Orne-Gliemann, J.; et al. Diagnostic advances in childhood tuberculosis—Improving specimen collection and yield of microbiological diagnosis for intrathoracic tuberculosis. Pathogens 2022, 11, 389. [Google Scholar] [CrossRef]
- Elabed, S.; Khaled, R.; Farhat, N.; Madkour, M.; Zadeh, S.A.M.; Shousha, T.; Taneera, J.; Semerjian, L.; Abass, K. Assessing Aflatoxin Exposure in the United Arab Emirates (UAE): Biomonitoring AFM1 levels in Urine Samples and their Association with Dietary Habits. Environ. Toxicol. Pharmacol. 2025, 114, 104644. [Google Scholar] [CrossRef]
- Nasir, U.; Naeem, I.; Asif, M.; Ismail, A.; Gong, Y.Y.; Routledge, M.N.; Amjad, A.; Fazal, A.; Ismail, Z. Assessment of Aflatoxins exposure through urinary biomarker approach and the evaluation of the impacts of Aflatoxins exposure on the selected health parameters of the children of Multan city of Pakistan. Food Control 2021, 123, 107863. [Google Scholar] [CrossRef]
- Kuempel, E.D.; Roberts, J.R.; Roth, G.; Dunn Kevin, L.; Zumwalde, R.D.; Hubbs, A.F.; Trout, D.; Holdsworth, G. Health Effects of Occupational Exposure to Silver Nanomaterials. 2021. Available online: https://www.cdc.gov/niosh/docs/2021-112/default.html (accessed on 29 April 2025).
- Liu, B.; Wang, Y.; Zhang, Y.; Yan, B. Mechanisms of protective effects of SGLT2 inhibitors in cardiovascular disease and renal dysfunction. Curr. Top. Med. Chem. 2019, 19, 1818–1849. [Google Scholar] [CrossRef]
- Panel, E.C.; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- Ouhibi, S.; Vidal, A.; Martins, C.; Gali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. LC-MS/MS methodology for simultaneous determination of patulin and citrinin in urine and plasma applied to a pilot study in colorectal cancer patients. Food Chem. Toxicol. 2020, 136, 110994. [Google Scholar] [CrossRef]
- Dabuo, B.; Avogo, E.W.; Koomson, G.O.; Akantibila, M.; Gbati, D.A. Aflatoxins: Toxicity, Occurrences and Chronic Exposure, in Aflatoxins-Occurrence, Detection and Novel Detoxification Strategies; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Turner, P.C.; Snyder, J.A. Development and limitations of exposure biomarkers to dietary contaminants mycotoxins. Toxins 2021, 13, 314. [Google Scholar] [CrossRef]
- Xue, K.S. Toxic Effects and Intervention Strategies for Co-Exposure to Aflatoxins and Fumonisins in Animals and High-Risk Human Populations. University of Georgia. 2017. Available online: https://getd.libs.uga.edu/pdfs/xue_kathy_s_201705_phd.pdf (accessed on 29 April 2025).
- Slobodchikova, I.; Vuckovic, D. Liquid chromatography–high resolution mass spectrometry method for monitoring of 17 mycotoxins in human plasma for exposure studies. J. Chromatogr. A 2018, 1548, 51–63. [Google Scholar] [CrossRef]
- Xue, K.S.; Cai, W.; Tang, L.; Wang, J.-S. Aflatoxin B1-lysine adduct in dried blood spot samples of animals and humans. Food Chem. Toxicol. 2016, 98, 210–219. [Google Scholar] [CrossRef]
- Adaku Chilaka, C.; Mally, A. Mycotoxin occurrence, exposure and health implications in infants and young children in Sub-Saharan Africa: A review. Foods 2020, 9, 1585. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, Y.; Ghorbani, R.; Taghavi, M.; Keramati, H.; Amanidaz, N.; Moradi, B.; Nazari, S.H.; Shariatifar, N.; Khaneghah, A.M. Concentration and prevalence of aflatoxin M1 in human breast milk in Iran: Systematic review, meta-analysis, and carcinogenic risk assessment: A review. J. Food Prot. 2019, 82, 785–795. [Google Scholar] [CrossRef]
- Min, L.; Fink-Gremmels, J.; Li, D.; Tong, X.; Tang, J.; Nan, X.; Yu, Z.; Chen, W.; Wang, G. An overview of aflatoxin B1 biotransformation and Aflatoxin M1 secretion in lactating dairy cows. Anim. Nutr. 2021, 7, 42–48. [Google Scholar] [CrossRef]
- Li, K.; Cai, H.; Luo, B.; Duan, S.; Yang, J.; Zhang, N.; He, Y.; Wu, A.; Liu, H. Recent Progress of Mycotoxin in Various Food Products—Human Exposure and Health Risk Assessment. Foods 2025, 14, 865. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Abia, W.A.; Braun, D.; Šarkanj, B.; Ayeni, K.I.; Oyedele, O.A.; Michael-Chikezie, E.C.; Ezekiel, V.C.; Mark, B.; Ahuchaogu, C.P.; et al. Comprehensive mycotoxin exposure biomonitoring in breastfed and non-exclusively breastfed Nigerian children. medRxiv 2020. [Google Scholar] [CrossRef]
