Abstract
Spasticity is a persistent and debilitating consequence of stroke and effective rehabilitation is a healthcare priority. Botulinum neurotoxin A (BoNT-A) with supportive therapy has increasingly been embedded within clinical practice for treatment of post-stroke spasticity. But the evidence for this approach has hitherto been limited to the findings of a limited number of small trials. The InTENSE trial was undertaken specifically to provide high-quality clinical trial evidence focusing on the effect of BoNT-A and adjunctive therapy on upper limb spasticity. While the clinical trial did not detect a significant impact upon clinical outcomes, there remains a need to evaluate any impact on the broader use of healthcare resources and overall cost-effectiveness. A detailed cost–utility analysis of the InTENSE trial was undertaken. The costs over the 12-month follow-up period were compared with quality-adjusted life years (QALY) gained using utilities generated from the EQ-5D three level (EQ-5D-3L) instrument. There were no significant differences in QALY gained between the intervention and control groups identified, or in the majority of health and community care costs. The Incremental Cost-Effectiveness Ratio per QALY gained was estimated at AU $63,947.11 (Australian dollars), which is well above accepted thresholds for cost-effectiveness in Australia. The study was unable to identify evidence for the cost-effectiveness of treatment approaches combining BoNT-A with adjunctive therapy.
Keywords:
stroke; rehabilitation; botulinum neurotoxin A; economic evaluation; cost–utility analysis; cost-effectiveness analysis; costs Key Contribution:
This study shows that the use of BoNT-A with adjunctive therapy in people with chronic stroke and limited upper limb function is not cost-effective.
1. Introduction
Stroke is among the top 10 leading causes of disability-adjusted life years lost in countries across the world [1]. One significant impact of a stroke can be spasticity, which has large implications for independence in activities of daily living for the person, and on caregiver burden for informal carers [2]. People living with spasticity experience significantly impaired quality of life compared to people post-stroke but not experiencing spasticity, and it commonly becomes a chronic condition requiring long-term management. Spasticity refers to a state of increased muscle tone with exaggerated reflexes and affects over 40% people following stroke [3]. It may impact only one part of a limb (known as focal) or can impact a range of areas of a limb or more than one limb itself. Treatment must, therefore, be individualised to target the combination of symptoms and their impact on an individual. While the effects of other deficits post-stroke, such as muscle weakness and loss of dexterity, can be significant, it is often muscle spasticity that has the most severe impact on an individual’s daily life, their independence, and engagement with the community. Additionally, spasticity can also be present with other complex post-stroke impairments, such as neglect, fatigue, and sensory or cognitive impairments. Therefore, long-term care can be complex and require input from multiple specialist health and allied health disciplines to achieve desired outcomes.
Effective treatments to maximise recovery of function following stroke, including for those experiencing spasticity, are crucial in practice but have been challenging to identify and translate into practice [4,5,6]. In the last decade, botulinum neurotoxin A (BoNT-A) with adjunctive therapy (i.e., treatment from a multidisciplinary therapy team including physiotherapy and occupational therapy primarily, with support from other health professionals such as orthotists and rehabilitation nurses as appropriate) has been embedded within national and international guidelines as treatment for post-stroke spasticity across the world [7,8]. Potentially, if the adjunctive therapy is successful in supporting the practice of beneficial movement patterns, strengthening abnormal muscle weakness, or reducing muscle overactivity in the short window post-injection during which the BoNT-A reduces muscle overactivity, the benefit from the injection could be maximised, for a relatively small additional cost. However, the evidence supporting this approach is limited, and recommendations have been based on expert opinion and extrapolated from a small number of trials rather than more high-quality clinical trials [7,9]. The InTENSE trial was conducted to evaluate the impact of BoNT-A treatment with specifically developed evidence-based adjunctive therapy compared with BoNT-A alone. This study focused on treatment of upper limb spasticity to ensure consistency in the outcome measures used across the sample. The clinical trial analysis found that the intervention did not have an effect on the primary or secondary clinical outcomes (in terms of the Goal Attainment Scale and the upper limb activity measured through the Box and Block Test) at 3 months [10,11], and a small gain in strength for the intervention group at 3 months was not maintained at the long-term 12-month follow-up [10]. However there remains a need to understand any impact on the use of healthcare resources and costs associated with the intervention, to inform a broader understanding of the cost-effectiveness of the InTENSE intervention.
