The Dissociation of Latrophilin Fragments by Perfluorooctanoic Acid (PFOA) Inhibits LTXN4C-Induced Neurotransmitter Release
Abstract
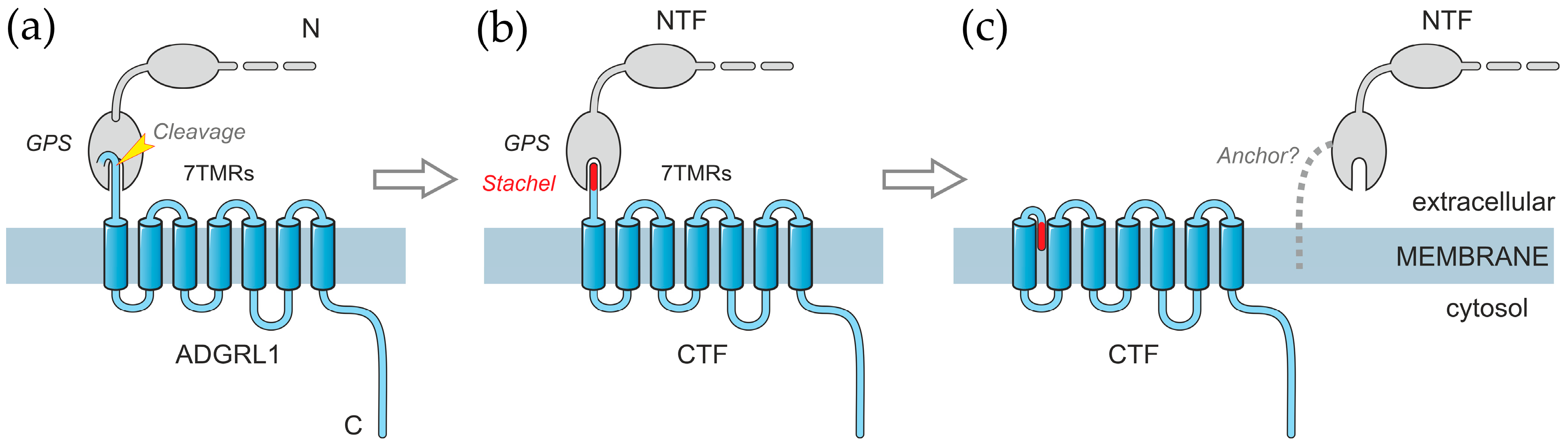
1. Introduction
2. Results
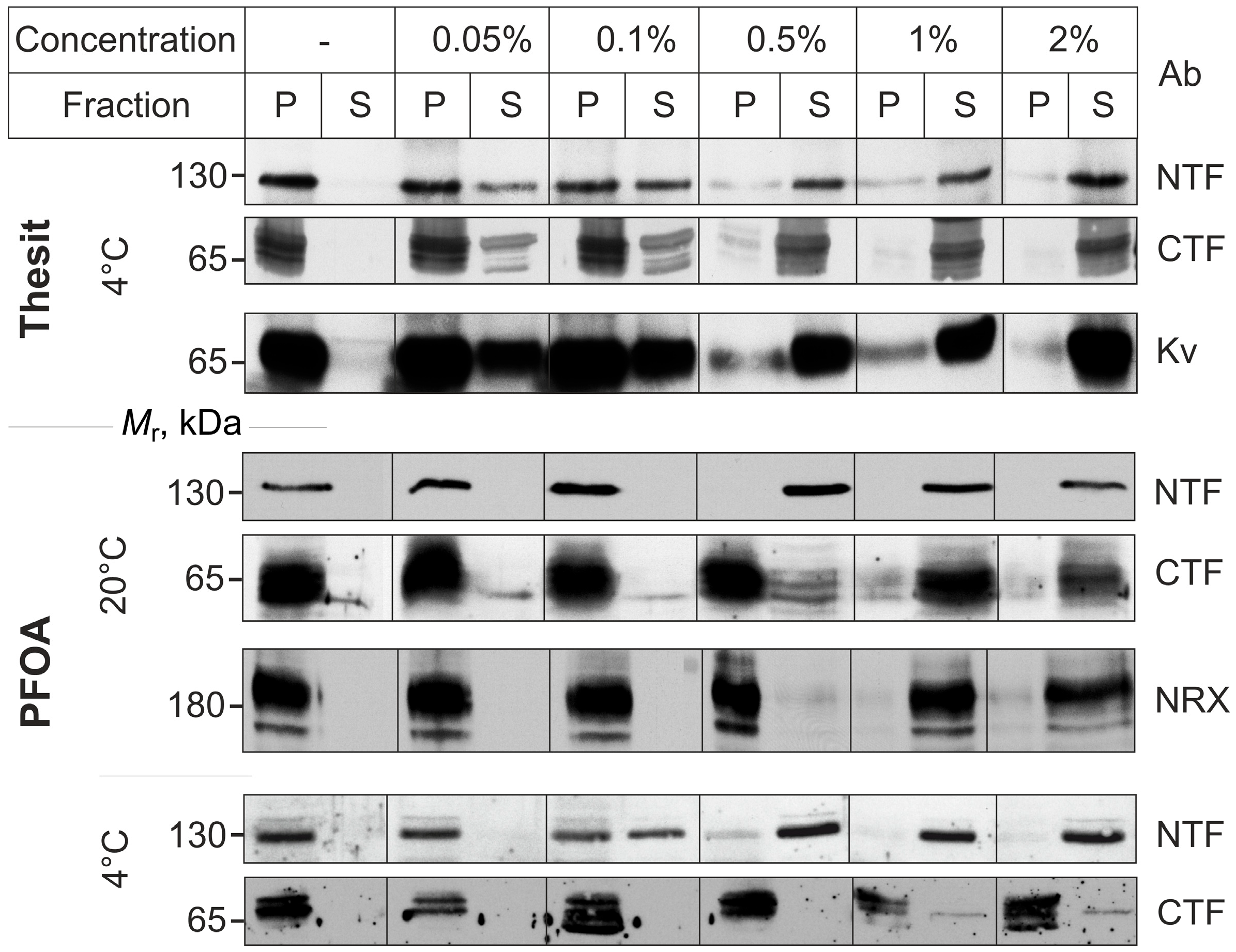
2.1. PFOA Disrupts the NTF-CTF Complexes
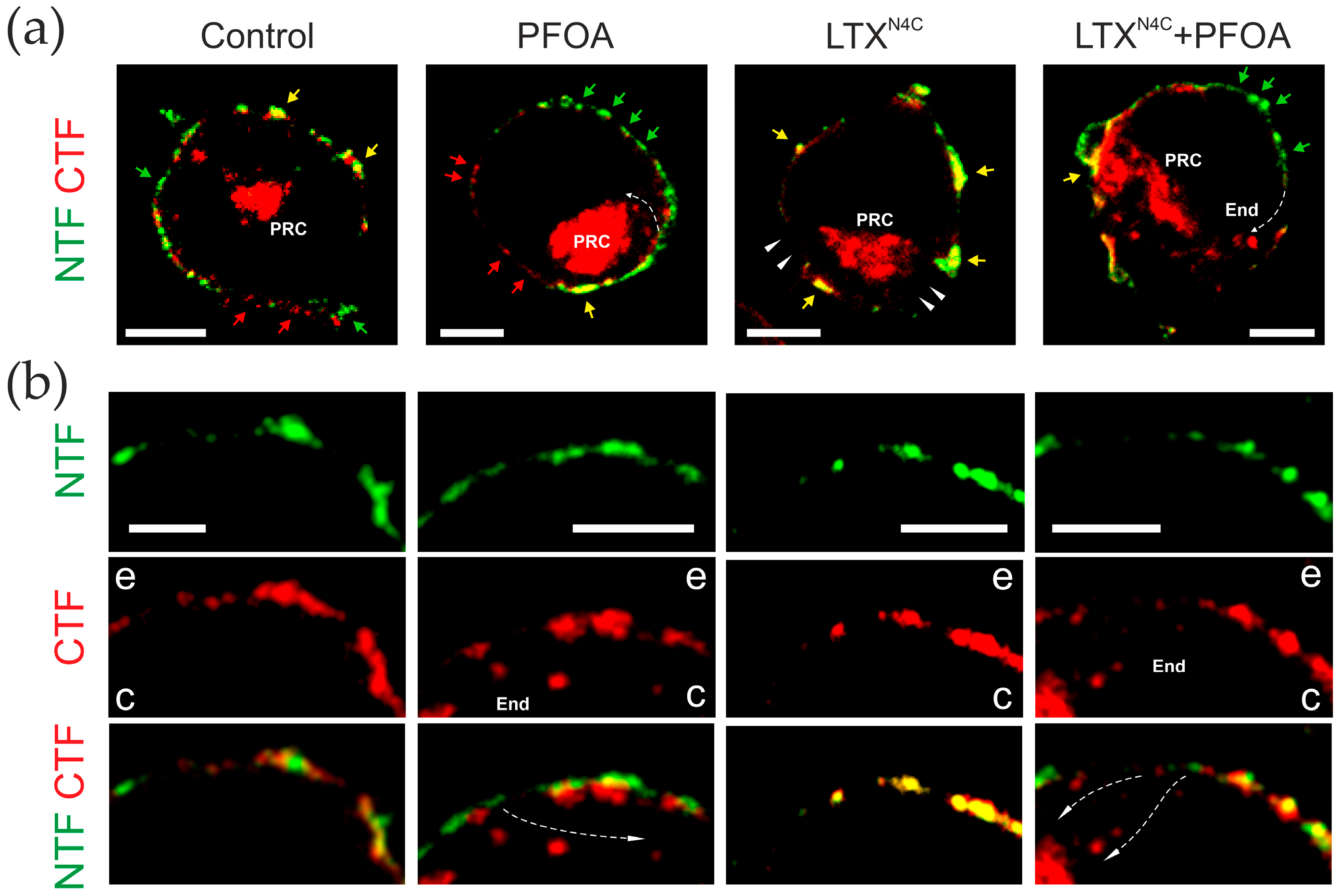
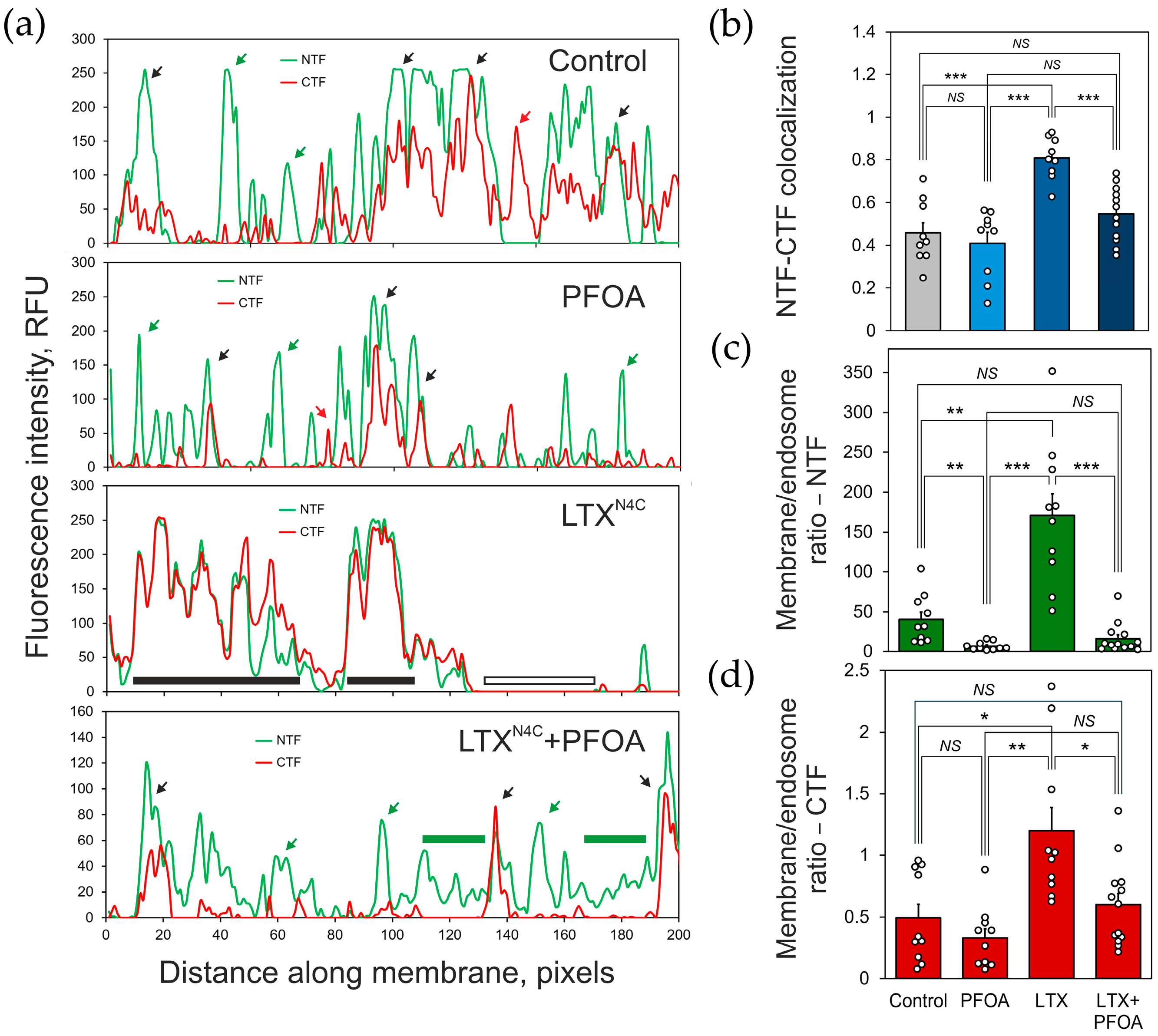
2.2. PFOA Induces NTF and CTF Redistribution in Cell Membrane
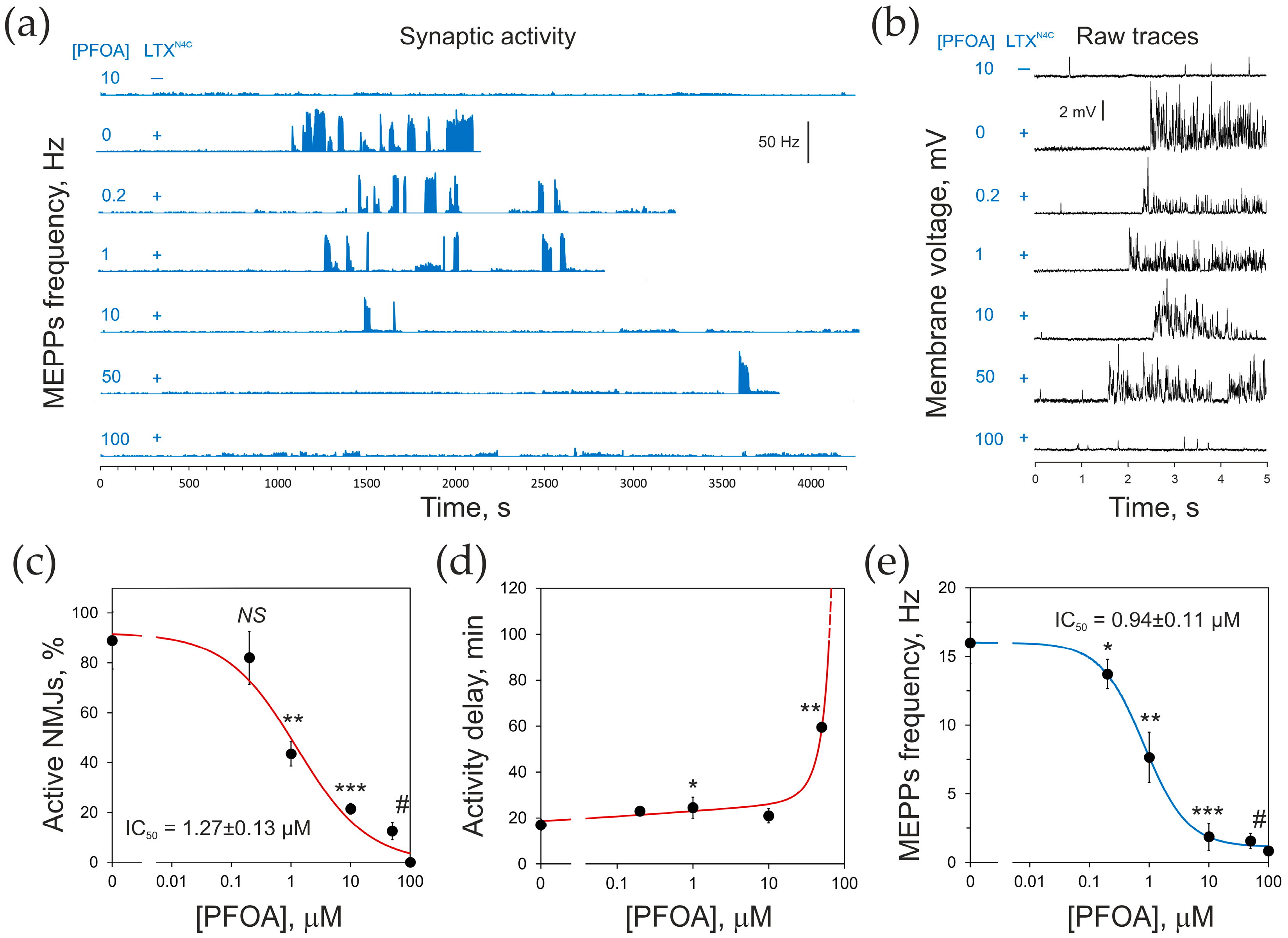
2.3. PFOA Inhibits LTXN4C-Induced Neurotransmitter Release
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Solubilization and Affinity Chromatography of ADGRL1 Fragments
4.3. Cell Culture
4.4. PFOA Treatment, LTX Binding, and Immunostaining of Cultured Cells
4.5. Confocal Microscopy
4.6. Neurotransmitter Release
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longenecker, H.E., Jr.; Hurlbut, W.P.; Mauro, A.; Clark, A.W. Effects of Black Widow Spider Venom on the Frog Neuromuscular Junction. Nature 1970, 225, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.-P.; Suckling, J.; Ushkaryov, Y. Penelope’s Web: Using α-Latrotoxin to Untangle the Mysteries of Exocytosis. J. Neurochem. 2009, 111, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, X. Recent Advances in Research on Widow Spider Venoms and Toxins. Toxins 2015, 7, 5055–5067. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.; Rubin, L.L.; Tzeng, M.C. Black Widow Spider Venom: Effect of Purified Toxin on Lipid Bilayer Membranes. Science 1976, 193, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, L.; Meldolesi, J. α-Latrotoxin and Related Toxins. Pharmacol. Ther. 1989, 42, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.W.; Mauro, A.; Longenecker, H.E.; Hurlbut, W.P. Effects of Black Widow Spider Venom on the Frog Neuromuscular Junction. Effects on the Fine Structure of the Frog Neuromuscular Junction. Nature 1970, 225, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Ushkaryov, Y.A.; Volynski, K.E.; Ashton, A.C. The Multiple Actions of Black Widow Spider Toxins and Their Selective Use in Neurosecretion Studies. Toxicon 2004, 43, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, M.C.; Siekevitz, P. The Binding Interaction between Alpha-Latrotoxin from Black Widow Spider Venom and a Dog Cerebral Cortex Synaptosomal Membrane Preparation. J. Neurochem. 1979, 33, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Ushkaryov, Y.A.; Rohou, A.; Sugita, S. α-Latrotoxin and Its Receptors. Handb. Exp. Pharmacol. 2008, 184, 171–206. [Google Scholar] [CrossRef]