- Lehmann, G.M.; LaKind, J.S.; Davis, M.H.; Hines, E.P.; Marchitti, S.A.; Alcala, C.; Lorber, M. Environmental chemicals in breast milk and formula: Exposure and risk assessment implications. Environ. Health Perspect. 2018, 126, 096001. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Mupunga, I.; van Rensburg, I.J.; Luthuli, N.; Abafe, O.A.; Shai, L.J.; Katerere, D.R. Analysis of aflatoxin biomarkers in the hair of experimental animals. Toxins 2021, 13, 570. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Q.; Wu, J.; Wu, W.; Jiang, J.; Yan, H.; Huang, J.; Sun, Y.; Deng, Y. The metabolism and biotransformation of AFB1: Key enzymes and pathways. Biochem. Pharmacol. 2022, 199, 115005. [Google Scholar] [CrossRef]
- Eaton, D.L.; Williams, D.E.; Coulombe, R.A. Species differences in the biotransformation of aflatoxin B1: Primary determinants of relative carcinogenic potency in different animal species. Toxins 2025, 17, 30. [Google Scholar] [CrossRef]
- Kensler, T.W.; Groopman, J.D. Aflatoxin Exposure, Human Liver Cancer Risk, and Chemoprevention. In Carcinogens, DNA Damage and Cancer Risk: Mechanisms of Chemical Carcinogenesis; World Scientific: Singapore, 2019; pp. 143–169. [Google Scholar]
- Hamzehloo-Moghadam, M.; Asri, N.; Sherafat, S.J.; Mansouri, V. Detection of aflatoxin contamination through biomarker discovery in human intestinal Caco-2 cells. Appl. Food Biotechnol. 2024, 11, e4. [Google Scholar]
- Giolo, M.P.; Oliveira, C.M.D.; Bertolini, D.A.; Lonardoni, M.V.C.; Gouveia, M.S.; Netto, D.P.; Nixdorf, S.L.; Machinski Junior, M. Aflatoxin M1 in the urine of non-carriers and chronic carriers of hepatitis B virus in Maringa, Brazil. Braz. J. Pharm. Sci. 2012, 48, 447–452. [Google Scholar] [CrossRef]
- de Cássia Romero, A.; Ferreira, T.R.B.; dos Santos Dias, C.T.; Calori-Domingues, M.A.; da Gloria, E.M. Occurrence of AFM1 in urine samples of a Brazilian population and association with food consumption. Food Control 2010, 21, 554–558. [Google Scholar] [CrossRef]
- Ediage, E.N.; Di Mavungu, J.D.; Song, S.; Sioen, I.; De Saeger, S. Multimycotoxin analysis in urines to assess infant exposure: A case study in Cameroon. Environ. Int. 2013, 57, 50–59. [Google Scholar] [CrossRef]
- Cao, X.; Wu, S.; Yue, Y.; Wang, S.; Wang, Y.; Tao, L.; Tian, H.; Xie, J.; Ding, H. A high-throughput method for the simultaneous determination of multiple mycotoxins in human and laboratory animal biological fluids and tissues by PLE and HPLC–MS/MS. J. Chromatogr. B 2013, 942, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Mykkänen, H.; Zhu, H.; Salminen, E.; Juvonen, R.O.; Ling, W.; Ma, J.; Polychronaki, N.; Kemiläinen, H.; Mykkänen, O.; Salminen, S. Fecal and urinary excretion of aflatoxin B1 metabolites (AFQ1, AFM1 and AFB-N7-guanine) in young Chinese males. Int. J. Cancer 2005, 115, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.; Njobeh, P.B.; Turner, P.C.; Kouanfack, C.; Eyongetah, M.; Dutton, M.; et al. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem. Toxicol. 2013, 62, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Groopman, J.D.; Wang, J.-S.; Kensler, T.W.; Friesen, M.D. Quantification of aflatoxin-b1-n7-guanine in human urine by high-performance liquid chromatography and isotope dilution tandem mass spectrometry1. Chem. Res. Toxicol. 2006, 19, 1191–1195. [Google Scholar] [CrossRef]
- Wang, J.-S.; Huang, T.; Su, J.; Liang, F.; Wei, Z.; Liang, Y.; Luo, H.; Kuang, S.-Y.; Qian, G.-S.; Sun, G. Hepatocellular carcinoma and aflatoxin exposure in Zhuqing village, Fusui county, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 2001, 10, 143–146. [Google Scholar]
- Ostry, V.; Malir, F.; Roubal, T.; Skarkova, J.; Ruprich, J.; Cerna, M.; Creppy, E.E. Monitoring of mycotoxin biomarkers in the Czech Republic. Mycotoxin Res. 2005, 21, 49–52. [Google Scholar] [CrossRef]
- Hatem, N.L.; Hassab, H.M.; Al-Rahman, E.M.A.; El-Deeb, S.A.; Ahmed, R.L.E.-S. Prevalence of Aflatoxins in blood and urine of Egyptian infants with protein–energy malnutrition. Food Nutr. Bull. 2005, 26, 49–56. [Google Scholar] [CrossRef]
