Cell Type-Specific Effects of Fusarium Mycotoxins on Primary Neurons and Astroglial Cells
Abstract
1. Introduction
2. Results
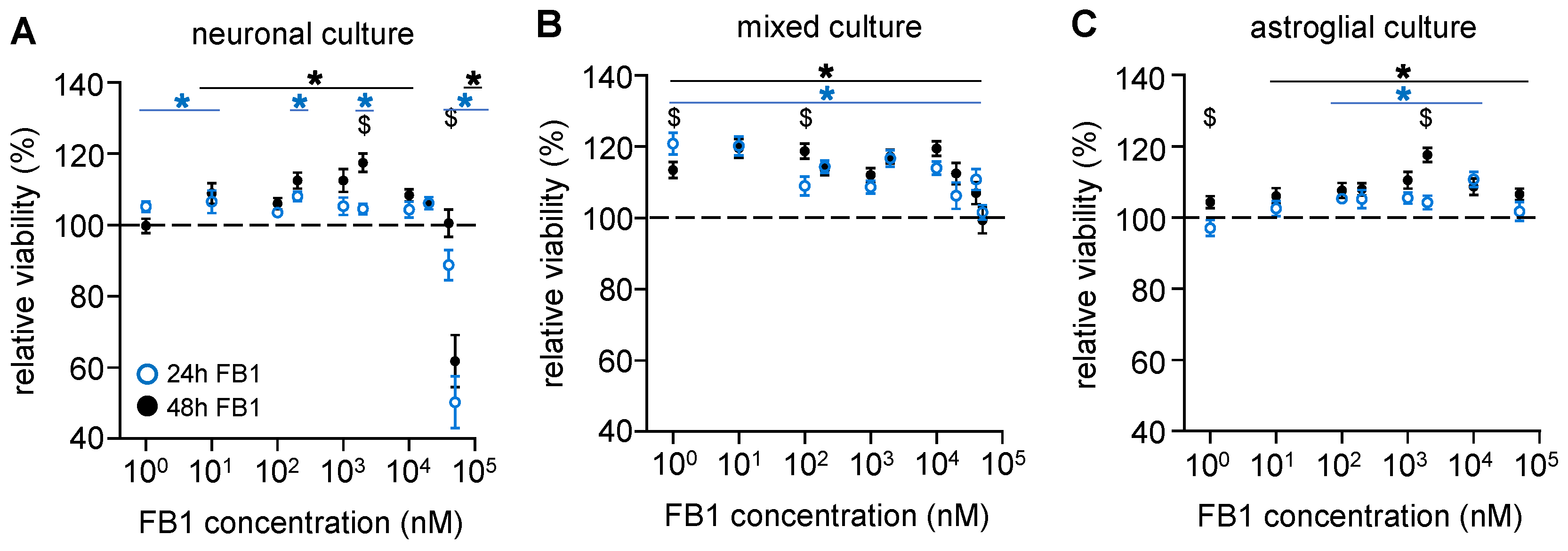
2.1. The Presence of Astroglial Cells Attenuates the Cytotoxic Effect of FB1
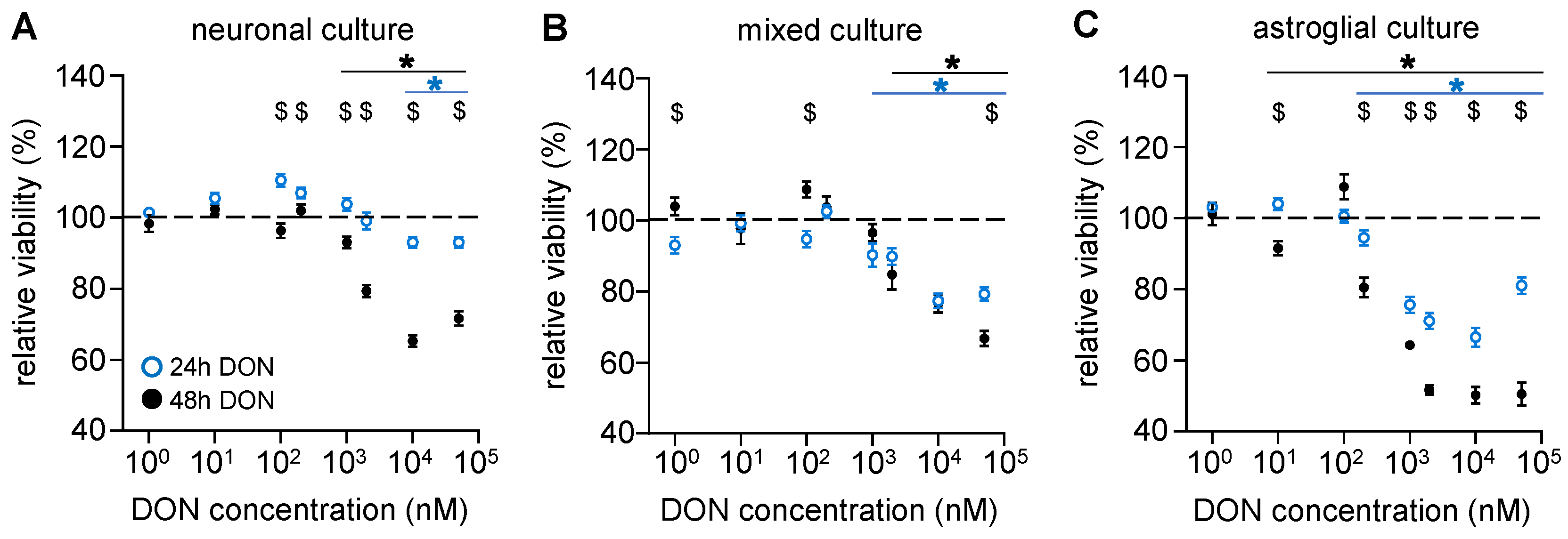
2.2. Astroglial Cells Are More Sensitive than Neurons to the Cytotoxic Effects of DON
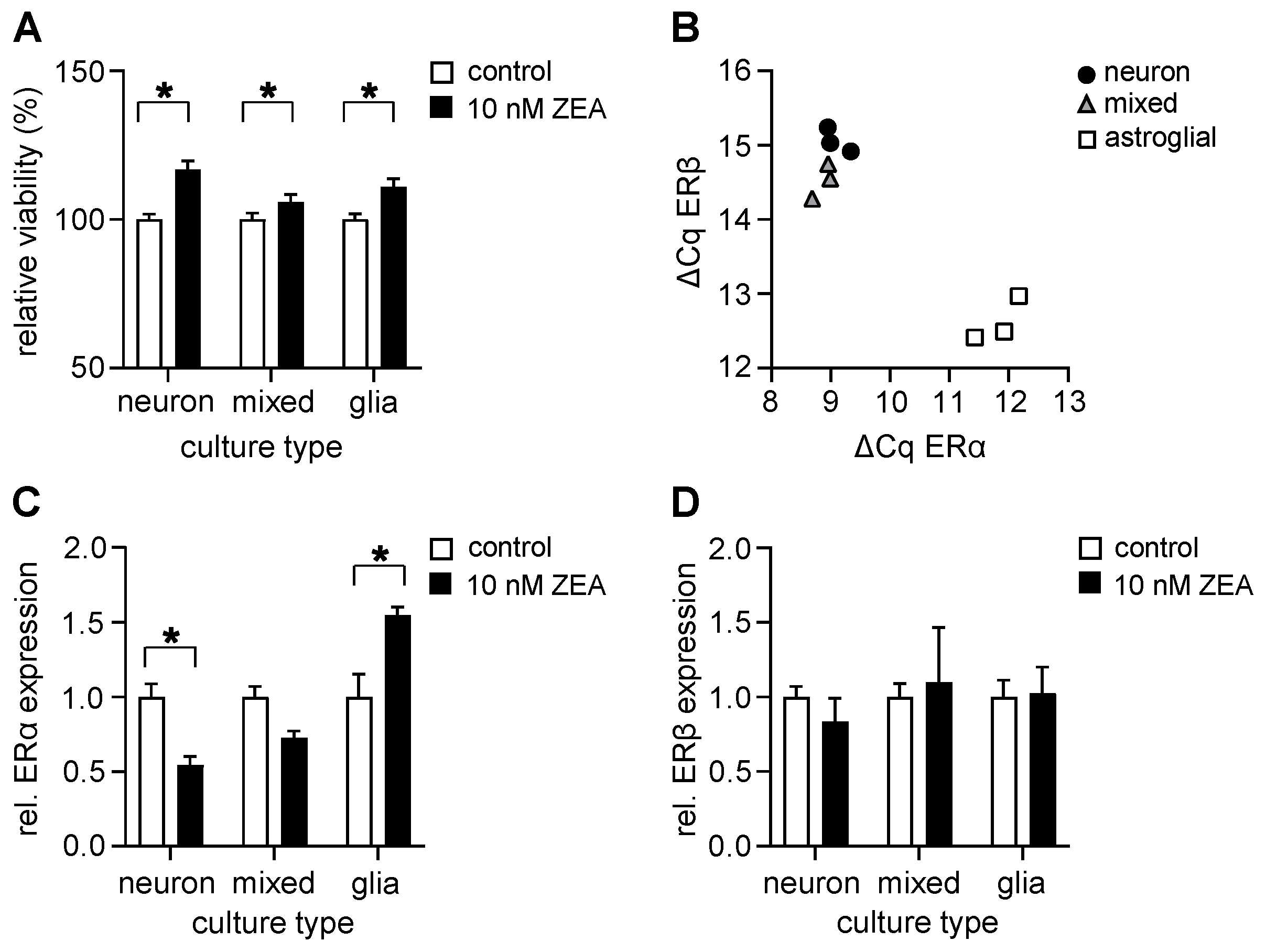
2.3. ZEA Increases Cell Viability in All Investigated Culture Types Even at Low Concentrations and Alters the Expression of ERα in a Cell Type-Specific Manner
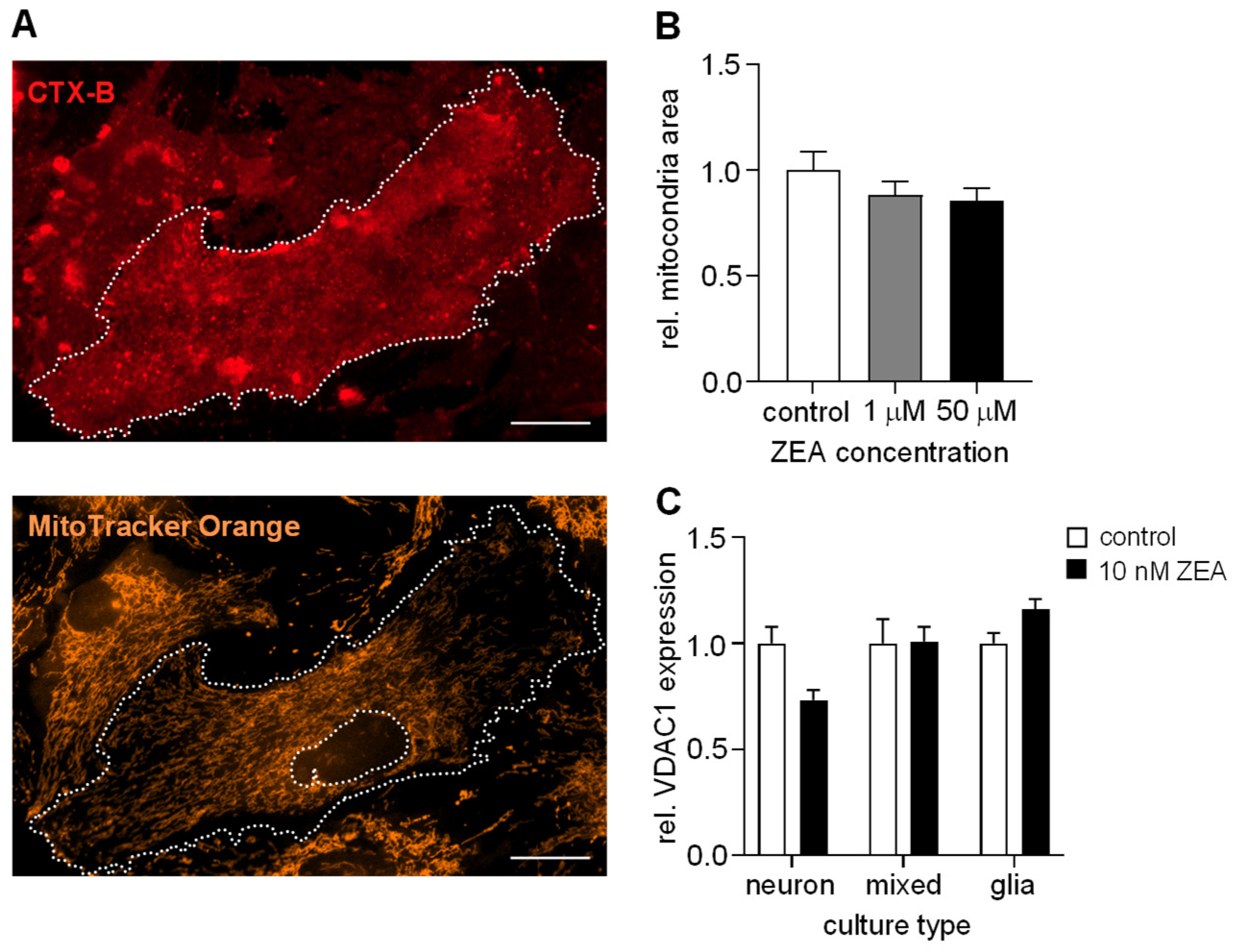
2.4. ZEA Does Not Affect the Number of Mitochondria in Astrocytes
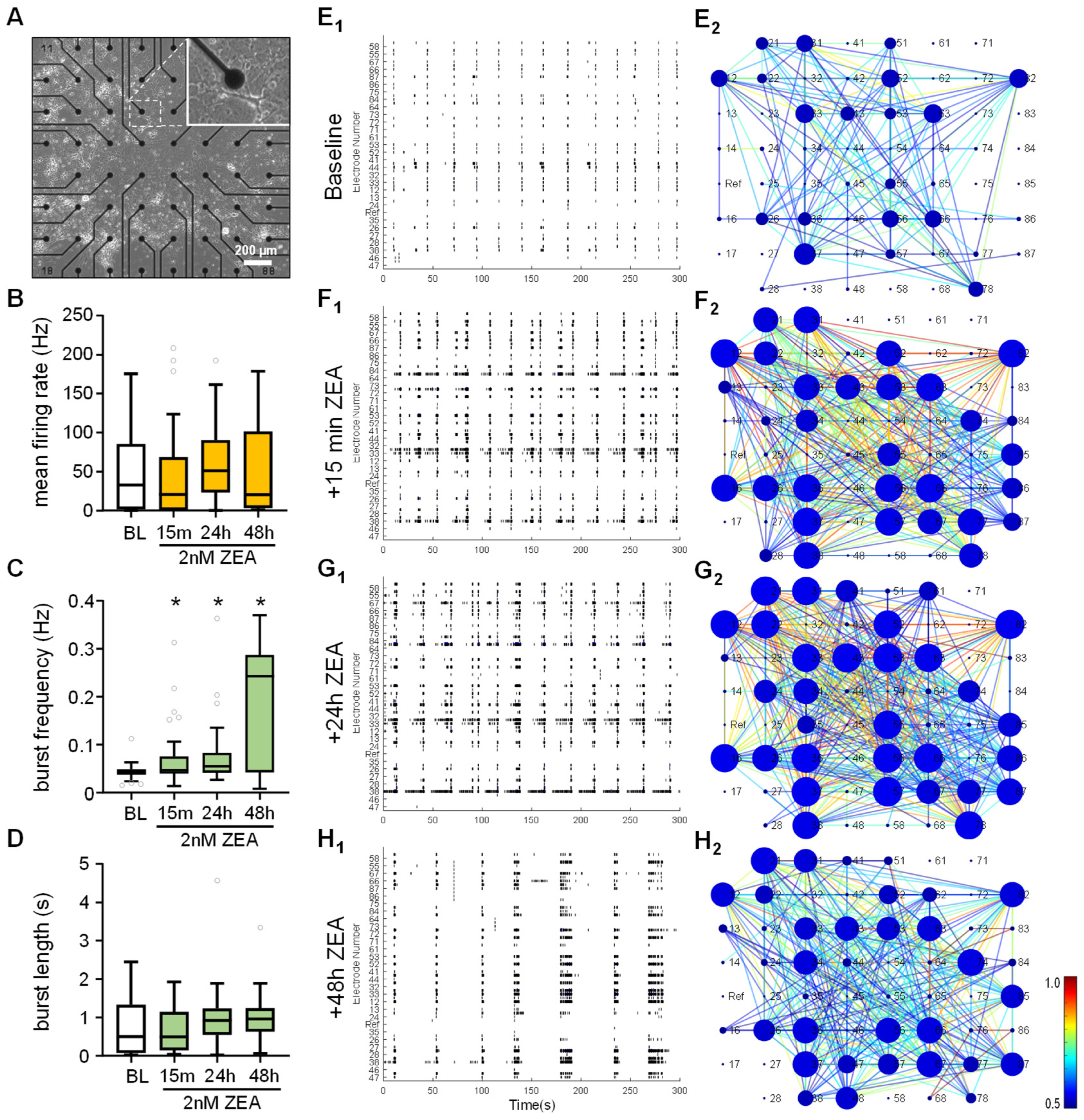
2.5. ZEA Alters Spontaneous Neuronal Activity in Mixed-Cell Cultures
3. Discussion
3.1. Effects of Fumonisin B1 on Neural Cultures
3.2. Effects of Deoxynivalenol on Neural Cultures
3.3. Effects of Zearalenone on Neural Cultures
4. Conclusions
5. Materials and Methods
5.1. Animal Handling
5.2. Drug Preparation
5.3. Preparation of Cell Cultures
5.4. Cell Viability Assay
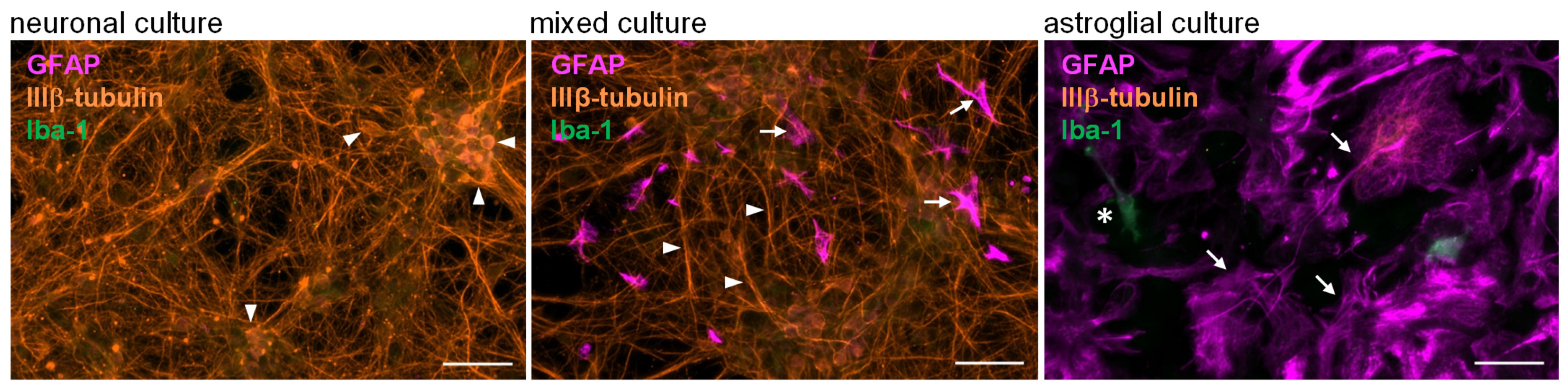
5.5. Immunocytochemistry and Microscopic Analysis
5.6. Quantitative Real-Time PCR (qRT-PCR)
5.7. MEA Recordings and Analysis
5.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FB1 | Fumonisin B1 |
| DON | Deoxynivalenol |
| ZEA | Zearalenon |
| ER | Estrogen receptor |
| MEA | Multi-electrode array |
Appendix A
| Gene of Interest | Primer | Sequence | Product Length (bp) |
|---|---|---|---|
| Mus musculus estrogen receptor 1 (alpha) (Esr1) (NM_007956.5) | Forward | ATTATGGGGTCTGGTCCTGC | 187 |
| Reverse | TCTTTCCGTATGCCGCCTTT | ||
| Mus musculus estrogen receptor 2 (beta) (Esr2) (NM_207707.1) | Forward | CATTACGGTGTCTGGTCCTGT | 189 |
| Reverse | TTCTCTCCTGGATCCACACTTG | ||
| Mus musculus ribosomal protein L13A (Rpl13a) (NM_207707.1) | Forward | AGGGGCAGGTTCTGGTATTG | 191 |
| Reverse | GGGGTTGGTATTCATCCGCT | ||
| Mus musculus voltage-dependent anion channel 1 (Vdac1)(NM_001362693.1) | Forward | GGCTACGGCTTTGGCTTAAT | 199 |
| Reverse | CAGTGATCTCAGTGCCCAGG | ||
| Mus musculus GAPDH (NM_008084.3) | Forward | TGGTGAAGGTCGGTGTGAAC | 106 |
| Reverse | ATGAAGGGGTCGTTGATGGC |
References
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Beczner, J.; Mohácsi-Farkas, C. Potential Impact of the Climate Change on the Risk of Mycotoxin Contamination of Agricultural Products in Southeast Central Europe A Mini-Review. Acta Univ. Sapientiae Aliment. 2011, 4, 89–96. [Google Scholar]
- Gelderblom, W.C.A.; Abel, S.; Smuts, C.M.; Marnewick, J.; Marasas, W.F.O.; Lemmer, E.R.; Ramljak, D. Fumonisin-Induced Hepatocarcinogenesis: Mechanisms Related to Cancer Initiation and Promotion. Environ. Health Perspect. 