- Henkel, A.W.; Sankaranarayanan, S. Mechanisms of Alpha-Latrotoxin Action. Cell Tissue Res. 1999, 296, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Ichtchenko, K.; Khvotchev, M.; Kiyatkin, N.; Simpson, L.; Sugita, S.; Südhof, T.C. α-Latrotoxin Action Probed with Recombinant Toxin: Receptors Recruit α-Latrotoxin but Do Not Transduce an Exocytotic Signal. EMBO J. 1998, 17, 6188–6199. [Google Scholar] [CrossRef] [PubMed]
- Volynski, K.E.; Capogna, M.; Ashton, A.C.; Thomson, D.; Orlova, E.V.; Manser, C.F.; Ribchester, R.R.; Ushkaryov, Y.A. Mutant α-Latrotoxin (LTXN4C) Does Not Form Pores and Causes Secretion by Receptor Stimulation. This Action Does Not Require Neurexins. J. Biol. Chem. 2003, 278, 31058–31066. [Google Scholar] [CrossRef] [PubMed]
- Capogna, M.; Volynski, K.E.; Emptage, N.J.; Ushkaryov, Y.A. The α-Latrotoxin Mutant LTXN4C Enhances Spontaneous and Evoked Transmitter Release in CA3 Pyramidal Neurons. J. Neurosci. 2003, 23, 4044–4053. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lee, D.; Wang, L.; Khvotchev, M.; Chiew, S.K.; Arunachalam, L.; Collins, T.; Feng, Z.P.; Sugita, S. N-Terminal Insertion and C-Terminal Ankyrin-like Repeats of α-Latrotoxin Are Critical for Ca2+-Dependent Exocytosis. J. Neurosci. 2005, 25, 10188–10197. [Google Scholar] [CrossRef] [PubMed]
- Déak, F.; Liu, X.; Khvotchev, M.; Li, G.; Kavalali, E.T.; Sugita, S.; Sudhof, T.C. α-Latrotoxin Stimulates a Novel Pathway of Ca2+-Dependent Synaptic Exocytosis Independent of the Classical Synaptic Fusion Machinery. J. Neurosci. 2009, 29, 8639–8648. [Google Scholar] [CrossRef] [PubMed]
- Volynski, K.E.; Silva, J.-P.P.; Lelianova, V.G.; Rahman, M.A.; Hopkins, C.; Ushkaryov, Y.A. Latrophilin Fragments Behave as Independent Proteins That Associate and Signal on Binding of LTXN4C. EMBO J. 2004, 23, 4423–4433. [Google Scholar] [CrossRef] [PubMed]