- Polychronaki, N.; Wild, C.P.; Mykkänen, H.; Amra, H.; Abdel-Wahhab, M.; Sylla, A.; Diallo, M.; El-Nezami, H.; Turner, P.C. Urinary biomarkers of aflatoxin exposure in young children from Egypt and Guinea. Food Chem. Toxicol. 2008, 46, 519–526. [Google Scholar] [CrossRef]
- Piekkola, S.; Turner, P.; Abdel-Hamid, M.; Ezzat, S.; El-Daly, M.; El-Kafrawy, S.; Savchenko, E.; Poussa, T.; Woo, J.; Mykkänen, H. Characterisation of aflatoxin and deoxynivalenol exposure among pregnant Egyptian women. Food Addit. Contam. Part A 2012, 29, 962–971. [Google Scholar] [CrossRef]
- Mohd Redzwan, S.; Rosita, J.; Mohd Sokhini, A.M.; Nurul Aqilah, A.R. Association between aflatoxin M 1 excreted in human urine samples with the consumption of milk and dairy products. Bull. Environ. Contam. Toxicol. 2012, 89, 1115–1119. [Google Scholar] [CrossRef]
- Warth, B.; Sulyok, M.; Fruhmann, P.; Mikula, H.; Berthiller, F.; Schuhmacher, R.; Hametner, C.; Abia, W.A.; Adam, G.; Fröhlich, J.; et al. Development and validation of a rapid multi-biomarker liquid chromatography/tandem mass spectrometry method to assess human exposure to mycotoxins. Rapid Commun. Mass Spectrom. 2012, 26, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ediage, E.N.; Wu, A.; De Saeger, S. Development and application of salting-out assisted liquid/liquid extraction for multi-mycotoxin biomarkers analysis in pig urine with high performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 111–120. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Warth, B.; Ogara, I.M.; Abia, W.A.; Ezekiel, V.C.; Atehnkeng, J.; Sulyok, M.; Turner, P.C.; Tayo, G.O.; Krska, R.; et al. Mycotoxin exposure in rural residents in northern Nigeria: A pilot study using multi-urinary biomarkers. Environ. Int. 2014, 66, 138–145. [Google Scholar] [CrossRef]
- Cao, X.; Li, X.; Li, J.; Niu, Y.; Shi, L.; Fang, Z.; Zhang, T.; Ding, H. Quantitative determination of carcinogenic mycotoxins in human and animal biological matrices and animal-derived foods using multi-mycotoxin and analyte-specific high performance liquid chromatography-tandem mass spectrometric methods. J. Chromatogr. B 2018, 1073, 191–200. [Google Scholar] [CrossRef]
- McMillan, A.; Renaud, J.B.; Burgess, K.M.; Orimadegun, A.E.; Akinyinka, O.O.; Allen, S.J.; Miller, J.D.; Reid, G.; Sumarah, M.W. Aflatoxin exposure in Nigerian children with severe acute malnutrition. Food Chem. Toxicol. 2018, 111, 356–362. [Google Scholar] [CrossRef]
- Koshiol, J.; Gao, Y.-T.; Dean, M.; Egner, P.; Nepal, C.; Jones, K.; Wang, B.; Rashid, A.; Luo, W.; Van Dyke, A.L.; et al. Association of aflatoxin and gallbladder cancer. Gastroenterology 2017, 153, 488–494.e1. [Google Scholar] [CrossRef]
- Mohammed, A.; Chala, A.; Dejene, M.; Fininsa, C.; Hoisington, D.A.; Sobolev, V.S.; Arias, R.S. Aspergillus and aflatoxin in groundnut (Arachis hypogaea L.) and groundnut cake in Eastern Ethiopia. Food Addit. Contam. Part B 2016, 9, 290–298. [Google Scholar] [CrossRef]
- Lauer, J.M.; Duggan, C.P.; Ausman, L.M.; Griffiths, J.K.; Webb, P.; Wang, J.S.; Xue, K.S.; Agaba, E.; Nshakira, N.; Ghosh, S. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Matern. Child Nutr. 2019, 15, e12701. [Google Scholar] [CrossRef]
- Leroy, J.L.; Sununtnasuk, C.; García-Guerra, A.; Wang, J.S. Low level aflatoxin exposure associated with greater linear growth in southern Mexico: A longitudinal study. Matern. Child Nutr. 2018, 14, e12619. [Google Scholar] [CrossRef]
- Leroy, J.L.; Wang, J.-S.; Jones, K. Serum aflatoxin B1-lysine adduct level in adult women from Eastern Province in Kenya depends on household socio-economic status: A cross sectional study. Soc. Sci. Med. 2015, 146, 104–110. [Google Scholar] [CrossRef]