Previous studies of the cost-effectiveness of botulinum neurotoxin A with or without adjunctive therapy have been mixed. Some previous cost-effectiveness studies indicated that use of Botox compared to no treatment is cost-effective, for example, Rychilk and Turcu [12,13]. However, it is relatively uncommon in most countries now that no treatment is offered to people with spasticity following stroke. Other studies have compared the use of botulinum toxin plus adjunctive therapy (such as movement training or physiotherapy) compared with adjunctive therapy alone. Studies have shown that the use of botulinum toxin and adjunctive therapy in the UK and Spain, compared with adjunctive therapy alone, was cost-effective [14,15,16]. In these studies, estimations were calculated using Markov or other models for post-stroke spasticity in general (i.e., upper or lower limbs or other sites). By comparison the BoTULS trial included a cost-effectiveness analysis conducted alongside their randomised clinical trial of botulinum toxin with an adjunctive therapy program compared with the toxin alone among those experiencing upper limb spasticity only [17]. The identified high costs in their treatment group and only small improvements in quality-adjusted life years (QALY) gained over a 3-month follow-up period from the health- and social care service perspective, and concluded the treatment was very unlikely to be cost-effective within the UK [17]. In an Australian-based study, Moore et al. [16] used a Markov model to estimate the cost-effectiveness of Abobulinumtoxin A and best supportive care compared with best supportive care alone for lower limb spasticity following stroke or traumatic brain injury. They identified an incremental cost-effectiveness ratio (ICER) of AU $35,721 per QALY gained, which was higher than previously estimated willingness-to-pay threshold of AU $28,000 per QALY gained in Australia [18]. A number of Markov models have been used to estimate the cost-effectiveness of newer forms of botulinum toxin A, such as incobotulinumtoxin A (Xeomin®, Merz Pharmaceuticals, Frankfurt am Main, Germany) or Abobotulinumtoxin A, which are similarly effective but currently cheaper and have less wastage than the alternative (Onabotulinumtoxin A or Botox®, Allergan Aesthetics, Dublin, Ireland), finding these to produce improved quality of life and/or reduced costs for the UK, Greek, or US health systems for treatment of spasticity and other conditions [15,16,17,19,20,21]. For Australia, Makino et al. [22] used a Markov model to estimate the cost-effectiveness of extending beyond the current limit of four treatment cycles of botulinum neurotoxin to unlimited treatment for those with post-stroke spasticity of an upper limb exhibiting continued adequate response to previous cycles [22]. They defined an adequate response as a greater than 1-point improvement in the Modified Ashworth Scale score (a common measure of spasticity). They estimated an ICER of AU $59,911 per QALY gained for the extended treatment program compared to the current treatment over a five-year period. Their model assumed a relatively high (although based on a previous clinical trial [23]) initial rate of adequate response to the Botox of 68.5%, followed by a 70% and 87.2% response rate in subsequent treatments for the previous responders.
There has been no evaluation of the cost-effectiveness of botulinum toxin A and adjunctive therapy compared with botulinum toxin A alone, particularly among those living with spasticity of an upper limb. Secondly, some Markov models produced used assumed effects on quality-of-life-improving utilities by 0.1 to 0.2 points and response rates of 60 to 70% among populations [12,14,16,20]. How realistic these assumptions are in the context of upper limb spasticity post-stroke in practice is unclear, where the extent of the disability experienced and the rate of recovery have hitherto been poor.
This study reports the findings of the cost–utility analysis and cost-effectiveness analysis of BoNT-A combined with an evidence-based adjunctive therapy treatment compared with Botox alone for people living with upper limb spasticity, alongside the InTENSE blinded-randomised controlled trial.
2. Materials and Methods
The clinical protocol for the trial, and the protocol for the economic evaluation have both previously been published in detail [24,25]. In brief, the economic evaluation was conducted alongside a Phase III clinical trial that aimed to determine the effectiveness of undertaking evidence-based movement training following a botulinum toxin A (BoNT-A) injection in adults with neurological spasticity. All participants received a program of injections with BoNT-A according to standard Australian practice recommendations, which, among other approaches, recommends guided injections to confirm the injection into the intended muscles [8]. The BoNT-A was supplied through the Pharmaceutical Benefits Scheme. The InTENSE program was administered to the intervention group by trained physical or occupational therapists immediately following their initial injection [25]. It included serial casting of the wrist, placing it in maximum extension for two weeks, followed by 10 weeks of movement training to reduce weakness and improve active movement (via a combination of electrical stimulation and progressive resistance exercises) and an intensive program of home exercises [26]. Participants received the intervention via a mix of in-clinic sessions, home visits, and phone calls for support.
2.1. Measurement, Valuation, and Analysis of Costs
A summary of the resources measured as part of the study, the cost values applied, and their sources are outlined in Table 1.

Table 1.
Unit costs and their sources.
All costs were updated to a standard reference year for analysis. Discounting was not undertaken as the length of the study was 1 year. Total costs of care for the participants in the intervention and control groups over the 12-month follow-up period were calculated. These were aggregated into intervention costs, total healthcare costs (including costs of hospitalisation, emergency department presentations, doctors’ attendances, and visits to allied health professionals). Two participants passed away within the 12-month follow-up period; both were allocated to the intervention group. However, no accounting for censoring was undertaken as the proportion of total observations missing due to censoring was less than 1% [34].
