Potential Compounds as Inhibitors of Staphylococcal Virulence Factors Involved in the Development of Thrombosis
Abstract
1. Introduction
2. Therapeutic Strategies in Neutralization of Virulence Factors
2.1. Natural Product
2.2. Antimicrobial Peptides
2.3. Monoclonal Antibodies and Vaccines
2.4. Nanoparticles as Alternative Therapeutic Compounds
3. Antithrombotic Action of Used Compounds
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-ES | ethoxysanguinarine |
| 6-MS | 6-methoxydihydrosanguinarine |
| ADP | adenosine diphosphate |
| AI-2 | autoinducer-2 |
| AMP | antimicrobial peptide |
| aPTT | activated partial thromboplastin time |
| ASA | acetylsalicylic acid |
| ASK1 | apoptosis signal-regulating kinase 1 |
| ATP | adenosine triphosphate |
| CH | chelerythrine |
| Cna | collagen adhesin |
| COX-2 | cyclooxygenase-2 |
| DICH | dihydrochelerythrine |
| DNA | deoxyribonucleic acid |
| EC | endothelial cell |
| FDA | Food and Drug Administration |
| FnBPA | fibronectin-binding protein A |
| FnBPB | fibronectin-binding protein B |
| HBD-1 | human β-defensin 1 |
| HD | human α-defensin |
| HDAC | histone deacetylase |
| Hla | α-hemolysin |
| HNP | human neutrophil peptide |
| HT | hydroxytyrosol |
| HT-AC | hydroxytyrosol acetate |
| HUVEC | human umbilical vein endothelial cell |
| iNOS | inducible nitric oxide synthase |
| IsdB | iron-regulated surface determinant protein B |
| IVIg | intravenous immunoglobulin |
| LDL | low-density lipoprotein |
| LukAB | leukocidin AB |
| MDR | multidrug-resistant |
| MIC | minimal inhibitory concentration |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSCRAMMs | microbial surface components recognizing adhesive matrix molecules |
| MSSA | meticillin-sensitive Staphylococcus aureus |
| NP | nanoparticle |
| OroA | oroxylin A |
| PAI-1 | plasminogen activator inhibitor type 1 |
| PGE2 | prostaglandin E2 |
| PGF1 | prostaglandin F1 |
| PGF2 | prostaglandin F2 |
| PIA | polysaccharide intercellular adhesin |
| PKC | protein kinase C |
| PLGA | polylactic-co-glycolic acid |
| PSMα | phenol-soluble modulin |
| PT | prothrombin time |
| PVL | Panton-Valentine leukocidin |
| QS | quorum sensing |
| rAT | recombinant α-toxoid |
| RBC | red blood cell |
| RM-PL | erythroliposome |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| rTSST-1v | toxic shock syndrome toxin 1 variant vaccine |
| SE | staphylococcal enterotoxin |
| SEA | staphylococcal enterotoxin A |
| SEB | staphylococcal enterotoxin B |
| SMC | vascular smooth muscle cell |
| SplB | Staphylococcus aureus-serine protease-like protein B |
| SrtA | sortase A |
| SSTIs | skin and soft tissue infections |
| TF | tissue factor |
| TNF-α | tumor necrosis factor-α |
| TP | TxA2 receptor |
| t-PA | tissue-type plasminogen activator |
| TSS | toxic shock syndrome |
| TSST-1 | toxic shock syndrome toxin-1 |
| TxA2 | thromboxane A2 |
| vWbp | vWF-binding protein |
| vWF | von Willebrand factor |
References
- Joshi, A.A.; Patil, R.H. Metal nanoparticles as inhibitors of enzymes and toxins of multidrug-resistant Staphylococcus aureus. Infect. Med. 2023, 2, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Olchowik-Grabarek, E.; Sekowski, S.; Bitiucki, M.; Dobrzynska, I.; Shlyonsky, V.; Ionov, M.; Burzynski, P.; Roszkowska, A.; Swiecicka, I.; Abdulladjanova, N.; et al. Inhibition of interaction between Staphylococcus aureus α-hemolysin and erythrocytes membrane by hydrolysable tannins: Structure-related activity study. Sci. Rep. 2020, 10, 11168. [Google Scholar] [CrossRef] [PubMed]
- Júnior, J.G.d.A.S.; Coutinho, H.D.M.; Rodrigues, J.P.V.; Ferreira, V.P.G.; Neto, J.B.d.A.; Costa da Silva, M.M.; Justino de Araújo, A.C.; Pereira, R.L.S.; Alexandre de Aquino, P.E.; Oliveira–Tintino, C.D.d.M.; et al. Liposome evaluation in inhibiting pump efflux of NorA of Staphylococcus aureus. Chem. Phys. Lipids 2022, 245, 105204. [Google Scholar] [CrossRef]
- Wójcik-Bojek, U.; Różalska, B.; Sadowska, B. Staphylococcus aureus—A Known Opponent against Host Defense Mechanisms and Vaccine Development—Do We Still Have a Chance to Win? Int. J. Mol. Sci. 2022, 23, 948. [Google Scholar] [CrossRef]