- Smith, L.E.; Mbuya, M.N.; Prendergast, A.J.; Turner, P.C.; Ruboko, S.; Humphrey, J.H.; Nelson, R.J.; Chigumira, A.; Kembo, G.; Stoltzfus, R.J. Determinants of recent aflatoxin exposure among pregnant women in rural Zimbabwe. Mol. Nutr. Food Res. 2017, 61, 1601049. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mitchell, N.J.; Gratz, J.; Houpt, E.R.; Gong, Y.; Egner, P.A.; Groopman, J.D.; Riley, R.T.; Showker, J.L.; Svensen, E.; et al. Exposure to aflatoxin and fumonisin in children at risk for growth impairment in rural Tanzania. Environ. Int. 2018, 115, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mahfuz, M.; Alam, M.A.; Fahim, S.M.; Gazi, M.A.; Raihan, M.J.; Hossain, M.; Egner, P.A.; Bessong, P.O.; Petri, W.A.; Groopman, J.D.; et al. Aflatoxin exposure in children living in Mirpur, Dhaka: Data from MAL-ED companion study. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 655–662. [Google Scholar] [CrossRef]
- Hernandez-Vargas, H.; Castelino, J.; Silver, M.J.; Dominguez-Salas, P.; Cros, M.-P.; Durand, G.; Calvez-Kelm, F.L.; Prentice, A.M.; Wild, C.P.; Moore, S.E.; et al. Exposure to aflatoxin B1 in utero is associated with DNA methylation in white blood cells of infants in The Gambia. Int. J. Epidemiol. 2015, 44, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Seetha, A.; Monyo, E.S.; Tsusaka, T.W.; Msere, H.W.; Madinda, F.; Chilunjika, T.; Sichone, E.; Mbughi, D.; Chilima, B.; Matumba, L. Aflatoxin-lysine adducts in blood serum of the Malawian rural population and aflatoxin contamination in foods (groundnuts, maize) in the corresponding areas. Mycotoxin Res. 2018, 34, 195–204. [Google Scholar] [CrossRef]
- Watson, S.; Chen, G.; Sylla, A.; Routledge, M.N.; Gong, Y.Y. Dietary exposure to aflatoxin and micronutrient status among young children from Guinea. Mol. Nutr. Food Res. 2016, 60, 511–518. [Google Scholar] [CrossRef]
- Watson, S.; Moore, S.E.; Darboe, M.K.; Chen, G.; Tu, Y.-K.; Huang, Y.-T.; Eriksen, K.G.; Bernstein, R.M.; Prentice, A.M.; Wild, C.P.; et al. Impaired growth in rural Gambian infants exposed to aflatoxin: A prospective cohort study. BMC Public Health 2018, 18, 1247. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, H.; Shen, J.; Peng, T.; Wang, J.; Yao, K.; Sun, S.; Shao, B.; Tan, J. Design of multifunctional nanostructure for ultrafast extraction and purification of Aflatoxins in foodstuffs. Anal. Chem. 2017, 89, 10556–10564. [Google Scholar] [CrossRef]
- Kang, M.-S.; Nkurunziza, P.; Muwanika, R.; Qian, G.; Tang, L.; Song, X.; Xue, K.; Nkwata, A.; Ssempebwa, J.; Lutalo, T. Longitudinal evaluation of aflatoxin exposure in two cohorts in south-western Uganda. Food Addit. Contam. Part A 2015, 32, 1322–1330. [Google Scholar] [CrossRef]
- Shirima, C.P.; Kimanya, M.E.; Routledge, M.N.; Srey, C.; Kinabo, J.L.; Humpf, H.-U.; Wild, C.P.; Tu, Y.-K.; Gong, Y.Y. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ. Health Perspect. 2015, 123, 173–178. [Google Scholar] [CrossRef]
- Turner, P.C.; Loffredo, C.; Kafrawy, S.E.; Ezzat, S.; Eissa, S.A.L.; Daly, M.E.; Nada, O.; Abdel-Hamid, M. Pilot survey of aflatoxin–albumin adducts in sera from Egypt. Food Addit. Contam. 2008, 25, 583–587. [Google Scholar] [CrossRef]
- Shuaib, F.M.; Jolly, P.E.; Ehiri, J.E.; Yatich, N.; Jiang, Y.; Funkhouser, E.; Person, S.D.; Wilson, C.; Ellis, W.O.; Wang, J.S.; et al. Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Trop. Med. Int. Health 2010, 15, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Sylla, A.; Gong, Y.Y.; Diallo, M.S.; Sutcliffe, A.E.; Hall, A.J.; Wild, C.P. Reduction in exposure to carcinogenic Aflatoxins by postharvest intervention measures in West Africa: A community-based intervention study. Lancet 2005, 365, 1950–1956. [Google Scholar] [CrossRef] [PubMed]
- Yard, E.E.; Daniel, J.H.; Lewis, L.S.; Rybak, M.E.; Paliakov, E.M.; Kim, A.A.; Montgomery, J.M.; Bunnell, R.; Abudo, M.U.; Akhwale, W.; et al. Human aflatoxin exposure in Kenya, 2007: A cross-sectional study. Food Addit. Contam. Part A 2013, 30, 1322–1331. [Google Scholar] [CrossRef]
- Leong, Y.-H.; Rosma, A.; Latiff, A.A.; Izzah, A.N. Associations of serum aflatoxin B1–lysine adduct level with socio-demographic factors and Aflatoxins intake from nuts and related nut products in Malaysia. Int. J. Hyg. Environ. Health 2012, 215, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Jonsyn-Ellis, F.E. Aflatoxins and ochratoxins in serum samples of school children. J. Nutr. Environ. Med. 2007, 16, 52–58. [Google Scholar] [CrossRef]
