Snake Venom Metalloproteinases from Puff Adder and Saw-Scaled Viper Venoms Cause Cytotoxic Effects in Human Keratinocytes
Abstract
1. Introduction
2. Results
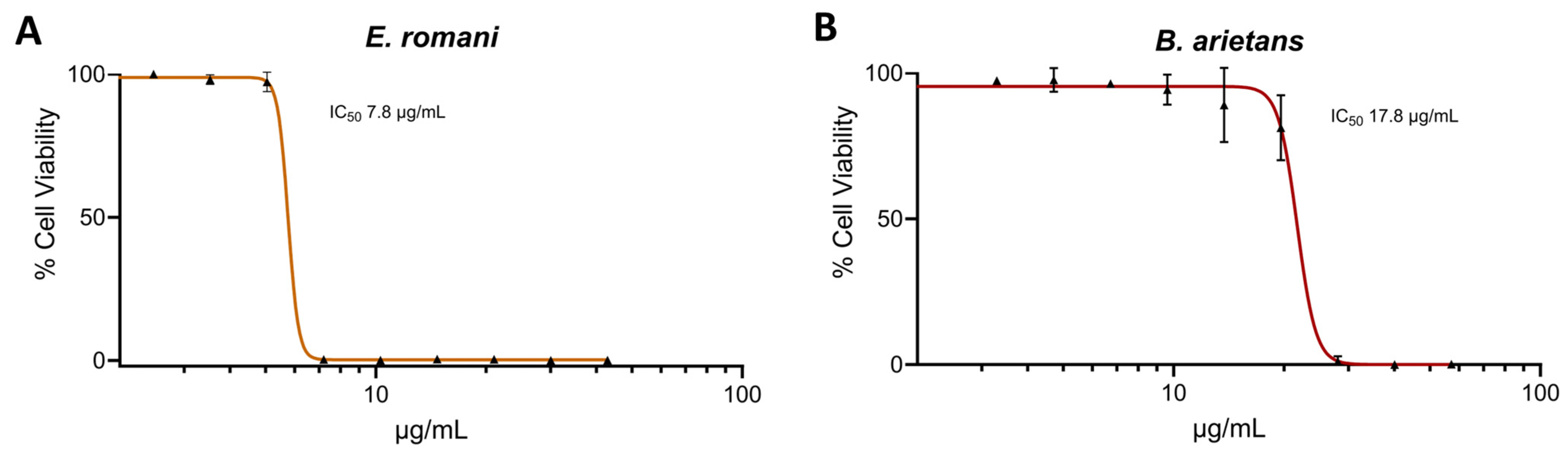
2.1. Saw-Scaled Viper and Puff Adder Venoms Cause Cell Cytotoxicity in Human Epidermal Keratinocytes