2001, 109, 291–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stockmann-Juvala, H.; Savolainen, K. A Review of the Toxic Effects and Mechanisms of Action of Fumonisin B 1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Toxicity, Mechanisms and Animal Health Risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of Action, Human Exposure, and Toxicological Relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of Oxidative Stress in Deoxynivalenol Induced Toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, L.; Peng, Z.; Wang, D.; Song, Y.; Wang, H.; Yao, P.; Yan, H.; Nüssler, A.K.; Liu, L.; et al. Embryotoxicity Caused by DON-Induced Oxidative Stress Mediated by Nrf2/HO-1 Pathway. Toxins 2017, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the Toxicity, Occurrence, Metabolism, Detoxification, Regulations and Intake of Zearalenone: An Oestrogenic Mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Dai, C.; Ma, Z.; Li, Q.; Lan, D.; Sun, C.; Wu, X.; Li, J.; Wang, S. Enhanced Glutathione Production Protects against Zearalenone-Induced Oxidative Stress and Ferroptosis in Female Reproductive System. Food Chem. Toxicol. 2024, 185, 114462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Jiang, S.; Huang, L.; Wang, Y.; Yang, W. Zearalenone Exposure Affects the Keap1–Nrf2 Signaling Pathway and Glucose Nutrient Absorption Related Genes of Porcine Jejunal Epithelial Cells. Toxins 2022, 14, 793. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Ben Ammar, R.; Al-Saeedi, F.J.; Mohamed, M.E.; Elnaggar, M.A.; Al-Ramadan, S.Y.; Bekhet, G.M.; Soliman, A.M. Kaempferol Inhibits Zearalenone-Induced Oxidative Stress and Apoptosis via the Pi3k/Akt-Mediated Nrf2 Signaling Pathway: In Vitro and in Vivo Studies. Int. J. Mol. Sci. 2021, 22, 217. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, F.; Tang, Z.; Yang, X.; Liu, Y.; Zhao, M.; Liu, S.; Han, S.; Zhang, Z.; Chen, B. Quercetagetin Alleviates Zearalenone-Induced Liver Injury in Rabbits through Keap1/Nrf2/ARE Signaling Pathway. Front. Pharmacol. 2023, 14, 1271384. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Shim, J.Y.; Sayama, K.; Tsubura, A.; Zhu, B.T.; Shimoi, K. Characterization of the Estrogenic Activities of Zearalenone and Zeranol in Vivo and in Vitro. J. Steroid Biochem. Mol. Biol. 2007, 103, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.M. Fumonisin B1: A Neurotoxic Mycotoxin. Arh. Hig. Rada Toksikol. 2012, 63, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.H.; Rothman, K.J.; Hendricks, K.A. Exposure to Fumonisins and the Occurence of Neutral Tube Defects along the Texas-Mexico Border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Harel, R.; Futerman, A.H. Inhibition of Sphingolipid Synthesis Affects Axonal Outgrowth in Cultured Hippocampal Neurons. J. Biol. Chem. 1993, 268, 14476–14481. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Ono, K.; Hirabayashi, Y. Sphingolipid Biosynthesis Is Necessary for Dendrite Growth and Survival of Cerebellar Purkinje Cells in Culture. J. Neurochem. 1995, 65, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Monnet-Tschudi, F.; Zurich, M.G.; Sorg, O.; Matthieu, J.M.; Honegger, P.; Schilter, B. The Naturally Occurring Food Mycotoxin Fumonisin B1 Impairs Myelin Formation in Aggregating Brain Cell Culture. Neurotoxicology 1999, 20, 41–48. [Google Scholar] [PubMed]