- Turner, P.C.; Collinson, A.C.; Cheung, Y.B.; Gong, Y.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int. J. Epidemiol. 2007, 36, 1119–1125. [Google Scholar] [CrossRef]
- Johnson, N.M.; Qian, G.; Xu, L.; Tietze, D.; Marroquin-Cardona, A.; Robinson, A.; Rodriguez, M.; Kaufman, L.; Cunningham, K.; Wittmer, J.; et al. Aflatoxin and PAH exposure biomarkers in a US population with a high incidence of hepatocellular carcinoma. Sci. Total Environ. 2010, 408, 6027–6031. [Google Scholar] [CrossRef]
- Polychronaki, N.; Turner, P.C.; Mykkänen, H.; Gong, Y.; Amra, H.; Abdel-Wahhab, M.; El-Nezami, H. Determinants of aflatoxin M1 in breast milk in a selected group of Egyptian mothers. Food Addit. Contam. 2006, 23, 700–708. [Google Scholar] [CrossRef]
- Gürbay, A.; Sabuncuoğlu, S.A.; Girgin, G.; Şahin, G.; Yiğit, Ş.; Yurdakök, M.; Tekinalp, G. Exposure of newborns to aflatoxin M1 and B1 from mothers’ breast milk in Ankara, Turkey. Food Chem. Toxicol. 2010, 48, 314–319. [Google Scholar] [CrossRef]
- Abdulrazzaq, Y.M.; Osman, N.; Yousif, Z.M.; Al-Falahi, S. Aflatoxin M1 in breast-milk of UAE women. Ann. Trop. Pediatr. 2003, 23, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, M.; Barlow, N.; Cross, A.; Hill, S.; Robson, A.; Taylor, A.; Tyson, J. Atomic spectrometry update: Review of advances in the analysis of clinical and biological materials, foods and beverages. J. Anal. At. Spectrom. 2021, 36, 452–511. [Google Scholar] [CrossRef]
- Sanchis, A.; Bosch-Orea, C.; Salvador, J.-P.; Marco, M.-P.; Farré, M. Development and validation of a multianalyte immunoassay for the quantification of environmental pollutants in seawater samples from the Catalonia coastal area. Anal. Bioanal. Chem. 2019, 411, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Devalloir, Q. Effects of Multi-Stressors (Pollution, Nutritional Quality) on the Immunocompetence of the Wood Mouse. Ph.D. Thesis, Université Bourgogne Franche-Comté, Besançon, France, 2023. Available online: https://theses.hal.science/tel-04598579 (accessed on 29 April 2025).
- Afzal, A.; Mahreen, N. Emerging insights into the impacts of heavy metals exposure on health, reproductive and productive performance of livestock. Front. Pharmacol. 2024, 15, 1375137. [Google Scholar] [CrossRef]
- Kraft, S.; Buchenauer, L.; Polte, T. Mold, mycotoxins and a dysregulated immune system: A combination of concern? Int. J. Mol. Sci. 2021, 22, 12269. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The compromised intestinal barrier induced by mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef]
- Gonkowski, S.; Gajęcka, M.; Makowska, K. Mycotoxins and the enteric nervous system. Toxins 2020, 12, 461. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, K.; Tang, Z.; Fan, K.; Meng, J.; Nie, D.; Zhao, Z.; Wu, Y.; Han, Z. Exposure assessment of multiple mycotoxins and cumulative health risk assessment: A biomonitoring-based study in the Yangtze River Delta, China. Toxins 2021, 13, 103. [Google Scholar] [CrossRef]
- Kyei, N.N.; Cramer, B.; Humpf, H.-U.; Degen, G.H.; Ali, N.; Gabrysch, S. Assessment of multiple mycotoxin exposure and its association with food consumption: A human biomonitoring study in a pregnant cohort in rural Bangladesh. Arch. Toxicol. 2022, 96, 2123–2138. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef]
- Sumon, A.H.; Islam, F.; Mohanto, N.C.; Kathak, R.R.; Molla, N.H.; Rana, S.; Degen, G.H.; Ali, N. The presence of Aflatoxin M1 in milk and milk products in Bangladesh. Toxins 2021, 13, 440. [Google Scholar] [CrossRef]
- Ritieni, A.; Santini, A.; Mussap, M.; Ferracane, R.; Bosco, P.; Gazzolo, D.; Galvano, F. Simultaneous determination of mycotoxins in biological fluids by LC-MS/MS. Front. Biosci. 2010, 2, 151–158. [Google Scholar] [CrossRef]