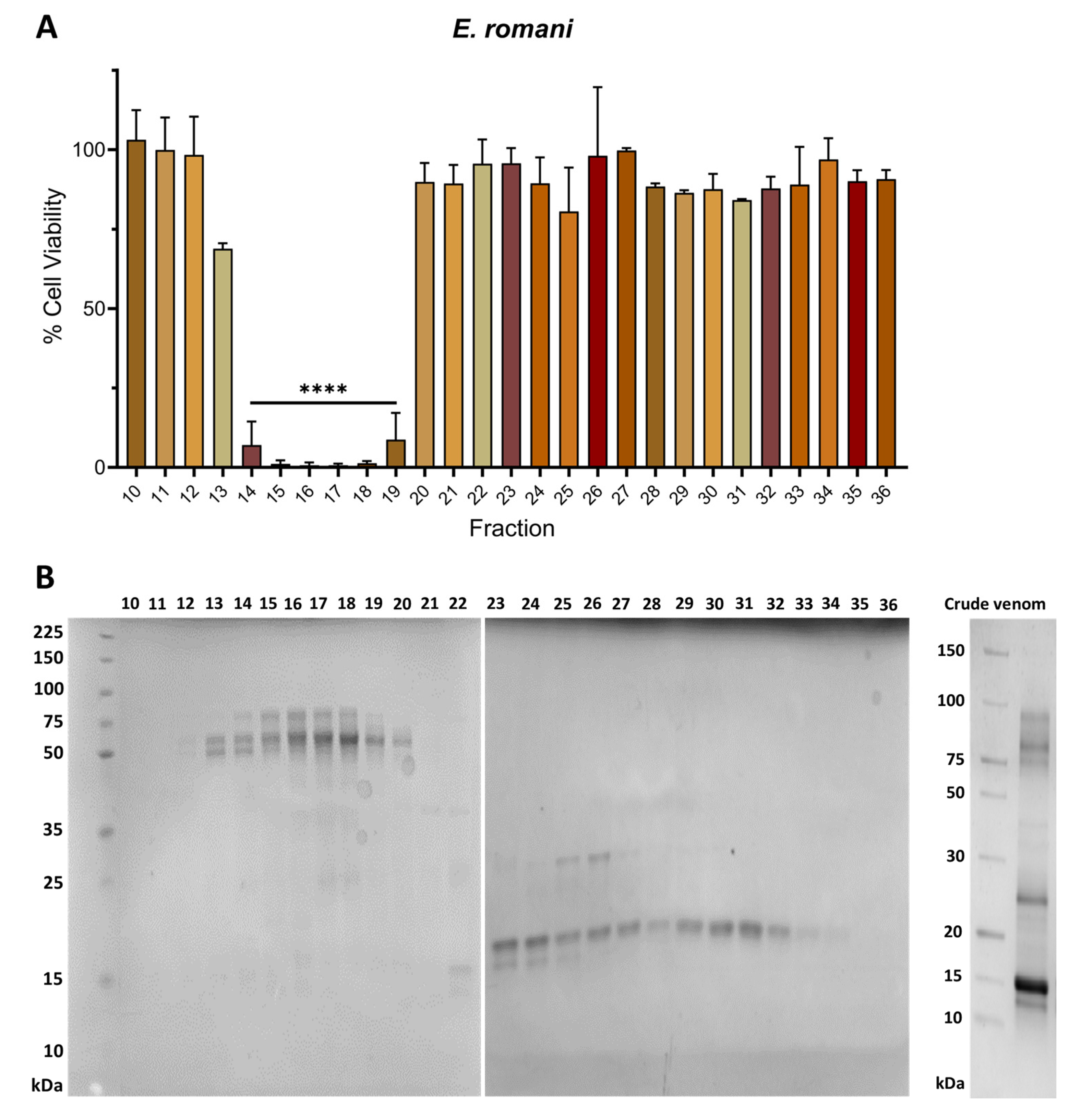
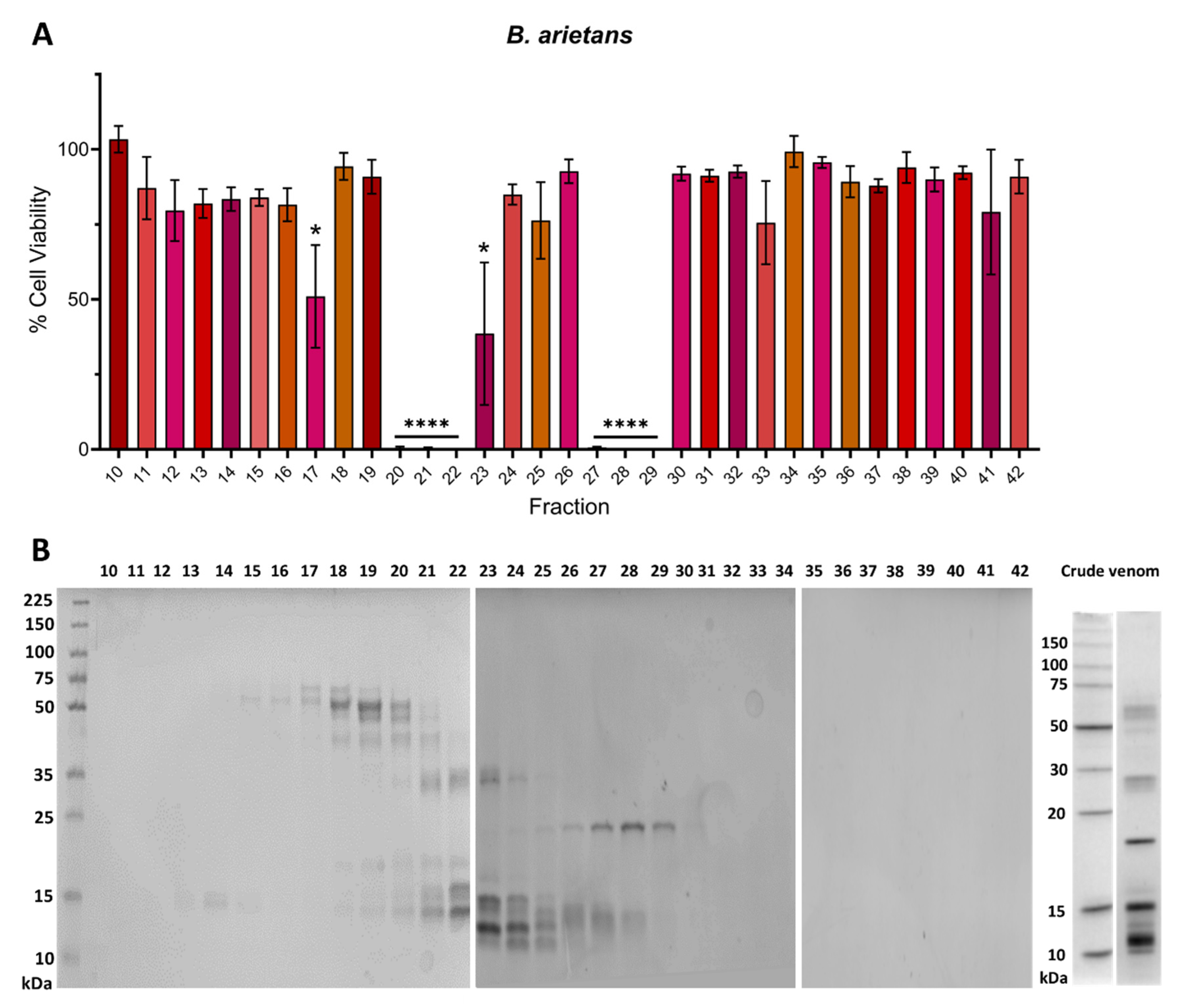
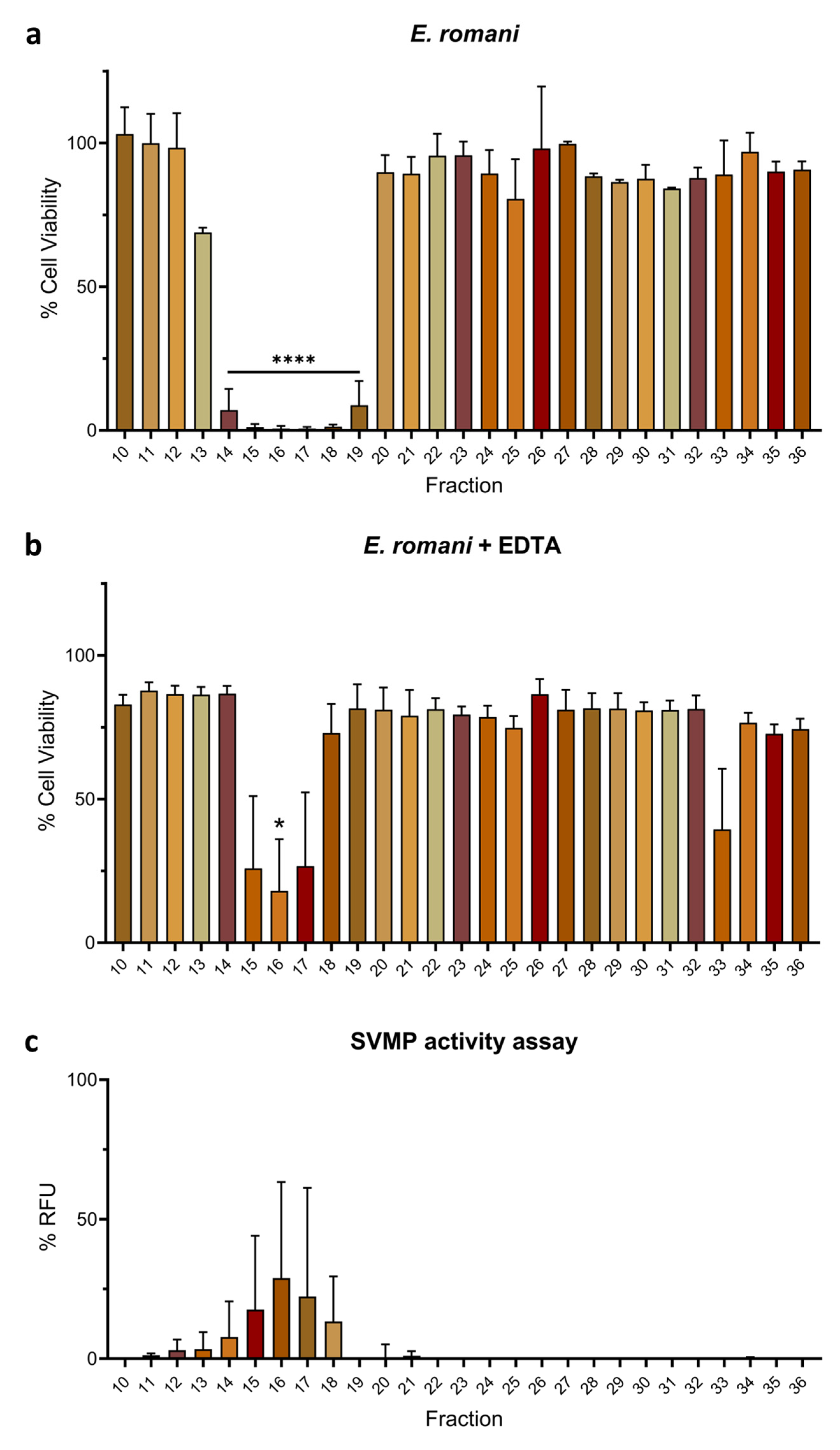
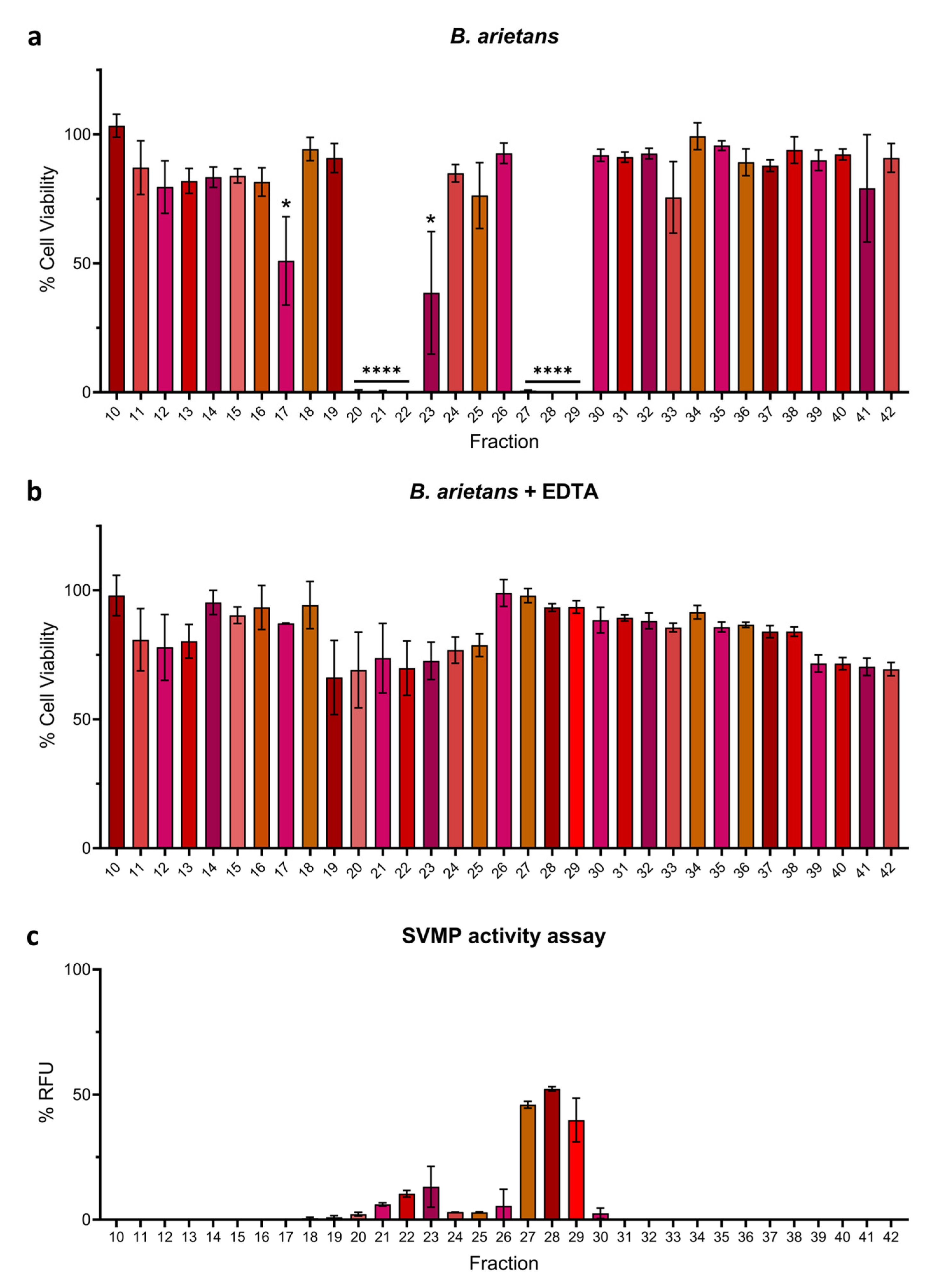
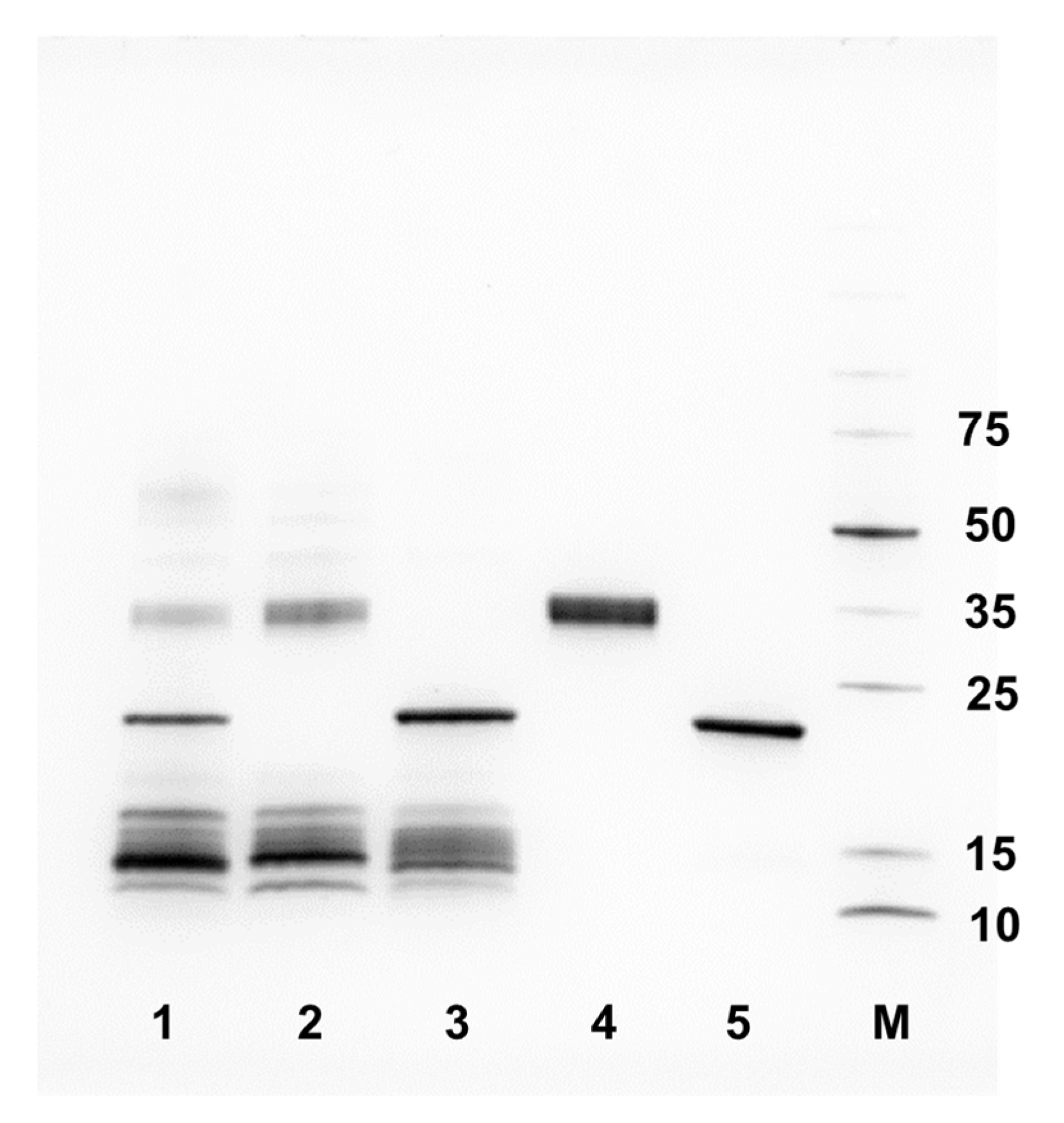
2.2. Venom Fractions Responsible for Causing Cell Cytotoxicity Are Rich in SVMP Toxins
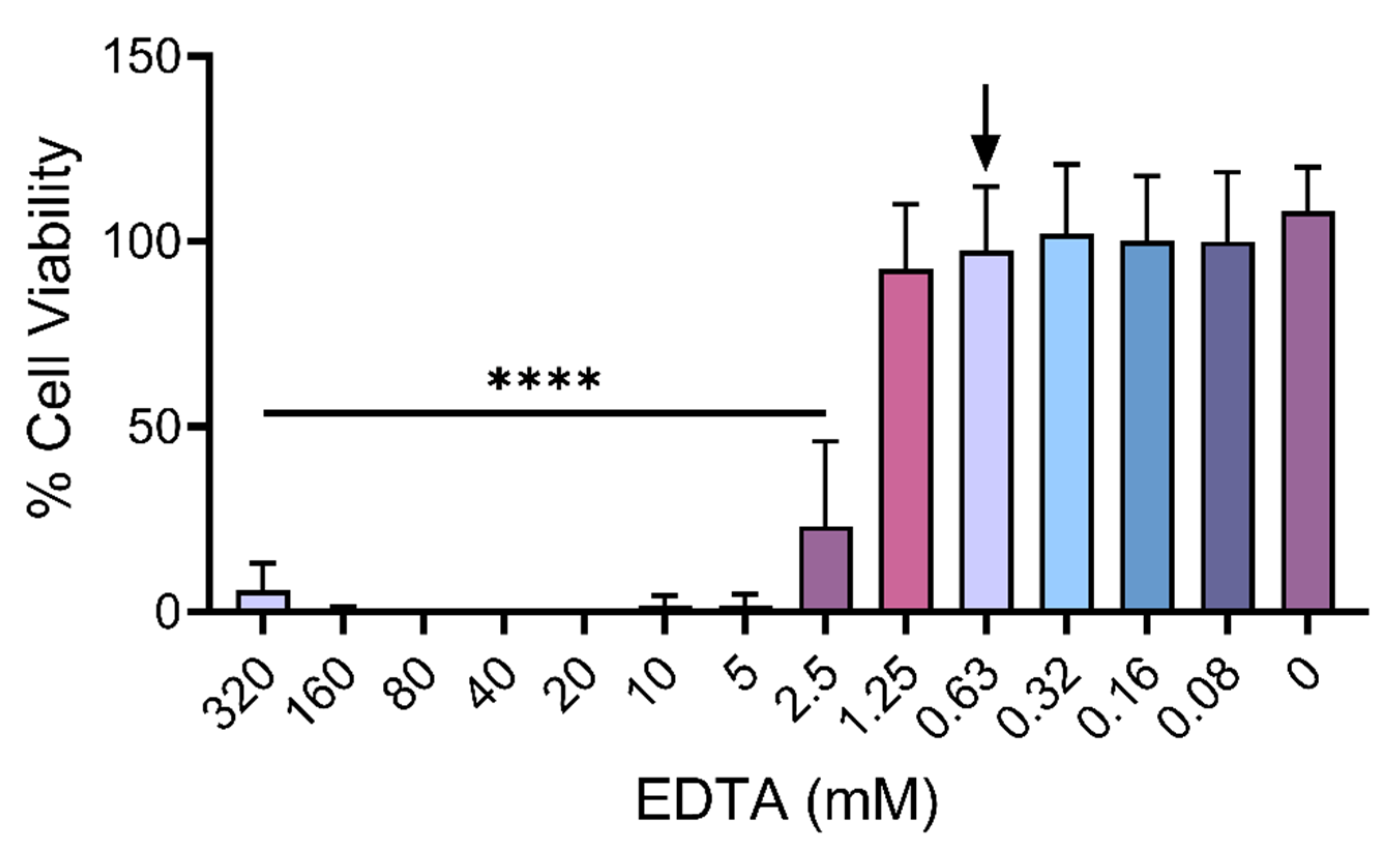