- Soriano, J.M.; González, L.; Catalá, A.I. Mechanism of Action of Sphingolipids and Their Metabolites in the Toxicity of Fumonisin B1. Prog. Lipid Res. 2005, 44, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Uetsuka, K. Mechanisms of Mycotoxin-Induced Neurotoxicity through Oxidative Stress-Associated Pathways. Int. J. Mol. Sci. 2011, 12, 5213–5237. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Islam, Z.; Amuzie, C.J. Immunochemical Assessment of Deoxynivalenol Tissue Distribution Following Oral Exposure in the Mouse. Toxicol. Lett. 2008, 178, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M. From the Gut to the Brain: Journey and Pathophysiological Effects of the Food-Associated Trichothecene Mycotoxin Deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and Metabolism of Deoxynivalenol in Animals and Humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef] [PubMed]
- Csikós, V.; Varró, P.; Bódi, V.; Oláh, S.; Világi, I.; Dobolyi, A. The Mycotoxin Deoxynivalenol Activates GABAergic Neurons in the Reward System and Inhibits Feeding and Maternal Behaviours. Arch. Toxicol. 2020, 94, 3297–3313. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and Its Toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Yeung, J.M.; Thompson, B.K.; Trenholm, H.L. Effect of Deoxynivalenol on Neurotransmitters in Discrete Regions of Swine Brain. Arch. Environ. Contam. Toxicol. 1992, 22, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Invited Review: Toxicology of Deoxynivalenol (Vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Mounien, L.; Trouslard, J.; et al. Central Inflammation and Sickness-like Behavior Induced by the Food Contaminant Deoxynivalenol: A PGE2-Independent Mechanism. Toxicol. Sci. 2011, 124, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Wanaverbecq, N.; Mounien, L.; et al. The Food-Contaminant Deoxynivalenol Modifies Eating by Targeting Anorexigenic Neurocircuitry. PLoS ONE 2011, 6, e26134. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.F.X.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Eppley, R.M.; Hines, F.A.; Rorie, J.; Ruggles, D.I. Effects of Deoxynivalenol (DON, Vomitoxin) on in Utero Development in Rats. Food Chem. Toxicol. 2006, 44, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.H.; Deng, H.D.; Deng, Y.T.; Deng, J.L.; Zuo, Z.C.; Yu, S.M.; Shen, L.H.; Cui, H.M.; Xu, Z.W.; Hu, Y.C. Effect of the Fusarium Toxins, Zearalenone and Deoxynivalenol, on the Mouse Brain. Environ. Toxicol. Pharmacol. 2016, 46, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, M.; Chandra Nayaka, S.; Anand, T.; Rajesh, R.; Aiyaz, M.; Divakara, S.T.; Murali, H.S.; Prakash, H.S.; Lakshmana Rao, P.V. Zearalenone Induced Toxicity in SHSY-5Y Cells: The Role of Oxidative Stress Evidenced by N-Acetyl Cysteine. Food Chem. Toxicol. 2014, 65, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Agahi, F.; Font, G.; Juan, C.; Juan-García, A. Individual and Combined Effect of Zearalenone Derivates and Beauvericin Mycotoxins on SH-SY5Y Cells. Toxins 2020, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Agahi, F.; Álvarez-Ortega, N.; Font, G.; Juan-García, A.; Juan, C. Oxidative Stress, Glutathione, and Gene Expression as Key Indicators in SH-SY5Y Cells Exposed to Zearalenone Metabolites and Beauvericin. Toxicol. Lett. 2020, 334, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wong, T.Y.; Chan, F.L.; Chen, S.; Leung, L.K. Assessing the Effect of Food Mycotoxins on Aromatase by Using a Cell-Based System. Toxicol. In Vitro 2014, 28, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jiang, A.; Yang, H.; Chen, H.; Wang, Y. Phytoestrogen α -Zearalanol Improves Memory Impairment and Hippocampal Neurogenesis in Ovariectomized Mice. Sci. World J. 2014, 2014, 862019. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.L.; Yue, Y.; Liu, F.H.; Lang, S.Y.; Zhang, X.C.; Dai, S.L.; Ge, Q.S.; Zuo, P.P. Treatment with Phytoestrogen α-Zearalanol Might Protect Neurons of Hippocampus in Ovariectomized Rats. Endocrine 2006, 30, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.L.; Zuo, P.P.; Li, Q.; Liu, F.H.; Dai, S.L.; Ge, Q.S. Protective Effects of Phytoestrogen α-Zearalanol on Beta Amyloid 25-35 Induced Oxidative Damage in Cultured Rat Hippocampal Neurons. Endocrine 2007, 32, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, R.; Jiang, A.; Xu, Z.; Wang, Y. Phytoestrogen Alpha-Zearalanol Attenuate Endoplasmic Reticulum Stress to against Cultured Rat Hippocampal Neurons Apoptotic Death Induced by Amyloid Beta25-35. Neuroendocrinol. Lett. 2017, 38, 353–359. [Google Scholar] [PubMed]
- Jocsak, G.; Kiss, D.S.; Toth, I.; Goszleth, G.; Bartha, T.; Frenyo, L.V.; Horvath, T.L.; Zsarnovszky, A. Comparison of Individual and Combined Effects of Four Endocrine Disruptors on Estrogen Receptor Beta Transcription in Cerebellar Cell Culture: The Modulatory Role of Estradiol and Triiodo-Thyronine. Int. J. Environ. Res. Public Health 2016, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Kiss, D.S.; Ioja, E.; Toth, I.; Barany, Z.; Jocsak, G.; Bartha, T.; Horvath, T.L.; Zsarnovszky, A. Comparative Analysis of Zearalenone Effects on Thyroid Receptor Alpha (TRα) and Beta (TRβ) Expression in Rat Primary Cerebellar Cell Cultures. Int. J. Mol. Sci. 2018, 19, 1440. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Gheux, A.; Coton, M.; Madec, S.; Hymery, N.; Coton, E. In Vitro Co-Culture Models to Evaluate Acute Cytotoxicity of Individual and Combined Mycotoxin Exposures on Caco-2, THP-1 and HepaRG Human Cell Lines. Chem. Biol. Interact. 2018, 281, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Juan-García, A.; Manyes, L.; Ruiz, M.J.; Font, G. Applications of Flow Cytometry to Toxicological Mycotoxin Effects in Cultured Mammalian Cells: A Review. Food Chem. Toxicol. 2013, 56, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.S.; Hong, S.H.; Bulitta, J.B.; Lee, J.B.; Hwang, S.W.; Kim, H.J.; Yang, S.D.; Yoon, H.S.; Kim, D.J.; Lee, B.M.; et al. Physiologically Based Pharmacokinetics of Zearalenone. J. Toxicol. Environ. Health—Part A Curr. Issues 2009, 72, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, B.; Li, X.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Bai, J.; Bian, J.; et al. Zearalenone Promotes Cell Proliferation or Causes Cell Death? Toxins 2018, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, X.; Liu, J.; Zheng, S.; Yin, Q.; Yu, Y. Growth-Promoting Effect of Environmental Endocrine Disruptors on Human Neuroblastoma SK-N-SH Cells. Environ. Toxicol. Pharmacol. 2007, 24, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.J.; Wiggins, J.P.; Wangsness, P.J. Effects of Zeranol on in Vitro Growth Hormone Release by Lamb and Rat Pituitary Cells. J. Anim. Sci. 1988, 66, 2614–2625. [Google Scholar] [CrossRef] [PubMed]
