The Emerging Mycotoxin 2-Amino-14, 16-Dimethyloctadecan-3-ol (AOD) Alters Transcriptional Regulation and Sphingolipid Metabolism and Undergoes N-Acylation by HepG2 Cells
Abstract
1. Introduction
2. Results
2.1. Effects of AOD, m18:0, and d18:0 on Cell Viability and the Cellular Content of Sphingoid Bases and AOD After Incubation with HepG2 Cells
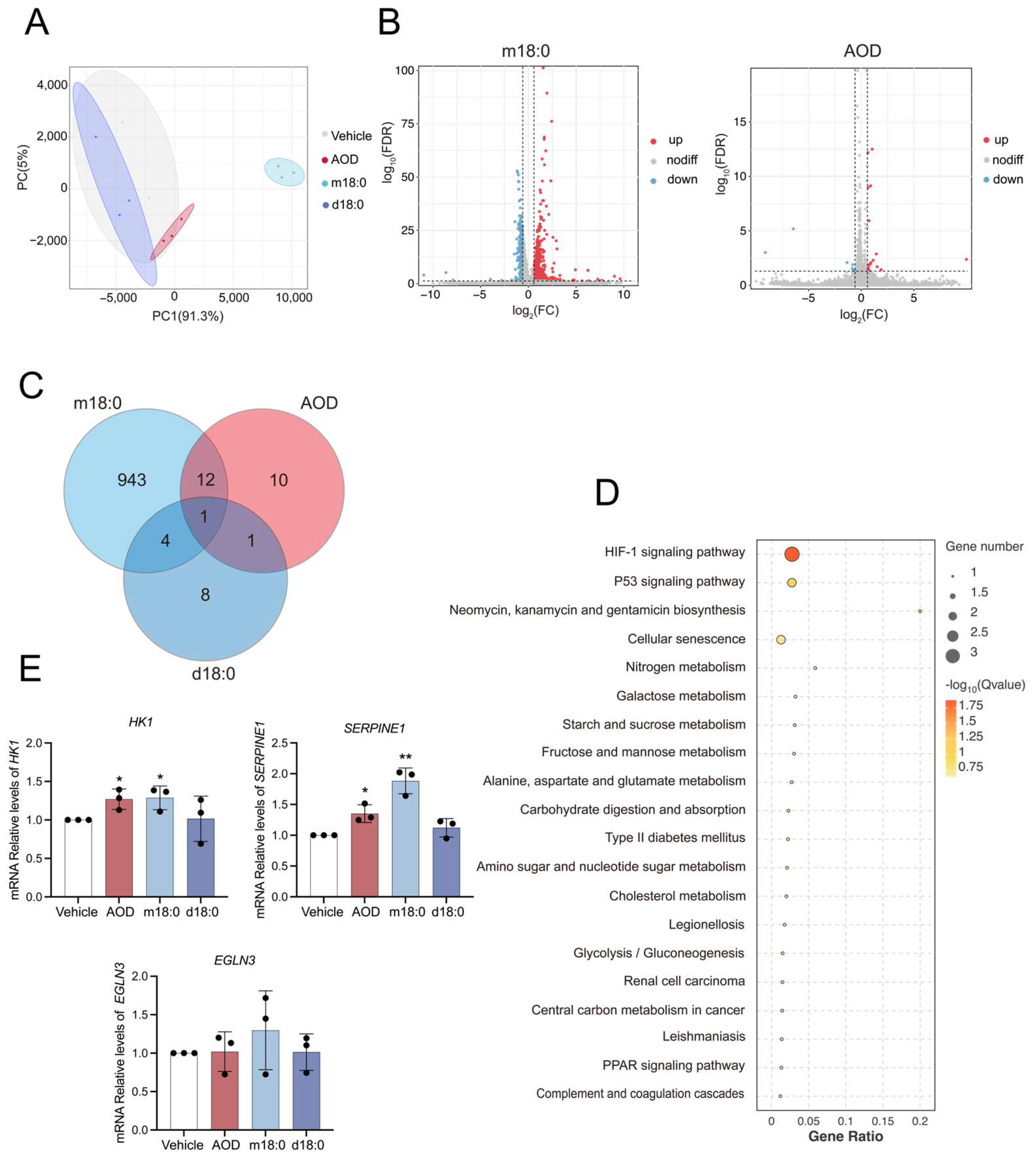
2.2. AOD Elicits Limited Transcriptomic Changes While Inducing Senescence-Associated Pathways
2.3. Transcriptional Analysis of Genes Related to Sphingolipid Metabolism in AOD-Treated HepG2 Cells
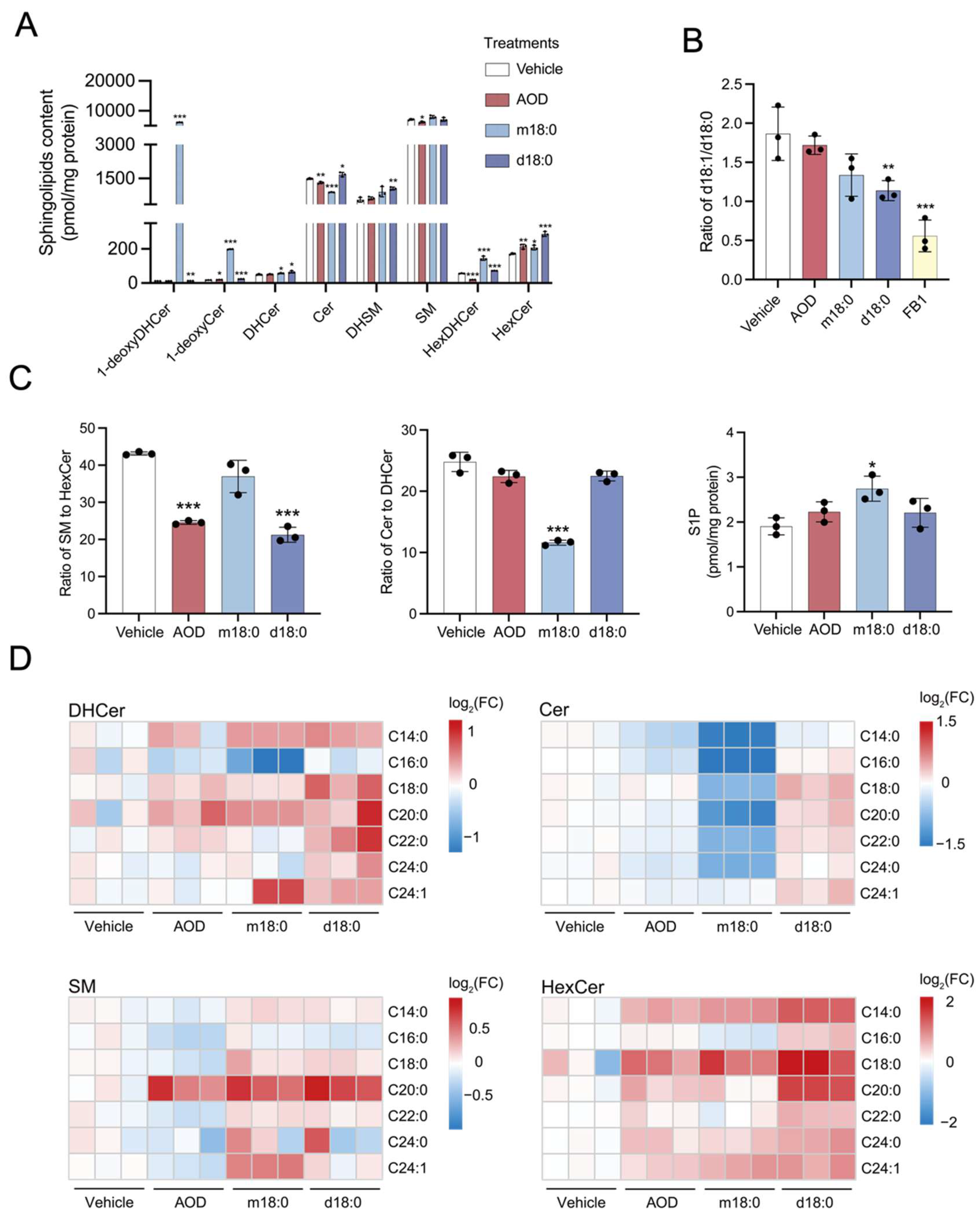
2.4. Sphingolipid Profile Remodeling of HepG2 Cells in Response to AOD and Comparison with m18:0 or d18:0 Treatment
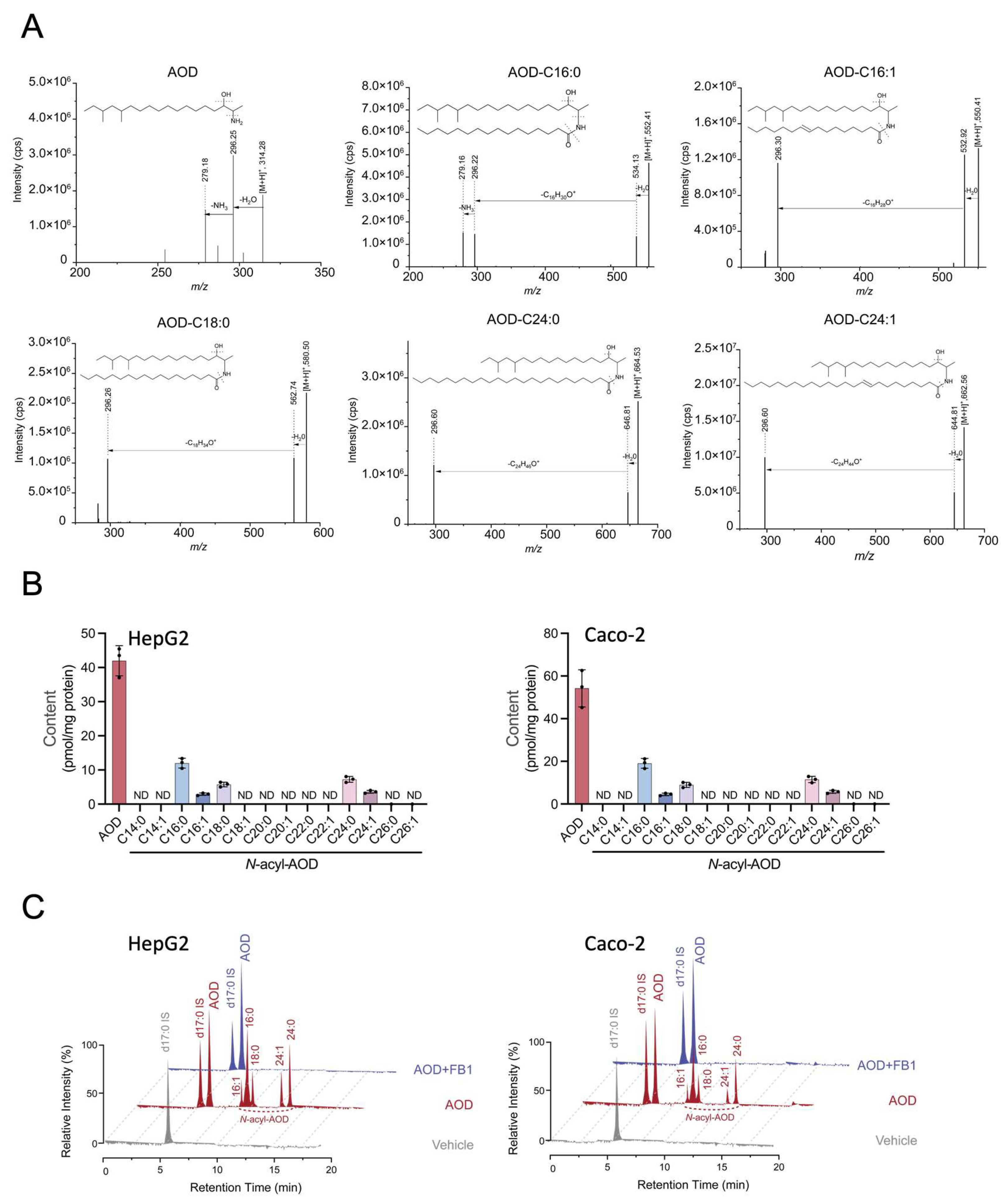
2.5. AOD Is Metabolized into 1-Deoxydihydroceramide-like Molecules, and This Is Inhibited by Fumonisin B1
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture Preparation and Treatment
4.3. Cell Viability Assay
4.4. Lipid Extraction
4.5. LC-MS/MS Analysis
4.6. Cellular RNA Sequencing
4.7. Quantitative Real-Time PCR
4.8. Western Blotting
4.9. SA-β-Gal Staining
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOD | 2-amino-14,16-dimethyloctadecan-3-ol |
| m18:0 | 1-deoxysphinganine |
| d18:0 | sphinganine |
| d18:1 | sphingosine |
| SPT | serine palmitoyltransferase(s) |
| CerS | ceramide synthase(s) |
| DEGS | dihydroceramide desaturase(s) |
| dhCerS | dihydroceramide synthase(s) |
| Cdase | ceramidase(s) |
| GCase | glucocerebrosidase(s) |
| GCS | glucosylceramide synthase(s) |
| SMS | sphingomyelin synthases(s) |
| SMase | sphingomyelinase(s) |
| SK | sphingosine kinase(s) |
| S1PP | S1P phosphatase(s) |
| HK1 | hexokinase 1 |
| EGLN3 | egl-9 family hypoxia-inducible factor 3 |
| SERPINE1 | serpin family E member 1 |
| CERS4 | ceramide synthase 4 |
| DEGS1 | dihydroceramide desaturase 1 |
| FASD3 | fatty acid desaturase 3 |
| SPHK1 | sphingosine kinase 1 |