- Ford, C.A.; Hurford, I.M.; Cassat, J.E. Antivirulence Strategies for the Treatment of Staphylococcus aureus Infections: A Mini Review. Front. Microbiol. 2021, 11, 632706. [Google Scholar] [CrossRef]
- Sabino, Y.N.V.; Cotter, P.D.; Mantovani, H.C. Anti-virulence compounds against Staphylococcus aureus associated with bovine mastitis: A new therapeutic option? Microbiol. Res. 2023, 271, 127345. [Google Scholar] [CrossRef]
- Garrett, S.R.; Palmer, T. The role of proteinaceous toxins secreted by Staphylococcus aureus in interbacterial competition. FEMS Microbes 2024, 5, xtae006. [Google Scholar] [CrossRef]
- Jahn, K.; Handtke, S.; Palankar, R.; Kohler, T.P.; Wesche, J.; Wolff, M.; Bayer, J.; Wolz, C.; Greinacher, A.; Hammerschmidt, S. α-hemolysin of Staphylococcus aureus impairs thrombus formation. J. Thromb. Haemost. 2022, 20, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Binsker, U.; Palankar, R.; Wesche, J.; Kohler, T.; Prucha, J.; Burchhardt, G.; Rohde, M.; Schmidt, F.; Bröker, B.; Mamat, U.; et al. Secreted Immunomodulatory Proteins of Staphylococcus aureus Activate Platelets and Induce Platelet Aggregation. Thromb. Haemost. 2018, 47, 745–757. [Google Scholar] [CrossRef]
- Spaan, A.N.; van Strijp, J.A.G.G.; Torres, V.J. Leukocidins: Staphylococcal bi-component pore-forming toxins find their receptors. Nat. Rev. Microbiol. 2017, 15, 435–447. [Google Scholar] [CrossRef]
- Martin, E.; Cevik, C.; Nugent, K. The role of hypervirulent Staphylococcus aureus infections in the development of deep vein thrombosis. Thromb. Res. 2012, 130, 302–308. [Google Scholar] [CrossRef]
- Escajadillo, T.; Nizet, V. Pharmacological Targeting of Pore-Forming Toxins as Adjunctive Therapy for Invasive Bacterial Infection. Toxins 2018, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K. Staphylococcus aureus Toxins: From Their Pathogenic Roles to Anti-virulence Therapy Using Natural Products. Biotechnol. Bioprocess Eng. 2019, 24, 424–435. [Google Scholar] [CrossRef]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Dal Piaz, F. Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, Y.; Zhang, L.; Liu, F.; Duan, G.; Chen, S.; Long, J.; Jin, Y.; Yang, H. Recent advances in the use of resveratrol against Staphylococcus aureus infections (Review). Med. Int. 2024, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Stecko, J.; Sobieraj, J.; Szymańska, N.; Kozłowska, J. Naringenin and Its Derivatives—Health-Promoting Phytobiotic against Resistant Bacteria and Fungi in Humans. Antibiotics 2022, 11, 1628. [Google Scholar] [CrossRef]
- Xia, F.; Li, X.; Wang, B.; Gong, P.; Xiao, F.; Yang, M.; Zhang, L.; Song, J.; Hu, L.; Cheng, M.; et al. Combination Therapy of LysGH15 and Apigenin as a New Strategy for Treating Pneumonia Caused by Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 87–94. [Google Scholar] [CrossRef]
- Mohan, R.; Venugopal, S. In silico Molecular Interaction Studies of Gamma-hemolysin of Staphylococcus aureus with Flavonoid Compounds. Trends Bioinforma. 2013, 6, 91–100. [Google Scholar] [CrossRef]
- Chowdhury, T. Virtual screening of compounds derived from Garcinia pedunculata as an inhibitor of gamma hemolysin component A of Staphylococcus aureus. Bangladesh J. Pharmacol. 2014, 9, 67–71. [Google Scholar] [CrossRef]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure–Activity Relationship of Flavonoids on Their Anti-Escherichia coli Activity and Inhibition of DNA Gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef]
- Liu, M.-H.; Otsuka, N.; Noyori, K.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Synergistic Effect of Kaempferol Glycosides Purified from Laurus nobilis and Fluoroquinolones on Methicillin-Resistant Staphylococcus aureus. Biol. Pharm. Bull. 2009, 32, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Huang, C.-C.; Chen, C.-C.; Yang, K.-J.; Huang, C.-Y. Inhibition of Staphylococcus aureus PriA Helicase by Flavonol Kaempferol. Protein J. 2015, 34, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Chan, E.W.L.; Gray, A.I.; Igoli, J.O.; Lee, S.M.; Goh, J.K. Galloylated flavonol rhamnosides from the leaves of Calliandra tergemina with antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Phytochemistry 2014, 107, 148–154. [Google Scholar] [CrossRef]