- Shier, W.T.; Shier, A.C.; Xie, W.; Mirocha, C.J. Structure-Activity Relationships for Human Estrogenic Activity in Zearalenone Mycotoxins. Toxicon 2001, 39, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, S.; Lelong, M.; Bourgine, G.; Efstathiou, T.; Saligaut, C.; Pakdel, F. Assessment of the Potential Activity of Major Dietary Compounds as Selective Estrogen Receptor Modulators in Two Distinct Cell Models for Proliferation and Differentiation. Toxicol. Appl. Pharmacol. 2017, 325, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Nikov, G.N.; Hopkins, N.E.; Boue, S.; Alworth, W.L. Interactions of Dietary Estrogens with Human Estrogen Receptors and the Effect on Estrogen Receptor-Estrogen Response Element Complex Formation. Environ. Health Perspect. 2000, 108, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and Toxicity of a Fusarium Mycotoxin, Zearalenone. Crit. Rev. Food Sci. Nutr. 2019, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H. Regulation of Estrogen Receptor Expression in the Hypothalamus by Sex Steroids: Implication in the Regulation of Energy Homeostasis. Int. J. Endocrinol. 2015, 2015, 949085. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.; Beyer, C. Neuroprotection by Estrogen in the Brain: The Mitochondrial Compartment as Presumed Therapeutic Target. J. Neurochem. 2009, 110, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Woolley, C.S. Acute Effects of Estrogen on Neuronal Physiology. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 657–680. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.F.M.; Gross, G.W.; Weiss, D.G.; Schroeder, O.H.U.; Gramowski, A.; Shafer, T.J. Microelectrode Arrays: A Physiologically Based Neurotoxicity Testing Platform for the 21st Century. Neurotoxicology 2010, 31, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Bódi, V.; Csikós, V.; Rátkai, E.A.; Szűcs, A.; Tóth, A.; Szádeczky-Kardoss, K.; Dobolyi, Á.; Schlett, K.; Világi, I.; Varró, P. Short-Term Neuronal Effects of Fumonisin B1 on Neuronal Activity in Rodents. Neurotoxicology 2020, 80, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Slikker, W.; Davies, D.L. Biochemical and Morphological Effects of Fumonisin B1 on Primary Cultures of Rat Cerebrum. Neurotoxicol. Teratol. 2000, 22, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Campisi, A.; Russo, A.; Galvano, G.; Palumbo, M.; Renis, M.; Barcellona, M.L.; Perez-Polo, J.R.; Vanella, A. DNA Damage in Astrocytes Exposed to Fumonisin B1. Neurochem. Res. 2002, 27, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Stockmann-Juvala, H.; Mikkola, J.; Naarala, J.; Loikkanen, J.; Elovaara, E.; Savolainen, K. Fumonisin B1-Induced Toxicity and Oxidative Damage in U-118MG Glioblastoma Cells. Toxicology 2004, 202, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Sharma, R.P. Fumonisin B1 Induces Necrotic Cell Death in BV-2 Cells and Murine Cultured Astrocytes and Is Antiproliferative in BV-2 Cells While N2A Cells and Primary Cortical Neurons Are Resistant. Neurotoxicology 2005, 26, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.M.; Kovac, S.; Abramov, A.Y. Impact of Fumonisin B 1 on Glutamate Toxicity and Low Magnesium-Induced Seizure Activity in Neuronal Primary Culture. Neuroscience 2012, 202, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.M.; Abramov, A.Y. Fumonisin B1 Inhibits Mitochondrial Respiration and Deregulates Calcium Homeostasis—Implication to Mechanism of Cell Toxicity. Int. J. Biochem. Cell Biol. 2011, 43, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Stockmann-Juvala, H.; Mikkola, J.; Naarala, J.; Loikkanen, J.; Elovaara, E.; Savolainen, K. Oxidative Stress Induced by Fumonisin B1 in Continuous Human and Rodent Neural Cell Cultures. Free Radic. Res. 2004, 38, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Hirrlinger, J. Glutathione Pathways in the Brain. Biol. Chem. 2003, 384, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, J.P. Bioenergetics and Redox Adaptations of Astrocytes to Neuronal Activity. J. Neurochem. 2016, 139, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Santos, C.L.; de Souza Almeida, R.R.; da Silva, A.; Thomaz, N.K.; Costa, N.L.F.; Weber, F.B.; Schmitz, I.; Medeiros, L.S.; Medeiros, L.; et al. Gliotoxicity and Glioprotection: The Dual Role of Glial Cells. Mol. Neurobiol. 2021, 58, 6577–6592. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cha, M.; Lee, B.H. Crosstalk between Neuron and Glial Cells in Oxidative Injury and Neuroprotection. Int. J. Mol. Sci. 2021, 22, 13315. [Google Scholar] [CrossRef] [PubMed]
- Oyarce, K.; Cepeda, M.Y.; Lagos, R.; Garrido, C.; Vega-Letter, A.M.; Garcia-Robles, M.; Luz-Crawford, P.; Elizondo-Vega, R. Neuroprotective and Neurotoxic Effects of Glial-Derived Exosomes. Front. Cell. Neurosci. 2022, 16, 920686. [Google Scholar] [CrossRef] [PubMed]
- Tima, H.; Rácz, A.; Guld, Z.; Mohácsi-Farkas, C.; Kiskó, G. Deoxynivalenol, Zearalenone and T-2 in Grain Based Swine Feed in Hungary. Food Addit. Contam. Part B 2016, 9, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Tima, H.; Brückner, A.; Mohácsi-Farkas, C.; Kiskó, G. Fusarium Mycotoxins in Cereals Harvested from Hungarian Fields. Food Addit. Contam. Part B Surveill. 2016, 9, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Gerding, J.; Cramer, B.; Humpf, H.U. Determination of Mycotoxin Exposure in Germany Using an LC-MS/MS Multibiomarker Approach. Mol. Nutr. Food Res. 2014, 58, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Sulyok, M.; Fruhmann, P.; Berthiller, F.; Schuhmacher, R.; Hametner, C.; Adam, G.; Fröhlich, J.; Krska, R. Assessment of Human Deoxynivalenol Exposure Using an LC-MS/MS Based Biomarker Method. Toxicol. Lett. 2012, 211, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Hartin, K.E.; Trenholm, H.L. Distribution of Deoxynivalenol in Cerebral Spinal Fluid Following Administration to Swine and Sheep. J. Environ. Sci. Health Part B 1990, 25, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Hüwel, S.; Galla, H.J.; Humpf, H.U. Blood-Brain Barrier Effects of the Fusarium Mycotoxins Deoxynivalenol, 3 Acetyldeoxynivalenol, and Moniliformin and Their Transfer to the Brain. PLoS ONE 2015, 10, e143640. [Google Scholar] [CrossRef] [PubMed]