- Omar, S.S. Incidence of aflatoxin M1 in human and animal milk in Jordan. J. Toxicol. Environ. Health Part A 2012, 75, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Kościelecka, K.; Kuć, A.; Kubik-Machura, D.; Męcik-Kronenberg, T.; Włodarek, J.; Radko, L. Endocrine effect of some mycotoxins on humans: A clinical review of the ways to mitigate the action of mycotoxins. Toxins 2023, 15, 515. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Cardwell, K.; Hounsa, A.; Egal, S.; Turner, P.C.; Hall, A.J.; Wild, C.P. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross-sectional study. Br. Med. J. 2002, 325, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Eaton, D.L. 65 Years on—Aflatoxin Biomarkers Blossoming: Whither Next? Toxins 2024, 16, 496. [Google Scholar] [CrossRef]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef]
- Davwar, P.; David, P.; Imoh, L.; Duguru, M.; Zawaya, K.; Tsok, Y.; Sagay, A.; Okeke, E. Aflatoxin exposure in a population of HIV patients at risk of hepatocellular carcinoma, North-Central, Nigeria. Afr. Health Sci. 2023, 23, 81–87. [Google Scholar] [CrossRef]
- Jolly, P.E.; Akinyemiju, T.F.; Sakhuja, S.; Sheth, R. Association of aflatoxin B1 levels with mean CD4 cell count and uptake of ART among HIV infected patients: A prospective study. PLoS ONE 2022, 17, e0260873. [Google Scholar] [CrossRef]
- Madeen, E.P.; Maldarelli, F.; Groopman, J.D. Environmental pollutants, mucosal barriers, and pathogen susceptibility; the case for aflatoxin b1 as a risk factor for hiv transmission and pathogenesis. Pathogens 2021, 10, 1229. [Google Scholar] [CrossRef]
- Bekker, L.-G.; Beyrer, C.; Mgodi, N.; Lewin, S.R.; Delany-Moretlwe, S.; Taiwo, B.; Masters, M.C.; Lazarus, J.V. HIV infection. Nat. Rev. Dis. Primers 2023, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Mutua, F.K.; Grace, D.; Watts, C. Food Safety Investments in East Africa; International Livestock Research Institute: Nairobi, Kenya, 2021; p. 12. [Google Scholar]
- Rasouli, H.; Nayeri, F.D.; Khodarahmi, R. May phytophenolics alleviate Aflatoxins-induced health challenges? A holistic insight on current landscape and future prospects. Front. Nutr. 2022, 9, 981984. [Google Scholar] [CrossRef] [PubMed]
- Dabuo, B.; Xorlali, N.; Amoliga, N.T.; Atibodu, Z.K.; Newman, P.M.; Mohammed, A.; Ali, R.A.; Abudu, A. Aspergillus and Aspergillosis in People with Chronic Diseases. 2023. Available online: https://www.intechopen.com/chapters/87304 (accessed on 29 April 2025).
- Gurunathan, S.; Lee, A.R.; Kim, J.H. Antifungal effect of nanoparticles against COVID-19 linked black fungus: A perspective on biomedical applications. Int. J. Mol. Sci. 2022, 23, 12526. [Google Scholar] [CrossRef] [PubMed]
- Malvandi, A.M.; Shahba, S.; Mehrzad, J.; Lombardi, G. Metabolic disruption by naturally occurring mycotoxins in circulation: A focus on vascular and bone homeostasis dysfunction. Front. Nutr. 2022, 9, 915681. [Google Scholar] [CrossRef]
- Ye, D.; Hao, Z.; Tang, S.; Velkov, T.; Dai, C. Aflatoxin exposure-caused male reproductive toxicity: Molecular mechanisms, detoxification, and future directions. Biomolecules 2024, 14, 1460. [Google Scholar] [CrossRef]
- Kannan, K.; George, J.A.; Sahadevan, R.; Kothari, M.; Sadhukhan, S. Insights into one drug, multi-target aspects of polyphenols for diabetes management: In vitro, in vivo, and clinical evidence. Phytochem. Rev. 2024, 1–49. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, C.; Li, L.; Zhang, H.; Zha, W.; Ma, L.; Chen, L.; Gan, J. The role of oxidative stress in the development and therapeutic intervention of hepatocellular carcinoma. Curr. Cancer Drug Targets 2023, 23, 792–804. [Google Scholar] [CrossRef]
- Hasan, M.I.; Hossain, M.A.; Bhuiyan, P.; Miah, M.S.; Rahman, M.H. A system biology approach to determine therapeutic targets by identifying molecular mechanisms and key pathways for type 2 diabetes that are linked to the development of tuberculosis and rheumatoid arthritis. Life Sci. 2022, 297, 120483. [Google Scholar] [CrossRef]
- Thenuwara, G.; Javed, B.; Singh, B.; Byrne, H.J.; Tian, F. Sex-and Gender-Specific Considerations in Mycotoxin Screening: Assessing Differential Exposure, Health Impacts, and Mitigation Strategies. Microbiol. Res. 2024, 15, 2455–2492. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, M.; Bhatt, S.; Saini, V.; Malik, A. Cancer causes and treatments. Int. J. Pharm. Sci. Res. 2020, 11, 3121–3134. [Google Scholar]