- Ushkaryov, Y.A.; Petrenko, A.G.; Geppert, M.; Sudhof, T.C. Neurexins: Synaptic Cell Surface Proteins Related to the α-Latrotoxin Receptor and Laminin. Science 1992, 257, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Geppert, M.; Ushkaryov, Y.A.; Hata, Y.; Davletov, B.; Petrenko, A.G.; Südhof, T.C. Neurexins. Cold Spring Harb. Symp. Quant. Biol. 1992, 57, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Davletov, B.A.; Shamotienko, O.G.; Lelianova, V.G.; Grishin, E.V.; Ushkaryov, Y.A. Isolation and Biochemical Characterization of a Ca2+-Independent α-Latrotoxin-Binding Protein. J. Biol. Chem. 1996, 271, 23239–23245. [Google Scholar] [CrossRef] [PubMed]
- Krasnoperov, V.G.; Beavis, R.; Chepurny, O.G.; Little, A.R.; Plotnikov, A.N.; Petrenko, A.G. The Calcium-Independent Receptor of α-Latrotoxin Is Not a Neurexin. Biochem. Biophys. Res. Commun. 1996, 227, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Krasnoperov, V.G.; Bittner, M.A.; Beavis, R.; Kuang, Y.; Salnikow, K.V.; Chepurny, O.G.; Little, A.R.; Plotnikov, A.N.; Wu, D.; Holz, R.W.; et al. α-Latrotoxin Stimulates Exocytosis by the Interaction with a Neuronal G-Protein-Coupled Receptor. Neuron 1997, 18, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Lelianova, V.G.; Davletov, B.A.; Sterling, A.; Rahman, M.A.; Grishin, E.V.; Totty, N.F.; Ushkaryov, Y.A. α-Latrotoxin Receptor, Latrophilin, Is a Novel Member of the Secretin Family of G Protein-Coupled Receptors. J. Biol. Chem. 1997, 272, 21504–21508. [Google Scholar] [CrossRef] [PubMed]
- Krasnoperov, V.; Bittner, M.A.; Mo, W.; Buryanovsky, L.; Neubert, T.A.; Holz, R.W.; Ichtchenko, K.; Petrenko, A.G. Protein-Tyrosine Phosphatase-σ Is a Novel Member of the Functional Family of α-Latrotoxin Receptors. J. Biol. Chem. 2002, 277, 35887–35895. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.-P.; Lelianova, V.G.; Ermolyuk, Y.S.; Vysokov, N.V.; Hitchen, P.G.; Berninghausen, O.; Rahman, M.A.; Zangrandi, A.; Fidalgo, S.; Tonevitsky, A.G.; et al. Latrophilin 1 and Its Endogenous Ligand Lasso/Teneurin-2 Form a High-Affinity Transsynaptic Receptor Pair with Signaling Capabilities. Proc. Natl. Acad. Sci. USA 2011, 108, 12113–12118. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.-P.; Lelianova, V.; Hopkins, C.; Volynski, K.E.; Ushkaryov, Y. Functional Cross-Interaction of the Fragments Produced by the Cleavage of Distinct Adhesion G-Protein-Coupled Receptors. J. Biol. Chem. 2009, 284, 6495–6506. [Google Scholar] [CrossRef] [PubMed]