2.3. Metalloproteinase Inhibitor EDTA Significantly Reduces Cytotoxic Effects of Venom Fractions
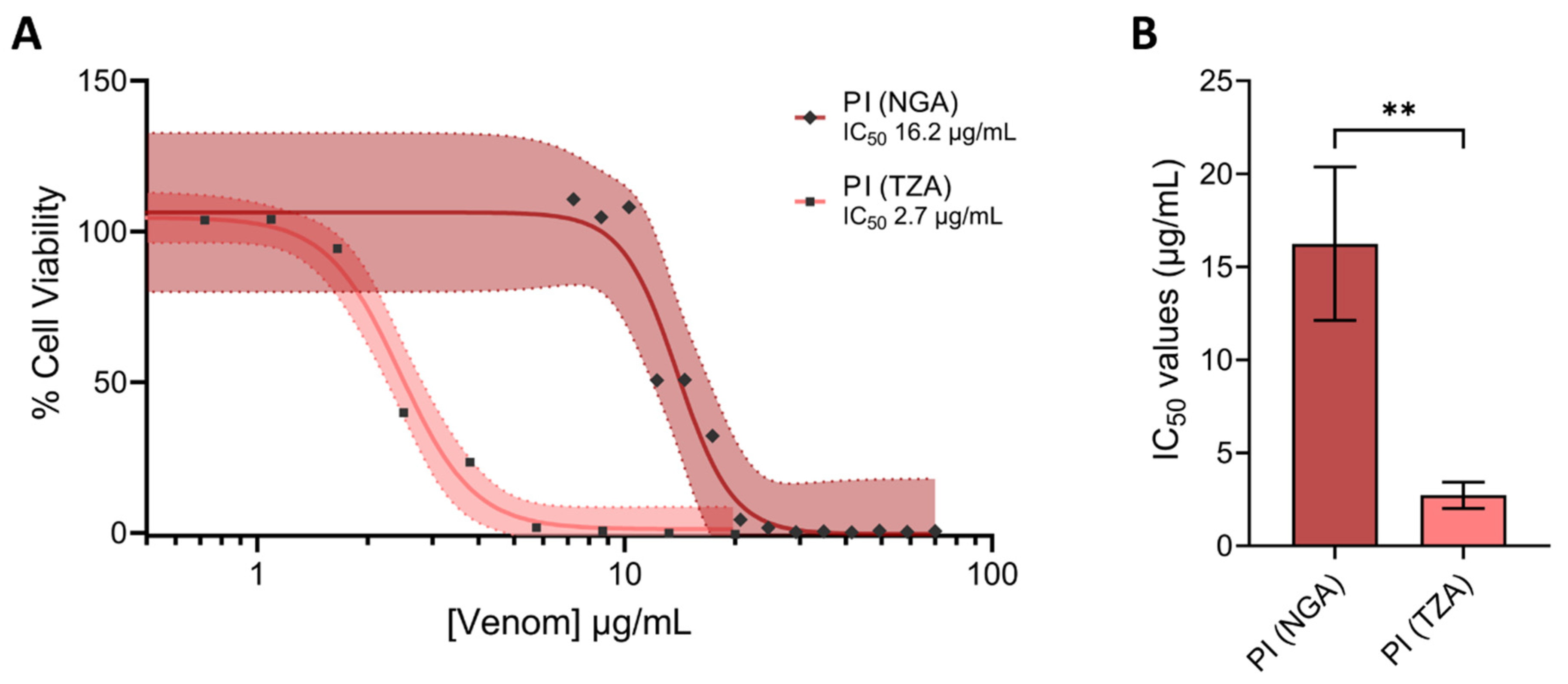
2.4. PI SVMPs from Puff Adders of Differing Geographical Origin Exhibit Substantial Differences in Cell Cytotoxicity
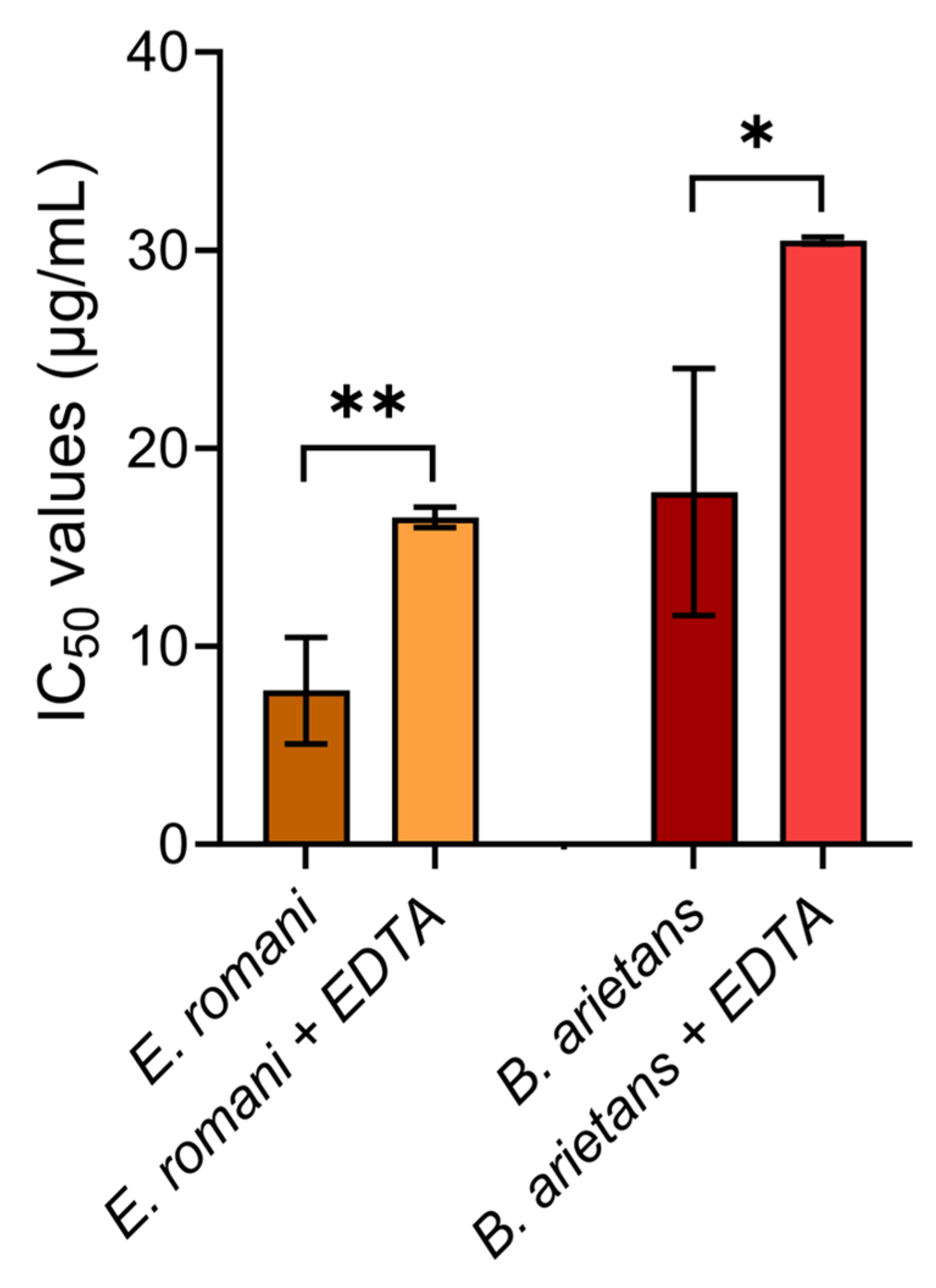
2.5. Metalloproteinase Inhibitor EDTA Significantly Reduces Cytotoxic Effects of Whole Venoms
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Snake Venoms
4.3. Venom Toxin Fractionation and Isolation
4.4. Cell Culture
4.5. MTT Measures of Cell Metabolic Activity
4.6. Inhibition of Cytotoxic Effects by EDTA
4.7. SDS-PAGE Gel Electrophoresis
4.8. Quantifying Snake Venom Metalloproteinase Activity
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Snakebite Envenoming: A Strategy for Prevention and Control. Available online: https://www.who.int/publications-detail/9789241515641 (accessed on 13 November 2024).