- Daly, S.M.; Elmore, B.O.; Kavanaugh, J.S.; Triplett, K.D.; Figueroa, M.; Raja, H.A.; El-Elimat, T.; Crosby, H.A.; Femling, J.K.; Cech, N.B.; et al. ω-Hydroxyemodin Limits Staphylococcus aureus Quorum Sensing-Mediated Pathogenesis and Inflammation. Antimicrob. Agents Chemother. 2015, 59, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yi, T.; Shen, Z.; Teng, Z.; Wang, J. Aloe-emodin Attenuates Staphylococcus aureus Pathogenicity by Interfering with the Oligomerization of α-Toxin. Front. Cell. Infect. Microbiol. 2019, 9, 157. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Wang, W.-M.; Zhang, Z.; Sun, L.; Wu, S.-C. Natural Antibacterial and Antivirulence Alkaloids From Macleaya cordata Against Methicillin-Resistant Staphylococcus aureus. Front. Pharmacol. 2022, 13, 813172. [Google Scholar] [CrossRef]
- Parlet, C.P.; Kavanaugh, J.S.; Crosby, H.A.; Raja, H.A.; El-Elimat, T.; Todd, D.A.; Pearce, C.J.; Cech, N.B.; Oberlies, N.H.; Horswill, A.R. Apicidin Attenuates MRSA Virulence through Quorum-Sensing Inhibition and Enhanced Host Defense. Cell Rep. 2019, 27, 187–198.e6. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, M.; Kang, J.; Kang, D. Effect of sub-lethal treatment of carvacrol and thymol on virulence potential and resistance to several bactericidal treatments of Staphylococcus aureus. J. Food Saf. 2022, 42, e13004. [Google Scholar] [CrossRef]
- Hayati, A.; Razavler, V.; Barin, A.; Langroudi, A. Evaluation of the effect of Carvacrol and Linalool on the growth of Staphylococcus aureus and E. coli enterotoxin and production of ent A and ent B toxins in Staphylococcus aureus and ST1 and ST2 toxins in E. coli enterotoxins. J. Complement. Med. Res. 2020, 11, 279. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Shi, L. A Carvacrol-Rich Essential Oil Extracted From Oregano (Origanum vulgare “Hot & Spicy”) Exerts Potent Antibacterial Effects Against Staphylococcus aureus. Front. Microbiol. 2021, 12, 741861. [Google Scholar] [CrossRef]
- Peng, Q.; Tang, X.; Dong, W.; Zhi, Z.; Zhong, T.; Lin, S.; Ye, J.; Qian, X.; Chen, F.; Yuan, W. Carvacrol inhibits bacterial polysaccharide intracellular adhesin synthesis and biofilm formation of mucoid Staphylococcus aureus: An in vitro and in vivo study. RSC Adv. 2023, 13, 28743–28752. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Permana, A.D.; Rodgers, A.M.; Donnelly, R.F.; Rehman, A. Enhancement in Site-Specific Delivery of Carvacrol against Methicillin Resistant Staphylococcus aureus Induced Skin Infections Using Enzyme Responsive Nanoparticles: A Proof of Concept Study. Pharmaceutics 2019, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Viljoen, A.M. Geraniol—A review update. S. Afr. J. Bot. 2022, 150, 1205–1219. [Google Scholar] [CrossRef]
- Lin, L.; Long, N.; Qiu, M.; Liu, Y.; Sun, F.; Dai, M. The Inhibitory Efficiencies of Geraniol as an Anti-Inflammatory, Antioxidant, and Antibacterial, Natural Agent Against Methicillin-Resistant Staphylococcus aureus Infection in vivo. Infect. Drug Resist. 2021, 14, 2991–3000. [Google Scholar] [CrossRef]
- Gu, K.; Ouyang, P.; Hong, Y.; Dai, Y.; Tang, T.; He, C.; Shu, G.; Liang, X.; Tang, H.; Zhu, L.; et al. Geraniol inhibits biofilm formation of methicillin-resistant Staphylococcus aureus and increase the therapeutic effect of vancomycin in vivo. Front. Microbiol. 2022, 13, 960728. [Google Scholar] [CrossRef]
- Solecki, O.; Mosbah, A.; Baudy Floc’h, M.; Felden, B. Converting a Staphylococcus aureus Toxin into Effective Cyclic Pseudopeptide Antibiotics. Chem. Biol. 2015, 22, 329–335. [Google Scholar] [CrossRef]
- Ganesan, N.; Mishra, B.; Felix, L.; Mylonakis, E. Antimicrobial Peptides and Small Molecules Targeting the Cell Membrane of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 2023, 87, e0003722. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Y.; Li, B.; Qu, G.; Li, A.; Peng, X.; Li, S.; Shao, C. Antimicrobial peptide biological activity, delivery systems and clinical translation status and challenges. J. Transl. Med. 2025, 23, 292. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, D.M. Antimicrobial peptides: Features, applications and the potential use against COVID-19. Mol. Biol. Rep. 2022, 49, 10039–10050. [Google Scholar] [CrossRef]