- Vysokov, N.V.; Silva, J.-P.; Lelianova, V.G.; Suckling, J.; Cassidy, J.; Blackburn, J.K.; Yankova, N.; Djamgoz, M.B.A.; Kozlov, S.V.; Tonevitsky, A.G.; et al. Proteolytically Released Lasso/Teneurin-2 Induces Axonal Attraction by Interacting with Latrophilin-1 on Axonal Growth Cones. Elife 2018, 7, e3793. [Google Scholar] [CrossRef] [PubMed]
- Arac, D.; Boucard, A.A.; Bolliger, M.F.; Nguyen, J.; Soltis, S.M.; Sudhof, T.C.; Brunger, A.T. A Novel Evolutionarily Conserved Domain of Cell-Adhesion GPCRs Mediates Autoproteolysis. EMBO J. 2012, 31, 1364–1378. [Google Scholar] [CrossRef] [PubMed]
- Krasnoperov, V.; Lu, Y.; Buryanovsky, L.; Neubert, T.A.; Ichtchenko, K.; Petrenko, A.G.; Ichtchenko, K.; Petrenko, A.G. Post-Translational Proteolytic Processing of the Calcium-Independent Receptor of a-Latrotoxin (CIRL), a Natural Chimera of the Cell Adhesion Protein and the G Protein-Coupled Receptor. Role of the G Protein-Coupled Receptor Proteolysis Site (GPS) Motif. J. Biol. Chem. 2002, 277, 46518–46526. [Google Scholar] [CrossRef] [PubMed]
- Hamann, J.; Aust, G.; Arac, D.; Engel, F.B.; Formstone, C.; Fredriksson, R.; Hall, R.A.; Harty, B.L.; Kirchhoff, C.; Knapp, B.; et al. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G Protein-Coupled Receptors. Pharmacol. Rev. 2015, 67, 338–367. [Google Scholar] [CrossRef] [PubMed]
- Barros-Álvarez, X.; Nwokonko, R.M.; Vizurraga, A.; Matzov, D.; He, F.; Papasergi-Scott, M.M.; Robertson, M.J.; Panova, O.; Yardeni, E.H.; Seven, A.B.; et al. The Tethered Peptide Activation Mechanism of Adhesion GPCRs. Nature 2022, 604, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhao, R.J.; Dong, Y.J.; Gao, M.; Chen, L.N.; Zhang, C.; Xiao, P.; Guo, J.; Qin, J.; Shen, D.D.; et al. Conformational Transitions and Activation of the Adhesion Receptor CD97. Mol. Cell 2024, 84, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Chiang, N.-Y.; Hu, C.-H.; Hsiao, C.-C.; Cheng, K.-F.; Tsai, W.-P.; Yona, S.; Stacey, M.; Gordon, S.; Chang, G.-W.; et al. Activation of Myeloid Cell-Specific Adhesion Class G Protein-Coupled Receptor EMR2 via Ligation-Induced Translocation and Interaction of Receptor Subunits in Lipid Raft Microdomains. Mol. Cell. Biol. 2012, 32, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Ramjeesingh, M.; Huan, L.J.; Garami, E.; Bear, C.E. Novel Method for Evaluation of the Oligomeric Structure of Membrane Proteins. Biochem. J. 1999, 342, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kedei, N.; Szabo, T.; Lile, J.D.; Treanor, J.J.; Olah, Z.; Iadarola, M.J.; Blumberg, P.M. Analysis of the Native Quaternary Structure of Vanilloid Receptor 1. J. Biol. Chem. 2001, 276, 28613–28619. [Google Scholar] [CrossRef] [PubMed]