- Santacroce, M.P.; Conversano, M.; Casalino, E.; Lai, O.; Zizzadoro, C.; Centoducati, G.; Crescenzo, G. Aflatoxins in aquatic species: Metabolism, toxicity and perspectives. Rev. Fish Biol. Fish. 2008, 18, 99–130. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Mary, V.S.; Theumer, M.G.; Arias, S.L.; Rubinstein, H.R. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology 2012, 302, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 toxicity and protective effects of curcumin: Molecular mechanisms and clinical implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front. Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef]
- Popescu, R.G.; Rădulescu, A.L.; Georgescu, S.E.; Dinischiotu, A. Aflatoxins in feed: Types, metabolism, health consequences in swine and mitigation strategies. Toxins 2022, 14, 853. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Sadiq, I.Z. Free radicals and oxidative stress: Signaling mechanisms, redox basis for human diseases, and cell cycle regulation. Curr. Mol. Med. 2023, 23, 13–35. [Google Scholar] [CrossRef]
- Malik, S.; Krishnaswamy, K.; Mustapha, A. Hazard analysis and risk-based preventive controls (HARPC): Current food safety and quality standards for complementary foods. Foods 2021, 10, 2199. [Google Scholar] [CrossRef]
- Mishra, R.; Madhav, S.; Dhaka, R.K.; Garg, P. (Eds.) Biomarkers in Environmental and Human Health Biomonitoring: An Integrated Perspective; Elsevier: Amsterdam, The Netherlands, 2024; pp. 150–151. [Google Scholar]
- Dropulic, L.K.; Lederman, H.M. Overview of infections in the immunocompromised host. Diagn. Microbiol. Immunocompromised Host 2016, 4. [Google Scholar] [CrossRef]
- Omar, S.S.; Haddad, M.A.; Parisi, S. Validation of HPLC and Enzyme-Linked Immunosorbent Assay (ELISA) techniques for detection and quantification of Aflatoxins in different food samples. Foods 2020, 9, 661. [Google Scholar] [CrossRef]
- Baratta, F.; Pastori, D.; Angelico, F.; Balla, A.; Paganini, A.M.; Cocomello, N.; Ferro, D.; Violi, F.; Sanyal, A.J.; Del Ben, M. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin. Gastroenterol. Hepatol. 2020, 18, 2324–2331.e4. [Google Scholar] [CrossRef]
- Wenndt, A.; Mutua, F.; Grace, D.; Thomas, L.F.; Lambertini, E. Quantitative assessment of aflatoxin exposure and hepatocellular carcinoma (HCC) risk associated with consumption of select Nigerian staple foods. Front. Sustain. Food Syst. 2023, 7, 1128540. [Google Scholar] [CrossRef]
- Visser, M.E.; Schoonees, A.; Ezekiel, C.N.; Randall, N.P.; Naude, C.E. Agricultural and nutritional education interventions for reducing aflatoxin exposure to improve infant and child growth in low-and middle-income countries. Cochrane Database Syst. Rev. 2020, 2020, CD013376. [Google Scholar] [CrossRef]
- Wild, C.P.; Miller, J.D.; Groopman, J.D. Intervention Strategies to Reduce Human Exposure to Aflatoxins and Fumonisins. Mycotoxin Control in Low-and Middle-Income Countries; International Agency for Research on Cancer (IARC): Lyon, Paris, 2015; pp. 103–112. [Google Scholar]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Jones, D.L.; Morgan, K.E.; Martinez, P.C.; Rodriguez, V.J.; Vazquez, A.; Raccamarich, P.D.; Alcaide, M.L. COVID-19 burden and risk among people with HIV/AIDS. J. Acquir. Immune Defic. Syndr. 2021, 87, 869–874. [Google Scholar] [CrossRef]
- Lee, M.; Quinn, R.; Pradhan, K.; Fedorov, K.; Levitz, D.; Fromowitz, A.; Thakkar, A.; Shapiro, L.C.; Kabarriti, R.; Ruiz, R.E.; et al. Impact of COVID-19 on case fatality rate of patients with cancer during the Omicron wave. Cancer Cell 2022, 40, 343–345. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
- Mupunga, I. A Comparative Study of Natural Contamination with Aflatoxins and Fumonisins in Selected Food Commodities from Botswana and Zimbabwe. University of South Africa. 2013. Available online: https://www.researchgate.net/publication/313888038_A_comparative_study_of_natural_contamination_with_aflatoxins_and_fumonisins_in_selected_food_commodities_from_Botswana_and_Zimbabwe (accessed on 29 April 2025).