- Yang, Z.; He, S.; Wu, H.; Yin, T.; Wang, L.; Shan, A. Nanostructured Antimicrobial Peptides: Crucial Steps of Overcoming the Bottleneck for Clinics. Front. Microbiol. 2021, 12, 710199. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Ren, K.; Xu, T.; Zhang, Z.; Xu, M.; Rao, Z.; Zhang, X. A Review of Antimicrobial Peptides: Structure, Mechanism of Action, and Molecular Optimization Strategies. Fermentation 2024, 10, 540. [Google Scholar] [CrossRef]
- Vieira, A.P.G.C.; de Souza, A.N.; Lima, W.G.; Brito, J.C.M.; Simião, D.C.; Gonçalves, L.V.R.; Cordeiro, L.P.B.; de Oliveira Scoaris, D.; Fernandes, S.O.A.; Resende, J.M.; et al. The Synthetic Peptide LyeTx I mn∆K, Derived from Lycosa erythrognatha Spider Toxin, Is Active against Methicillin-Resistant Staphylococcus aureus (MRSA) In Vitro and In Vivo. Antibiotics 2024, 13, 248. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Beck, C.; Nøhr-Meldgaard, K.; Peschel, A.; Kretschmer, D.; Ingmer, H.; Vestergaard, M. Inhibition of the ATP synthase sensitizes Staphylococcus aureus towards human antimicrobial peptides. Sci. Rep. 2020, 10, 11391. [Google Scholar] [CrossRef] [PubMed]
- Schlievert, P.M.; Kilgore, S.H.; Beck, L.A.; Yoshida, T.; Klingelhutz, A.J.; Leung, D.Y.M. Host Cationic Antimicrobial Molecules Inhibit S. aureus Exotoxin Production. mSphere 2023, 8, e00576-22. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Rogozhin, E.A. Sub-inhibitory Effects of Antimicrobial Peptides. Front. Microbiol. 2019, 10, 1160. [Google Scholar] [CrossRef]
- Cogen, A.L.; Yamasaki, K.; Muto, J.; Sanchez, K.M.; Crotty Alexander, L.; Tanios, J.; Lai, Y.; Kim, J.E.; Nizet, V.; Gallo, R.L. Staphylococcus epidermidis Antimicrobial δ-Toxin (Phenol-Soluble Modulin-γ) Cooperates with Host Antimicrobial Peptides to Kill Group A Streptococcus. PLoS ONE 2010, 5, e8557. [Google Scholar] [CrossRef]
- Toor, H.G.; Banerjee, D.I.; Chauhan, J.B. In Silico Evaluation of Human Cathelicidin LL-37 as a Novel Therapeutic Inhibitor of Panton-Valentine Leukocidin Toxin of Methicillin-Resistant Staphylococcus aureus. Microb. Drug Resist. 2021, 27, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Dlozi, P.N.; Gladchuk, A.; Crutchley, R.D.; Keuler, N.; Coetzee, R.; Dube, A. Cathelicidins and defensins antimicrobial host defense peptides in the treatment of TB and HIV: Pharmacogenomic and nanomedicine approaches towards improved therapeutic outcomes. Biomed. Pharmacother. 2022, 151, 113189. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chu, A.J.; Tsang, T.F.; Zheng, Y.; Lam, N.M.; Li, K.S.L.; Ip, M.; Yang, X.; Ma, C. Synthesis and biological evaluation of nusbiarylin derivatives as bacterial rRNA synthesis inhibitor with potent antimicrobial activity against MRSA and VRSA. Bioorg. Chem. 2022, 124, 105863. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, G. Staphylococcus aureus Infection: Pathogenesis and Antimicrobial Resistance. Int. J. Mol. Sci. 2023, 24, 8182. [Google Scholar] [CrossRef]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus Vaccine Research and Development: The Past, Present and Future, Including Novel Therapeutic Strategies. Front. Immunol. 2021, 12, 705360. [Google Scholar] [CrossRef]
- Liu, F.; Guan, Z.; Liu, Y.; Li, J.; Liu, C.; Gao, Y.; Ma, Y.; Feng, J.; Shen, B.; Yang, G. Identification of a Human Anti-Alpha-Toxin Monoclonal Antibody Against Staphylococcus aureus Infection. Front. Microbiol. 2021, 12, 692279. [Google Scholar] [CrossRef]
- Hua, L.; Cohen, T.S.; Shi, Y.; Datta, V.; Hilliard, J.J.; Tkaczyk, C.; Suzich, J.; Stover, C.K.; Sellman, B.R. MEDI4893* Promotes Survival and Extends the Antibiotic Treatment Window in a Staphylococcus aureus Immunocompromised Pneumonia Model. Antimicrob. Agents Chemother. 2015, 59, 4526–4532. [Google Scholar] [CrossRef]
- Kailasan, S.; Kant, R.; Noonan-Shueh, M.; Kanipakala, T.; Liao, G.; Shulenin, S.; Leung, D.W.; Alm, R.A.; Adhikari, R.P.; Amarasinghe, G.K.; et al. Antigenic landscapes on Staphylococcus aureus pore-forming toxins reveal insights into specificity and cross-neutralization. MAbs 2022, 14, 2083467. [Google Scholar] [CrossRef]