2.2. Measurement and Valuation of Benefit
The primary economic evaluation framework applied was the cost–utility analysis (CUA). This approach was chosen for a number of reasons. Firstly, the trial provided two clear alternatives for comparison (a fundamental requirement for undertaking a CUA) [35]. Additionally, the potential benefits of the intervention were expected to be across multiple clinical domains (e.g., spasticity, pain, function, physical and mental wellbeing) rather than a single clearly defined clinical domain. Therefore, measuring the benefits of the intervention using the concept of quality-adjusted life years (QALY), which incorporates benefits across a number of areas of a person’s life simultaneously, was considered most appropriate for this study, leading to the selection of the CUA framework. The benefit of the intervention was primarily measured via quality-adjusted life years (QALY) gained by participants over the 12-month follow-up period. Health-related quality of life (HRQoL) was measured using the EQ-5D-3L instrument [36]. The EQ-5D-3L measures HRQoL across 5 dimensions using three levels of possible response for each dimension. Participants were asked to rate their HRQoL during the last week using the EQ-5D-3L, at baseline, 3-month follow-up, and 12-month follow-up. These responses were then used to calculate the individual utility scores for participants using the preference-based scoring algorithm developed for Australia [37]. The utility scores created are on a scale where 0 represents a health state equivalent to death, 1 represents full health, and negative values are possible indicating a health state considered worse than death. These individual utility scores were then combined with the time that participants spent in each of these health states and used to calculate the overall QALY gain for participants using the area-under-the-curve method [35].
A secondary analysis was undertaken using the Goal Attainment Scale (GAS) scores over the 12-month follow-up period as the measure of benefit in a cost-effectiveness analysis (CEA). The GAS is a validated measure of achievement of rehabilitation specific goals identified by the participants themselves, usually related to activity and participation in meaningful tasks, and was chosen as it is a recommended outcome measure in Australian Clinical Guidelines [8]. Participants then score themselves for current and expected levels of performance (ranging from −2 to +2), with t-scores then calculated as described by Kiresuk et al. [38].
2.3. Cost–Utility Analysis
The economic evaluation was undertaken on an intention to treat basis. The difference in healthcare costs between the intervention and control groups was divided by the difference in QALY gain to give an incremental cost-effectiveness ratio (ICER) of cost per QALY gained (i.e., ICER = Ca − Cb/Ea − Eb, where Ca is the cost of the intervention, Cb is the cost of the control, Ea is the effectiveness of the intervention, and Eb is the effectiveness of the control). We also planned to undertake a subgroup analysis using those participants with some function of the upper extremity at baseline (i.e., those who could move 1 or more blocks on the Box and Block test at baseline). However, given only 31 participants met this criterion, we did not perform the subgroup analysis.
2.4. Statistical Analysis
A statistical analysis was undertaken using Stata version 17.0. Although cost and quality-of-life data were skewed (as is common and well described with this type of data), an approach reporting mean values was utilised, as has been recommended previously. Missing data on costs and selected outcomes were addressed using multiple imputation by chained equations (MICE) in Stata (version 17.0), implemented in wide format. MICE specifies an imputation model for each variable, iteratively predicting missing values using the available data until convergence is achieved [39]. The imputation model targeted missing values in monthly cost data (diary costs from month 5 to month 11) and two outcome variables (EQ-5D-3L scores at 3 months and 12 months). The number of complete cases available for analysis is presented by group allocation in Supplementary Information Table S1. In summary, 132 participants had complete data for EQ-5D-5L at the 12-month time point, 134 had complete Medicare Benefits Schedule (doctor’s appointments, laboratory tests, radiology, and allied health appointments) and Pharmaceutical Benefit Scheme (prescription pharmaceuticals) data (which comprises the majority of community-based healthcare in Australia [40]). However, completion of the monthly diary was much lower, declining from 99 to 57 of participants over time, necessitating the focus on this in the imputation model. We used predictive mean matching (PMM) with 12 nearest neighbours to account for the non-normal distribution of cost data. Thirty imputations were created using 300 iterations with a 10-iteration burn-in. The following demographics and clinical baseline characteristics variables were included as predictors: age, gender, centre of treatment, ambulation status, mental health status, baseline Tardieu scale at wrist, baseline Box and Block test score, days since stroke, and baseline EQ-5D score. The stability and convergence of the imputation model were assessed using trace plots, comparisons between observed and imputed distributions, and missing data diagnostics. All analyses were conducted using mi estimate, and final cost-effectiveness results were pooled across the 30 imputed datasets using Rubin’s rules [41] to account for both between-imputation and within-imputation variance components associated with the estimates.
Following imputation, mean incremental costs and mean incremental quality-adjusted life years (QALY) were calculated using Seemingly Unrelated Regression (SUR) models to account for the correlation between costs and outcomes [42]. Non-parametric bootstrap methods with 10,000 replications were also employed to generate the joint distribution of costs and outcomes, which were used to populate the cost-effectiveness plane and cost-effectiveness acceptability curve.
3. Results
As has been previously reported, 140 people with stroke were recruited to the study (69 randomised to the intervention and 71 to the control group) [11]. There were no significant differences between the intervention and control groups at baseline in any demographic or clinical characteristic tested for (see Table 2).

Table 2.
Baseline characteristics.