- Razafimanjato, H.; Benzaria, A.; Taïeb, N.; Guo, X.J.; Vidal, N.; di Scala, C.; Varini, K.; Maresca, M. The Ribotoxin Deoxynivalenol Affects the Viability and Functions of Glial Cells. Glia 2011, 59, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, M.; Chu, X.; Zhang, Y.; ur Rahman, S.; Jiang, Y.; Chen, X.; Zhu, D.; Feng, S.; Li, Y.; et al. Deoxynivalenol Induces Toxicity and Apoptosis in Piglet Hippocampal Nerve Cells via the MAPK Signaling Pathway. Toxicon 2018, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, W.; Fan, M.; Meng, T.; Chen, X.; Jiang, Y.; Zhu, D.; Hu, W.; Gong, J.; Feng, S.; et al. Deoxynivalenol Induces Apoptosis in PC12 Cells via the Mitochondrial Pathway. Environ. Toxicol. Pharmacol. 2016, 43, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Belcher, S.M. Regulated Expression of Estrogen Receptor α and β MRNA in Granule Cells during Development of the Rat Cerebellum. Dev. Brain Res. 1999, 115, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.Å. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors and α and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C. Estrogen and the Developing Mammalian Brain. Anat. Embryol. 1999, 199, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Castrillo, A.; Arevalo, M.A. Microglial and Astrocytic Function in Physiological and Pathological Conditions: Estrogenic Modulation. Int. J. Mol. Sci. 2020, 21, 3219. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and Is Neuroprotective Following Ischemic Brain Injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Westberry, J.M. Regulation of Oestrogen Receptor Gene Expression: New Insights and Novel Mechanisms. J. Neuroendocrinol. 2009, 21, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Westberry, J.M.; Trout, A.L. Estrogen Receptor-Alpha Gene Expression in the Cortex: Sex Differences during Development and in Adulthood. Horm. Behav. 2011, 59, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Da Su, J.; Qiu, J.; Zhong, Y.P.; Chen, Y.Z. Expression of Estrogen Receptor -α and -β Immunoreactivity in the Cultured Neonatal Suprachiasmatic Nucleus: With Special Attention to GABAergic Neurons. Neuroreport 2001, 12, 1955–1959. [Google Scholar] [CrossRef]
- Piechota, M.; Korostynski, M.; Golda, S.; Ficek, J.; Jantas, D.; Barbara, Z.; Przewlocki, R. Transcriptional Signatures of Steroid Hormones in the Striatal Neurons and Astrocytes. BMC Neurosci. 2017, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Frago, L.M.; Canelles, S.; Freire-Regatillo, A.; Argente-Arizón, P.; Barrios, V.; Argente, J.; Garcia-Segura, L.M.; Chowen, J.A. Estradiol Uses Different Mechanisms in Astrocytes from the Hippocampus of Male and Female Rats to Protect against Damage Induced by Palmitic Acid. Front. Mol. Neurosci. 2017, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Schreihofer, D.A. Transcriptional Regulation by Phytoestrogens in Neuronal Cell Lines. Mol. Cell. Endocrinol. 