- Rouha, H.; Weber, S.; Janesch, P.; Maierhofer, B.; Gross, K.; Dolezilkova, I.; Mirkina, I.; Visram, Z.C.; Malafa, S.; Stulik, L.; et al. Disarming Staphylococcus aureus from destroying human cells by simultaneously neutralizing six cytotoxins with two human monoclonal antibodies. Virulence 2018, 9, 231–247. [Google Scholar] [CrossRef]
- Wood, J.B.; Jones, L.S.; Soper, N.R.; Nagarsheth, M.; Creech, C.B.; Thomsen, I.P. Commercial Intravenous Immunoglobulin Preparations Contain Functional Neutralizing Antibodies against the Staphylococcus aureus Leukocidin LukAB (LukGH). Antimicrob. Agents Chemother. 2017, 61, e00968-17. [Google Scholar] [CrossRef]
- Tanaka, J.; Hirayama, F.; Yanase, S.; Uno, S.; Nakae, T.; Kamizono, A.; Hanaki, H. Effective concentration of intravenous immunoglobulin for neutralizing Panton-Valentine leukocidin in human blood. J. Infect. Chemother. 2018, 24, 383–388. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Z.; Ge, S.; Zhang, J.; Xu, L.; Yang, F.; Lu, D.; Luo, P.; Gu, J.; Zou, Q.; et al. Determining the immunological characteristics of a novel human monoclonal antibody developed against staphylococcal enterotoxin B. Hum. Vaccin. Immunother. 2020, 16, 1708–1718. [Google Scholar] [CrossRef]
- Aguilar, J.L.; Varshney, A.K.; Pechuan, X.; Dutta, K.; Nosanchuk, J.D.; Fries, B.C. Monoclonal antibodies protect from Staphylococcal Enterotoxin K (SEK) induced toxic shock and sepsis by USA300 Staphylococcus aureus. Virulence 2017, 8, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Fowler, V.G.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153. [Google Scholar] [CrossRef] [PubMed]
- Wardenburg, J.B.; Schneewind, O. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 2008, 205, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Scafa-Udriste, A.; Popa, M.-I.; Popa, G.-L. Updates on Staphylococcal Vaccines. Microbiol. Res. (Pavia) 2023, 15, 137–151. [Google Scholar] [CrossRef]
- Tomaszewski, K.L.; Blanchard, M.; Olaniyi, R.; Brenton, H.R.; Hayes, S.; Fatma, F.; Amarasinghe, G.K.; Cho, B.-K.; Goo, Y.A.; DeDent, A.C.; et al. Enhanced Staphylococcus aureus protection by uncoupling of the α-toxin-ADAM10 interaction during murine neonatal vaccination. Nat. Commun. 2024, 15, 8702. [Google Scholar] [CrossRef]
- Adhikari, R.P.; Kort, T.; Shulenin, S.; Kanipakala, T.; Ganjbaksh, N.; Roghmann, M.-C.; Holtsberg, F.W.; Aman, M.J. Antibodies to S. aureus LukS-PV Attenuated Subunit Vaccine Neutralize a Broad Spectrum of Canonical and Non-Canonical Bicomponent Leukotoxin Pairs. PLoS ONE 2015, 10, e0137874. [Google Scholar] [CrossRef]
- Landrum, M.L.; Lalani, T.; Niknian, M.; Maguire, J.D.; Hospenthal, D.R.; Fattom, A.; Taylor, K.; Fraser, J.; Wilkins, K.; Ellis, M.W.; et al. Safety and immunogenicity of a recombinant Staphylococcus aureus α -toxoid and a recombinant Panton-Valentine leukocidin subunit, in healthy adults. Hum. Vaccin. Immunother. 2017, 13, 791–801. [Google Scholar] [CrossRef]
- Venkatasubramaniam, A.; Liao, G.; Cho, E.; Adhikari, R.P.; Kort, T.; Holtsberg, F.W.; Elsass, K.E.; Kobs, D.J.; Rudge, T.L.; Kauffman, K.D.; et al. Safety and Immunogenicity of a 4-Component Toxoid-Based Staphylococcus aureus Vaccine in Rhesus Macaques. Front. Immunol. 2021, 12, 621754. [Google Scholar] [CrossRef]
- Wu, X.; Wang, H.; Xiong, J.; Yang, G.-X.; Hu, J.-F.; Zhu, Q.; Chen, Z. Staphylococcus aureus biofilm: Formulation, regulatory, and emerging natural products-derived therapeutics. Biofilm 2024, 7, 100175. [Google Scholar] [CrossRef] [PubMed]
- Roetzer, A.; Stich, N.; Model, N.; Schwameis, M.; Firbas, C.; Jilma, B.; Eibl, M.M. High Titer Persistent Neutralizing Antibodies Induced by TSST-1 Variant Vaccine Against Toxic Shock Cytokine Storm. Toxins 2020, 12, 640. [Google Scholar] [CrossRef] [PubMed]
- Schoergenhofer, C.; Gelbenegger, G.; Hasanacevic, D.; Schöner, L.; Steiner, M.M.; Firbas, C.; Buchtele, N.; Derhaschnig, U.; Tanzmann, A.; Model, N.; et al. A randomized, double-blind study on the safety and immunogenicity of rTSST-1 variant vaccine: Phase 2 results. eClinicalMedicine 2024, 67, 102404. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Zhang, Y.; Lee, J.H.; Escajadillo, T.; Gong, H.; Fang, R.H.; Gao, W.; Nizet, V.; Zhang, L. Broad-Spectrum Neutralization of Pore-Forming Toxins with Human Erythrocyte Membrane-Coated Nanosponges. Adv. Healthc. Mater. 2018, 7, 1701366. [Google Scholar] [CrossRef]