Table 3 provides the mean utility scores for the two groups including both the raw and the imputed values. At baseline, the raw utility scores derived from the responses to the EQ-5D-3L were similar (0.04 difference between the two in favour of the intervention group). At 12 months, while both groups improved in their utility scores, the difference remained consistent between the two groups (0.03) and not statistically significant. Table 4 gives the mean use of Australian Medical Benefits Schedule (MBS) and Australian Pharmaceutical Benefits Scheme (PBS) resources for the participants in the two groups over the 12-month trial period. There were no significant differences in the use of these resources over the trial period and the control group also used substantial resources. Table 5 gives the costs for the health- and social care services used by participants over the 12-month trial period. As expected, the intervention group incurred significantly higher costs as part of the intervention. But apart from this, there were no significant differences in costs of health service use between the two groups, excluding for use of podiatry, where there was an increased cost among the intervention group (difference AU $96.80, p = 0.047).

Table 3.
Mean outcomes per participant.

Table 4.
Mean MBS and PBS resource use per participant over 12 months (raw non-imputed values).

Table 5.
Mean costs (Australian dollars) per participant over 12 months (complete cases only, no imputations).
Table 6 gives the results of the base case costs and QALY gain over the 12-month trial period for both groups, and the ICER for the final imputed values. The intervention group incurred significantly higher total costs over the 12-month trial period compared to the control (difference AU $5111.04, p = 0.02). However, there was no significant difference between the two groups in QALY gain. This resulted in a ICER of AU $63,974 per QALY gained for the InTENSE program, a value well above the estimated WTP for a QALY in Australia of AU $28,000 [18]. A sensitivity analysis was undertaken using only cases with complete data (n = 53) with a similar finding (ICER of AU $65,792.80).

Table 6.
Primary and secondary analysis of Incremental Cost-Effectiveness Ratios over 12 months (all costs in Australian Dollars).
Table 6 also gives the results of the secondary analysis using the GAS as the measure of benefit across the 12-month follow-up period. Similar to the findings with QALY as a measure of benefit, there were not significant differences in GAS scores over the 12-month time period using either the imputed values or when considering only complete cases (sensitivity analysis). The estimated ICER for this analysis was AU $1,667.51 per point improvement in GAS between the two groups for the imputed values, with a similar finding (AU $1,563.76) when only complete cases were considered.
The cost-effectiveness plane presenting the non-parametric bootstrapped estimates of the ICER is presented for the estimates with the QALY gain as the measure of benefit (Figure 1a) and with the GAS as the measure of benefit (Figure 2) as a graphical representation of the uncertainty around the estimates of the ICER. It can be seen that, for both figures, there is a relatively large scatter of points in the top north-east quadrant of the plane (which indicates the intervention is more effective but also more costly than the control) across into the north-west quadrant of the plane (indicating that the intervention is less effective than the control and also more costly). The relatively large spread of data points indicates there is currently a large amount of uncertainty around the point estimates of the ICER (supported by wide 95% CI reported in Table 6). Additionally, a cost-effectiveness acceptability curve (CEAC) is presented representing the probability that the intervention would be considered cost-effective compared to the control for a range of potential values of cost-effectiveness thresholds for the primary analysis (i.e., ICER cost/QALY gained over 12 months). This figure indicates a relatively low likelihood of cost-effectiveness across the range of presented thresholds, reaching only just over 60% probability at a willingness-to-pay threshold of AU $70,000 per QALY gained, well beyond accepted thresholds in Australia. The figures displaying the cost-effectiveness plane and CEAC for the complete case data are presented in Supplementary Materials (Figures S1 and S2).
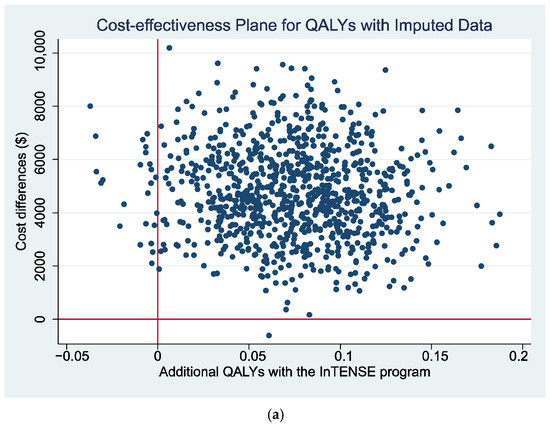
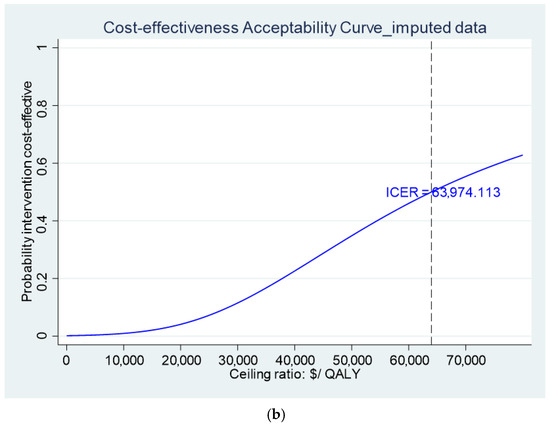
Figure 1.
(a) Cost-effectiveness plane and (b) CEAC using QALYs gain over 12 months as measure of benefit.