- Florentin, A.; Deblonde, T.; Diguio, N.; Hautemaniere, A.; Hartemann, P. Impacts of Two Perfluorinated Compounds (PFOS and PFOA) on Human Hepatoma Cells: Cytotoxicity but No Genotoxicity? Int. J. Hyg. Environ. Health 2011, 214, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Kim, J.H.; Park, J.K.; Lee, K.M.; Kim, E.; Jeon, W.B. Cytotoxicity and Inhibition of Intercellular Interaction in N2a Neurospheroids by Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid. Food Chem. Toxicol. 2013, 60, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Maso, L.; Trande, M.; Liberi, S.; Moro, G.; Daems, E.; Linciano, S.; Sobott, F.; Covaceuszach, S.; Cassetta, A.; Fasolato, S.; et al. Unveiling the Binding Mode of Perfluorooctanoic Acid to Human Serum Albumin. Protein Sci. 2021, 30, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Manser, C.; Benlaouer, O.; Suckling, J.; Blackburn, J.K.; Silva, J.P.; Ushkaryov, Y.A. C-Terminal Phosphorylation of Latrophilin-1/ADGRL1 Affects the Interaction between Its Fragments. Ann. N. Y. Acad. Sci. 2019, 1456, 122–143. [Google Scholar] [CrossRef] [PubMed]
- Elrick, D.B.; Charlton, M.P. Alpha-Latrocrustatoxin Increases Neurotransmitter Release by Activating a Calcium Influx Pathway at Crayfish Neuromuscular Junction. J. Neurophysiol. 1999, 82, 3550–3562. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.S.; Schnellmann, R.G. Measurement of Cell Death in Mammalian Cells. Curr. Protoc. 2021, 1, e210. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring Cell Death by Propidium Iodide Uptake and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Costes, S.V.; Daelemans, D.; Cho, E.H.; Dobbin, Z.; Pavlakis, G.; Lockett, S. Automatic and Quantitative Measurement of Protein-Protein Colocalization in Live Cells. Biophys. J. 2004, 86, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Innamorati, G.; Le Gouill, C.; Balamotis, M.; Birnbaumer, M. The Long and the Short Cycle. Alternative Intracellular Routes for Trafficking of G-Protein-Coupled Receptors. J. Biol. Chem. 2001, 276, 13096–13103. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Paing, M.M.; Temple, B.R.S.; Trejo, J. G Protein-Coupled Receptor Sorting to Endosomes and Lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 601–629. [Google Scholar] [CrossRef] [PubMed]