- Rao, L.; Tian, R.; Chen, X. Cell-Membrane-Mimicking Nanodecoys against Infectious Diseases. ACS Nano 2020, 14, 2569–2574. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Fang, R.H.; Copp, J.; Luk, B.T.; Zhang, L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013, 8, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Liu, C.; Ruan, S.; He, Y.; Li, X.; Zhu, Y.; Wang, H.; Huang, H.; Pang, Z. Broad-spectrum and powerful neutralization of bacterial toxins by erythroliposomes with the help of macrophage uptake and degradation. Acta Pharm. Sin. B 2022, 12, 4235–4248. [Google Scholar] [CrossRef]
- Wolfmeier, H.; Wardell, S.J.T.; Liu, L.T.; Falsafi, R.; Draeger, A.; Babiychuk, E.B.; Pletzer, D.; Hancock, R.E.W. Targeting the Pseudomonas aeruginosa Virulence Factor Phospholipase C With Engineered Liposomes. Front. Microbiol. 2022, 13, 867449. [Google Scholar] [CrossRef]
- Bhatia, E.; Sharma, S.; Jadhav, K.; Banerjee, R. Combinatorial liposomes of berberine and curcumin inhibit biofilm formation and intracellular methicillin resistant Staphylococcus aureus infections and associated inflammation. J. Mater. Chem. B 2021, 9, 864–875. [Google Scholar] [CrossRef]
- Jabir, M.S.; Rashid, T.M.; Nayef, U.M.; Albukhaty, S.; AlMalki, F.A.; Albaqami, J.; AlYamani, A.A.; Taqi, Z.J.; Sulaiman, G.M. Inhibition of Staphylococcus aureus α-Hemolysin Production Using Nanocurcumin Capped Au@ZnO Nanocomposite. Bioinorg. Chem. Appl. 2022, 2022, 2663812. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Qi, Y.; Guo, S.; Hao, K.; Zhao, M.; Guo, N. Effect of AgWPA nanoparticles on the inhibition of Staphylococcus aureus growth in biofilms. Food Control 2019, 100, 240–246. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef]
- Neubauer, K.; Zieger, B. Endothelial cells and coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int J Hematol Oncol Stem Cell Res. 2017, 11, 319–327. [Google Scholar] [PubMed]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef]
- Mackman, N. New insights into the mechanisms of venous thrombosis. J. Clin. Investig. 2012, 122, 2331–2336. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Parastatidou, S.; Tsantes, E.A.; Bonova, E.; Tsante, K.A.; Mantzios, P.G.; Vaiopoulos, A.G.; Tsalas, S.; Konstantinidi, A.; Houhoula, D.; et al. Sepsis-Induced Coagulopathy: An Update on Pathophysiology, Biomarkers, and Current Guidelines. Life 2023, 13, 350. [Google Scholar] [CrossRef]
- Ushio, N.; Wada, T.; Ono, Y.; Yamakawa, K. Sepsis-induced disseminated intravascular coagulation: An international estrangement of disease concept. Acute Med. Surg. 2023, 10, e843. [Google Scholar] [CrossRef]
- Cieza, M.Y.R.; Bonsaglia, E.C.R.; Rall, V.L.M.; Santos, M.V.d.; Silva, N.C.C. Staphylococcal Enterotoxins: Description and Importance in Food. Pathogens 2024, 13, 676. [Google Scholar] [CrossRef]
- Ortega, E.; Abriouel, H.; Lucas, R.; Gálvez, A. Multiple Roles of Staphylococcus aureus Enterotoxins: Pathogenicity, Superantigenic Activity, and Correlation to Antibiotic Resistance. Toxins 2010, 2, 2117–2131. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, L.; Mo, H.; Kong, T. Increased T Cell Chemotaxis Response to Staphylococcus Enterotoxin B Mediated Human Endothelial Cell Damage In Vitro. Scand. J. Immunol. 2012, 75, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T.; Stiles, B.G. The staphylococcal enterotoxin (SE) family. Virulence 2013, 4, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Pöhlmann-Dietze, P.; Ulrich, M.; Kiser, K.B.; Döring, G.; Lee, J.C.; Fournier, J.-M.; Botzenhart, K.; Wolz, C. Adherence of Staphylococcus aureus to Endothelial Cells: Influence of Capsular Polysaccharide, Global Regulator agr, and Bacterial Growth Phase. Infect. Immun. 2000, 68, 4865–4871. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.M.; Crosby, H.A.; Valotteau, C.; Hippensteel, J.A.; Nayak, M.K.; Chauhan, A.K.; Schmidt, E.P.; Dufrêne, Y.F.; Horswill, A.R. Staphylococcus aureus adhesion in endovascular infections is controlled by the ArlRS–MgrA signaling cascade. PLoS Pathog. 2019, 15, e1007800. [Google Scholar] [CrossRef]