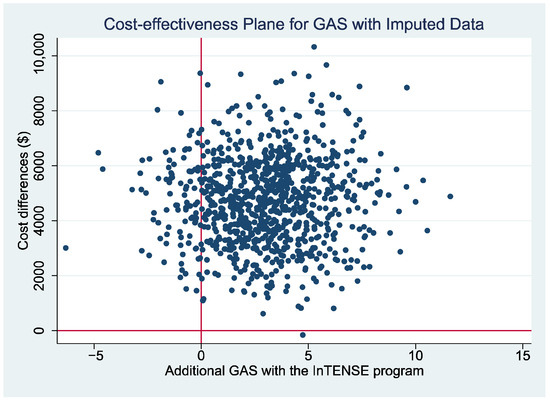
Figure 2.
Cost-effectiveness plane using difference in GAS score between the intervention and usual care groups at 12 months as measure of benefit.
4. Discussion
This study reports the findings of a detailed economic evaluation alongside the InTENSE clinical trial [9,10] applying a cost–utility and cost-effectiveness analysis framework. Significantly higher costs related to the provision of the intervention were identified, but no significant differences were observed in other health- and social care costs for the sample of people living with moderate or severe upper limb spasticity post-stroke. The limited improvement in HRQOL over the 12-month follow-up period across both groups, and the lack of significant difference between the groups, resulted in a relatively high estimated ICER (over AU $63,000), which is above the current estimated WTP fora QALY in Australia (AU $28,000) [18].
Previous studies evaluating the cost-effectiveness of BoNT-A are relatively few, and none currently provide a direct comparison to the intervention and control in this study. Within the Australian setting, Makino et al. [22] undertook a cost-effectiveness analysis of extending access to BoNT-A funded by the Pharmaceutical Benefits Scheme in Australia among people with upper limb spasticity beyond the currently approved four treatment cycles for those who have an adequate response to previous treatment cycles. They concluded that the extension in access may be cost-effective if provided to patients most likely to continue to see treatment gains. They estimated an ICER per QALY gained for the extended treatment among those who had demonstrated a successful response to previous treatment of AU $59,911. It should be noted that, similar to the current study, the ICER for this study was well above current estimates of willingness to pay for aQALY in Australia. This study also differed from the current study, which was based on data collected directly alongside a clinical trial. Instead, it was estimated using a Markov-state transition model developed using estimated treatment effects from previously published studies and, in this case, predominantly from a single randomised controlled trial and extension study conducted in Germany [23]. Additionally, this model was based on around 70% of patients improving following treatment with BoNT-A. However, we did not see this level of improvement in either group in the InTENSE trial [10].
In comparison, while the current study identified a similar ICER per QALY gained, cost-effectiveness cannot be concluded given it is significantly above current published estimates of willingness to pay (WTP) for a QALY for Australia. The current study also did not show any impact on health- or community care costs between the two groups, which if it did occur, could have been considered a benefit to the Australian taxpayer, who bears the vast majority of the costs of providing this intervention. This study is, therefore, unable to recommend this intervention as value for money for the Australian health system currently for people in the chronic phase after stroke with limited or no function in the affected upper extremity. The use of BoNT-A only (the control group) is also costly and demonstrated no clinical efficacy [43].
This economic evaluation does have some limitations, which should be considered alongside the findings. While the study was able to include a wide variety of health and community care costs incurred for participants, it did not collect information on informal carer costs (for example, informal carer time providing assistance with daily tasks, loss of productivity from informal carers leaving the workforce, etc.). While informal carer costs have typically been given haphazard consideration by health economists in the past, in recent years, the significant economic impact of informal caring has been recognised. There is significant support provided by carers that the current social care system simply would be incapable of providing. In addition, there is increasing acknowledgement of inequity in the impact of informal caregiving. A large proportion of informal care is provided by women, who, if they left the workforce to provide this care, potentially experience a reduction in income, not only at the time of providing care, but via follow-on effects through reduced contribution to retirement funding (which in Australia at this time is substantially supported through superannuation contributions accrued over time in the workforce). Additionally, there is also the potential for an intervention to have an impact on the quality of life and wellbeing of informal carers. While traditionally, CUA has focused heavily on the impact on the individual participants, more recently, a model for incorporating ‘spillovers’ from the individual participant to their family networks has been proposed [44]. Future economic evaluations should consider informal caregiver costs and impact on quality of life and wellbeing to provide greater understanding of the potential impact of interventions on informal caregivers, to give a true societal perspective of cost-effectiveness. Additionally, we found relatively low completion rates of monthly participant cost diaries in our sample and this completion declined over time. Other studies have also experienced challenges in collecting self-report healthcare utilisation data, particularly over long periods of time [45,46]. However, general engagement with the trial follow-up measures in the current trial was excellent, with over 94% of participants completing follow-up assessments at 3 and 12 months, and very low levels of missing data for administrative linked data. This provides data on the majority of community healthcare, which in Australia is government-funded through the Medicare Benefits Schedule and Pharmaceutical Benefits Scheme. Future studies should continue to prioritize the use of administrative linked datasets for the collection of healthcare costs in Australia [40].