| SGPP1 | sphingosine-1-phosphate phosphatase 1 |
| UGCG | UDP-glucosylceramide glucosyltransferase |
| S1P | sphingosine-1-phosphate |
| Cer | ceramide |
| SM | sphingomyelin |
| HexCer | hexosylceramide |
| DHCer | dihydroceramide |
| DHSM | dihydrosphingomyelin |
| HexDHCer | dihydrohexosylceramide |
| FB1 | fumonisin B1 |
| PCA | principal component analysis |
| DEGs | differentially expressed genes |
| SNA | Synthetischer Nährstoffarmer Agar |
| LC–MS/MS | liquid chromatography tandem mass spectrometry |
| UPLC | ultra-high-performance liquid chromatography |
| sMRM | scheduled multiple reaction monitoring |
| TPM | transcripts per million |
| RNA-seq | RNA sequencing |
| PBS | phosphate-buffered saline |
| SA-β-gal | senescence-associated β-galactosidase |
References
- Christ, D.S.; Gödecke, R.; von Tiedemann, A.; Varrelmann, M. Pathogenicity, symptom development, and mycotoxin formation in wheat by Fusarium species frequently isolated from sugar beet. Phytopathology 2011, 101, 1338–1345. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Phipps, R.K.; Nielsen, K.F.; Schroers, H.J.; Frank, J.; Thrane, U. Analysis of Fusarium avenaceum metabolites produced during wet apple core rot. J. Agric. Food Chem. 2009, 57, 1632–1639. [Google Scholar] [CrossRef]
- Uhlig, S.; Petersen, D.; Flåøyen, A.; Wilkins, A. 2-Amino-14,16-dimethyloctadecan-3-ol, a new sphingosine analogue toxin in the fungal genus Fusarium. Toxicon 2005, 46, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Gutleb, A.C.; Thrane, U.; Flåøyen, A. Identification of cytotoxic principles from Fusarium avenaceum using bioassay-guided fractionation. Toxicon 2005, 46, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Eriksen, G.S.; Hofgaard, I.S.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a changing climate: Semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.M.; González, L.; Catalá, A.I. Mechanism of action of sphingolipids and their metabolites in the toxicity of fumonisin B1. Prog. Lipid Res. 2005, 44, 345–356. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Duan, J.; Merrill, A.H., Jr. 1-Deoxysphingolipids Encountered Exogenously and Made de Novo: Dangerous Mysteries inside an Enigma. J. Biol. Chem. 2015, 290, 15380–15389. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. Don’t Be Surprised When These Surprise You: Some Infrequently Studied Sphingoid Bases, Metabolites, and Factors That Should Be Kept in Mind During Sphingolipidomic Studies. Int. J. Mol. Sci. 2025, 26, 650. [Google Scholar] [CrossRef]
- Gai, Z.; Gui, T.; Alecu, I.; Lone, M.A.; Hornemann, T.; Chen, Q.; Visentin, M.; Hiller, C.; Hausler, S.; Kullak-Ublick, G.A. Farnesoid X receptor activation induces the degradation of hepatotoxic 1-deoxysphingolipids in non-alcoholic fatty liver disease. Liver Int. 2020, 40, 844–859. [Google Scholar] [CrossRef]
- Rosarda, J.D.; Giles, S.; Harkins-Perry, S.; Mills, E.A.; Friedlander, M.; Wiseman, R.L.; Eade, K.T. Imbalanced unfolded protein response signaling contributes to 1-deoxysphingolipid retinal toxicity. Nat. Commun. 2023, 14, 4119. [Google Scholar] [CrossRef]
- Gantner, M.L.; Eade, K.; Wallace, M.; Handzlik, M.K.; Fallon, R.; Trombley, J.; Bonelli, R.; Giles, S.; Harkins-Perry, S.; Heeren, T.F.C.; et al. Serine and Lipid Metabolism in Macular Disease and Peripheral Neuropathy. N. Engl. J. Med. 2019, 381, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, M.; Cuevas, C.; Alonso, J.L.; Otero, G.; Faircloth, G.; Fernandez-Sousa, J.M.; Avila, J.; Wandosell, F. The marine sphingolipid-derived compound ES 285 triggers an atypical cell death pathway. Apoptosis 2007, 12, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Penno, A.; Reilly, M.M.; Houlden, H.; Laurá, M.; Rentsch, K.; Niederkofler, V.; Stoeckli, E.T.; Nicholson, G.; Eichler, F.; Brown, R.H., Jr.; et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 2010, 285, 11178–11187. [Google Scholar] [CrossRef] [PubMed]