- Kramko, N.; Sinitski, D.; Seebach, J.; Löffler, B.; Dieterich, P.; Heilmann, C.; Peters, G.; Schnittler, H.-J. Early Staphylococcus aureus-induced changes in endothelial barrier function are strain-specific and unrelated to bacterial translocation. Int. J. Med. Microbiol. 2013, 303, 635–644. [Google Scholar] [CrossRef]
- Alfeo, M.J.; Pagotto, A.; Barbieri, G.; Foster, T.J.; Vanhoorelbeke, K.; De Filippis, V.; Speziale, P.; Pietrocola, G. Staphylococcus aureus iron-regulated surface determinant B (IsdB) protein interacts with von Willebrand factor and promotes adherence to endothelial cells. Sci. Rep. 2021, 11, 22799. [Google Scholar] [CrossRef]
- Powers, M.E.; Kim, H.K.; Wang, Y.; Bubeck Wardenburg, J. ADAM10 Mediates Vascular Injury Induced by Staphylococcus aureus α-Hemolysin. J. Infect. Dis. 2012, 206, 352–356. [Google Scholar] [CrossRef]
- Tran, P.M.; Tang, S.S.; Salgado-Pabón, W. Staphylococcus aureus β-Toxin Exerts Anti-angiogenic Effects by Inhibiting Re-endothelialization and Neovessel Formation. Front. Microbiol. 2022, 13, 840236. [Google Scholar] [CrossRef]
- Lubkin, A.; Lee, W.L.; Alonzo, F.; Wang, C.; Aligo, J.; Keller, M.; Girgis, N.M.; Reyes-Robles, T.; Chan, R.; O’Malley, A.; et al. Staphylococcus aureus Leukocidins Target Endothelial DARC to Cause Lethality in Mice. Cell Host Microbe 2019, 25, 463–470.e9. [Google Scholar] [CrossRef]
- Li, L.; Pian, Y.; Chen, S.; Hao, H.; Zheng, Y.; Zhu, L.; Xu, B.; Liu, K.; Li, M.; Jiang, H.; et al. Phenol-soluble modulin α4 mediates Staphylococcus aureus-associated vascular leakage by stimulating heparin-binding protein release from neutrophils. Sci. Rep. 2016, 6, 29373. [Google Scholar] [CrossRef]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7, 10.1128. [Google Scholar] [CrossRef] [PubMed]
- McAdow, M.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus Secretes Coagulase and von Willebrand Factor Binding Protein to Modify the Coagulation Cascade and Establish Host Infections. J. Innate Immun. 2012, 4, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Niemann, S.; Bertling, A.; Brodde, M.F.; Fender, A.C.; Van de Vyver, H.; Hussain, M.; Holzinger, D.; Reinhardt, D.; Peters, G.; Heilmann, C.; et al. Panton-Valentine Leukocidin associated with S. aureus osteomyelitis activates platelets via neutrophil secretion products. Sci. Rep. 2018, 8, 2185. [Google Scholar] [CrossRef] [PubMed]
- Cicalău, G.; Babes, P.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.; Ganea, M.; Scrobotă, I. Anti-Inflammatory and Antioxidant Properties of Carvacrol and Magnolol, in Periodontal Disease and Diabetes Mellitus. Molecules 2021, 26, 6899. [Google Scholar] [CrossRef]
- El Azab, E.F.; Saleh, A.M.; Yousif, S.O.; Mazhari, B.B.Z.; Abu Alrub, H.; Elfaki, E.M.; Hamza, A.; Abdulmalek, S. New insights into geraniol’s antihemolytic, anti-inflammatory, antioxidant, and anticoagulant potentials using a combined biological and in silico screening strategy. Inflammopharmacology 2022, 30, 1811–1833. [Google Scholar] [CrossRef]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2016, 38, 785–791. [Google Scholar] [CrossRef]
- Nieswandt, B.; Aktas, B.; Moers, A.; Sachs, U.J.H. Platelets in atherothrombosis: Lessons from mouse models. J. Thromb. Haemost. 2005, 3, 1725–1736. [Google Scholar] [CrossRef]
- Marumo, M.; Ekawa, K.; Wakabayashi, I. Resveratrol inhibits Ca2+ signals and aggregation of platelets. Environ. Health Prev. Med. 2020, 25, 70. [Google Scholar] [CrossRef]
- Wiciński, M.; Socha, M.; Walczak, M.; Wódkiewicz, E.; Malinowski, B.; Rewerski, S.; Górski, K.; Pawlak-Osińska, K. Beneficial Effects of Resveratrol Administration—Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients 2018, 10, 1813. [Google Scholar] [CrossRef]