5. Conclusions
In conclusion, this study was unable to find evidence for the cost-effectiveness of BoNT-A combined with adjunctive therapy, compared with BoNT-A only, in a population of people living with upper limb spasticity and limited upper limb function post-stroke.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins17070341/s1, Figure S1: Cost-effectiveness plane and CEAC for QALYs with complete case data, Figure S2: Cost-effectiveness plane for GAS with the complete case data; Table S1: Complete cases available by variable, timeframe, and group allocation.
Author Contributions
Conceptualization, J.R., I.D.C. and N.A.L.; methodology, R.M., J.S., S.D., J.R. and N.A.L.; validation, R.M., J.S. and S.D.; formal analysis, R.M., J.S., S.D. and J.R.; investigation, J.R., I.D.C., M.C., L.A., C.E. and N.A.L.; data curation, R.M., J.S., S.D. and J.R.; writing—original draft preparation, R.M., I.D.C. and N.A.L.; writing—review and editing, J.S., S.D., J.R., M.C., L.A. and C.E.; visualization, R.M. and J.R.; supervision, J.R.; project administration, N.A.L.; funding acquisition, J.R., I.D.C., M.C., L.A., C.E. and N.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by a project grant from the National Health and Medical Research Council, Australia (#1079542). N.A.L. is supported by the National Heart Foundation of Australia (#106762).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Alfred Health Human Research Ethics Committee (protocol code 442/12 on 18 November 2014) for studies involving humans.
Informed Consent Statement
Written informed consent was obtained from all study participants and/or their legal guardian(s) before data collection began.
Data Availability Statement
The data presented in this study are available on request from the corresponding author, as approval for raw data release has not been approved by the Ethics Committee.
Acknowledgments
We acknowledge the InTENSE Trial Group.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BoNT-A | Botulinum neurotoxin A |
| CI | Confidence interval |
| DOAJ | Directory of Open Access Journals |
| EQ-5D | Euroqol five dimension |
| EQ-5D-3L | Euroqol five dimension three level |
| GAS | Goal Attainment Scale |
| GP | General practitioner |
| HRQOL | Health-related quality of life |
| ICER | Incremental cost-effectiveness ratio |
| LD | Linear dichroism |
| MAR | Missing at random |
| MBS | Medicare Benefits Schedule |
| MICE | Multiple imputation by chained equations |
| MDPI | Multidisciplinary Digital Publishing Institute |
| OT | Occupational therapist |
| PBS | Pharmaceutical Benefits Scheme |
| PMM | Predictive mean matching |
| PT | Physiotherapist |
| QALYs | Quality-adjusted life years |
| SD | Standard deviation |
| SUR | Seemingly unrelated regression |
| UK | United Kingdom |
| US | United States of America |
| WTP | Willingness to pay |
References
- Global Burden of Disease 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Gillard, P.J.; Sucharew, H.; Kleindorfer, D.; Belagaje, S.; Varon, S.; Alwell, K.; Moomaw, C.J.; Woo, D.; Khatri, P.; Flaherty, M.L.; et al. The negative impact of spasticity on the health-related quality of life of stroke survivors: A longitudinal cohort study. Health Qual. Life Outcomes 2015, 13, 159. [Google Scholar] [CrossRef]
- Urban, P.P.; Wolf, T.; Uebele, M.; Marx, J.J.; Vogt, T.; Stoeter, P.; Bauermann, T.; Weibrich, C.; Vucurevic, G.D.; Schneider, A.; et al. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke 2010, 41, 2016–2020. [Google Scholar] [CrossRef]
- Corbetta, D.; Sirtori, V.; Castellini, G.; Moja, L.; Gatti, R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst. Rev. 2015, 2015, CD004433. [Google Scholar] [CrossRef]
- Coupar, F.; Pollock, A.; Legg, L.A.; Sackley, C.; van Vliet, P. Home-based therapy programmes for upper limb functional recovery following stroke. Cochrane Database Syst. Rev. 2012, 2012, CD006755. [Google Scholar] [CrossRef]
- Winter, J.; Hunter, S.; Sim, J.; Crome, P. Hands-on therapy interventions for upper limb motor dysfunction following stroke. Cochrane Database Syst. Rev. 2011, 2011, CD006609. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Stroke Rehabilitation in Adults; NICE guideline NG236; National Institute for Health and Care Excellence: London, UK, 2023. Available online: www.nice.org.uk/guidance/ng236 (accessed on 20 April 2025).