| Food Commodity | Type of Aflatoxin | Analytical Method | Sample Size | % Contamination | Concentration Range (ppb) | References |
|---|---|---|---|---|---|---|
| Maize | AFs | HPLC | 200 | 80 | LOD-40.31 | [27] |
| Groundnut | AFs | HPLC | 180 | 100 | LOD-162.40 | [27] |
| Breakfast cereal | AFB1 | HPLC-FD | 26 | 69 | LOD-0.13 | [58] |
| maize | AFs | HPLC | 800 | 52 | LOD-1369 | [59] |
| Maize fermented dough | AFs | HPLC | 32 | 97 | LOD-310 | [60] |
| Maize | AFs | HPLC | - | 100 | 20–355 | [60] |
| Maize | AFB1 | LC-MS/MS | 114 | 47 | 1–149 | [61] |
| Ginger | AFs | HPLC, ELISA | 100 | 100 | 3.63–411.1 | [62] |
| Processed maize | AFB1 | HPLC | 176 | 50 | - | [63] |
| Home-brewed beer | AFs | HPLC/TLC | 29 | 28 | 200–400 | [64] |
| Groundnut | AFs | ELISA | 75 | 93 | LOD-43.23 | [65] |
| Dried beef | AFB1 | LC-MS/MS | 108 | 66 | 3.91–295.41 | [66] |
| UHT Milk | AFM1 | HPLC | 11 | 54.5 | 0.013–0.219 | [67] |
| Dairy products | AFM1 | HPLC | 156 | 45.5 | 0.015–7.350 | [67] |
| Rice and maize | AFs | ELISA | 32 | 74 | 1.75–173.3 | [68] |
| Cowpea | AFs | LC-MS/MS | 81 | - | LOD-209 | [69] |
| Sorghum | AFs | ELISA | 20 | 100 | 4.80–42.60 | [70] |
| Millet | AFs | ELISA | 20 | 100 | 4.80–45.60 | [70] |
| Yam flour | AFs | ELISA | 20 | 100 | 5.0–39.45 | [70] |
| Garri | AFs | ELISA | 20 | 100 | 2.60–55.40 | [70] |
| Rice | AFs | ELISA | 62 | 100 | 2.10–248.20 | [70] |
| Milk | AFM1 | HPLC-FD | 372 | 56.1 | LOD-345.8 | [71] |
| Maize | AFs | HPLC-FD | 180 | 57 | 1.3–91.4 | [72] |
| Maize | AFs | 3000 | 5–72 | LOD-76.2 | [73] | |
| Milk and dairy products | AFM1 | ELISA | 160 | 100 | 0.137–0.319 | [55] |
| Environment | Analytical Method | Sample Size | % Contamination | Contamination Range (ppb) | References |
|---|---|---|---|---|---|
| Sugar production factory | ELISA | 15 | - | 6–11 | [101] |
| Sugar and paper-making factory | ELISA | 181 | 56 | 5.9–10.4 | [102] |
| Warehouses for green coffee, black pepper, and cocoa beans | HPLC | 44 | - | LOD-0.023 | [103] |
| Farms handling grains | HPLC | 24 | - | [104] | |
| Animal feed production | ELISA | 45 | 20 | LOD-8 | [105] |
| Waste industry | ELISA | 41 | 100 | 2.5–25.9 | [106] |
| Poultry production | ELISA | 31 | 59 | 1–4.23 | [107] |
| Textile industry | 58 | 33 | [108] | ||
| Swine production | HPLC-MS/MS | 25 | 16 | [92] | |
| Waste management | ELISA | 41 | - | - | [109] |
| Feed mill workers | HPLC | 28 | 100 | 73.4–189.2 | [110] |
| Condition | Population | Biomarkers Assessed | Type of AF | AF Levels ppb | Method Used | References |
|---|---|---|---|---|---|---|
| Diabetes | 15–55 | Glucose levels, Glycosylated, Heamoglobin (HbA1c) | AFM1 | 1.86 | Elisa kits, Microplate Elisa readers, Spectrophotometric methods | [23] |
| Pregnant women | ||||||
| Hepatitis | Children, age 5–12 | Liver enzymes (ALT, AST) | AFM1 | 15 | Serum Biochemical Tests, Enzyme Immunoassays, Liver Function Tests | [251] |
| HIV | Adults with HIV-positive | CD4 count | AFB1 | 10 | Flow Cytometry, Immunoassays, Laboratory Blood Tests | [252] |
| Cancer | Cancer patients, various types | Tumor markers | AFB1 | 25 | Serum Biochemical Tests, ELISA | [253] |
| HIV | HIV | HIV Viral Load | AFB1 | 400 ng/kg body weight/day | Serum Biochemical Tests | [220] |
| Cancer | Cancer patients in European population | AFB1 | 10 ng/kg body weight per day | Margin of Exposure (MOE) approach | ||
| Cancer | Hepatocellular Carcinoma in sub-Saharan Africa | HCC | AFB1 | 5 to 500 | Quantitative cancer risk assessment | [254] |
| Cancer | Hepatocellular carcinoma in Southern Africa | HCC | AFB1 | Peanut butter (6.8–250.) Peanut (6.6–622.1) | (HPLC) | [255] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagbohun, T.R.; Nji, Q.N.; Okechukwu, V.O.; Adelusi, O.A.; Nyathi, L.A.; Awong, P.; Njobeh, P.B. Aflatoxin Exposure in Immunocompromised Patients: Current State and Future Perspectives. Toxins 2025, 17, 414. https://doi.org/10.3390/toxins17080414
Fagbohun TR, Nji QN, Okechukwu VO, Adelusi OA, Nyathi LA, Awong P, Njobeh PB. Aflatoxin Exposure in Immunocompromised Patients: Current State and Future Perspectives. Toxins. 2025; 17(8):414. https://doi.org/10.3390/toxins17080414
Chicago/Turabian StyleFagbohun, Temitope R., Queenta Ngum Nji, Viola O. Okechukwu, Oluwasola A. Adelusi, Lungani A. Nyathi, Patience Awong, and Patrick B. Njobeh. 2025. "Aflatoxin Exposure in Immunocompromised Patients: Current State and Future Perspectives" Toxins 17, no. 8: 414. https://doi.org/10.3390/toxins17080414
APA StyleFagbohun, T. R., Nji, Q. N., Okechukwu, V. O., Adelusi, O. A., Nyathi, L. A., Awong, P., & Njobeh, P. B. (2025). Aflatoxin Exposure in Immunocompromised Patients: Current State and Future Perspectives. Toxins, 17(8), 414. https://doi.org/10.3390/toxins17080414