- Eichler, F.S.; Hornemann, T.; McCampbell, A.; Kuljis, D.; Penno, A.; Vardeh, D.; Tamrazian, E.; Garofalo, K.; Lee, H.J.; Kini, L.; et al. Overexpression of the wild-type SPT1 subunit lowers desoxysphingolipid levels and rescues the phenotype of HSAN1. J. Neurosci. 2009, 29, 14646–14651. [Google Scholar] [CrossRef]
- Garofalo, K.; Penno, A.; Schmidt, B.P.; Lee, H.J.; Frosch, M.P.; von Eckardstein, A.; Brown, R.H.; Hornemann, T.; Eichler, F.S. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Investig. 2011, 121, 4735–4745. [Google Scholar] [CrossRef]
- Auranen, M.; Toppila, J.; Suriyanarayanan, S.; Lone, M.A.; Paetau, A.; Tyynismaa, H.; Hornemann, T.; Ylikallio, E. Clinical and metabolic consequences of L-serine supplementation in hereditary sensory and autonomic neuropathy type 1C. Cold Spring Harb. Mol. Case Stud. 2017, 3, a002212. [Google Scholar] [CrossRef]
- Ivanova, L.; Uhlig, S. A bioassay for the simultaneous measurement of metabolic activity, membrane integrity, and lysosomal activity in cell cultures. Anal. Biochem. 2008, 379, 16–19. [Google Scholar] [CrossRef]
- Solhaug, A.; Karlsøen, L.M.; Holme, J.A.; Kristoffersen, A.B.; Eriksen, G.S. Immunomodulatory effects of individual and combined mycotoxins in the THP-1 cell line. Toxicol. Vitr. 2016, 36, 120–132. [Google Scholar] [CrossRef]
- Solhaug, A.; Torgersen, M.L.; Holme, J.A.; Wiik-Nilsen, J.; Thiede, B.; Eriksen, G.S. The Fusarium mycotoxin, 2-Amino-14,16-dimethyloctadecan-3-ol (AOD) induces vacuolization in HepG2 cells. Toxicology 2020, 433–434, 152405. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, Y.; Wang, X.; Deng, Y.; Wang, X.; Li, S.; Ding, X.; Duan, J. Accumulation of Fatty Acylated Fusarium Toxin 2-Amino-14,16-dimethyloctadecan-3-ol, a Class of Novel 1-Deoxysphingolipid Analogues, during Food Storage. J. Agric. Food Chem. 2022, 70, 5151–5158. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr.; Nimkar, S.; Menaldino, D.; Hannun, Y.A.; Loomis, C.; Bell, R.M.; Tyagi, S.R.; Lambeth, J.D.; Stevens, V.L.; Hunter, R.; et al. Structural requirements for long-chain (sphingoid) base inhibition of protein kinase C in vitro and for the cellular effects of these compounds. Biochemistry 1989, 28, 3138–3145. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Norred, W.P.; Showker, J.; Riley, R.T. Elevated sphingoid bases and complex sphingolipid depletion as contributing factors in fumonisin-induced cytotoxicity. Toxicol. Appl. Pharmacol. 1996, 138, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Ivanova, L.; Bernhoft, A.; Eriksen, G.S. 2-Amino-14,16-dimethyloctadecan-3-ol: In vitro bioactivity and bio-production by the fungus Fusarium avenaceum. World Mycotoxin J. 2008, 1, 49–58. [Google Scholar] [CrossRef]
- Khan, S.Y.; Awad, E.M.; Oszwald, A.; Mayr, M.; Yin, X.; Waltenberger, B.; Stuppner, H.; Lipovac, M.; Uhrin, P.; Breuss, J.M. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Sci. Rep. 2017, 7, 39501. [Google Scholar] [CrossRef]
- Grenier, B.; Schwartz-Zimmermann, H.E.; Caha, S.; Moll, W.D.; Schatzmayr, G.; Applegate, T.J. Dose-dependent effects on sphingoid bases and cytokines in chickens fed diets prepared with fusarium verticillioides culture material containing fumonisins. Toxins 2015, 7, 1253–1272. [Google Scholar] [CrossRef]
- Grenier, B.; Schwartz-Zimmermann, H.E.; Gruber-Dorninger, C.; Dohnal, I.; Aleschko, M.; Schatzmayr, G.; Moll, W.D.; Applegate, T.J. Enzymatic hydrolysis of fumonisins in the gastrointestinal tract of broiler chickens. Poult. Sci. 2017, 96, 4342–4351. [Google Scholar] [CrossRef]
- Enongene, E.N.; Sharma, R.P.; Bhandari, N.; Voss, K.A.; Riley, R.T. Disruption of sphingolipid metabolism in small intestines, liver and kidney of mice dosed subcutaneously with fumonisin B (1). Food Chem. Toxicol. 2000, 38, 793–799. [Google Scholar] [CrossRef]
- Wegner, M.S.; Schiffmann, S.; Parnham, M.J.; Geisslinger, G.; Grösch, S. The enigma of ceramide synthase regulation in mammalian cells. Prog. Lipid Res. 2016, 63, 93–119. [Google Scholar] [CrossRef]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef]