- Gupta, A.D.; Baguley, I.; Estell, J.; Geffon, S.; Goh, K.; Rawicki, B.; De Graaf, S.; Olver, J. Statement of the Rehabilitation Medicine Society of Australia and New Zealand for the therapeutic use of botulinum toxin A in spasticity management. Intern. Med. J. 2024, 54, 178–182. [Google Scholar] [CrossRef]
- Demetrios, M.; Khan, F.; Turner-Stokes, L.; Brand, C.; McSweeney, S. Multidisciplinary rehabilitation following botulinum toxin and other focal intramuscular treatment for post-stroke spasticity. Cochrane Database Syst. Rev. 2013, 2013, CD009689. [Google Scholar] [CrossRef]
- Lannin, N.A.; Ada, L.; English, C.; Ratcliffe, J.; Faux, S.; Palit, M.; Gonzales, S.; Olver, J.; Schneider, E.; Crotty, M.; et al. Long-term effect of additional rehabilitation following botulinum toxin-A on upper limb activity in chronic stroke: The InTENSE randomised trial. BMC Neurol. 2022, 22, 154. [Google Scholar] [CrossRef]
- Lannin, N.A.; Ada, L.; English, C.; Ratcliffe, J.; Faux, S.; Palit, M.; Gonzales, S.; Olver, J.; Cameron, I.; Crotty, M. Effect of Additional Rehabilitation After Botulinum Toxin-A on Upper Limb Activity in Chronic Stroke: The InTENSE Trial. Stroke 2020, 51, 556–562. [Google Scholar] [CrossRef]
- Turcu-Stiolica, A.; Subtirelu, M.S.; Bumbea, A.M. Cost-Utility Analysis of Incobotulinumtoxin-A Compared with Conventional Therapy in the Management of Post-Stroke Spasticity in Romania. Front. Pharmacol. 2019, 10, 1516. [Google Scholar] [CrossRef]
- Rychlik, R.; Kreimendahl, F.; Schnur, N.; Lambert-Baumann, J.; Dressler, D. Quality of life and costs of spasticity treatment in German stroke patients. Health Econ. Rev. 2016, 6, 1–9. [Google Scholar]
- Rodriguez, M.E.; Fernandez, M.; Del Llano, J.; Nuno-Solinis, R. Systematic review and cost-effectiveness analysis of the treatment of post-stroke spasticity with abobotulinumtoxinA compared to physiotherapy. Farm. Hosp. 2023, 47, 201–209. [Google Scholar] [CrossRef]
- Fheodoroff, K.; Danchenko, N.; Whalen, J.; Balcaitiene, J.; Magalhães, B.; Szulc, E.; Zaffalon, A.; Burchakova, M.; Nechiporenko, D.; Robbins, S. Modelling Long-Term Outcomes and Risk of Death for Patients with Post-Stroke Spasticity Receiving Abobotulinumtoxina Treatment and Rehabilitation Therapy. J. Rehabil. Med. 2022, 54, 2422. [Google Scholar] [CrossRef]
- Moore, P.; Danchenko, N.; Weidlich, D.; Tijerina, A.R. Cost-effectiveness of abobotulinumtoxinA plus best supportive care compared with best supportive care alone for early treatment of adult lower limb spasticity following an acute event. PLoS ONE 2024, 19, e0296340. [Google Scholar] [CrossRef]
- Shackley, P.; Shaw, L.; Price, C.; van Wijck, F.; Barnes, M.; Graham, L.; Ford, G.A.; Steen, N.; Rodgers, H. Cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type a: Results from the botulinum toxin for the upper limb after stroke (BoTULS) trial. Toxins 2012, 4, 1415–1426. [Google Scholar] [CrossRef]
- Edney, L.C.; Haji Ali Afzali, H.; Cheng, T.C.; Karnon, J. Estimating the Reference Incremental Cost-Effectiveness Ratio for the Australian Health System. Pharmacoeconomics 2018, 36, 239–252. [Google Scholar] [CrossRef]
- Kazerooni, R.; Howard, I.M.; Keener, A.M.; Bounthavong, M. Real-World Six-Year National Cost-Minimization Analysis of IncobotulinumtoxinA and OnabotulinumtoxinA in the VA/DoD Healthcare Systems. Clin. Outcomes Res. 2021, 13, 603–609. [Google Scholar] [CrossRef]
- Danchenko, N.; Johnston, K.M.; Whalen, J. The cost-effectiveness of abobotulinumtoxinA (Dysport) and onabotulinumtoxinA (Botox) for managing spasticity of the upper and lower limbs, and cervical dystonia. J. Med. Econ. 2022, 25, 919–929. [Google Scholar] [CrossRef]
- Nomikos, N.; Eleftheriou, C.; Athanasakis, K. A Cost-Effectiveness and Budget Impact Analysis of AbobotulinumtoxinA in Greece. Toxins 2023, 15, 561. [Google Scholar] [CrossRef]
- Makino, K.; Tilden, D.; Guarnieri, C.; Mudge, M.; Baguley, I.J. Cost Effectiveness of Long-Term Incobotulinumtoxin-A Treatment in the Management of Post-stroke Spasticity of the Upper Limb from the Australian Payer Perspective. Pharmacoecon. Open 2018, 3, 93–102. [Google Scholar] [CrossRef]
- Kanovský, P.; Slawek, J.; Denes, Z.; Platz, T.; Sassin, I.; Comes, G.; Grafe, S. Efficacy and safety of botulinum neurotoxin NT 201 in poststroke upper limb spasticity. Clin. Neuropharmacol. 2009, 32, 259–265. [Google Scholar] [CrossRef]
- Milte, R.; Ratcliffe, J.; Ada, L.; English, C.; Crotty, M.; Lannin, N.A. Protocol for the economic evaluation of the InTENSE program for rehabilitation of chronic upper limb spasticity. BMC Health Serv. Res. 2020, 20, 1–6. [Google Scholar] [CrossRef]
- Lannin, N.A.; Ada, L.; English, C.; Ratcliffe, J.; Crotty, M. Effect of adding upper limb rehabilitation to botulinum toxin-A on upper limb activity after stroke: Protocol for the InTENSE trial. Int. J. Stroke 2018, 13, 648–653. [Google Scholar] [CrossRef]
- Ada, L.; Dorsch, S.; Canning, C.G. Strengthening interventions increase strength and improve activity after stroke: A systematic review. Aust. J. Physiother. 2006, 52, 241–248. [Google Scholar] [CrossRef]
- Australian Government Department of Veterans’ Affairs. Dental and Allied Health Fee Schedules Canberra, Australia: Australian Government Department of Veterans’ Affairs. Available online: https://www.dva.gov.au/get-support/providers/fees-forms-claims-providers/fee-schedules/dental-and-allied-health-fee-schedules (accessed on 5 March 2025).