- Ralph, R.; Sharma, S.K.; Faiz, M.A.; Ribeiro, I.; Rijal, S.; Chappuis, F.; Kuch, U. The Timing Is Right to End Snakebite Deaths in South Asia. BMJ 2019, 364, k5317. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Johansen, K.H.; Engmark, M.; Andersen, M.R. Recombinant Snakebite Antivenoms: A Cost-Competitive Solution to a Neglected Tropical Disease? PLoS Neglected Trop. Dis. 2017, 11, e0005361. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Pucca, M.B.; Jürgensen, J.A.; Janke, R.; Ledsgaard, L.; Schoof, E.M.; Sørensen, C.V.; Çalışkan, F.; Laustsen, A.H. An In Vitro Methodology for Discovering Broadly-Neutralizing Monoclonal Antibodies. Sci. Rep. 2020, 10, 10765. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, L.O.; Hale, M.S.; Ainsworth, S.; Alsolaiss, J.; Crittenden, E.; Calvete, J.J.; Evans, C.; Wilkinson, M.C.; Harrison, R.A.; Kool, J.; et al. Preclinical Validation of a Repurposed Metal Chelator as an Early-Intervention Therapeutic for Hemotoxic Snakebite. Sci. Transl. Med. 2020, 12, eaay8314. [Google Scholar] [CrossRef]
- Lewin, M.R.; Carter, R.W.; Matteo, I.A.; Samuel, S.P.; Rao, S.; Fry, B.G.; Bickler, P.E. Varespladib in the Treatment of Snakebite Envenoming: Development History and Preclinical Evidence Supporting Advancement to Clinical Trials in Patients Bitten by Venomous Snakes. Toxins 2022, 14, 783. [Google Scholar] [CrossRef]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutiérrez, J.M. Toxicovenomics and Antivenom Profiling of the Eastern Green Mamba Snake (Dendroaspis angusticeps). J. Proteom. 2016, 136, 248–261. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Arrahman, A.; Xie, C.; Casewell, N.R.; Lewis, R.J.; Kool, J.; Cardoso, F.C. Multifunctional Toxins in Snake Venoms and Therapeutic Implications: From Pain to Hemorrhage and Necrosis. Front. Ecol. Evol. 2019, 7, 218. [Google Scholar] [CrossRef]
- Liu, C.C.; Chou, Y.S.; Chen, C.Y.; Liu, K.L.; Huang, G.J.; Yu, J.S.; Wu, C.J.; Liaw, G.W.; Hsieh, C.H.; Chen, C. Pathogenesis of Local Necrosis Induced by Naja atra Venom: Assessment of the Neutralization Ability of Taiwanese Freeze-Dried Neurotoxic Antivenom in Animal Models. PLoS Neglected Trop. Dis. 2020, 14, e0008054. [Google Scholar] [CrossRef]
- Chippaux, J.P. Estimate of the Burden of Snakebites in Sub-Saharan Africa: A Meta-Analytic Approach. Toxicon 2011, 57, 586–599. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for the Prevention and Clinical Management of Snakebite in Africa; World Health Organization: Geneva, The Switzerland, 2010.
- Megale, A.A.A.; Portaro, F.C.; Da Silva, W.D. Bitis arietans Snake Venom Induces an Inflammatory Response Which Is Partially Dependent on Lipid Mediators. Toxins 2020, 12, 594. [Google Scholar] [CrossRef]
- Pook, C.E.; Joger, U.; Stümpel, N.; Wüster, W. When Continents Collide: Phylogeny, Historical Biogeography and Systematics of the Medically Important Viper Genus Echis (Squamata: Serpentes: Viperidae). Mol. Phylogenet. Evol. 2009, 53, 792–807. [Google Scholar] [CrossRef] [PubMed]
- Trape, J.F. Partition d’Echis ocellatus Stemmler, 1970 (Squamata, Viperidae), Avec La Description d’une Espèce Nouvelle. Bull. Société Herpétologique Fr. 2018, 167, 13–34. [Google Scholar]
- Habib, A.G. Venomous Snakes and Snake Envenomation in Nigeria. In Clinical Toxinology in Asia Pacific and Africa; Gopalakrishnakone, P., Faiz, A., Fernando, R., Gnanathasan, C.A., Habib, A.G., Yang, C., Eds.; Toxinology; Springer: Dordrecht, The Netherlands, 2015; Volume 2, pp. 275–298. [Google Scholar]
- Habib, A.G. Public Health Aspects of Snakebite Care in West Africa: Perspectives from Nigeria. J. Venom. Anim. Toxins Trop. Dis. 2013, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, S.C.; Sanz, L.; Juarez, P.; Harrison, R.A.; Calvete, J.J. Combined Snake Venomics and Venom Gland Transcriptomic Analysis of the Ocellated Carpet Viper, Echis ocellatus. J. Proteom. 2009, 71, 609–623. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wagstaff, S.C.; Wüster, W.; Cook, D.A.N.; Bolton, F.M.S.; King, S.I.; Pla, D. Medically Important Differences in Snake Venom Composition Are Dictated by Distinct Postgenomic Mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 9205–9210. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Dingwoke, E.J.; Adamude, F.A.; Mohamed, G.; Klein, A.; Salihu, A.; Abubakar, M.S.; Sallau, A.B. Venom Proteomic Analysis of Medically Important Nigerian Viper Echis ocellatus and Bitis arietans Snake Species. Biochem. Biophys. Rep. 2021, 28, 101164. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.A.; Bartlett, K.E.; Wilkinson, M.C.; Ainsworth, S.; Albulescu, L.O.; Kazandjian, T.D.; Hall, S.R.; Westhorpe, A.P.; Clare, R.H.; Wagstaff, S.C.; et al. Intraspecific Venom Variation in the Medically Important Puff Adder (Bitis arietans): Comparative Venom Gland Transcriptomics, In Vitro Venom Activity and Immunological Recognition by Antivenom. PLoS Neglected Trop. Dis. 2024, 18, e0012570. [Google Scholar] [CrossRef]
- Olaoba, O.T.; dos Santos, P.K.; Selistre-de-Araujo, H.S.; de Souza, D.H.F. Snake Venom Metalloproteinases (SVMPs): A Structure-Function Update. Toxicon X 2020, 7, 100052. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. The Myth of Cobra Venom Cytotoxin: More than Just Direct Cytolytic Actions. Toxicon X 2022, 14, 100123. [Google Scholar] [CrossRef]