- Sugawara, T.; Kinoshita, M.; Ohnishi, M.; Tsuzuki, T.; Miyazawa, T.; Nagata, J.; Hirata, T.; Saito, M. Efflux of sphingoid bases by P-glycoprotein in human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2004, 68, 2541–2546. [Google Scholar] [CrossRef]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef]
- Cordes, T.; Kuna, R.S.; McGregor, G.H.; Khare, S.V.; Gengatharan, J.; Muthusamy, T.; Metallo, C.M. 1-Deoxysphingolipid synthesis compromises anchorage-independent growth and plasma membrane endocytosis in cancer cells. J. Lipid Res. 2022, 63, 100281. [Google Scholar] [CrossRef]
- Esaki, K.; Sayano, T.; Sonoda, C.; Akagi, T.; Suzuki, T.; Ogawa, T.; Okamoto, M.; Yoshikawa, T.; Hirabayashi, Y.; Furuya, S. L-Serine Deficiency Elicits Intracellular Accumulation of Cytotoxic Deoxysphingolipids and Lipid Body Formation. J. Biol. Chem. 2015, 290, 14595–14609. [Google Scholar] [CrossRef]
- Othman, A.; Bianchi, R.; Alecu, I.; Wei, Y.; Porretta-Serapiglia, C.; Lombardi, R.; Chiorazzi, A.; Meregalli, C.; Oggioni, N.; Cavaletti, G.; et al. Lowering plasma 1-deoxysphingolipids improves neuropathy in diabetic rats. Diabetes 2015, 64, 1035–1045. [Google Scholar] [CrossRef]
- Truman, J.P.; Ruiz, C.F.; Montal, E.; Garcia-Barros, M.; Mileva, I.; Snider, A.J.; Hannun, Y.A.; Obeid, L.M.; Mao, C. 1-Deoxysphinganine initiates adaptive responses to serine and glycine starvation in cancer cells via proteolysis of sphingosine kinase. J. Lipid Res. 2022, 63, 100154. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kim, J.; Huang, Z.; St Clair, J.R.; Brown, D.A.; London, E. Efficient replacement of plasma membrane outer leaflet phospholipids and sphingolipids in cells with exogenous lipids. Proc. Natl. Acad. Sci. USA 2016, 113, 14025–14030. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; You, Y.; Yu, B.; Deng, Z.; Liu, M.; Hu, Z.; Duan, J. Loss of the ceramide synthase HYL-2 from Caenorhabditis elegans impairs stress responses and alters sphingolipid composition. J. Biol. Chem. 2024, 300, 107320. [Google Scholar] [CrossRef] [PubMed]