- Lobingier, T.B.; von Zastrow, M. When Trafficking and Signaling Mix: How Subcellular Location Shapes G Protein-Coupled Receptor Activation of Heterotrimeric G Proteins. Traffic 2019, 20, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Crilly, S.E.; Puthenveedu, M.A. Compartmentalized GPCR Signaling from Intracellular Membranes. J. Membr. Biol. 2021, 254, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Anderson, P.J.; Rajagopal, S.; Lefkowitz, R.J.; Rockman, H.A. G Protein-Coupled Receptors: A Century of Research and Discovery. Circ. Res. 2024, 135, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Demberg, L.M.; Winkler, J.; Wilde, C.; Simon, K.U.; Schön, J.; Rothemund, S.; Schöneberg, T.; Prömel, S.; Liebscher, I. Activation of Adhesion G Protein-Coupled Receptors: Agonist Specificity of Stachel Sequence-Derived Peptides. J. Biol. Chem. 2017, 292, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Arac, D.; Strater, N.; Seiradake, E. Understanding the Structural Basis of Adhesion GPCR Functions. In Adhesion G Protein-coupled Receptors, Handbook of Experimental Pharmacology 234; Langenhan, T., Schoneberg, T., Eds.; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 67–82. [Google Scholar] [CrossRef]
- Seufert, F.; Chung, Y.K.; Hildebrand, P.W.; Langenhan, T. 7TM Domain Structures of Adhesion GPCRs: What’s New and What’s Missing? Trends Biochem. Sci. 2023, 48, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Wilde, C.; Fischer, L.; Lede, V.; Kirchberger, J.; Rothemund, S.; Schöneberg, T.; Liebscher, I. The Constitutive Activity of the Adhesion GPCR GPR114/ADGRG5 Is Mediated by Its Tethered Agonist. FASEB J. 2016, 30, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Huang, W.; Tang, Q.; Niu, M.; Guo, S.; Langenhan, T.; Song, G.; Yan, J. Unveiling Mechanical Activation: GAIN Domain Unfolding and Dissociation in Adhesion GPCRs. Nano Lett. 2023, 23, 9179–9186. [Google Scholar] [CrossRef] [PubMed]