- Escalante, T.; Rucavado, A.; Pinto, A.F.M.; Terra, R.M.S.; Gutiérrez, J.M.; Fox, J.W. Wound Exudate as a Proteomic Window to Reveal Different Mechanisms of Tissue Damage by Snake Venom Toxins. J. Proteome Res. 2009, 8, 5120–5131. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A Comprehensive View of the Structural and Functional Alterations of Extracellular Matrix by Snake Venom Metalloproteinases (SVMPs): Novel Perspectives on the Pathophysiology of Envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef]
- Slagboom, J.; Kool, J.; Harrison, R.A.; Casewell, N.R. Haemotoxic Snake Venoms: Their Functional Activity, Impact on Snakebite Victims and Pharmaceutical Promise. Br. J. Haematol. 2017, 177, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M.T. Insights into and Speculations about Snake Venom Metalloproteinase (SVMP) Synthesis, Folding and Disulfide Bond Formation and Their Contribution to Venom Complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef] [PubMed]
- Freitas-de-Sousa, L.A.; Colombini, M.; Lopes-Ferreira, M.; Serrano, S.M.T.; Moura-da-Silva, A.M. Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase. Toxins 2017, 9, 239. [Google Scholar] [CrossRef]
- Moretto Del-Rei, T.H.; Sousa, L.F.; Rocha, M.M.T.; Freitas-de-Sousa, L.A.; Travaglia-Cardoso, S.R.; Grego, K.; Sant’Anna, S.S.; Chalkidis, H.M.; Moura-da-Silva, A.M. Functional Variability of Bothrops atrox Venoms from Three Distinct Areas across the Brazilian Amazon and Consequences for Human Envenomings. Toxicon 2019, 164, 61–70. [Google Scholar] [CrossRef]
- Khan, S.A.; Ilies, M.A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef]
- Lomonte, B.; Angulo, Y.; Rufini, S.; Cho, W.; Giglio, J.R.; Ohno, M.; Daniele, J.J.; Geoghegan, P.; Gutiérrez, J.M. Comparative Study of the Cytolytic Activity of Myotoxic Phospholipases A2 on Mouse Endothelial (tEnd) and Skeletal Muscle (C2C12) Cells In Vitro. Toxicon 1999, 37, 145–158. [Google Scholar] [CrossRef]
- Gasanov, S.E.; Dagda, R.K.; Rael, E.D. Snake Venom Cytotoxins, Phospholipase A2s, and Zn2+-Dependent Metalloproteinases: Mechanisms of Action and Pharmacological Relevance. J. Clin. Toxicol. 2014, 4, 1000181. [Google Scholar] [CrossRef]
- Araya, C.; Lomonte, B. Antitumor Effects of Cationic Synthetic Peptides Derived from Lys49 Phospholipase A2 Homologues of Snake Venoms. Cell Biol. Int. 2007, 31, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bryan-Quiros, W.; Fernandez, J.; Gutierrez, J.M.; Lewin, M.R.; Lomonte, B. Neutralizing Properties of LY315920 toward Snake Venom Group I and II Myotoxic Phospholipases A2. Toxicon 2019, 157, 1–7. [Google Scholar] [CrossRef]
- Ghazaryan, N.A.; Ghulikyan, L.; Kishmiryan, A.; Andreeva, T.V.; Utkin, Y.N.; Tsetlin, V.I.; Lomonte, B.; Ayvazyan, N.M. Phospholipases A2 from Viperidae Snakes: Differences in Membranotropic Activity between Enzymatically Active Toxin and Its Inactive Isoforms. Biochim. Biophys. Acta 2015, 1848, 463–468. [Google Scholar] [CrossRef]
- Fernández, J.; Gutiérrez, J.M.; Angulo, Y.; Sanz, L.; Juárez, P.; Calvete, J.J.; Lomonte, B. Isolation of an Acidic Phospholipase A2 from the Venom of the Snake Bothrops Asper of Costa Rica: Biochemical and Toxicological Characterization. Biochimie 2010, 92, 273–283. [Google Scholar] [CrossRef]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic Toxins from Snake Venom: Structural Characterization and Mechanism of Catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Costal-Oliveira, F.; Stransky, S.; Guerra-Duarte, C.; Naves de Souza, D.L.; Vivas-Ruiz, D.E.; Yarlequé, A.; Sanchez, E.F.; Chávez-Olórtegui, C.; Braga, V.M.M. L-Amino Acid Oxidase from Bothrops atrox Snake Venom Triggers Autophagy, Apoptosis and Necrosis in Normal Human Keratinocytes. Sci. Rep. 2019, 9, 781. [Google Scholar] [CrossRef]
- Abdelkafi-Koubaa, Z.; Elbini-Dhouib, I.; Souid, S.; Jebali, J.; Doghri, R.; Srairi-Abid, N.; Essafi-Benkhadir, K.; Micheau, O.; Marrakchi, N. Pharmacological Investigation of CC-LAAO, an L-Amino Acid Oxidase from Cerastes cerastes Snake Venom. Toxins 2021, 13, 904. [Google Scholar] [CrossRef]
- Bartlett, K.E.; Hall, S.R.; Rasmussen, S.A.; Crittenden, E.; Dawson, C.A.; Albulescu, L.O.; Laprade, W.; Harrison, R.A.; Saviola, A.J.; Modahl, C.M.; et al. Dermonecrosis Caused by Spitting Cobra Snakebite Is the Result of Toxin Potentiation and Is Prevented by the Repurposed Drug Varespladib. Proc. Natl. Acad. Sci. USA 2024, 121, e2315597121. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.R.; Rasmussen, S.A.; Crittenden, E.; Dawson, C.A.; Bartlett, K.E.; Westhorpe, A.P.; Albulescu, L.; Kool, J.; Gutiérrez, J.M.; Casewell, N.R. Repurposed Drugs and Their Combinations Prevent Morbidity-Inducing Dermonecrosis Caused by Diverse Cytotoxic Snake Venoms. Nat. Commun. 2023, 14, 7812. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In Vitro Cytotoxicity Assays: Comparison of LDH, Neutral Red, MTT and Protein Assay in Hepatoma Cell Lines Following Exposure to Cadmium Chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.C.; Modahl, C.; Saviola, A.; Albulesco, L.O.; Tianyi, F.L.; Harrison, R.A.; Casewell, N.R. Complex Compositional and Functional Diversity of Venom Metalloproteinases in African Puff Adders [Preprint]. BioRxiv 2025. Available online: https://www.biorxiv.org/content/10.1101/2024.05.31.596867v3 (accessed on 17 June 2024).
- Wilkinson, M.C.; Modahl, C.; Saviola, A.; Albulesco, L.O.; Tianyi, F.L.; Casewell, N.R. Isolation and Characterisation of Serine Proteases and Metalloproteases from the Venom of African Puff Adders [Preprint]. BioRxiv 2024. Available online: https://www.biorxiv.org/content/10.1101/2024.05.31.596867v2?versioned=true (accessed on 17 June 2024).