- Shaner, R.L.; Allegood, J.C.; Park, H.; Wang, E.; Kelly, S.; Haynes, C.A.; Sullards, M.C.; Merrill, A.H., Jr. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J. Lipid Res. 2009, 50, 1692–1707. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinform. 2021, 19, 578–583. [Google Scholar] [CrossRef]
- Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022, 50, D27–D38. [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, S.; Hu, Z.; Zhu, H.; Yu, B.; Chen, X.; Chen, Y.; Merrill, A.H., Jr.; Duan, J. The Emerging Mycotoxin 2-Amino-14, 16-Dimethyloctadecan-3-ol (AOD) Alters Transcriptional Regulation and Sphingolipid Metabolism and Undergoes N-Acylation by HepG2 Cells. Toxins 2025, 17, 413. https://doi.org/10.3390/toxins17080413
Mo S, Hu Z, Zhu H, Yu B, Chen X, Chen Y, Merrill AH Jr., Duan J. The Emerging Mycotoxin 2-Amino-14, 16-Dimethyloctadecan-3-ol (AOD) Alters Transcriptional Regulation and Sphingolipid Metabolism and Undergoes N-Acylation by HepG2 Cells. Toxins. 2025; 17(8):413. https://doi.org/10.3390/toxins17080413
Chicago/Turabian StyleMo, Shenlong, Zhenying Hu, Huaiyi Zhu, Boming Yu, Xiaoyan Chen, Yu Chen, Alfred H. Merrill, Jr., and Jingjing Duan. 2025. "The Emerging Mycotoxin 2-Amino-14, 16-Dimethyloctadecan-3-ol (AOD) Alters Transcriptional Regulation and Sphingolipid Metabolism and Undergoes N-Acylation by HepG2 Cells" Toxins 17, no. 8: 413. https://doi.org/10.3390/toxins17080413
APA StyleMo, S., Hu, Z., Zhu, H., Yu, B., Chen, X., Chen, Y., Merrill, A. H., Jr., & Duan, J. (2025). The Emerging Mycotoxin 2-Amino-14, 16-Dimethyloctadecan-3-ol (AOD) Alters Transcriptional Regulation and Sphingolipid Metabolism and Undergoes N-Acylation by HepG2 Cells. Toxins, 17(8), 413. https://doi.org/10.3390/toxins17080413