- Australian Government Department of Health. MBS Online Medicare Benefits Schedule; Commonwealth of Australia: Canberra, Australia, 2018. Available online: http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home (accessed on 20 March 2021).
- Department of Health and Aged Care. The Pharmaceutical Benefits Scheme; Australian Government: Canberra, Australia, 2025. Available online: https://www.pbs.gov.au/pbs/home (accessed on 20 January 2025).
- Independent Hospital Pricing Authority. National Hospital Cost Data Collection Report, Public Sector, Round 23 (Financial Year 2018–2019); Independent Hospital Pricing Authority: Sydney, Australia, 2021. [Google Scholar]
- Department of Health and Aged Care. Commonwealth Home Support Programme (CHSP)—Payment in Arrears and Unit Pricing Fact Sheet; Australian Government: Canberra, Australia, 2021. Available online: https://www.health.gov.au/resources/publications/commonwealth-home-support-programme-chsp-payment-in-arrears-and-unit-pricing-fact-sheet?language=en (accessed on 20 January 2021).
- Department of Health and Aged Care. Home Care Package Fees; Australian Government: Canberra, Australia, 2022. Available online: https://www.health.gov.au/our-work/hcp/fees (accessed on 13 March 2022).
- Department of Health and Aged Care. Schedule of Fees and Charges for Residential and Home Care; Australian Government: Canberra, Australia, 2022. Available online: https://www.health.gov.au/our-work/residential-aged-care/charging/fees (accessed on 13 March 2022).
- Glick, H.A.; Doshi, J.A.; Sonnad, S.S.; Polsky, D. Economic Evaluation in Clinical Trials, 2nd ed.; Univeristy Press: Oxford, UK, 2015. [Google Scholar]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes, 4th ed.; Univeristy Press: Oxford, UK, 2015. [Google Scholar]
- The EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Viney, R.; Norman, R.; King, M.T.; Cronin, P.; Street, D.J.; Knox, S.; Ratcliffe, J. Time trade-off derived EQ-5D weights for Australia. Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 2011, 14, 928–936. [Google Scholar] [CrossRef]
- Kiresuk, T.; Smith, A.; Cardillo, J. Goal Attainment Scaling: Applications, Theory, and Measurement; Taylor & Francis: New York, NY, USA, 2014. [Google Scholar]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Health Expenditure Australia 2018–2019; AIHW: Canberra, Australia, 2020. [Google Scholar]
- Little, R.; Rubin, D. Statistical Analysis with Missing Data; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Zellner, A.; Huang, D.S. Further properties of efficient estimators for seemingly unrelated regression equations. Int. Econ. Rev. 1962, 3, 300–313. [Google Scholar] [CrossRef]
- Cameron, I.D.; Ada, L.; Crotty, M.; Palit, M.; Huang, L.; Olver, J.; Faux, S.G.; Gonzales, S.; Anthonisz, B.; Bowman, M.; et al. The Lack of Effect of Botulinum Toxin-A on Upper Limb Activity in Chronic Stroke: A Short Report from the InTENSE Trial. Toxins 2024, 16, 510. [Google Scholar] [CrossRef]
- Al-Janabi, H.; van Exel, J.; Brouwer, W.; Coast, J. A Framework for Including Family Health Spillovers in Economic Evaluation. Med. Decis. Making 2016, 36, 176–186. [Google Scholar] [CrossRef]
- Unangst, J.; Lewis, T.; Laflamme, E.; Prachand, N.; Weaver, K. Transitioning the Healthy Chicago Survey from a Telephone Mode to Self-administered by Mail Mode. J. Public Health Manag. Pract. 2022, 28, 309–316. [Google Scholar] [CrossRef]
- Sinclair, M.; O’Toole, J.; Malawaraarachchi, M.; Leder, K. Comparison of response rates and cost-effectiveness for a community-based survey: Postal, internet and telephone modes with generic or personalised recruitment approaches. BMC Med. Res. Methodol. 2012, 12, 132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).