- Vizurraga, A.; Adhikari, R.; Yeung, J.; Yu, M.; Tall, G.G. Mechanisms of Adhesion G Protein–Coupled Receptor Activation. J. Biol. Chem. 2020, 295, 14065–14083. [Google Scholar] [CrossRef] [PubMed]
- Nazarko, O.; Kibrom, A.; Winkler, J.; Leon, K.; Stoveken, H.; Salzman, G.; Merdas, K.; Lu, Y.; Narkhede, P.; Tall, G.; et al. A Comprehensive Mutagenesis Screen of the Adhesion GPCR Latrophilin-1/ADGRL1. iScience 2018, 3, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Salzman, G.S.; Zhang, S.; Gupta, A.; Koide, A.; Koide, S.; Araç, D. Stachel-Independent Modulation of GPR56/ADGRG1 Signaling by Synthetic Ligands Directed to Its Extracellular Region. Proc. Natl. Acad. Sci. USA 2017, 114, 10095–10100. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Vuković, L.; Narayan, M. An Atomic and Molecular Insight into How PFOA Reduces α-Helicity, Compromises Substrate Binding, and Creates Binding Pockets in a Model Globular Protein. J. Am. Chem. Soc. 2024, 146, 12766–12777. [Google Scholar] [CrossRef] [PubMed]
- Vitobello, A.; Mazel, B.; Lelianova, V.G.; Zangrandi, A.; Petitto, E.; Suckling, J.; Salpietro, V.; Meyer, R.; Elbracht, M.; Kurth, I.; et al. ADGRL1 Haploinsufficiency Causes a Variable Spectrum of Neurodevelopmental Disorders in Humans and Alters Synaptic Activity and Behavior in a Mouse Model. Am. J. Hum. Genet. 2022, 109, 1436–1457. [Google Scholar] [CrossRef] [PubMed]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; Dewitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- And Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Eze, C.G.; Okeke, E.S.; Nwankwo, C.E.; Nyaruaba, R.; Anand, U.; Okoro, O.J.; Bontempi, E. Emerging Contaminants in Food Matrices: An Overview of the Occurrence, Pathways, Impacts and Detection Techniques of per- and Polyfluoroalkyl Substances. Toxicol. Reports 2024, 12, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Seals, R.; Bartell, S.M.; Steenland, K. Accumulation and Clearance of Perfluorooctanoic Acid (PFOA) in Current and Former Residents of an Exposed Community. Environ. Health Perspect. 2011, 119, 119–124. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Our Current Understanding of the Human Health and Environmental Risks of PFAS. Available online: https://www.epa.gov/pfas/our-current-understanding-human-health-and-environmental-risks-pfas (accessed on 13 July 2025).