- Huang, M.; Deng, M.; Nie, W.; Zou, D.; Wu, H.; Xu, D. Naringenin Inhibits Platelet Activation and Arterial Thrombosis Through Inhibition of Phosphoinositide 3-Kinase and Cyclic Nucleotide Signaling. Front. Pharmacol. 2021, 12, 722257. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Núñez, L.; Lozano, M.L.; Palomo, M.; Martínez, C.; Vicente, V.; Castillo, J.; Benavente-García, O.; Diaz-Ricart, M.; Escolar, G.; Rivera, J. Apigenin Inhibits Platelet Adhesion and Thrombus Formation and Synergizes with Aspirin in the Suppression of the Arachidonic Acid Pathway. J. Agric. Food Chem. 2008, 56, 2970–2976. [Google Scholar] [CrossRef]
- Janardhanan, S. Antimicrobial Effects of Garcinia Mangostana on Cariogenic Microorganisms. J. Clin. Diagn Res. 2017, 11, ZC19–ZC22. [Google Scholar] [CrossRef]
- Espirito Santo, B.L.S.d.; Santana, L.F.; Kato Junior, W.H.; de Araújo, F.d.O.; Bogo, D.; Freitas, K.d.C.; Guimarães, R.d.C.A.; Hiane, P.A.; Pott, A.; Filiú, W.F.d.O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef]
- Jantan, I.; Jumuddin, F.A.; Saputri, F.C.; Rahman, K. Inhibitory effects of the extracts of Garcinia species on human low-density lipoprotein peroxidation and platelet aggregation in relation to their total phenolic contents. J. Med. Plants Res. 2011, 5, 2699–2709. [Google Scholar]
- González Correa, J.A.; López-Villodres, J.A.; Asensi, R.; Espartero, J.L.; Rodríguez-Gutiérez, G.; De La Cruz, J.P. Virgin olive oil polyphenol hydroxytyrosol acetate inhibits in vitro platelet aggregation in human whole blood: Comparison with hydroxytyrosol and acetylsalicylic acid. Br. J. Nutr. 2008, 101, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Vijakumaran, U.; Yazid, M.D.; Hj Idrus, R.B.; Abdul Rahman, M.R.; Sulaiman, N. Molecular Action of Hydroxytyrosol in Attenuation of Intimal Hyperplasia: A Scoping Review. Front. Pharmacol. 2021, 12, 663266. [Google Scholar] [CrossRef]
- Choo, M.-K.; Park, E.-K.; Yoon, H.-K.; Kim, D.-H. Antithrombotic and Antiallergic Activities of Daidzein, a Metabolite of Puerarin and Daidzin Produced by Human Intestinal Microflora. Biol. Pharm. Bull. 2002, 25, 1328–1332. [Google Scholar] [CrossRef]
- Ku, S.-K.; Bae, J.-S. Antithrombotic activities of wogonin and wogonoside via inhibiting platelet aggregation. Fitoterapia 2014, 98, 27–35. [Google Scholar] [CrossRef]
- Basu, A.; Kumar, G.S. Interaction of the putative anticancer alkaloid chelerythrine with nucleic acids: Biophysical perspectives. Biophys. Rev. 2020, 12, 1369–1386. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, T.; Wang, X.-A.; Tong, X.; Ding, J. Histone deacetylases and atherosclerosis. Atherosclerosis 2015, 240, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Bergh, N.; Lu, E.; Ulfhammer, E.; Magnusson, M.; Wåhlander, K.; Karlsson, L.; Jern, S. Histone deacetylase inhibitors stimulate tissue-type plasminogen activator production in vascular endothelial cells. J. Thromb. Thrombolysis 2013, 35, 185–192. [Google Scholar] [CrossRef] [PubMed]
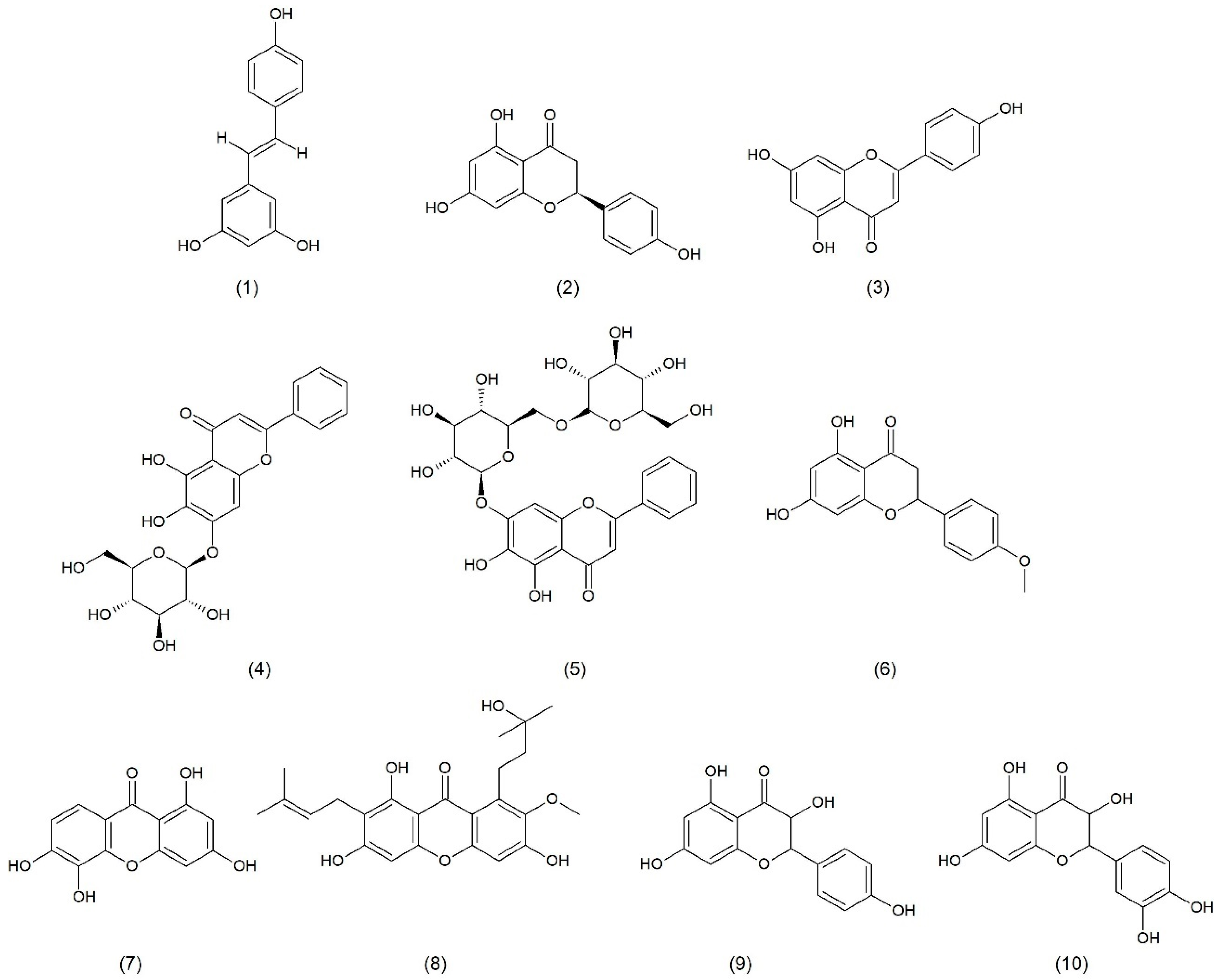

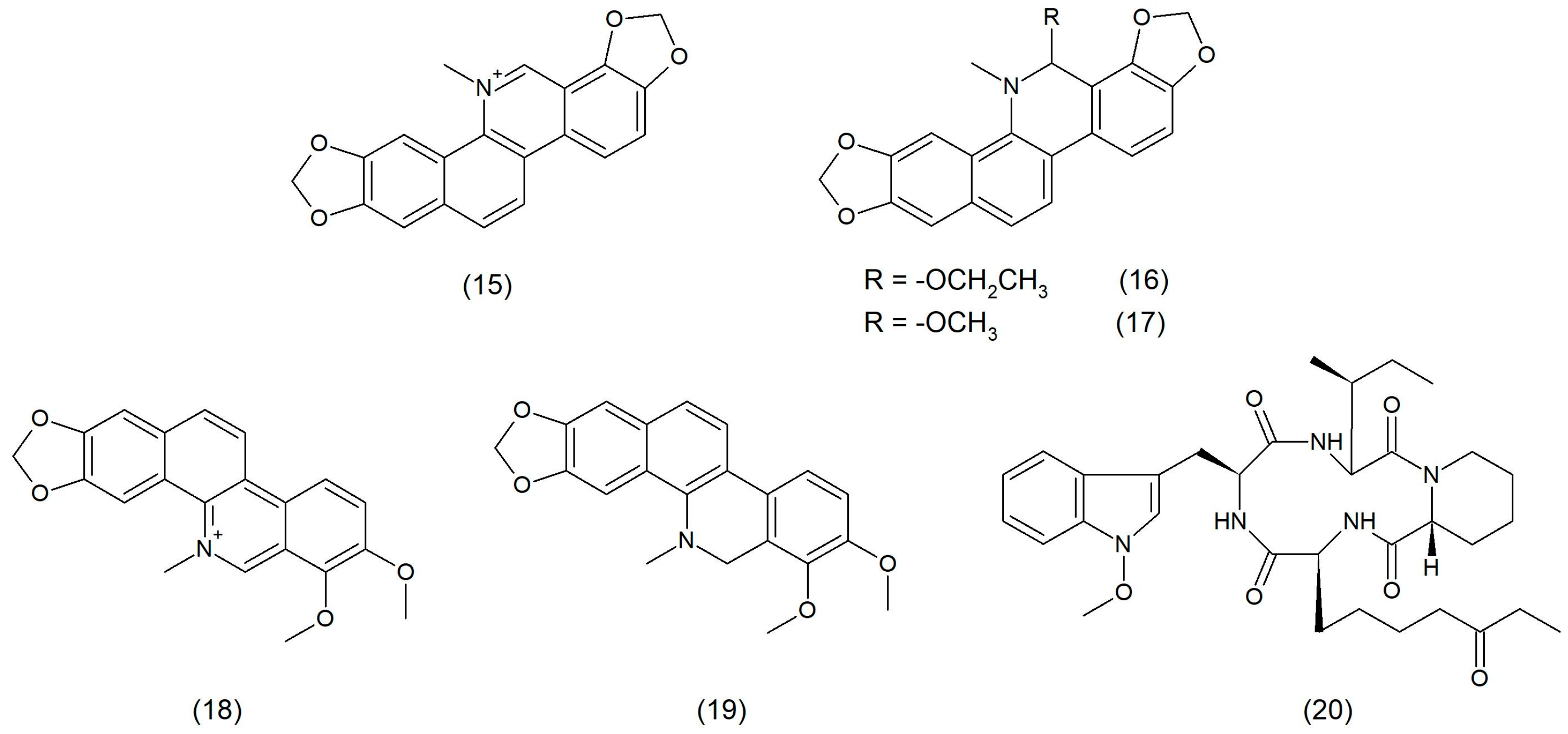

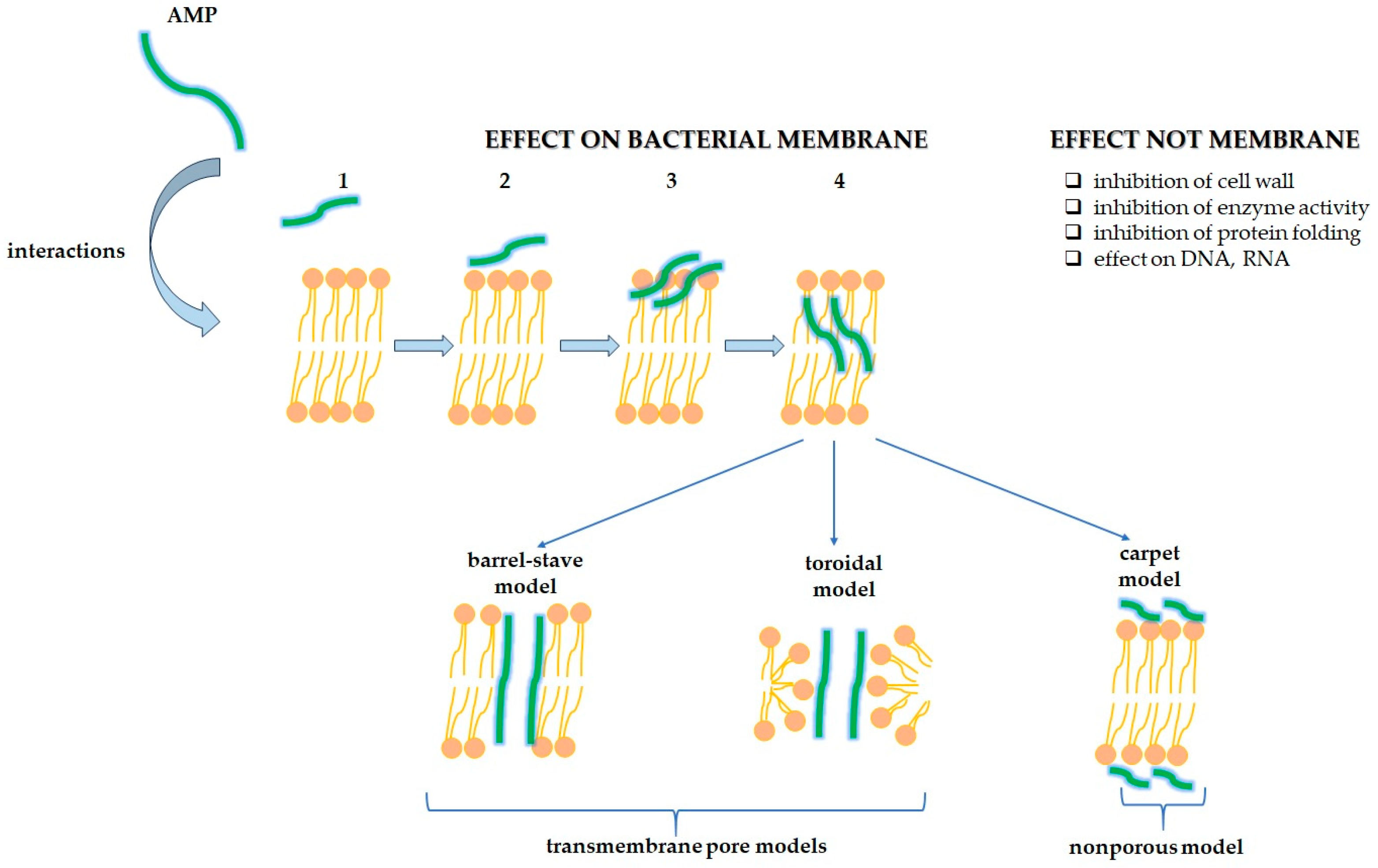
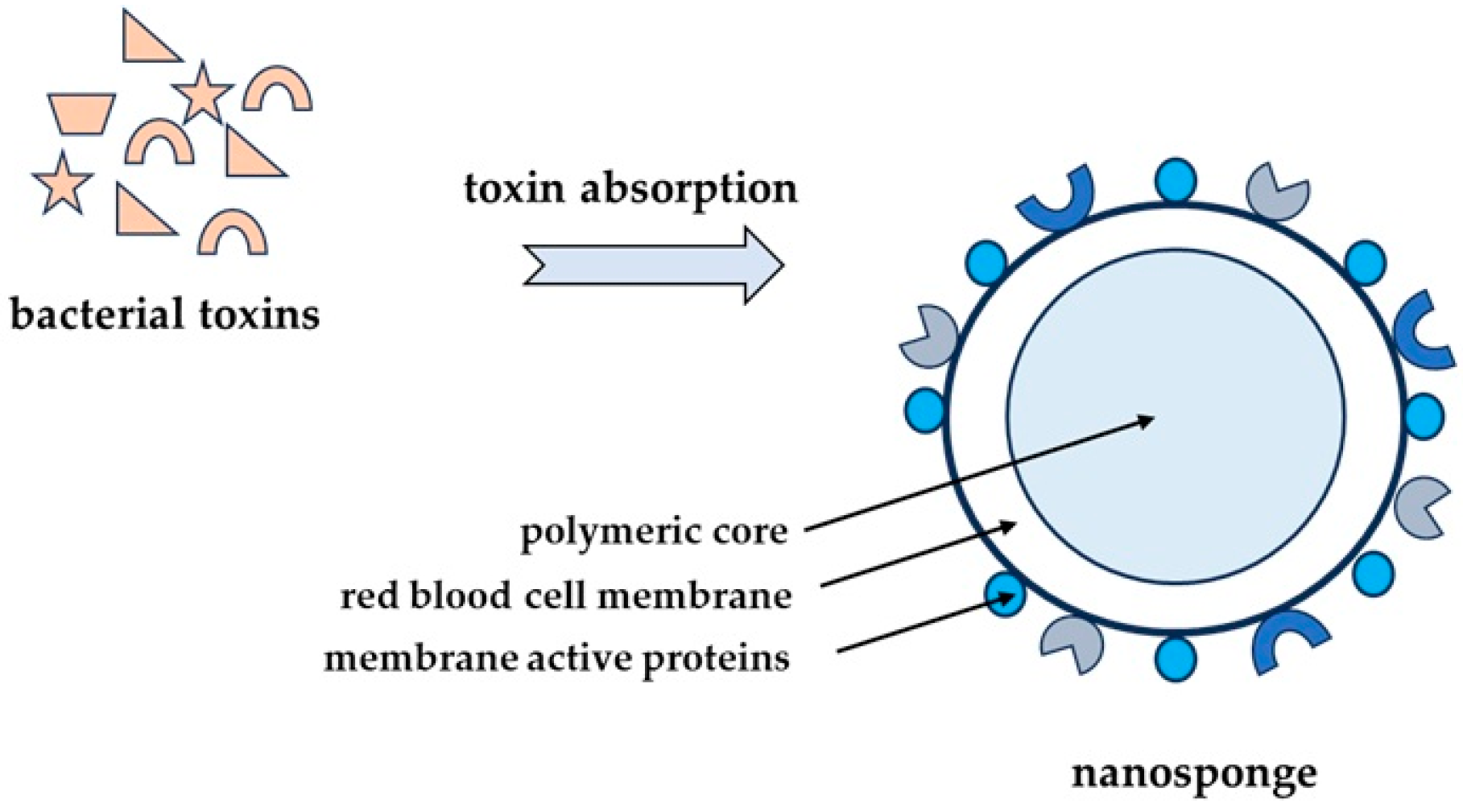

| Virulence Factors | Role of Virulence Factors on Host Cell’s | References |
|---|---|---|
| Enterotoxins |
| [90,91,92,93] |
| Adhesins |
| [94,95,96,97] |
| Exotoxins |
| [98,99,100,101] |
| Exoenzymes |
| [102,103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichota, A.; Gwozdzinski, K.; Sienkiewicz, M. Potential Compounds as Inhibitors of Staphylococcal Virulence Factors Involved in the Development of Thrombosis. Toxins 2025, 17, 340. https://doi.org/10.3390/toxins17070340
Lichota A, Gwozdzinski K, Sienkiewicz M. Potential Compounds as Inhibitors of Staphylococcal Virulence Factors Involved in the Development of Thrombosis. Toxins. 2025; 17(7):340. https://doi.org/10.3390/toxins17070340
Chicago/Turabian StyleLichota, Anna, Krzysztof Gwozdzinski, and Monika Sienkiewicz. 2025. "Potential Compounds as Inhibitors of Staphylococcal Virulence Factors Involved in the Development of Thrombosis" Toxins 17, no. 7: 340. https://doi.org/10.3390/toxins17070340
APA StyleLichota, A., Gwozdzinski, K., & Sienkiewicz, M. (2025). Potential Compounds as Inhibitors of Staphylococcal Virulence Factors Involved in the Development of Thrombosis. Toxins, 17(7), 340. https://doi.org/10.3390/toxins17070340