2005, 231, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Tatay, E.; Espín, S.; García-Fernández, A.J.; Ruiz, M.J. Oxidative Damage and Disturbance of Antioxidant Capacity by Zearalenone and Its Metabolites in Human Cells. Toxicol. In Vitro 2017, 45, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.S.; Hong, S.H.; Bulitta, J.B.; Hwang, S.W.; Kim, H.J.; Lee, J.B.; Yang, S.D.; Kim, J.E.; Yoon, H.S.; Kim, D.J.; et al. Disposition, Oral Bioavailability, and Tissue Distribution of Zearalenone in Rats at Various Dose Levels. J. Toxicol. Environ. Health—Part A Curr. Issues 2009, 72, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Suresh, J.; Radojicic, M.; Pesce, L.L.; Bhansali, A.; Wang, J.; Tryba, A.K.; Marks, J.D.; Van Drongelen, W. Network Burst Activity in Hippocampal Neuronal Cultures: The Role of Synaptic and Intrinsic Currents. J. Neurophysiol. 2016, 115, 3073–3089. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Boehler, M.D.; Jones, T.T.; Wheeler, B.C. NbActiv4 Medium Improvement to Neurobasal/B27 Increases Neuron Synapse Densities and Network Spike Rates on Multielectrode Arrays. J. Neurosci. Methods 2008, 170, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU; The European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union: Luxembourg, 2010.
- Tárnok, K.; Kiss, E.; Luiten, P.G.M.; Nyakas, C.; Tihanyi, K.; Schlett, K.; Eisel, U.L.M. Effects of Vinpocetine on Mitochondrial Function and Neuroprotection in Primary Cortical Neurons. Neurochem Int 2008, 53, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Staack, A.; Donjacour, A.A.; Brody, J.; Cunha, G.R.; Carroll, P. Mouse Urogenital Development: A Practical Approach. Differentiation 2003, 71, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Song, J.; Xue, K.; Han, H.; Ding, F.; Li, S.; Dai, Y.; Li, N. Rapid Atraumatic Sex Identification of Developmental Day 14-16 Mice. Biotech. Histochem. 2015, 90, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Tárnok, K.; Szilágyi, L.; Berki, T.; Németh, P.; Gráf, L.; Schlett, K. Anoxia Leads to a Rapid Translocation of Human Trypsinogen 4 to the Plasma Membrane of Cultured Astrocytes. J. Neurochem. 2010, 115, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Dastgheyb, R.M.; Yoo, S.W.; Haughey, N.J. MEAnalyzer—A Spike Train Analysis Tool for Multi Electrode Arrays. Neuroinformatics 2020, 18, 163–179. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szentgyörgyi, V.; Tagscherer-Micska, B.; Rátkai, A.; Schlett, K.; Bencsik, N.; Tárnok, K. Cell Type-Specific Effects of Fusarium Mycotoxins on Primary Neurons and Astroglial Cells. Toxins 2025, 17, 368. https://doi.org/10.3390/toxins17080368
Szentgyörgyi V, Tagscherer-Micska B, Rátkai A, Schlett K, Bencsik N, Tárnok K. Cell Type-Specific Effects of Fusarium Mycotoxins on Primary Neurons and Astroglial Cells. Toxins. 2025; 17(8):368. https://doi.org/10.3390/toxins17080368
Chicago/Turabian StyleSzentgyörgyi, Viktória, Brigitta Tagscherer-Micska, Anikó Rátkai, Katalin Schlett, Norbert Bencsik, and Krisztián Tárnok. 2025. "Cell Type-Specific Effects of Fusarium Mycotoxins on Primary Neurons and Astroglial Cells" Toxins 17, no. 8: 368. https://doi.org/10.3390/toxins17080368
APA StyleSzentgyörgyi, V., Tagscherer-Micska, B., Rátkai, A., Schlett, K., Bencsik, N., & Tárnok, K. (2025). Cell Type-Specific Effects of Fusarium Mycotoxins on Primary Neurons and Astroglial Cells. Toxins, 17(8), 368. https://doi.org/10.3390/toxins17080368