- Agency for Toxic Substancies and Disease Registry. Fast Facts: PFAS in the U.S. Population. Available online: https://www.atsdr.cdc.gov/pfas/data-research/facts-stats/ (accessed on 10 July 2025).
- Johansson, N.; Fredriksson, A.; Eriksson, P. Neonatal Exposure to Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) Causes Neurobehavioural Defects in Adult Mice. Neurotoxicology 2008, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Johansson, N.; Eriksson, P.; Viberg, H. Neonatal Exposure to PFOS and PFOA in Mice Results in Changes in Proteins Which Are Important for Neuronal Growth and Synaptogenesis in the Developing Brain. Toxicol. Sci. 2009, 108, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Franklin, J.N.; Bryan, I.; Morris, E.; Wood, A.; DeWitt, J.C. Does Developmental Exposure to Perflurooctanoic Acid (PFOA) Induce Immunopathologies Commonly Observed in Neurodevelopmental Disorders? Neurotoxicology 2012, 33, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Nilsson, S.; Shepherd, C.E.; Zammit, I.; Suryana, E.; Mueller, N.; Halliday, G.; Wang, X.; Symeonides, C.; Dunlop, S.; et al. Number of Carbons Is a Critical Parameter for Accumulation of Per- and Polyfluoroalkyl Substances in the Human Brain. Environ. Sci. Technol. 2025, 59, 3366–3375. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Liberi, S.; Vascon, F.; Linciano, S.; De Felice, S.; Fasolato, S.; Foresta, C.; De Toni, L.; Di Nisio, A.; Cendron, L.; et al. Investigation of the Interaction between Human Serum Albumin and Branched Short-Chain Perfluoroalkyl Compounds. Chem. Res. Toxicol. 2022, 35, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Ashton, A.C.; Rahman, M.A.; Volynski, K.E.; Manser, C.; Orlova, E.V.; Matsushita, H.; Davletov, B.A.; Van Heel, M.; Grishin, E.V.; Ushkaryov, Y.A. Tetramerisation of α-Latrotoxin by Divalent Cations Is Responsible for Toxin-Induced Non-Vesicular Release and Contributes to the Ca2+-Dependent Vesicular Exocytosis from Synaptosomes. Biochimie 2000, 82, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Shamotienko, O.G.; Parcej, D.N.; Dolly, J.O. Subunit Combinations Defined for K+ Channel Kv1 Subtypes in Synaptic Membranes from Bovine Brain. Biochemistry 1997, 36, 8195–8201. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Hall, M.A.; Holmes, G.; Kirkby, R.; Pfahringer, B.; Witten, I.H.; Trigg, L. Weka—A Machine Learning Workbench for Data Mining. In Data Mining and Knowledge Discovery Handbook; Maimon, O., Rokach, L., Eds.; Springer: Boston, MA, USA, 2009. [Google Scholar]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of Co-localization of Objects in Dual-colour Confocal Images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lau, A.; Morris, T.J.; Guo, L.; Fordyce, C.B.; Stanley, E.F. A Syntaxin 1, Gαo, and N-Type Calcium Channel Complex at a Presynaptic Nerve Terminal: Analysis by Quantitative Immunocolocalization. J. Neurosci. 2004, 24, 4070–4081. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The Arrive Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, 6–10. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The Arrive Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, 1–12. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petitto, E.; Blackburn, J.K.; Rahman, M.A.; Ushkaryov, Y.A. The Dissociation of Latrophilin Fragments by Perfluorooctanoic Acid (PFOA) Inhibits LTXN4C-Induced Neurotransmitter Release. Toxins 2025, 17, 359. https://doi.org/10.3390/toxins17070359
Petitto E, Blackburn JK, Rahman MA, Ushkaryov YA. The Dissociation of Latrophilin Fragments by Perfluorooctanoic Acid (PFOA) Inhibits LTXN4C-Induced Neurotransmitter Release. Toxins. 2025; 17(7):359. https://doi.org/10.3390/toxins17070359
Chicago/Turabian StylePetitto, Evelina, Jennifer K. Blackburn, M. Atiqur Rahman, and Yuri A. Ushkaryov. 2025. "The Dissociation of Latrophilin Fragments by Perfluorooctanoic Acid (PFOA) Inhibits LTXN4C-Induced Neurotransmitter Release" Toxins 17, no. 7: 359. https://doi.org/10.3390/toxins17070359
APA StylePetitto, E., Blackburn, J. K., Rahman, M. A., & Ushkaryov, Y. A. (2025). The Dissociation of Latrophilin Fragments by Perfluorooctanoic Acid (PFOA) Inhibits LTXN4C-Induced Neurotransmitter Release. Toxins, 17(7), 359. https://doi.org/10.3390/toxins17070359