- Ainsworth, S.; Slagboom, J.; Alomran, N.; Pla, D.; Alhamdi, Y.; King, S.I.; Bolton, F.M.S. The Paraspecific Neutralisation of Snake Venom Induced Coagulopathy by Antivenoms. Commun. Biol. 2018, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gutiérrez, J.M.; Ovadia, M. Inhibition of the Hemorrhagic Activity of Bothrops asper Venom by a Novel Neutralizing Mixture. Toxicon 1997, 35, 865–877. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Chaves, F.; Rucavado, A.; León, G.; Franceschi, A.; Ovadia, M.; Escalante, T.; Cury, Y. Inhibition of Local Hemorrhage and Dermonecrosis Induced by Bothrops asper Snake Venom: Effectiveness of Early in Situ Administration of the Peptidomimetic Metalloproteinase Inhibitor Batimastat and the Chelating Agent CaNa2EDTA. Am. J. Trop. Med. Hyg. 2000, 63, 313–319. [Google Scholar] [CrossRef]
- Howes, J.M.; Theakston, R.D.G.; Laing, G.D. Neutralization of the Haemorrhagic Activities of Viperine Snake Venoms and Venom Metalloproteinases Using Synthetic Peptide Inhibitors and Chelators. Toxicon 2007, 49, 734–739. [Google Scholar] [CrossRef]
- Menzies, S.K.; Clare, R.H.; Xie, C.; Westhorpe, A.; Hall, S.R.; Edge, R.J.; Alsolaiss, J.; Crittenden, E.; Marriott, A.E.; Harrison, R.A.; et al. In Vitro and in Vivo Preclinical Venom Inhibition Assays Identify Metalloproteinase Inhibiting Drugs as Potential Future Treatments for Snakebite Envenoming by Dispholidus typus. Toxicon X 2022, 14, 100118. [Google Scholar] [CrossRef]
- Currier, R.B.; Harrison, R.A.; Rowley, P.D.; Laing, G.D.; Wagstaff, S.C. Intra-Specific Variation in Venom of the African Puff Adder (Bitis arietans): Differential Expression and Activity of Snake Venom Metalloproteinases (SVMPs). Toxicon Off. J. Int. Soc. Toxinology 2010, 55, 864–873. [Google Scholar] [CrossRef]
- Warrell, D.A.; Williams, D.J. Clinical Aspects of Snakebite Envenoming and Its Treatment in Low-Resource Settings. Lancet 2023, 401, 1382–1398. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Casewell, N.R.; Laustsen, A.H. Progress and Challenges in the Field of Snakebite Envenoming Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2024, 65, 465–485. [Google Scholar] [CrossRef]
- Warrell, D.A.; Davidson, N.M.; Greenwood, B.M.; Ormerod, L.D.; Pope, H.M.; Watkins, B.J.; Prentice, C.R.M. Poisoning by Bites of the Saw-Scaled or Carpet Viper (Echis carinatus) in Nigeria. QJM Int. J. Med. 1977, 46, 33–62. [Google Scholar] [CrossRef]
- Fernandez, S.; Hodgson, W.; Chaisakul, J.; Kornhauser, R.; Konstantakopoulos, N.; Smith, A.I.; Kuruppu, S. In Vitro Toxic Effects of Puff Adder (Bitis arietans) Venom, and Their Neutralization by Antivenom. Toxins 2014, 6, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Coto, J.; Segura, Á.; Vargas, M.; Solano, G.; Herrera, M.; Villalta, M.; Estrada, R.; Gutiérrez, J.M.; León, G. Effect of Geographical Variation of Echis ocellatus, Naja nigricollis and Bitis arietans Venoms on Their Neutralization by Homologous and Heterologous Antivenoms. Toxicon 2015, 108, 80–83. [Google Scholar] [CrossRef]
- Bhatia, S.; Vasudevan, K. Comparative Proteomics of Geographically Distinct Saw-Scaled Viper (Echis carinatus) Venoms from India. Toxicon X 2020, 7, 100048. [Google Scholar] [CrossRef] [PubMed]
- Senji, L.R.R.; Attarde, S.; Khochare, S.; Suranse, V.; Martin, G.; Casewell, N.R.; Whitaker, R.; Sunagar, K. Biogeographical Venom Variation in the Indian Spectacled Cobra (Naja naja) Underscores the Pressing Need for Pan-India Efficacious Snakebite Therapy. PLoS Neglected Trop. Dis. 2021, 15, e0009150. [Google Scholar] [CrossRef]
- Mora-Obando, D.; Salazar-Valenzuela, D.; Pla, D.; Lomonte, B.; Guerrero-Vargas, J.A.; Ayerbe, S.; Gibbs, H.L.; Calvete, J.J. Venom Variation in Bothrops asper Lineages from North-Western South America. J. Proteom. 2020, 229, 103945. [Google Scholar] [CrossRef]
- Silva-Júnior, L.N.; Abreu, L.D.S.; Rodrigues, C.F.B.; Galizio, N.D.C.; Aguiar, W.D.S.; Serino-Silva, C.; Santos, V.S.D.; Costa, I.A.; Oliveira, L.V.F.; Sant’Anna, S.S.; et al. Geographic Variation of Individual Venom Profile of Crotalus durissus Snakes. J. Venom. Anim. Toxins Trop. Dis. 2020, 26, e20200016. [Google Scholar] [CrossRef]
- Smith, C.F.; Nikolakis, Z.L.; Ivey, K.; Perry, B.W.; Schield, D.R.; Balchan, N.R.; Parker, J.; Hansen, K.C.; Saviola, A.J.; Castoe, T.A.; et al. Snakes on a Plain: Biotic and Abiotic Factors Determine Venom Compositional Variation in a Wide-Ranging Generalist Rattlesnake. BMC Biol. 2023, 21, 136. [Google Scholar] [CrossRef]
- Tianyi, F.L.; Ngari, C.; Wilkinson, M.C.; Parkurito, S.; Chebet, E.; Mumo, E.; Trelfa, A.; Otundo, D.; Crittenden, E.; Kephah, G.M.; et al. Clinical Features of Puff Adder Envenoming: Case Series of Bitis arietans Snakebites in Kenya and a Scoping Review of the Literature. PLoS Neglected Trop. Dis. 2025, 19, e0012845. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Mackessy, S.P. Functional Basis of a Molecular Adaptation: Prey-Specific Toxic Effects of Venom from Sistrurus Rattlesnakes. Toxicon 2009, 53, 672–679. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and Snake Venom Evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Zancolli, G.; Baker, T.; Barlow, A.; Bradley, R.; Calvete, J.; Carter, K.; De Jager, K.; Owens, J.; Price, J.; Sanz, L.; et al. Is Hybridization a Source of Adaptive Venom Variation in Rattlesnakes? A Test, Using a Crotalus scutulatus × Viridis Hybrid Zone in Southwestern New Mexico. Toxins 2016, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.G. Growth and Differentiation of HaCaT Keratinocytes. In Epidermal Cells: Methods and Protocols; Springer: New York, NY, USA, 2014; Volume 1195. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartlett, K.E.; Westhorpe, A.; Wilkinson, M.C.; Casewell, N.R. Snake Venom Metalloproteinases from Puff Adder and Saw-Scaled Viper Venoms Cause Cytotoxic Effects in Human Keratinocytes. Toxins 2025, 17, 328. https://doi.org/10.3390/toxins17070328
Bartlett KE, Westhorpe A, Wilkinson MC, Casewell NR. Snake Venom Metalloproteinases from Puff Adder and Saw-Scaled Viper Venoms Cause Cytotoxic Effects in Human Keratinocytes. Toxins. 2025; 17(7):328. https://doi.org/10.3390/toxins17070328
Chicago/Turabian StyleBartlett, Keirah E., Adam Westhorpe, Mark C. Wilkinson, and Nicholas R. Casewell. 2025. "Snake Venom Metalloproteinases from Puff Adder and Saw-Scaled Viper Venoms Cause Cytotoxic Effects in Human Keratinocytes" Toxins 17, no. 7: 328. https://doi.org/10.3390/toxins17070328
APA StyleBartlett, K. E., Westhorpe, A., Wilkinson, M. C., & Casewell, N. R. (2025). Snake Venom Metalloproteinases from Puff Adder and Saw-Scaled Viper Venoms Cause Cytotoxic Effects in Human Keratinocytes. Toxins, 17(7), 328. https://doi.org/10.3390/toxins17070328




